Abstract
Previous studies have shown that the reduction in CD8 T cell immunity observed during high-dose influenza virus infection (IAV) is mediated via lymph node (LN) dendritic cells (DC) that express FasL and drive FasL:Fas (DC:T) induced apoptosis. However, the specific DC subset(s) within the LN and the additional factors required for DC-mediated elimination of IAV-specific CD8 T cells remain unknown. Herein, we demonstrate that plasmacytoid DC (pDC) which downregulate FasL during sublethal, but not lethal, IAV-infection accumulate to greater numbers within the LN of lethal dose infected mice. Further our findings show that pDC from lethal, but not sublethal dose IAV infections, drive elimination of Fas+ CD8 T cells and that this elimination only occurs in the absence of TCR recognition of IAV-peptide-MHC class I complexes. Together these results suggest that pDC play a heretofore unknown deleterious role during lethal dose IAV-infections by limiting the CD8 T cell response.
Introduction
Clearance of primary influenza virus (IAV) infections is greatly enhanced by the generation of an effector CD8 T cell response (1–3). Initially, these CD8 T cells are primed by DC within lung draining lymph nodes (LN) (4–7) and then subsequently traffic to the lung where they eliminate virally infected cells via effector mechanisms including; perforin, FasL and tumor necrosis factor related apoptosis inducing ligand (TRAIL) (2, 8). Previously, we have demonstrated a novel regulatory mechanism whereby lymph node resident dendritic cells (LNDC) directly mediate apoptosis of IAV-specific CD8 T cells within the LN during lethal, but not sublethal, IAV infections (9). This loss of IAV-specific CD8 T cells leads to decreased numbers of CTLs that enter the lungs, resulting in a failure to clear the infection and ultimately the death of the host. Such elimination of Fas+ IAV-specific CD8 T cells occurs subsequent to the initial priming of CD8 T cells on days three and four post-infection (p.i.). In contrast to lethal dose IAV infections, during sublethal infections LNDC downregulate FasL expression allowing the developing Fas+ IAV-specific CD8 T cells to escape apoptosis and traffic into the lungs in sufficient numbers to clear the infection (9).
At least six distinct populations of DC have been described within the lung-draining LN. There are four LN resident DC subsets that can be identified phenotypically as CD4+ DC, CD8α+ DC and CD4−CD8α− DC, also known as double negative (DN) DC (10–12). Furthermore, in response to infection plasmacytoid DC (pDC) are recruited into the LN from the blood (13, 14). In addition to these LN resident DC, there are also at least two respiratory DC (rDC) populations that migrate from the lung into the draining LN during infections (4–7). Once they enter the LN these migratory rDC are thought to share Ag with LNDC, particularly with CD8α+ DC, allowing this LNDC subset to also participate in activation of naïve CD8 T cells (7, 15). Interestingly, although Ag may be shared with all LNDC subsets, not all LNDC subsets are able to present IAV antigens to naïve CD8 T cells as pDC purified from the LN of IAV infected mice are unable to activate CD8 T cells directly ex vivo (4, 5, 7, 15). In support of the idea that pDC do not participate in the activation of naïve CD8 T cells, when pDC are depleted in vivo during sublethal IAV infections there is no diminution of the CD8 T cell response (5, 16). However, in contrast when pDC are IAV-infected or pulsed with IAV peptides in vitro they are now able to activate naïve CD8 T cells (17, 18). Together these results suggest that while inherently capable of presenting viral antigens to naïve CD8 T cells, pDC may not present IAV-antigens via MHC class I in the LN due to inefficient processing of acquired viral proteins in vivo (5, 16).
While our previous work has demonstrated that LNDC eliminate IAV-specific CD8 T cells through a FasL-mediated pathway during lethal dose infections, it did not pinpoint the individual DC subset(s) involved, nor what role MHC class I presentation of viral antigens by these DC subsets might play in the LNDC FasL:Fas-mediated elimination of IAV-specific CD8 T cells. This report demonstrates that whereas all LN resident DC subsets were FasL+ during lethal dose IAV infection, only pDC were able to eliminate activated IAV-specific CD8 T cells. In contrast, pDC isolated from sublethal dose IAV infected mice downregulated FasL and were therefore unable to eliminate activated Fas+ CD8 T cells. Interestingly, our findings also demonstrate that the recruitment of pDC into the lung draining LN was increased during lethal versus sublethal dose IAV infections elevating the putative in vivo E:T ratio (i.e. pDC:T cell) and that transfer of FasL+ pDC into mice deficient in functional FasL (i.e. gld mice) was sufficient to reverse the previously described protection of gld mice from lethal dose IAV infections (9). Finally, this report demonstrates that lethal dose pDC elimination of IAV-specific CD8 T cells occurs in the absence of direct viral-peptide MHC class I presentation. Taken together these data suggest that pDC are the cell type responsible for dampening the CD8 T cell response during lethal IAV infection in vivo and that such elimination occurs in the absence of cognate IAV antigen presentation.
Materials and Methods
Mice
Wild-type BALB/c mice were purchased from the National Cancer Institute (Frederick, MD). BALB/c CD90.1 congenic mice were kind gifts from Dr. Richard Enelow (Dartmouth College, Hanover, NH) and Dr. John T. Harty (University of Iowa, Iowa City, IA). Clone-4 (CL-4) TCR transgenic mice specific for the HA533/HA529 epitope of H1 and H2 IAV viruses, respectively were a kind gift from Dr. Linda Sherman (Scripps Research Institute, La Jolla, CA). BALB/c gld mice (CPt.C3-Tnfsf6gld/J) were obtained from the Jackson Laboratory (Bar Habor, ME). DUC18 TCR transgenic mice specific for mutated tERK136–144 were kindly provided by Dr. Paul Allen (Washington University, St. Louis, MO). All experiments were performed in accordance with federal and institutional guidelines approved by the University of Iowa Animal Care and Use Committee.
Virus Infection
6–10 week old BALB/c mice were anesthetized by halothane or isofluorane and infected i.n. with either a 10LD50 or a 0.1LD50 dose of mouse-adapted A/JAPAN/305/57 in 50µl of Iscoves media. Viruses were grown and stored as previously described (9).
MHC I Tetramers
Tetramers HA204 (H-2K(d)/LYQNVGTYV), HA529 (H-2K(d)/IYATVAGSL), and NP147 (H2K(d)/TYQRTRALV) were obtained from National Institute of Allergy and Infectious Disease MHC Tetramer Core Facility (Atlanta, GA).
Flow Cytometry
LN cells were stained with the following monoclonal antibodies: rat anti-mouse CD8α (53-6.7), hamster anti-mouse CD11c (HL3), rat anti-mouse CD3ε (145-2C11), rat anti-mouse CD4 (CT-CD4), and rat anti-mouse CD43 (S7) purchased from Becton Dickinson; mouse anti-mouse CD90.2 (5a-8), rat anti-mouse CD45R (RA3-6B2), rat anti-mouse DX5, and rat anti-mouse CD19 (6D5) purchased from Caltag (Invtrogen). Anti-FasL CD95L (MFL3) was purchased from eBioscience. For FasL staining, cells were blocked with 1:100 rat serum, 1:100 hamster serum and 1:400 free streptavadin (Molecular Probes) on ice for 25 mins. Cells were then washed twice and stained with 2× biotin-congugated anti-FasL (MFL3) followed by streptavadin-PE purchased from Becton Dickinson. For surface staining, isolated cells (106) were stained with antibody, and then fixed using BD FACS Lysing Solution (BD Biosciences). All flow cytometry data were acquired on a BD FACS Calibur or BD FACS Canto II (BD Immunocytometry Systems) and analyzed using FlowJo software (TreeStar, Ashland, OR).
CD8 CL-4 T cell purification and adoptive transfer
Spleens from CL-4 mice were removed and processed into single-cell suspensions. Cells were then labeled with anti-CD8α Microbeads and purified according to manufacturer’s instructions (Miltenyi Biotec). The purified CD90.2+ CL-4 cells (2 ×106) were then adoptively transferred i.v. into BALB/c CD90.1+ mice. 24 hrs post-transfer the host mice were infected i.n. with a 0.1LD50 of IAV as described above. For isolation of activated CL-4 CD8 T cells lung draining LN from IAV-infected CD90.1-CL-4 transferred host mice were removed on day 3 p.i. and digested as described above. CD8 T cells were enriched using anti-CD8α beads according to manufacturer’s instructions (Miltenyi Biotech). CD8α+ cells were then stained with antibodies to CD90.2 and CD43 and activated CD90.2+CD43+ CL-4 cells were sort purified using a FACS DIVA.
LN Dendritic Cell Purification
Lung draining LN (peribronchiolar and mediastinal) were removed and digested with 4000 units of type IV collagenase (Worthington) and 600 units of DNase 1 (Sigma) in Iscoves media for 10 mins at room temperature. LN were then processed into a single-cell suspension and red blood cells lysed using NH4Cl-Tris. Cells were then stained with anti-CD3ε-PE and anti-CD19-PE mAb followed by anti-PE microbeads according to manufacture’s instructions (Miltenyi Biotech). Labeled cells were isolated using an autoMACS. The negative fraction was saved, stained with antibodies to CD11c, CD45R (B220), CD8α, CD4 and sorted into CD11cmodCD45R+CD8+ cells (i.e. pDC); CD11c+CD45R−CD8+ cells (i.e. CD8α+ DC); CD11c+CD45R−CD8−CD4−cells (i.e. DN DC); and CD11c+CD45R− CD8− CD4+ cells (i.e. CD4+DC) using a FACS DIVA.
Ex vivo LNDC killing assay
Purified LNDC subsets (104) were co-incubated with 104 activated CL-4 cells and 2.5µg of rmFas-human Fc (R&D Systems), 1µM HA529 peptide (Biosyn), or control media for 18 hours at 37°C. To determine viability, cells were resuspended in 1X annexin binding buffer and stained with Annexin-V-APC and 7-AAD (40% as per BD recommendation for 106 cells). The degree of DC killing of T cells was determined by measuring the fraction of CD8 T cells that were non-apoptotic (i.e. Annexin-V− 7-AAD−, live cells) and normalizing this value to CD8 T cells cultured alone.
In vivo pDC transfer studies
Spleens from naïve wild-type or gld mice were removed, and single cell suspensions stained with anti-PDCA-1 microbeads and purified according to manufacturer’s instructions (Miltenyi Biotec). 2 × 106 wild-type or gld pDC were then adoptively transferred i.v. into gld mice 18 hours post lethal IAV infection. Some groups of pDC were also pulsed with 1µM HA529 peptide for 30 mins at 37°C. Mice were monitored daily for weight loss and mortality or on day 4 p.i. lung draining LN were removed to determine IAV-specific T cell responses in the LN.
Statistical Analysis
Statistical analysis between 2 data sets was performed using a one tailed student’s T test. Differences were considered to be statistically significant at p values at or below 0.05. Statistical analysis for mortality experiments was performed using Kaplan-Meier survival analysis.
Results
CD8α+ DC and pDC downregulate FasL expression during lethal dose IAV infections
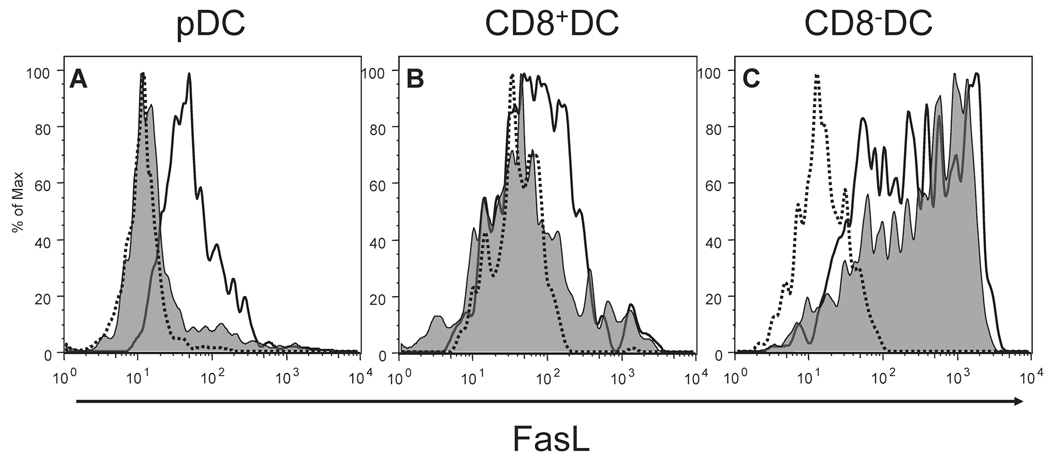
Our previous studies have demonstrated that the reduction in IAV-specific CD8 T cell numbers in lethal dose IAV infections (Fig. S1) is linked to FasL expression on DC within the LN (9). This FasL expression on LNDC decreases during sublethal, compared to lethal IAV infection, therein allowing activated effector IAV-specific CD8 T cells to leave the LN and traffic to the lungs to fight the infection. However the decrease in FasL expression on LNDC during sublethal IAV infections was not uniform with some LNDC maintaining high levels of FasL expression. This finding suggested that a unique LNDC subset(s) may be triggering the elimination of CD8 T cells during lethal dose IAV infection (9). In order to determine which LNDC subset(s) differentially modulate FasL expression during IAV infections, mice were infected with either a lethal or sublethal dose of IAV and the level of FasL on individual LNDC subsets determined on day 3 p.i., the time-point where LNDC-mediated elimination of IAV-specific CD8 T cells begins (9). Interestingly, while both CD8α+ DC and pDC decreased FasL expression during sublethal compared to lethal dose IAV infection, CD8α− DC, including both the CD4+ and DN DC subsets, did not modulate FasL expression between the two IAV infection doses (Fig. 1). Further, the rDC subsets, which have migrated from the lungs to the LN during IAV infection, remained FasL− during both lethal and sublethal IAV infection (data not shown). Given that the pDC and CD8α+ DC subsets are the only LNDC populations to down-modulate FasL expression during sublethal dose IAV infections and IAV-specific CD8 T cell responses are rescued at this dose of infection (Fig. S1) despite the remaining CD8α− DC FasL expression (Fig. S1)(9), it suggests that CD8α− DC are not the cells responsible for the elimination of CD8 T cells during lethal dose IAV infections. Further, these results importantly indicate that either the pDC and/or CD8α+ DC are likely responsible for mediating FasL driven CD8 T cell apoptosis during lethal dose IAV infections.
Figure 1.
pDC and CD8α+ DC modulate FasL expression during IAV infection. Mice were infected with either a 10LD50 (black line) or 0.1LD50 (shaded histogram) of IAV and on day 3 p.i. cells from draining LN (pooled) from each group examined for FasL expression. Dotted line represents staining with an isotype control mAb. (A) FasL expression on CD11cmodCD45R+CD8+ cells (i.e. pDC); 10LD50 M.F.I.=60.5 and 0.1LD50 M.F.I.=28.3. (B) FasL expression on CD11c+CD45R−CD8+ cells (i.e. CD8α+ DC); 10LD50 M.F.I.=139 and 0.1LD50 M.F.I.=118. (C) FasL expression on CD11c+CD45R−CD8− cells (i.e. CD8α− DC); 10LD50 M.F.I.=618 and 0.1LD50 M.F.I.=496.2. The MFI of staining with isotype control mAb has been subtracted from the FasL MFI for the DC subsets to yield the MFI reported above. Data are representative of 5 independent experiments.
pDC accumulate in the lung draining LN in greater numbers during lethal dose IAV infections
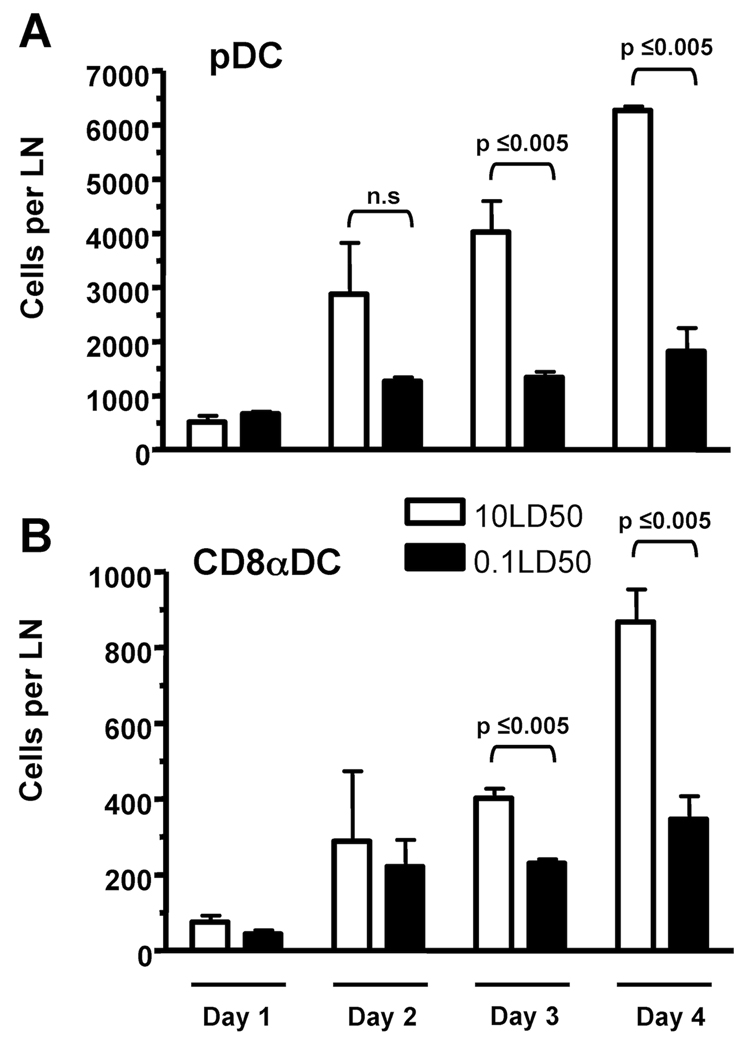
Since our above results suggested the involvement of CD8α+DC and/or pDC in the induction of CD8 T cell apoptosis during lethal dose IAV infections, we next determined the kinetics and magnitude of their recruitment/expansion within the lung draining LN during lethal and sublethal dose IAV infections. Our results show that the number of both pDC and CD8α+DC increases within the LN between day 2 and 4 (Fig. 2), i.e. the time-point during LNDC-mediated induction of CD8 T cell apoptosis occurs within the LN (9). Specifically, the number of pDC substantionally increased in the LN during lethal versus sublethal IAV infections between day 2 and 4 reaching significant differences by day 3 p.i. (Fig. 2A). Importantly, the number of pDC present in the LN was approximately six times greater than the number of CD8α+DC present during lethal dose IAV infections. These results together with the fact that there are approximately 6500 activated CD43+CD8 T cells present in the LN on day 4 p.i. during lethal IAV infection (data not shown) indicate a putative in vivo E:T (i.e. DC:activated T cell) ratio for pDC is conservativley ~1:1 compared to ~1:6 for CD8α+DC (data not shown). Given that pDC exhibited enhanced LN recruitment during lethal dose IAV infections (Fig 2) and that FasL expression on pDC was dependent on the dose of IAV infection (Fig 1), these data suggest that pDC may be the predominant LNDC population responsible for elimination of IAV-specific CD8 T cells during lethal IAV infections.
Figure 2.
pDC preferentially accumulate in the lung draining LN of lethal dose IAV infected mice. Mice were infected with IAV as in Fig. 1 and on day 1-4 p.i. LN from each group were pooled and the number of (A) CD11cmodCD45R+ cells (i.e. pDC) and (B) CD11c+CD45R−CD8+ cells (i.e. CD8α+ DC) in the LN was determined. Data are representative of 3 independent experiments with 2–3 mice per group.
pDC directly kill IAV-specific CD8 T cells during lethal dose IAV infections
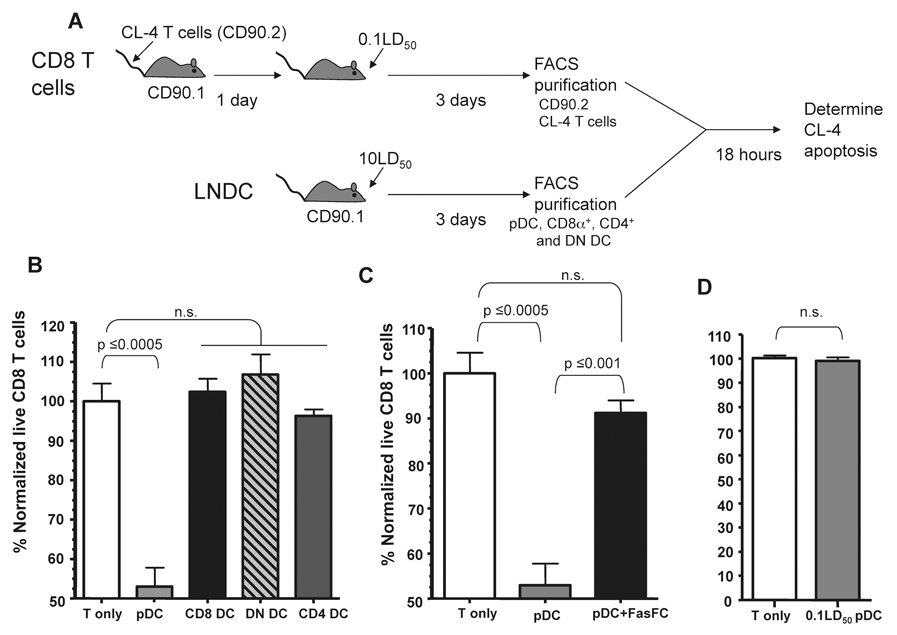
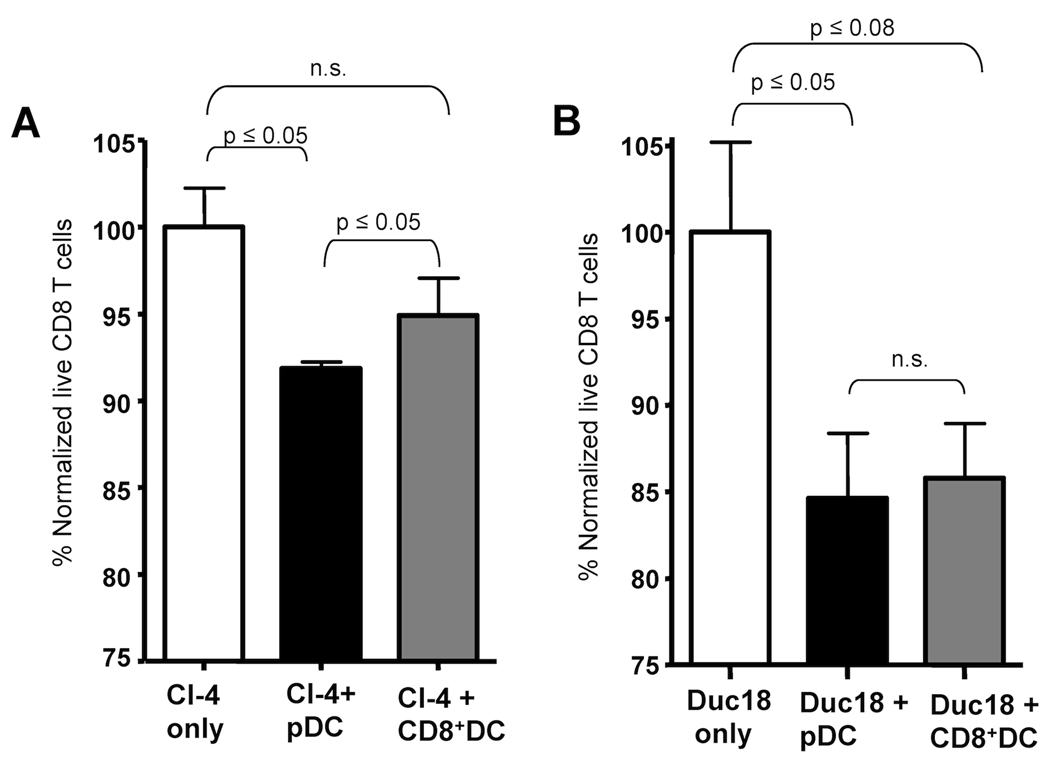
To directly determine the LNDC subset responsible for driving apoptosis of IAV-specific CD8 T cells during lethal dose IAV infections we utilized an ex vivo apoptosis assay. Briefly, transgenic CD90.2+ Clone-4 T cells (Cl-4), which are specific for the HA529 epitope of IAV, were transferred into CD90.1 hosts which were then infected with a sublethal dose of IAV. Activated donor CD90.2+ CL-4 cells, which express higher levels of Fas (Fig. S2), were then purified on day 3 p.i. (i.e. the time directly before T cell apoptosis occurs during lethal dose IAV infections) (9). These activated CD90.2+ CL-4 T cells were then incubated with CD90.1+ LNDC subsets purified from day 3 lethal dose IAV infected mice. Subsequent CL-4 CD8 T cell apoptosis was measured following 18 hours of coculture (Fig. 3). Interestingly, despite the fact that all lethal dose LNDC subsets express FasL at this time-point (Fig. 1), only pDC induced statistically significant levels of apoptosis of the IAV-specific CL-4 CD8 T cells after coculture (Fig. 3B). The T cell apoptosis induced by pDC was FasL:Fas dependent as coculture in the presence of Fas-Fc, which blocks FasL-mediated apoptosis, abrogated the ability of pDC to drive T cell apoptosis (Fig. 3C). Given that pDC during sublethal IAV infection downregulate FasL expression (Fig. 1) we next determined if pDC from sublethal dose IAV-infected mice showed a similar ability to induce apoptosis of activated IAV-specific CD8 T cells. Consistent with their downregulation of FasL during sublethal IAV infection, pDC from sublethal dose IAV-infected mice were unable to induce apoptosis of IAV-specific CD8 T cells (Fig. 3D).
Figure 3.
pDC from lethal dose IAV infected mice kill activated IAV-specific CD8 T cells. (A) Experimental setup for LNDC:CD8 T cell ex vivo apoptosis assay. (B) 104 of the indicated purified LNDC subsets were incubated with 104 activated CL-4 T cells and incubated at 37°C for 18 hours. After incubation the percentage of CD90.2+Annexin V−7-AAD− live CL-4 T cells was determined and normalized to CD8 T cells incubated alone (~70% live). Data are representative of 3 independent experiments. (C) pDC and CL-4 T cells were purified and incubated +/− Fas-Fc and the percentage of live CD8 T cells was determined as described above. Data are representative of 2 independent experiments. (D) 104 pDC from 0.1LD50 IAV-infected mice were incubated with 104 activated CL-4 T cells and apoptosis measured as in B. Data are representative of 3 independent experiments. n.s. = not significant.
pDC contribute to increased mortality during lethal IAV infection
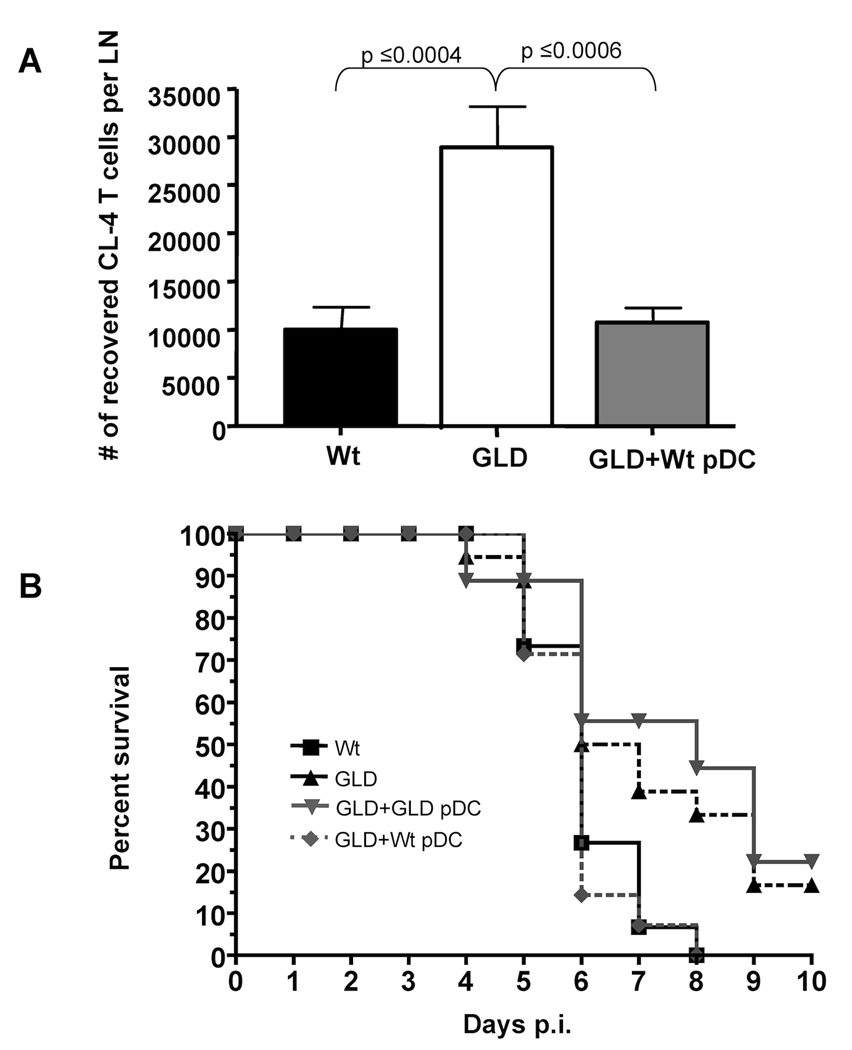
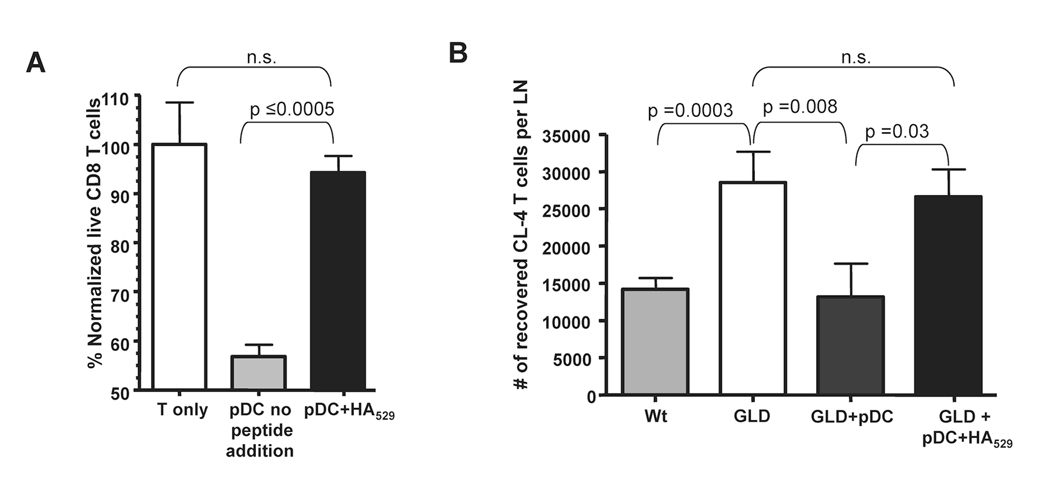
Our previous studies have shown that gld mice (i.e. mice lacking functional FasL) exhibit increased IAV-specific CD8 T cell expansion and protection during lethal dose IAV infections (9). Therefore we used gld mice as hosts for pDC in order to directly determine if wild-type pDC (i.e. FasL+) could mediate a similar reduction in CD8 T cell responses in vivo. Indeed, when wild-type pDC were adoptively transferred into gld mice, the number of CL-4 T cells within the LN on day 4 p.i. was significantly reduced (~65%) (Fig. 4A). In fact this reduction mirrored the number of IAV-specific T cells found in the LN of lethal dose infected wild-type mice. Further when wild-type pDC were transferred into lethal dose IAV infected gld mice it resulted in an enhanced mortality that was statistically similar to lethal dose IAV-infected wild-type mice (Fig. 4B). This increase in disease severity was FasL-dependent as adoptive transfer of gld pDC into lethal dose IAV-infected gld mice did not increase the severity of disease. Importantly in these latter experiments, the transferred donor pDC are the only cells expressing FasL and therefore the only LNDC population able to induce FasL:Fas-mediated apoptosis of IAV-specific CD8 T cells. Together these data suggest that pDC are sufficient to dampen in vivo the magnitude of the IAV-specific CD8 T cell response ultimately leading to an enhanced mortality.
Figure 4.
Donnor wild-type pDC reduce IAV-specific T cell numbers and cause enhanced mortality in mice deficient in functional FasL. (A) CD90.2 wild type (Wt) and gld mice received 2×106 CD90.1 CL-4 T cells and 24 hours post transfer were infected with a 10LD50 dose of IAV. 24 hours p.i. one group of gld mice received 2×106 Wt pDC. On day 4 p.i. lung draining LN were removed and CD3+CD8+CD90.1+ Cl-4 T cells enumerated. Data are pooled from 2 independent experiments with 8–10 mice per group. (B) gld or wild type mice were infected with a 2.5LD50 of IAV. 24 hours p.i. pDC were purified from naïve wild-type or gld mice and then transferred (2×106) into IAV-infected gld mice. Mortality was then monitored for 10 days p.i.. p values are as follows: Wt vs GLD=0.0285, Wt vs (GLD+ Wt pDC) =0.7013, GLD vs. (GLD+Wt pDC) =0.0157, (GLD+ Wt pDC) vs (GLD+ GLD pDC) =0.0103 and (GLD+ GLD pDC) vs GLD=0.5722. Data are pooled from 3 independent experiments with n values equaling; Wt=15, GLD+Wt pDC =14, GLD+ GLD pDC=9, GLD=18.
pDC eliminate IAV-specific CD8 T cells through FasL:Fas interactions in the absence of cognate IAV-antigen presentation
Given that both CD8α+DC and pDC downregulate FasL expression in sublethal dose IAV infected mice, it was surprising that only the pDC eliminated IAV-specific CD8 T cells directly ex vivo. Further, both CD4 and DN DC expressed FasL during lethal dose IAV infections yet did not lead to any detectable apoptosis. We therefore next undertook experiments to determine the mechanism regulating this differential killing. Previous studies have demonstrated that pDC isolated directly ex vivo from IAV-infected mice are unable to stimulate naïve CD8 T cells suggesting that pDC do not present IAV antigens via MHC class I in vivo (4, 5, 7, 15). In contrast to pDC, CD8α+ DC and CD8α−DC purified from the LN of IAV-infected mice are able to induce proliferation of naïve CD8 T cells directly ex vivo (4, 7, 15). Given this differential ability of LNDC subsets to present IAV-antigen to CD8 T cells, we hypothesized that IAV-peptide-MHC I presentation might rescue the IAV-specific CD8 T cells from FasL-mediated apoptosis. To test this hypothesis activated CL-4 T cells were incubated with pDC from the LN of day 3 lethal dose IAV-infected mice in the presence or absence of exogenous IAV-peptide and the ability of pDC to drive apoptosis of IAV-specific CD8 T cells was determined. Strikingly, culturing pDC with IAV-peptide abrogated their ability to eliminate IAV-specific CD8 T cells (Fig. 5A). To determine if antigen presentation by pDC ablates their ability to eliminate IAV-specific CD8 T cells in vivo, pDC were pulsed with IAV peptide, transferred into gld mice and the number of transferred CL-4 cells measured following IAV-infection on day 4 p.i.. Unlike unpulsed pDC (Figure 4A and Figure 5B), pDC pulsed with a cognate IAV peptide epitope did not eliminate IAV-specific CD8 T cells leading to a similar T cell response to that observed in gld mice that did not receive pDC (Fig. 5B). These data suggest that concomitant viral antigen peptide:MHC I presentation overrides the ability of pDC to induce FasL-mediated apoptosis in vitro and in vivo and suggest that pDC elimination of IAV-specific CD8 T cells is critically tied to a lack of IAV-antigen presentation. Therefore the lack of apoptosis induction by CD8α+ and CD8α− DC may relate to their presentation of IAV-antigens. Consistent with this idea, pDC from lethal dose IAV infected mice were also able to mediate the apoptosis of in vitro activated DUC18 (i.e. a non-IAV-specific T cell that display an activation phenotype similar to in vivo activated CL-4 T cells (Fig. S3)) (Fig. 6B) similar to their elimination of in vitro activated CL-4 cells (Fig. 6A). Furthermore while CD8α+DC purified from IAV-infected mice were unable to eliminate IAV-specific transgenic T cells (Fig. 3 and 6A), they were capable of eliminating non-IAV-specific transgenic T cells (Fig. 6B) suggesting that CD8α+DC can mediate elimination of activated T cells in the absence of antigen presentation. Together these data support the idea that elimination of IAV-specific T cells during lethal IAV infection does not require engagement of T cell receptors and in fact TCR engagement may inhibit such apoptosis. Therefore CD8 T cell apoptosis may instead relate to the overall T cell activation state and Fas expression.
Figure 5.
pDC induction of apoptosis in IAV-specific CD8 T cells is abrogated by cognate viral peptide:MHC I presentation. (A) CL-4 T cells and pDC were purified as described in Fig. 4, co-cultured +/− 1mM HA529 peptide and then the percentage of live CD8 T cells determined as in Fig. 4. (B) pDC were purified as in Fig. 4 and pulsed with 1µM HA529 peptide and transferred into IAV-infected mice and CL-4 T cell numbers measured as in Fig. 4. Data are representative of 2–3 independent experiments. n.s. = not significant.
Figure 6.
pDC from lethal dose IAV infected mice eliminate IAV-specific and non IAV-specific activated CD8 T cells. CD8 T cells from the spleens of naïve CL-4 (A) and DUC18 (B) transgenic mice were purified and cultured for 3 days in the presence of αCD3/CD28 antibodies in order to activate the T cells. Activated transgenic T cells (104) were incubated with pDC or CD8α+DC (104) from lethal dose IAV-infected mice at 37°C for 18 hours as described in Figure 3. After incubation the percentage of CD90.2+Annexin V−7-AAD− live transgenic T cells was determined and normalized to CD8 T cells incubated alone. n.s.= p > 0.1. Data are representative of 2 independent experiments.
Discussion
DC elimination of T cells through FasL:Fas interactions has been previously described by multiple investigators (19–21). Suss and Shortman demonstrated that CD8α+ splenic DC expressing FasL were able to eliminate CD4 T cells during a mixed leukocyte reaction. Additionally, multiple groups have used adoptive transfer of DC transfected with FasL to control T cell numbers in a variety of disease settings including autoimmunity, cancer and viral infection (19, 21, 22). Herein we have shown that LN resident pDC can mediate the elimination of IAV-specific CD8 T cells during lethal dose IAV infections. Together, our results, suggest that elimination of activated Fas+ T cells by FasL+pDC may represent an integral mechanism for dampening T cell numbers.
While DC-mediated reduction of the effector T cell response in autoimmune reactions or at the conclusion of an immune response would be beneficial, the loss of effector CD8 T cells here is clearly detrimental to survival during a high dose IAV infection (9). Indeed, our previous studies have shown that in the absence of functional FasL, sufficient numbers of CD8 T cells develop to control the high dose IAV inoculum (Fig. 4) (9). Thus, the enhanced recruitment of pDC into the LN observed during lethal dose IAV infections (Fig. 2), coupled with pDC-mediated elimination of the IAV-specific CD8 T cell response (Fig. 3 and Fig. 4), allows the virus to escape adaptive immune control leading to death of the infected host. In this regard, recent studies have shown that individuals infected with highly pathogenic Avian (H5N1) IAV have dampened or reduced CD4 and CD8 T cell responses (23). Similarly, mice and monkeys infected with highly pathogenic H5N1 IAV develop T cell lymphopenia (24–26) with the loss of CD8 T cells in the lungs and lymph nodes associated with enhanced levels of apoptosis (24). The exact pathway(s) and cell type mediating the apoptosis responsible for T cell lymphopenia remain poorly understood at this time. However, given our results, it will be important to determine what role FasL expression by pDC plays in the lymphopenia associated with high pathogenic avian H5N1 IAV infections.
Surprisingly, LNDC elimination of IAV-specific CD8 T cells does not require cognate MHC class I:antigen presentation. In fact the rescue of these T cells from FasL+ DC induced apoptosis during recognition of viral peptide-MHC I complexes may be due in part due to TCR mediated upregulation of NKκB, which also has been shown to protect T cells, macrophages and B cells from Fas mediated apoptosis (27–30). Interestingly signals through the B cell receptor, which upregulate NFκB, result in a transient (less than 24h) protection from Fas-mediated apoptosis (28). In our studies, pDC driven apoptosis occurs in the LN at a time point concomitant with DC-mediated antigen presentation. Signaling through Fas on T cells during activation can act as a co-stimulatory molecule and has been demonstrated to increase T cell proliferation and activation (31–34). Together this suggests the possibility that during a narrow window immediately following the activation of naive T cells within the LN, cognate MHC class I:antigen-TCR interactions in the presence of FasL:Fas engagement may lead to enhanced T cells responses rather than apoptosis. Conversely, FasL:Fas engagement alone or after egress from the draining LN would mediate death. Consistent with our results showing that co-culturing of pDC and CL-4 T cells with IAV-peptide epitopes reverses the pDC-mediated loss of the T cells (Fig. 5A), a recent report has demonstrated that antigen-pulsed FasL-transfected DC enhance antigen-specific CD8 T cell responses rather than induce apoptosis. This finding suggests that the presentation of antigen by these transfected DC inhibits their ability to drive elimination of cognate T cells (35). In contrast to this report, and our own findings, other groups have suggested that DC FasL-mediated elimination of CD4 and CD8 T cells can occur in an antigen-dependent manner (19, 21, 36). The reason for these differences is not clear at this time; however, in contrast to our own studies, these latter experiments utilized conventional bone-marrow derived DC or DC cell lines rather than pDC obtained from the LN. In addition, these studies used effector DC transfected with FasL cDNA resulting in constitutively high levels of FasL.
Our results suggest that pDC-mediated induction of apoptosis in activated T cells during lethal dose IAV infections occurs independent of IAV-antigen presentation (Fig. 5). In agreement with this idea recent reports have demonstrated that although LN resident pDC contain IAV-proteins following infection, they are unable to stimulate naïve or memory CD8 T cells and may instead regulate B cell responses (5). Unlike pDC, LN resident CD8α+ DC acquire IAV-antigen (likely from migratory rDC) and cross-present this antigen during IAV infections (15). Interestingly while our results show that CD8α+ DC are also able to regulate FasL expression in an IAV dose-dependent manner (see Fig. 1), they do not eliminate IAV-specific CD8 T cells during lethal dose IAV infections (Fig. 3). This appears to be due to their presentation of viral peptides as our preliminary results suggest that CD8α+ DC from lethally infected β2m−/− mice induce substantial apoptosis of IAV-specific T cells (data not shown). Together these results along with those demonstrating that pDC from lethal-dose IAV infected mice kill both activated non-IAV specific and IAV-specific CD8 T cells with the same efficiency (Fig. 6A) suggests that pDC elimination of effector CD8 T cells during lethal dose IAV infections is independent of T cell receptor engagement. Rather, pDC-mediated elimination of the T cells is associated with the T cells activation state and Fas expression. Thus Fas expressing activated or memory CD8 T cells of any specificity might be susceptible to pDC-mediated apoptosis during lethal dose IAV infections.
In addition to the differential FasL expression on pDC from lethal and sublethal dose IAV-infected mice, our results demonstrate an enhanced recruitment of pDC into the LN of lethal-dose infected mice (Fig. 2). pDC recruitment into LN through high endothelial venules (HEV) is thought to be mediated in part in response to CXCL12 and CXCL9 expression (13, 14). Interestingly, CXCL9 is upregulated in response to IL-1 and IFNγ expression (37). Consistent with these findings, our preliminary experiments have demonstrated increased secretion of IFNγ, IL-1α, and IL1β from in vitro cultured LN obtained from lethal compared with sublethal dose IAV infected mice (data not shown). Importantly, as pDC leave the HEV and enter the into LN, the increased recruitment of pDC during lethal IAV infections will also raise the local in vivo E:T ratio. Even without factoring in a co-localization of these recruited pDC into areas of the LN that accumulate newly activated Fas+ T cells (38), our data suggest that in vivo there is ~1 pDC available for every activated CD8 T cell. In our ex vivo analysis (Fig. 3), pDC cultured at a 1:1 ratio with T cells were able to reduce a static number of T cells by ~45% in the span of 18 hours. Therefore the ~80–95% reduction of the endogenous T cell response observed in vivo on days 5 and 6 p.i. (see (9) and Fig. S1) may relate to either the fact that elimination of activated T cells would also reduce the subsequent burst size of the total response and/or an increased local LN in vivo E:T ratio.
All together the data presented herein describe a novel role for pDC during lethal dose IAV-infection- namely the elimination of activated CD8 T cells leading to enhanced mortality. Given the emerging threat of highly pathogenic pandemic IAV and the detrimental role FasL+ pDC can play during a lethal IAV infection our findings suggest that pDC and FasL may be strong candidates for therapeutic blockade during highly virulent IAV infections.
Supplementary Material
Acknowledgements
We would like to thank Drs. J Harty, J Heusel, T Waldschmidt and S Varga for critical reading of this manuscript, the University of Iowa Flow Cytometry Facility for expert assistance and J McGill and B VanOosten-Anderson for technical assistance.
Grant Support
This work was supported by NIH AI-071085, AI-076989 and Department of Pathology Start-Up Funds to K.L.L.
Abbreviations
- pDC
plasmacytoid dendritic cell
- IAV
influenza A virus
- LN
lymph nodes
- DC
dendritic cells
- TRAIL
tumor necrosis factor apoptosis inducing ligand
- p.i.
post-infection
- DN
double negative
- LNDC
lymph node dendritic cell
- rDC
respiratory dendritic cell
Footnotes
“This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (JI). The American Association of Immunologists, INC (AAI), publisher of The JI, holds the copyright to this manuscript. This manuscript has not yet been copy edited or subjected to editorial proofreading by The JI; hence it may differ from the final version published in The JI (online and print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the United States National Institutes of Health or any other third party. The final, citable version of record can be found at www.Jimmunol.org”
References
- 1.Eichelberger M, Allan W, Zijlstra M, Jaenisch R, Doherty PC. Clearance of influenza virus respiratory infection in mice lacking class I major histocompatibility complex-restricted CD8+ T cells. J. Exp. Med. 1991;174:875–880. doi: 10.1084/jem.174.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J. Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 3.Graham MB, Braciale TJ. Resistance to and recovery from lethal influenza virus infection in B lymphocyte-deficient mice. J. Exp. Med. 1997;186:2063–2068. doi: 10.1084/jem.186.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim TS, Braciale TJ. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS ONE. 2009;4:e4204. doi: 10.1371/journal.pone.0004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GeurtsvanKessel CH, Willart MA, van Rijt LS, Muskens F, Kool M, Baas C, Thielemans K, Bennett C, Clausen BE, Hoogsteden HC, Osterhaus AD, Rimmelzwaan GF, Lambrecht BN. Clearance of influenza virus from the lung depends on migratory langerin+CD11b− but not plasmacytoid dendritic cells. J. Exp. Med. 2008;205:1621–1634. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Legge KL, Braciale TJ. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity. 2003;18:265–277. doi: 10.1016/s1074-7613(03)00023-2. [DOI] [PubMed] [Google Scholar]
- 7.Belz GT, Smith CM, Kleinert L, Reading P, Brooks A, Shortman K, Carbone FR, Heath WR. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8670–8675. doi: 10.1073/pnas.0402644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brincks EL, Katewa A, Kucaba TA, Griffith TS, Legge KL. CD8 T cells utilize TRAIL to control influenza virus infection. J. Immunol. 2008;181:4918–4925. doi: 10.4049/jimmunol.181.7.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Legge KL, Braciale TJ. Lymph node dendritic cells control CD8+ T cell responses through regulated FasL expression. Immunity. 2005;23:649–659. doi: 10.1016/j.immuni.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Maldonado-Lopez R, De Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8alpha+ and CD8alpha− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maldonado-Lopez R, De Smedt T, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Maliszewski CR, Moser M. Role of CD8alpha+ and CD8alpha− dendritic cells in the induction of primary immune responses in vivo. J. Leukoc. Biol. 1999;66:242–246. doi: 10.1002/jlb.66.2.242. [DOI] [PubMed] [Google Scholar]
- 12.Henri S, Vremec D, Kamath A, Waithman J, Williams S, Benoist C, Burnham K, Saeland S, Handman E, Shortman K. The dendritic cell populations of mouse lymph nodes. J. Immunol. 2001;167:741–748. doi: 10.4049/jimmunol.167.2.741. [DOI] [PubMed] [Google Scholar]
- 13.Yoneyama H, Matsuno K, Zhang Y, Nishiwaki T, Kitabatake M, Ueha S, Narumi S, Morikawa S, Ezaki T, Lu B, Gerard C, Ishikawa S, Matsushima K. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int. Immunol. 2004;16:915–928. doi: 10.1093/intimm/dxh093. [DOI] [PubMed] [Google Scholar]
- 14.Krug A, Uppaluri R, Facchetti F, Dorner BG, Sheehan KC, Schreiber RD, Cella M, Colonna M. IFN-producing cells respond to CXCR3 ligands in the presence of CXCL12 and secrete inflammatory chemokines upon activation. J. Immunol. 2002;169:6079–6083. doi: 10.4049/jimmunol.169.11.6079. [DOI] [PubMed] [Google Scholar]
- 15.Belz GT, Bedoui S, Kupresanin F, Carbone FR, Heath WR. Minimal activation of memory CD8+ T cell by tissue-derived dendritic cells favors the stimulation of naive CD8+ T cells. Nat. Immunol. 2007;8:1060–1066. doi: 10.1038/ni1505. [DOI] [PubMed] [Google Scholar]
- 16.Wolf AI, Buehler D, Hensley SE, Cavanagh LL, Wherry EJ, Kastner P, Chan S, Weninger W. Plasmacytoid dendritic cells are dispensable during primary influenza virus infection. J. Immunol. 2009;182:871–879. doi: 10.4049/jimmunol.182.2.871. [DOI] [PubMed] [Google Scholar]
- 17.Fonteneau JF, Gilliet M, Larsson M, Dasilva I, Munz C, Liu YJ, Bhardwaj N. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood. 2003;101:3520–3526. doi: 10.1182/blood-2002-10-3063. [DOI] [PubMed] [Google Scholar]
- 18.Di Pucchio T, Chatterjee B, Smed-Sorensen A, Clayton S, Palazzo A, Montes M, Xue Y, Mellman I, Banchereau J, Connolly JE. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nat. Immunol. 2008;9:551–557. doi: 10.1038/ni.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsue H, Matsue K, Walters M, Okumura K, Yagita H, Takashima A. Induction of antigen-specific immunosuppression by CD95L cDNA-transfected 'killer' dendritic cells. Nat. Med. 1999;5:930–937. doi: 10.1038/11375. [DOI] [PubMed] [Google Scholar]
- 20.Suss G, Shortman K. A subclass of dendritic cells kills CD4 T cells via Fas/Fas-ligand-induced apoptosis. J. Exp. Med. 1996;183:1789–1796. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolfe T, Asseman C, Hughes A, Matsue H, Takashima A, von Herrath MG. Reduction of antiviral CD8 lymphocytes in vivo with dendritic cells expressing Fas ligand-increased survival of viral (lymphocytic choriomeningitis virus) central nervous system infection. J. Immunol. 2002;169:4867–4872. doi: 10.4049/jimmunol.169.9.4867. [DOI] [PubMed] [Google Scholar]
- 22.Kim SH, Bianco N, Menon R, Lechman ER, Shufesky WJ, Morelli AE, Robbins PD. Exosomes derived from genetically modified DC expressing FasL are anti-inflammatory and immunosuppressive. Mol. Ther. 2006;13:289–300. doi: 10.1016/j.ymthe.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 23.de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, Qui PT, Cam BV, Ha do Q, Guan Y, Peiris JS, Chinh NT, Hien TT, Farrar J. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tumpey TM, Lu X, Morken T, Zaki SR, Katz JM. Depletion of lymphocytes and diminished cytokine production in mice infected with a highly virulent influenza A (H5N1) virus isolated from humans. J. Virol. 2000;74:6105–6116. doi: 10.1128/jvi.74.13.6105-6116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perrone LA, Plowden JK, Garcia-Sastre A, Katz JM, Tumpey TM. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000115. e1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baskin CR, Bielefeldt-Ohmann H, Tumpey TM, Sabourin PJ, Long JP, Garcia-Sastre A, Tolnay AE, Albrecht R, Pyles JA, Olson PH, Aicher LD, Rosenzweig ER, Murali-Krishna K, Clark EA, Kotur MS, Fornek JL, Proll S, Palermo RE, Sabourin CL, Katze MG. Early and sustained innate immune response defines pathology and death in nonhuman primates infected by highly pathogenic influenza virus. Proc. Natl. Acad. Sci. U. S. A. 2009;106:3455–3460. doi: 10.1073/pnas.0813234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dudley E, Hornung F, Zheng L, Scherer D, Ballard D, Lenardo M. NF-kappaB regulates Fas/APO-1/CD95- and TCR- mediated apoptosis of T lymphocytes. Eur. J. Immunol. 1999;29:878–886. doi: 10.1002/(SICI)1521-4141(199903)29:03<878::AID-IMMU878>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 28.Carey GB, Donjerkovic D, Mueller CM, Liu S, Hinshaw JA, Tonnetti L, Davidson W, Scott DW. B-cell receptor and Fas-mediated signals for life and death. Immunol. Rev. 2000;176:105–115. doi: 10.1034/j.1600-065x.2000.00502.x. [DOI] [PubMed] [Google Scholar]
- 29.Lu B, Wang L, Medan D, Toledo D, Huang C, Chen F, Shi X, Rojanasakul Y. Regulation of Fas (CD95)-induced apoptosis by nuclear factor-kappaB and tumor necrosis factor-alpha in macrophages. Am. J. Physiol. Cell Physiol. 2002;283:C831–C838. doi: 10.1152/ajpcell.00045.2002. [DOI] [PubMed] [Google Scholar]
- 30.Schram BR, Rothstein TL. NF-kappa B is required for surface Ig-induced Fas resistance in B cells. J Immunol. 2003;170:3118–3124. doi: 10.4049/jimmunol.170.6.3118. [DOI] [PubMed] [Google Scholar]
- 31.Chun DH, Jung KC, Park WS, Lee IS, Choi WJ, Kim CJ, Park SH, Bae Y. Costimulatory effect of Fas in mouse T lymphocytes. Mol. Cells. 2000;10:642–646. doi: 10.1007/s10059-000-0642-z. [DOI] [PubMed] [Google Scholar]
- 32.Maksimow M, Soderstrom TS, Jalkanen S, Eriksson JE, Hanninen A. Fas costimulation of naive CD4 T cells is controlled by NF-kappaB signaling and caspase activity. J. Leukoc. Biol. 2006;79:369–377. doi: 10.1189/jlb.0505238. [DOI] [PubMed] [Google Scholar]
- 33.Maksimow M, Santanen M, Jalkanen S, Hanninen A. Responding naive T cells differ in their sensitivity to Fas engagement: early death of many T cells is compensated by costimulation of surviving T cells. Blood. 2003;101:4022–4028. doi: 10.1182/blood-2002-06-1904. [DOI] [PubMed] [Google Scholar]
- 34.Siegel RM, Chan FK, Chun HJ, Lenardo MJ. The multifaceted role of Fas signaling in immune cell homeostasis and autoimmunity. Nat. Immunol. 2000;1:469–474. doi: 10.1038/82712. [DOI] [PubMed] [Google Scholar]
- 35.Buonocore S, Haddou NO, Moore F, Florquin S, Paulart F, Heirman C, Thielemans K, Goldman M, Flamand V. Neutrophil-dependent tumor rejection and priming of tumoricidal CD8+ T cell response induced by dendritic cells overexpressing CD95L. J. Leukoc. Biol. 2008;84:713–720. doi: 10.1189/jlb.0108075. [DOI] [PubMed] [Google Scholar]
- 36.Kusuhara M, Matsue K, Edelbaum D, Loftus J, Takashima A, Matsue H. Killing of naive T cells by CD95L-transfected dendritic cells (DC): in vivo study using killer DC-DC hybrids and CD4(+) T cells from DO11.10 mice. Eur. J. Immunol. 2002;32:1035–1043. doi: 10.1002/1521-4141(200204)32:4<1035::AID-IMMU1035>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 37.Sanmiguel JC, Olaru F, Li J, Mohr E, Jensen LE. Interleukin-1 regulates keratinocyte expression of T cell targeting chemokines through interleukin-1 receptor associated kinase-1 (IRAK1) dependent and independent pathways. Cell Signal. 2009;21:685–694. doi: 10.1016/j.cellsig.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barchet W, Blasius A, Cella M, Colonna M. Plasmacytoid dendritic cells: in search of their niche in immune responses. Immunol. Res. 2005;32:75–83. doi: 10.1385/IR:32:1-3:075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.