Abstract
Objectives
The objective of this study was to identify existing clinical compounds that either possess a fungicidal activity alone or can act synergistically with fungistatic antifungals.
Methods
We screened a clinical compound library for drugs that exhibited anti-Aspergillus activity. Among selected compounds, the cationic peptide antibiotic polymyxin B was chosen for further characterization because it can be used parenterally and topically. The fungicidal effect of polymyxin B and its synergistic interactions with azole antifungals were tested against a variety of fungal species. The toxicity of the drug combination of polymyxin B and fluconazole was compared with that of each drug alone in mammalian cell cultures.
Results
We found that polymyxin B possesses a broad-spectrum antifungal activity at relatively high concentrations. However, because of its synergistic interactions with azole antifungals, polymyxin B at much lower concentrations exerts a potent fungicidal effect against Cryptococcus neoformans, Candida albicans and non-albicans Candida species and moulds when combined with azoles. The combination of polymyxin B and fluconazole at concentrations within susceptible breakpoints is particularly potent against C. neoformans isolates, including fluconazole-resistant strains. The drug combination displayed no additional toxicity compared with polymyxin B alone when tested in cell culture.
Conclusions
The combination of polymyxin B and fluconazole has the potential to be used in the clinic to treat systemic cryptococcosis. Our findings suggest that combining cationic peptide antibiotics with azole antifungals could provide a new direction for developing novel antifungal therapies.
Keywords: antifungal, combination therapy, antibiotic, fungal membrane
Introduction
Systemic fungal infections have drastically increased over the past three decades due to the rising immunocompromised population as a result of transplantation, cancer chemotherapy, steroid therapy and, in particular, HIV infection (AIDS).1–5 Unfortunately, the outcome of current antifungal therapy is far from satisfactory. For example, the three major global invasive mycoses, aspergillosis, candidiasis and cryptococcosis caused by fungal species of Aspergillus, Candida and Cryptococcus, respectively, typically have mortality rates ranging from 10% to 90%.6–13 This poor outcome is in part due to the limited number of clinically available antifungals and the fact that many antifungals either lack potency or are toxic to the host.14–16 The emergence of resistant fungal strains to current antifungals, which is exacerbated by the necessity for long-term usage of antifungals in immunocompromised individuals, causes additional difficulty in treatment.15,17–24 For example, a recent global survey of close to 3000 Cryptococcus neoformans isolates indicated that >11% of the isolates are resistant to fluconazole.25 Therefore, there is an urgent need for new therapies.
Through a screen of a clinical compound library, we have found that polymyxin B, an antibiotic used to treat bacterial infections, possesses fungicidal activity. Similar to what has been observed previously,26–28 polymyxin B is fungicidal at relatively high concentrations. Interestingly, in combination with fluconazole or itraconazole, polymyxin B at low concentrations demonstrates a killing effect against Aspergillus fumigatus, Rhizopus oryzae, Candida albicans and non-albicans Candida species. The combination at clinically relevant low concentrations is particularly potent against C. neoformans, including strains of varied resistance to fluconazole.
Materials and methods
Strains and media
For the initial drug screening, the Aspergillus nidulans strain R2129 was grown on YAG medium (0.5% yeast extract, 2% agar and 2% glucose). For microscopic analyses of the effects of drugs, R21 and the A. fumigatus strain B523330 were grown on YG liquid medium (0.5% yeast extract and 2% glucose) supplemented with 0.12% uridine and uracil (Y + UU). All yeast strains were maintained on yeast peptone dextrose (YPD) medium. Drug disc diffusion and microdilution assays were performed using RPMI 1640 medium buffered with MOPS.
Clinical compound library screening
The Johns Hopkins Clinical Compound Library (JHCCL version 1.0), a collection of 1514 FDA-approved (1082) and foreign approved (432) drugs (FAD),31 was screened for drugs with an inhibitory effect on A. nidulans colony growth. The library was assembled in a 96-well plate format with 25 µL aliquots of 10 mM stocks of each compound in either water or DMSO. An aliquot of 2 µL from each well was spotted onto a YAG plate and ∼1 × 105 spores of A. nidulans strain R21 were point-inoculated onto the spots of the original drug application. The cells were incubated at 37°C for 2 days. Drugs that either significantly or completely inhibited A. nidulans colony formation were selected. Selected drugs at 2 µM were tested again for an inhibitory effect on A. nidulans in liquid medium. A. nidulans spores (∼105) were inoculated into 500 µL of drug-containing liquid medium and incubated at 37°C overnight in the 8-chambered Lab-Tek Borosilicate Coverglass System. Twenty-three compounds that significantly inhibited colony growth of A. nidulans strain R21 at this concentration were identified. Inhibition of spore germination or germ tube extension of the A. nidulans and A. fumigatus isolates was examined microscopically in the presence of polymyxin B at the indicated concentrations for the time period indicated in the text. Viability of those inhibited cells was examined microscopically after they had been cultured in drug-free medium for 24 h.
Disc diffusion halo assay for antifungal activity
Briefly, yeast cells at a cell density of ∼5 × 106 were spread onto RPMI 1640 agar medium with l-glutamine and without sodium bicarbonate. The plates were allowed to solidify and dry. Whatman paper discs (7 mm) containing water, fluconazole, polymyxin B and their combination at various concentrations were dried and placed on the solidified agar surface. The cells were incubated for 24–48 h at 37°C.
Microdilution assay for antifungal activity
The microdilution assay was performed according to the CLSI (formerly NCCLS) standard32 except that the cells were incubated at 37°C. Briefly, yeast cells at a final concentration of ∼1 × 103 cells/mL (or Aspergillus or Rhizopus spores at a final concentration of ∼5 × 104 cells/mL) were inoculated in the RPMI 1640 liquid medium with serial (2×) dilutions of each drug being tested. The concentrations used for polymyxin B and fluconazole are indicated in the text. Wells that contained no drugs or no yeast inoculation were included as positive and negative controls. The MIC100 of polymyxin B or the combination was defined as the lowest drug concentration that resulted in a 100% decrease in absorbance compared with that of the control in drug-free medium. The MIC90 of fluconazole was defined as the lowest drug concentration that resulted in a 90% decrease in absorbance compared with that of the control in drug-free medium. MICs were read after incubation without agitation for 24 h for Candida strains and 48 h for the Aspergillus, Rhizopus or Cryptococcus isolates. Fungicidal effect was examined by counting the cfu of the suspension from each well after plating it onto the YPD medium and incubating for 24 (for Candida strains) to 48 h (for Cryptococcus, Aspergillus and Rhizopus strains). The minimal fungicidal concentration (MFC) was defined as the minimal drug concentration that causes at least 99% of cells to be killed compared with the original inoculum. A synergistic fungicidal effect between fluconazole and polymyxin B for each strain was calculated based on the fractional fungicidal concentration index (FFCI), FFCI = [F]/MFCF + [P]/MFCP, where MFCF and MFCP are the MFCs of fluconazole and polymyxin B, respectively, and [F] and [P] are the concentrations at which fluconazole and polymyxin B, in combination, are fungicidal. FFCIs ≤0.5 indicate synergistic interactions, FFCIs >0.5–4.0 indicate no interaction and FFCIs >4.0 indicate antagonistic interactions.33
Generation of fluconazole-resistant H99FR
C. neoformans H99 reference strain was cultured in yeast nitrogen base (YNB) liquid medium with fluconazole at an inhibitory but sublethal concentration of 2.4 mg/L. The culture was maintained at 37°C with shaking. An aliquot of the culture was transferred to fresh YNB medium with fluconazole every other day and continued for 16 weeks.
Proliferation of HeLa and human monocytic THP-1 cells in the presence of the drugs
HeLa cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM l-glutamine and 100 U/L each of penicillin and streptomycin. THP-1 cells were grown in RPMI 1640 medium with the same supplements. HeLa cells (1 × 103) were seeded in a well of a 96-well culture plate with 50 µL of DMEM and were grown overnight in a 37°C incubator with 5% CO2. Polymyxin B and/or fluconazole were added to the medium to reach final concentrations of 40 and 100 mg/L, respectively. At 0, 24, 48 and 72 h after drug addition, cell growth was examined microscopically and quantified. One hundred thousand THP-1 cells were grown in 1 mL of medium and treated similarly. Triplicate samples were taken for each treatment at each timepoint. The experiment was repeated twice.
Results
The antibiotic polymyxin B showed anti-A. nidulans activity
The Johns Hopkins Clinical Compound Library,31 consisting of 1514 FADs and FDA-approved drugs was screened for anti-A. nidulans activity. Twenty-three drugs were found to either significantly or completely inhibit the colony growth of the A. nidulans strain R21 when 2 µL aliquots of the drugs from 10 mM stock were directly spotted onto each point inoculum containing ∼1 × 105 spores. Eleven compounds significantly inhibited spore germination in liquid medium at a concentration of 2 µM. The majority of them were known antifungals belonging to the azole family. One antibiotic (polymyxin B sulphate) and three antiseptic compounds were also identified. Because polymyxin B has the potential to treat systemic infections, it was chosen for further studies. The effect of polymyxin B on A. nidulans and its pathogenic relative A. fumigatus is fungicidal. Both spores and pre-germinated germ tubes inhibited by polymyxin B failed to re-grow after transfer to fresh drug-free medium (Figure 1a–d and data not shown).
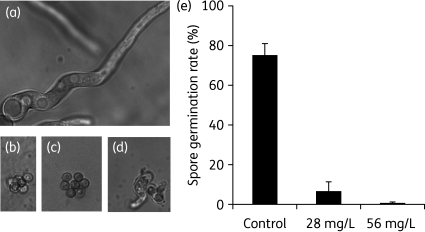
Figure 1.
Polymyxin B is fungicidal against Aspergillus fumigatus. (a) A. fumigatus spores germinated and formed long hyphae after overnight growth in drug-free medium. (b) Polymyxin B at 20 µM (28 mg/L) final concentration in Y + UU liquid medium inhibited germination of the majority of A. fumigatus spores. (c) A. fumigatus conidia transferred to drug-free medium after an overnight treatment with 20 µM polymyxin B did not germinate after 24 h of incubation at 37°C. (d) Pre-germinated germ tubes of A. fumigatus did not grow in the presence of 20 µM polymyxin B. In this experiment, germ tubes were formed after a 7 h incubation of spores in a drug-free medium, and then subjected to an overnight treatment with 20 µM polymyxin B, and subsequently followed by an incubation in drug-free medium for 24 h. Note that the germ tubes did not become longer hyphae, in contrast to the non-treated cells shown in (a). (e) Quantitative analysis of the effectiveness of polymyxin B at 20 µM (28 mg/L) or 40 µM (56 mg/L) in inhibiting the germination of A. fumigatus spores. Spores of A. fumigatus strain B5233 were incubated at 37°C for 9.5 h. One hundred and fifty cells were counted for each treatment. The averages and standard deviations based on three independent experiments are shown in the graph.
Polymyxin B alone is fungicidal at relatively high concentrations
Because A. nidulans is rarely pathogenic to humans, the susceptibility to polymyxin B of the pathogenic filamentous fungi A. fumigatus and R. oryzae and pathogenic yeasts including C. neoformans, C. albicans, Candida glabrata, Candida krusei and Candida parapsilosis was examined using the standard microdilution assay. Microscopic examination of polymyxin-treated A. fumigatus (B5233) revealed that polymyxin B at 28 or 56 mg/L significantly inhibited germination of A. fumigatus spores compared with the untreated control (Figure 1e). Those spores failed to grow even after removal of the drug, consistent with the fungicidal effect of polymyxin B (Figure 1c and data not shown). Pre-germinated germ tubes also failed to grow in the presence of polymyxin B as evidenced by lack of long hyphae (Figure 1d). However, some spores present in the population were resistant and were able to form hyphae in the presence of the drug after an overnight incubation. Thus, as expected, both B5233 and Af293 isolates showed strong resistance to polymyxin B in the disc diffusion assay (data not shown) and the microdilution assay (MIC100 > 1000 mg/L) based on visual examination. In contrast, the R. oryzae strain 99-880 is more susceptible, with a MIC100 of 32 mg/L. The pathogenic yeast strains tested also showed varied susceptibility to polymyxin B, with the MIC100 ranging from 8 to 256 mg/L (Table 1, columns 1 and 2). This range is consistent with MICs reported in the literature.27,28,34 Given that bacterial strains with MICs >8 mg/L are considered resistant to polymyxin B,35 the relatively high MICs observed in these fungal species preclude the clinical use of polymyxin B as a monotherapy in treating fungal infections.
Table 1.
Polymyxin B and fluconazole exhibit a synergistic fungicidal effect (mg/L)
| Yeast strains | MIC100 PMB | MFC PMB | MIC90 FLC | MFC FLC | MFC PMB/FLC | FFCI |
|---|---|---|---|---|---|---|
| Cryptococcus neoformans H99α76 | 8 | 8 | 1 | 8 | 1–2 | 0.266 |
| Candida albicans SC5314a | 128 | >256 | 0.2 | 64 | 6–8 | 0.125 |
| Candida glabrata PAT2ISO3a | 256 | 256 | 8 | >64 | 25–15 | 0.236 |
| Candida krusei DUMC132.91a | 32 | 32 | 64 | 64 | 8–10 | 0.160 |
| Candida parapsilosis MMRL1594a | 128 | 256 | >64 | >64 | 10–10 | 0.078 |
| Saccharomyces cerevisiae BY474177 | 32 | 64 | 4 | 16 | 2–4 | 0.252 |
| Filamentous fungal strains | MIC100 PMB | MFC PMB | MIC90 ITC | MFC ITC | MFC PMB/ITC | FFCI |
| Aspergillus fumigatus Af29378 | >1000 | >1000 | 0.4 | 12.8 | 12–1.6 | 0.137 |
| Rhizopus oryzae 99–88079 | 32 | 32 | 0.4 | 1.6 | 4–0.4 | 0.375 |
aStrains were obtained from Duke University Medical Center.
MFC is defined as the lowest drug concentration at which at least 99% of cells were killed compared with the original inocula. Suspensions from the microdilution assay after 24 or 48 h (for Cryptococcus) of incubation were plated on drug-free medium to obtain the numbers of cfu to determine the MFC. Successive 2× serial dilutions of the drugs were used. When fluconazole was used alone, sometimes the MFC could not be achieved in the concentration range tested and is indicated by ‘>’.
PMB stands for polymyxin B (susceptible breakpoint for bacteria is 2 mg/L according to the BSAC or 4 mg/L according to the CLSI); FLC, fluconazole (susceptible breakpoint for yeasts is 8 mg/L); ITC, itraconazole (susceptible breakpoint for moulds is 1.0 mg/L). The highest concentrations tested for PMB, FLC and ITC were 256 (except for A. fumigatus, where 1000 mg/L was the highest concentration tested), 64 and 6.4 mg/L, respectively.
The fractional fungicidal concentration index (FFCI) = [FLC]/MFCFLC + [PMB]/MFCPMB, where MFCFLC and MFCPMB are the concentrations of fluconazole and polymyxin B, respectively, and [FLC] and [PMP] are the concentrations at which fluconazole and polymyxin B, in combination, killed at least 99% of cells compared with the original inocula. When the MFC was not achieved (> is used in these cases), the concentration 2× that of the highest concentration tested is used for FFCI calculation. FFCIs ≤0.5 indicate synergistic interactions, FFCIs between 0.5 and 4.0 suggest no interaction and FFCIs >4.0 indicate antagonistic interactions.33 The same formulation was used to calculate the FFCI for the interaction between polymyxin B and itraconazole.
In combination with fluconazole, polymyxin B at lower concentrations is fungicidal for all yeast strains tested
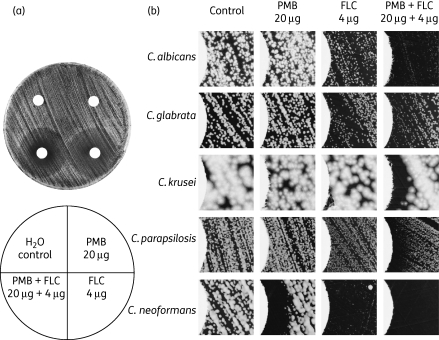
Polymyxins act against Gram-negative bacteria by binding lipopolysaccharide (LPS) and anionic phospholipids in the bacterial membrane, disrupting membrane integrity.36–40 It is possible that polymyxin B binds the fungal membrane in an analogous manner, but with reduced efficiency because eukaryotic membranes have low membrane potentials, high levels of sterols and higher contents of neutral lipid.41–43 The azole antifungals target the lanosterol 14α-demethylase Erg11 in the fungal ergosterol biosynthesis pathway.44 This results in a reduced ergosterol level and altered membrane property.14,45–47 We reason that polymyxin B and azole antifungals may have synergistic interactions against fungi. To test this hypothesis, the susceptibility of C. neoformans, C. albicans, C. glabrata, C. krusei and C. parapsilosis strains to fluconazole, polymyxin B and a combination of these two compounds was analysed by disc diffusion halo assay. As shown in Figure 2, the strains demonstrated varied susceptibility to fluconazole, with the C. krusei strain DUMC132.91 and the C. parapsilosis strain MMRL1594 being highly resistant. Polymyxin B alone at 20 µg per disc had no or minimal fungicidal activity against the strains tested (Figure 2b). Interestingly, when fluconazole was combined with polymyxin B, the halo surrounding the disc was significantly more clear (Figure 2a and b), an indication of potential fungicidal activity.
Figure 2.
Synergistic interaction between fluconazole and polymyxin B against Candida and Cryptococcus. Discs containing water, polymyxin B, fluconazole and the drug combination were dried and overlaid on a lawn of yeast cells derived from the strains indicated. Cells were incubated for 24 h (Candida species) or 48 h (Cryptococcus). Inhibition of fungal growth in regions surrounding the disc produces a halo. A completely clear halo indicates fungicidal activity. (a) Disc diffusion halo assays of the C. albicans strain SC5314 produced a clear halo when fluconazole and polymyxin B were used in combination. (b) Microscopic observations reveal significant clearing of the zone of inhibition (halo) when fluconazole and polymyxin B were used in combination. The left-hand side of each image shows the edge of the disc. PMB, polymyxin B; FLC, fluconazole.
Because the disc diffusion method was not quantitative in determining the susceptibility to polymyxin B due to the relatively large size of this molecule (mol. wt >1300 Da),48 the synergy of the drug combination was further examined using the microdilution assay. Cells from the microdilution assay after incubation with polymyxin B alone, fluconazole alone or the combination at various concentrations were also plated on drug-free medium to count the cfu for determination of the MFC. As shown in Table 1, the strains showed varied susceptibility to polymyxin B and fluconazole, and there is no apparent correlation between the susceptibility towards polymyxin B and the susceptibility towards fluconazole. Consistent with results from the disc diffusion assays, the C. krusei strain DUMC132.91 and the C. parapsilosis strain MMRL1594 are highly resistant to fluconazole (the susceptibility breakpoint for fluconazole is 8 mg/L according to the CLSI standard). The MFC of polymyxin B for each strain tested is similar to the MIC100 (columns 2 and 3), supporting its fungicidal property. Conversely, the MFC of fluconazole can be much higher than the MIC90 (columns 4 and 5), and complete cell killing is often not achievable. A synergistic fungicidal interaction between polymyxin B and fluconazole was observed against all fungal strains tested (last two columns). Similar synergistic interactions against the C. neoformans H99 strain were also observed for polymyxin B and itraconazole (another azole antifungal), and for polymyxin B and amphotericin B combinations (data not shown).
The susceptibility of the filamentous fungal (mould) pathogens A. fumigatus strain Af293 and R. oryzae strain 99-880 towards polymyxin B alone, itraconazole alone and the drug combination was also examined using the microdilution assay. As shown at the bottom of Table 1, synergistic interactions between itraconazole and polymyxin B were also observed.
The combination of polymyxin B and fluconazole at clinically relevant concentrations is effective against Cryptococcus strains with varied fluconazole resistance
Because the C. neoformans strain H99 is more susceptible to polymyxin B than the Candida strains tested (Table 1), we decided to further examine whether the susceptibility to polymyxin B and to the drug combination is specific to the H99 strain or is a general trait of Cryptococcus. C. neoformans has three serotypes (A, D and the less common AD hybrids). Serotype A causes >95% of cryptococcosis cases, and cryptococcosis caused by serotype A is also more severe.49 Serotype A isolates are in general more resistant to antifungals compared with serotype D.50 Thus, additional clinical and environmental C. neoformans serotype A isolates that are genetically distinct were examined (Table 1). These strains belong to three major C. neoformans molecular types of serotype A (VNI, VNII or VNB) and they are of either a or α mating type (Table 2).51–53 Despite the variations in susceptibility towards polymyxin or fluconazole, the combination of polymyxin B at 2 mg/L and fluconazole at 8 mg/L was able to achieve >99% cell killing for all strains tested except for two strains, Bt81 and A2-102-5, where 93–96% killing was achieved. We also obtained a relatively fluconazole-resistant strain H99FR by culturing susceptible H99 in the presence of sublethal fluconazole for 16 weeks. The strain H99FR showed a 16-fold increase in MIC90 of fluconazole (Table 2) and significantly increased resistance to itraconazole (data not shown). Again, a synergistic interaction against H99FR was observed between polymyxin B and fluconazole, and the combination of polymyxin B at 2 mg/L and fluconazole at 8 mg/L was again effective (Table 2).
Table 2.
In combination with fluconazole, polymyxin B at low concentrations is effective against different Cryptococcus neoformans isolates
| Strains | Genotypea | Sourcea | Virulencea | MIC100 PMB | MFC PMB | MIC90 FLC | MFC FLC | MFC PMB/FLC |
|---|---|---|---|---|---|---|---|---|
| H99 (α)76 | VNI (A1) | clinical | high | 8 | 8 | 1 | 4 | 1–2 |
| C45 (α)51 | VNII | clinical | high | 20 | 24 | 2 | 6 | 2–8 |
| A7-35-23 (α)51 | VNII | environmental | low | 16 | 20 | 4 | >64 | 2–8 |
| C23 (α)51 | VNI (A1) | clinical | high | 8 | 20 | 8 | 64 | 2–8 |
| Bt65 (a)52,53 | VNB (A15) | clinical | ― | 12 | 12 | 8 | 12 | 2–8 |
| Bt31 (α)52,53 | VNB (A4) | clinical | ― | 8 | 8 | 12 | 32 | 2–8 |
| Bt81 (a)52,53 | VNB (A15) | clinical | ― | 24 | 32 | 12 | >64 | 2–8b |
| Bt85 (a)52,53 | VNB (A21) | clinical | ― | 8 | 8 | 12 | >64 | 2–8 |
| A2-102-5 (α)51 | VNI (A2) | environmental | low | 10 | 10 | 16 | 32 | 2–8b |
| Bt70 (a)52,53 | VNB (A19) | clinical | ― | 8 | 8 | 16 | >64 | 2–8 |
| H99FR | VNI (A1) | laboratory | ― | 5 | 6 | 16 | 48 | 2–8 |
| Bt50 (α)52,53 | VNB (A4) | clinical | ― | 6 | 8 | 32 | >64 | 2–8 |
PMB, polymyxin B; FLC, fluconazole.
The highest concentration of fluconazole tested was 64 mg/L.
MICs/MFCs are given in terms of mg/L.
aInformation was obtained from previous reports.51–53 VNI, VNII and VNB indicate the three molecular types of C. neoformans. An upper case ‘A’ followed by a number indicates further genotypic classification based on the amplified fragment length polymorphism (‘AFLP’) genetic typing pattern. The symbol ‘―’ indicates that no information is available.
bThe combination at the indicated concentrations achieved 93%–96% rather than >99% killing.
The combination of polymyxin B and fluconazole displays no adverse effect on the growth and proliferation of HeLa and THP-1 cells when used at a concentration >10 times higher than that required for anti-Cryptococcus activity
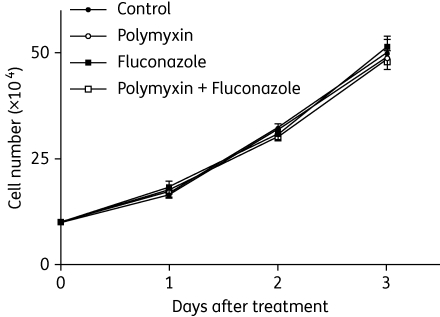
Although both polymyxin B and fluconazole have been used in human populations to treat infectious diseases for years and their toxicity profiles are known, the potential side effects caused by the combination of these two drugs in animals or humans remain unknown. As polymyxin B is accumulated to a much higher level in the kidney (nephrotoxicity), we decided to test the toxicity of the drug combination at higher doses than those required for the anti-Cryptococcus activity in tissue culture. Here, we examined the effect of polymyxin B alone at 40 mg/L (≥20-fold anti-Cryptococcus concentrations), fluconazole alone at 100 mg/L (>12-fold anti-Cryptococcus concentrations) and the combination of both drugs at the above concentrations on the growth of HeLa cells and human monocytic THP-1 cells. None of the treatments displayed any adverse effect on the growth or the morphology of HeLa cells or THP-1 cells (Figure 3 and data not shown).
Figure 3.
The combination does not produce any additional adverse effect on host cells. The growth of human monocytic THP-1 cells was not affected by the combination of polymyxin B sulphate and fluconazole at final concentrations of 40 and 100 mg/L, respectively. For all three timepoints, there are no statistical differences based on Student's t-tests between the control and treatments, with P values no greater than 0.01.
Discussion
Here we showed that the combination of polymyxin B and fluconazole (or itraconazole) at low concentrations is fungicidal against a variety of pathogenic fungal species and also relatively fluconazole-resistant strains, indicating the potential of this combination in treating systemic and localized fungal infections. Because polymyxin B (∼1000 mg/L) is already used in eye drop/ointment and in creams to treat bacterial infections, it could be combined with azole antifungals to treat non-systemic fungal infections, such as fungal keratitis and mucocutaneous candidiasis (oral thrush, vaginal candidiasis) that affect the general as well as the immunocompromised populations.
This drug combination could also potentially be an effective treatment for cryptococcosis that involves the brain. Polymyxin B can penetrate the brain tissue with a low but appreciable efficiency,54,55 and has been used to treat bacterial meningitis when administered intravenously, intrathecally or intraventricularly.35,54,56–62 Thus, it is conceivable that the combination of polymyxin B and fluconazole could be efficacious against cryptococcal meningitis. Even if polymyxin B could not reach a level at which it can act synergistically with fluconazole in the brain tissue when it is administered intravenously, more effective clearance/reduction of the fungal burden in other tissues/organs may exert a sink effect that could help further reduce brain fungal burden. Assessing the efficacy of the drug combination against systemic cryptococcosis with different routes of administration in animal models in the future will provide valuable information regarding the potential efficacy of this drug combination in the clinic.
Based on the well-known mechanism by which polymyxin B kills Gram-negative bacteria, we hypothesize that polymyxin B kills fungi through binding anionic lipids on fungal membrane and disruption of membrane integrity. This cationic heptapeptide with a hydrophobic tail derives its bactericidal activity from its electrostatic interaction with negatively charged lipids (LPS or anionic phospholipids) and its hydrophobic interaction with membrane lipids. These interactions allow polymyxin B to form channels to destroy the integrity of the cytoplasmic membrane.41,63–67 The observation that magainin 2, another antibiotic that is structurally different from polymyxin B but shares a similar mode of action against bacteria,63,68,69 is also fungicidal and acts synergistically with fluconazole against H99 (data not shown) indicates a conserved mechanism of cationic peptide antibiotics against fungi.
The lower efficiency of polymyxin B alone (or other cationic peptide antibiotics such as magainin 2) against eukaryotes compared with bacteria28,42,70,71 could be partly due to the presence of sterols in eukaryotic membrane, as sterols have been shown to reduce the insertion of cationic peptides into anionic mixed membranes to form pores.72 The fact that azole treatment lowers fungal ergosterol levels and renders fungi more susceptible to polymyxin B supports this hypothesis.
Although our toxicity study in cell culture suggests that the specificity of azoles against fungal ergosterol biosynthesis (without affecting mammalian cholesterol biosynthesis) may prevent increased toxicity of the drug combination towards hosts compared with polymyxin B alone, the safety concern of the current formulation of polymyxin B might limit its use in the clinic to treat systemic fungal infections. However, the resurging interest in using polymyxin B to treat bacterial infections caused by multidrug-resistant isolates may drive the development of safer and more effective new formulations. Regardless of the clinical potential of polymyxin B per se in treating fungal infections, given the recent discoveries of cationic peptides as potential antifungals,73–75 investigation into the fungicidal activity of polymyxin B and the synergy between polymyxin B and azoles will probably have a far-reaching impact on the development of novel, effective and safer antifungal therapies.
Funding
This work was supported by: the Department of Biology of Texas A&M University (the startup fund to X. L.); CNP Department of Defense (grant G171IM to X. X.); and National Institutes of Health (grant numbers GM069527 to X. X. and CA115884 to C.-Z. G.).
Transparency declarations
None to declare.
Acknowledgements
We thank Drs David Sullivan and Jun Liu for providing the clinical compound library, Drs Joseph Heitman, Alexander Idnurm and Yun Chang for providing strains, and Drs Ron Morris, Joseph Heitman, Alexander Idnurm, David Sullivan, Gudrun Ihrke and Yun Chang for discussions.
References
- 1.Anaissie EJ. Diagnosis and therapy of fungal infection in patients with leukemia—new drugs and immunotherapy. Best Pract Res Clin Haematol. 2008;21:683–90. doi: 10.1016/j.beha.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Meunier F, Lukan C. The First European Conference on Infections in Leukaemia—ECIL1: a current perspective. Eur J Cancer. 2008;44:2112–7. doi: 10.1016/j.ejca.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Black KE, Baden LR. Fungal infections of the CNS: treatment strategies for the immunocompromised patient. CNS Drugs. 2007;21:293–318. doi: 10.2165/00023210-200721040-00004. [DOI] [PubMed] [Google Scholar]
- 4.Bohme A, Karthaus M. Systemic fungal infections in patients with hematologic malignancies: indications and limitations of the antifungal armamentarium. Chemotherapy. 1999;45:315–24. doi: 10.1159/000007222. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell TG, Perfect JR. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8:515–48. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinbach WJ, Stevens DA. Review of newer antifungal and immunomodulatory strategies for invasive aspergillosis. Clin Infect Dis. 2003;37(Suppl 3):S157–87. doi: 10.1086/376523. [DOI] [PubMed] [Google Scholar]
- 7.van der Horst CM, Saag MS, Cloud GA, et al. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. National Institute of Allergy and Infectious Diseases Mycoses Study Group and AIDS Clinical Trials Group. N Engl J Med. 1997;337:15–21. doi: 10.1056/NEJM199707033370103. [DOI] [PubMed] [Google Scholar]
- 8.Pitisuttithum P, Tansuphasawadikul S, Simpson AJ, et al. A prospective study of AIDS-associated cryptococcal meningitis in Thailand treated with high-dose amphotericin B. J Infect. 2001;43:226–33. doi: 10.1053/jinf.2001.0916. [DOI] [PubMed] [Google Scholar]
- 9.Filioti J, Spiroglou K, Panteliadis CP, et al. Invasive candidiasis in pediatric intensive care patients: epidemiology, risk factors, management, and outcome. Intensive Care Med. 2007;33:1272–83. doi: 10.1007/s00134-007-0672-5. [DOI] [PubMed] [Google Scholar]
- 10.Powderly WG. Recent advances in the management of cryptococcal meningitis in patients with AIDS. Clin Infect Dis. 1996;22(Suppl 2):S119–23. doi: 10.1093/clinids/22.supplement_2.s119. [DOI] [PubMed] [Google Scholar]
- 11.Latgé J-P, Steinbach WJ. Aspergillus fumigatus and Aspergillosis. Washington, DC: ASM Press; 2009. [Google Scholar]
- 12.Longley N, Muzoora C, Taseera K, et al. Dose response effect of high-dose fluconazole for HIV-associated cryptococcal meningitis in southwestern Uganda. Clin Infect Dis. 2008;47:1556–61. doi: 10.1086/593194. [DOI] [PubMed] [Google Scholar]
- 13.Pukkila-Worley R, Mylonakis E. Epidemiology and management of cryptococcal meningitis: developments and challenges. Expert Opin Pharmacother. 2008;9:1–10. doi: 10.1517/14656566.9.4.551. [DOI] [PubMed] [Google Scholar]
- 14.Odds FC, Brown AJ, Gow NA. Antifungal agents: mechanisms of action. Trends Microbiol. 2003;11:272–9. doi: 10.1016/s0966-842x(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 15.Georgopapadakou NH. Antifungals: mechanism of action and resistance, established and novel drugs. Curr Opin Microbiol. 1998;1:547–57. doi: 10.1016/s1369-5274(98)80087-8. [DOI] [PubMed] [Google Scholar]
- 16.Kontoyiannis DP, Mantadakis E, Samonis G. Systemic mycoses in the immunocompromised host: an update in antifungal therapy. J Hosp Infect. 2003;53:243–58. doi: 10.1053/jhin.2002.1278. [DOI] [PubMed] [Google Scholar]
- 17.Lamb D, Kelly D, Kelly S. Molecular aspects of azole antifungal action and resistance. Drug Resist Updat. 1999;2:390–402. doi: 10.1054/drup.1999.0112. [DOI] [PubMed] [Google Scholar]
- 18.Perlin DS. Resistance to echinocandin-class antifungal drugs. Drug Resist Updat. 2007;10:121–30. doi: 10.1016/j.drup.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sar B, Monchy D, Vann M, et al. Increasing in vitro resistance to fluconazole in Cryptococcus neoformans Cambodian isolates: April 2000 to March 2002. J Antimicrob Chemother. 2004;54:563–5. doi: 10.1093/jac/dkh361. [DOI] [PubMed] [Google Scholar]
- 20.Berg J, Clancy CJ, Nguyen MH. The hidden danger of primary fluconazole prophylaxis for patients with AIDS. Clin Infect Dis. 1998;26:186–7. doi: 10.1086/517056. [DOI] [PubMed] [Google Scholar]
- 21.Armengou A, Porcar C, Mascaro J, et al. Possible development of resistance to fluconazole during suppressive therapy for AIDS-associated cryptococcal meningitis. Clin Infect Dis. 1996;23:1337–8. doi: 10.1093/clinids/23.6.1337-a. [DOI] [PubMed] [Google Scholar]
- 22.Paugam A, Dupouy-Camet J, Blanche P, et al. Increased fluconazole resistance of Cryptococcus neoformans isolated from a patient with AIDS and recurrent meningitis. Clin Infect Dis. 1994;19:975–6. doi: 10.1093/clinids/19.5.975-a. [DOI] [PubMed] [Google Scholar]
- 23.Bicanic T, Harrison T, Niepieklo A, et al. Symptomatic relapse of HIV-associated cryptococcal meningitis after initial fluconazole monotherapy: the role of fluconazole resistance and immune reconstitution. Clin Infect Dis. 2006;43:1069–73. doi: 10.1086/507895. [DOI] [PubMed] [Google Scholar]
- 24.Friese G, Discher T, Fussle R, et al. Development of azole resistance during fluconazole maintenance therapy for AIDS-associated cryptococcal disease. AIDS. 2001;15:2344–5. doi: 10.1097/00002030-200111230-00026. [DOI] [PubMed] [Google Scholar]
- 25.Pfaller MA, Diekema DJ, Gibbs DL, et al. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: 10.5-year analysis of susceptibilities of noncandidal yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J Clin Microbiol. 2009;47:117–23. doi: 10.1128/JCM.01747-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicholls MW. Polymyxin sensitivity of Candida tropicalis. J Med Microbiol. 1970;3:529–38. doi: 10.1099/00222615-3-3-529. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz SN, Medoff G, Kobayashi GS, et al. Antifungal properties of polymyxin B and its potentiation of tetracycline as an antifungal agent. Antimicrob Agents Chemother. 1972;2:36–40. doi: 10.1128/aac.2.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falla TJ, Karunaratne DN, Hancock RE. Mode of action of the antimicrobial peptide indolicidin. J Biol Chem. 1996;271:19298–303. doi: 10.1074/jbc.271.32.19298. [DOI] [PubMed] [Google Scholar]
- 29.Xiang X, Zuo W, Efimov VP, et al. Isolation of a new set of Aspergillus nidulans mutants defective in nuclear migration. Curr Genet. 1999;35:626–30. doi: 10.1007/s002940050461. [DOI] [PubMed] [Google Scholar]
- 30.Tsai HF, Wheeler MH, Chang YC, et al. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J Bacteriol. 1999;181:6469–77. doi: 10.1128/jb.181.20.6469-6477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chong CR, Chen X, Shi L, et al. A clinical drug library screen identifies astemizole as an antimalarial agent. Nat Chem Biol. 2006;2:415–6. doi: 10.1038/nchembio806. [DOI] [PubMed] [Google Scholar]
- 32.National Committee for Clinical Laboratory Standards. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts—Second Edition: Approved Standard M27-A2. Wayne, PA, USA: NCCLS; 2002. [Google Scholar]
- 33.Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 34.Moneib NA. In-vitro activity of commonly used antifungal agents in the presence of rifampin, polymyxin B and norfloxacin against Candida albicans. J Chemother. 1995;7:525–9. doi: 10.1179/joc.1995.7.6.525. [DOI] [PubMed] [Google Scholar]
- 35.Kwa A, Kasiakou SK, Tam VH, et al. Polymyxin B: similarities to and differences from colistin (polymyxin E) Expert Rev Anti Infect Ther. 2007;5:811–21. doi: 10.1586/14787210.5.5.811. [DOI] [PubMed] [Google Scholar]
- 36.Srimal S, Surolia N, Balasubramanian S, et al. Titration calorimetric studies to elucidate the specificity of the interactions of polymyxin B with lipopolysaccharides and lipid A. Biochem J. 1996;315:679–86. doi: 10.1042/bj3150679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaara M. Agents that increase the permeability of the outer membrane. Microbiol Rev. 1992;56:395–411. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hancock RE, Chapple DS. Peptide antibiotics. Antimicrob Agents Chemother. 1999;43:1317–23. doi: 10.1128/aac.43.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Storm DR, Rosenthal KS, Swanson PE. Polymyxin and related peptide antibiotics. Annu Rev Biochem. 1977;46:723–63. doi: 10.1146/annurev.bi.46.070177.003451. [DOI] [PubMed] [Google Scholar]
- 40.Teuber M, Bader J. Action of polymyxin B on bacterial membranes. Binding capacities for polymyxin B of inner and outer membranes isolated from Salmonella typhimurium G30. Arch Microbiol. 1976;109:51–8. doi: 10.1007/BF00425112. [DOI] [PubMed] [Google Scholar]
- 41.Hancock RE. Peptide antibiotics. Lancet. 1997;349:418–22. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 42.HsuChen CC, Feingold DS. The mechanism of polymyxin B action and selectivity toward biologic membranes. Biochemistry. 1973;12:2105–11. doi: 10.1021/bi00735a014. [DOI] [PubMed] [Google Scholar]
- 43.Teuber M, Miller IR. Selective binding of polymyxin B to negatively charged lipid monolayers. Biochim Biophys Acta. 1977;467:280–9. doi: 10.1016/0005-2736(77)90305-4. [DOI] [PubMed] [Google Scholar]
- 44.Jandrositz A, Turnowsky F, Hogenauer G. The gene encoding squalene epoxidase from Saccharomyces cerevisiae: cloning and characterization. Gene. 1991;107:155–60. doi: 10.1016/0378-1119(91)90310-8. [DOI] [PubMed] [Google Scholar]
- 45.Anderson JB. Evolution of antifungal-drug resistance: mechanisms and pathogen fitness. Nat Rev Microbiol. 2005;3:547–56. doi: 10.1038/nrmicro1179. [DOI] [PubMed] [Google Scholar]
- 46.Cowen LE. The evolution of fungal drug resistance: modulating the trajectory from genotype to phenotype. Nat Rev Microbiol. 2008;6:187–98. doi: 10.1038/nrmicro1835. [DOI] [PubMed] [Google Scholar]
- 47.Lupetti A, Danesi R, Campa M, et al. Molecular basis of resistance to azole antifungals. Trends Mol Med. 2002;8:76–81. doi: 10.1016/s1471-4914(02)02280-3. [DOI] [PubMed] [Google Scholar]
- 48.Gales AC, Reis AO, Jones RN. Contemporary assessment of antimicrobial susceptibility testing methods for polymyxin B and colistin: review of available interpretative criteria and quality control guidelines. J Clin Microbiol. 2001;39:183–90. doi: 10.1128/JCM.39.1.183-190.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Casadevall A, Perfect JR. Cryptococcus neoformans. Washington, DC: ASM Press; 1998. [Google Scholar]
- 50.Dannaoui E, Abdul M, Arpin M, et al. Results obtained with various antifungal susceptibility testing methods do not predict early clinical outcome in patients with cryptococcosis. Antimicrob Agents Chemother. 2006;50:2464–70. doi: 10.1128/AAC.01520-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Litvintseva AP, Mitchell TG. Most environmental isolates of Cryptococcus neoformans var. grubii (serotype A) are not lethal for mice. Infect Immun. 2009;77:3188–95. doi: 10.1128/IAI.00296-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Litvintseva AP, Marra RE, Nielsen K, et al. Evidence of sexual recombination among Cryptococcus neoformans serotype A isolates in sub-Saharan Africa. Eukaryot Cell. 2003;2:1162–8. doi: 10.1128/EC.2.6.1162-1168.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Litvintseva AP, Thakur R, Vilgalys R, et al. Multilocus sequence typing reveals three genetic subpopulations of Cryptococcus neoformans var. grubii (serotype A), including a unique population in Botswana. Genetics. 2006;172:2223–38. doi: 10.1534/genetics.105.046672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jimenez-Mejias ME, Pichardo-Guerrero C, Marquez-Rivas FJ, et al. Cerebrospinal fluid penetration and pharmacokinetic/pharmacodynamic parameters of intravenously administered colistin in a case of multidrug-resistant Acinetobacter baumannii meningitis. Eur J Clin Microbiol Infect Dis. 2002;21:212–4. doi: 10.1007/s10096-001-0680-2. [DOI] [PubMed] [Google Scholar]
- 55.Jimenez-Mejias ME, Becerril B, Marquez-Rivas FJ, et al. Successful treatment of multidrug-resistant Acinetobacter baumannii meningitis with intravenous colistin sulfomethate sodium. Eur J Clin Microbiol Infect Dis. 2000;19:970–1. doi: 10.1007/s100960000400. [DOI] [PubMed] [Google Scholar]
- 56.Segal-Maurer S, Mariano N, Qavi A, et al. Successful treatment of ceftazidime-resistant Klebsiella pneumoniae ventriculitis with intravenous meropenem and intraventricular polymyxin B: case report and review. Clin Infect Dis. 1999;28:1134–8. doi: 10.1086/514754. [DOI] [PubMed] [Google Scholar]
- 57.Benifla M, Zucker G, Cohen A, et al. Successful treatment of Acinetobacter meningitis with intrathecal polymyxin E. J Antimicrob Chemother. 2004;54:290–2. doi: 10.1093/jac/dkh289. [DOI] [PubMed] [Google Scholar]
- 58.Michalopoulos A, Falagas ME. Colistin and polymyxin B in critical care. Crit Care Clin. 2008;24:377–91. doi: 10.1016/j.ccc.2007.12.003. x. [DOI] [PubMed] [Google Scholar]
- 59.Evans ME, Feola DJ, Rapp RP. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant gram-negative bacteria. Ann Pharmacother. 1999;33:960–7. doi: 10.1345/aph.18426. [DOI] [PubMed] [Google Scholar]
- 60.Levin AS, Barone AA, Penco J, et al. Intravenous colistin as therapy for nosocomial infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clin Infect Dis. 1999;28:1008–11. doi: 10.1086/514732. [DOI] [PubMed] [Google Scholar]
- 61.Garrod LP, O'Grady F, Barber M. Antibiotic and Chemotherapy. Baltimore: Williams and Wilkins; 1971. [Google Scholar]
- 62.Stein A, Raoult D. Colistin: an antimicrobial for the 21st century? Clin Infect Dis. 2002;35:901–2. doi: 10.1086/342570. [DOI] [PubMed] [Google Scholar]
- 63.Savage PB, Li C, Taotafa U, et al. Antibacterial properties of cationic steroid antibiotics. FEMS Microbiol Lett. 2002;217:1–7. doi: 10.1111/j.1574-6968.2002.tb11448.x. [DOI] [PubMed] [Google Scholar]
- 64.Vaara M, Vaara T. Sensitization of Gram-negative bacteria to antibiotics and complement by a nontoxic oligopeptide. Nature. 1983;303:526–8. doi: 10.1038/303526a0. [DOI] [PubMed] [Google Scholar]
- 65.Sanders WE, Jr, Sanders CC. Toxicity of antibacterial agents: mechanism of action on mammalian cells. Annu Rev Pharmacol Toxicol. 1979;19:53–83. doi: 10.1146/annurev.pa.19.040179.000413. [DOI] [PubMed] [Google Scholar]
- 66.Stansly PG, Schlosser ME. Studies on polymyxin: isolation and identification of Bacillus polymyxa and differentiation of polymyxin from certain known antibiotics. J Bacteriol. 1947;54:549–56. doi: 10.1128/jb.54.5.549-556.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li J, Nation RL, Milne RW, et al. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int J Antimicrob Agents. 2005;25:11–25. doi: 10.1016/j.ijantimicag.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 68.Matsuzaki K, Nakamura A, Murase O, et al. Modulation of magainin 2-lipid bilayer interactions by peptide charge. Biochemistry. 1997;36:2104–11. doi: 10.1021/bi961870p. [DOI] [PubMed] [Google Scholar]
- 69.Matsuzaki K, Sugishita K, Harada M, et al. Interactions of an antimicrobial peptide, magainin 2, with outer and inner membranes of Gram-negative bacteria. Biochim Biophys Acta. 1997;1327:119–30. doi: 10.1016/s0005-2736(97)00051-5. [DOI] [PubMed] [Google Scholar]
- 70.Bearer EL, Friend DS. Anionic lipid domains: correlation with functional topography in a mammalian cell membrane. Proc Natl Acad Sci USA. 1980;77:6601–5. doi: 10.1073/pnas.77.11.6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ogita A, Nagao Y, Fujita K, et al. Amplification of vacuole-targeting fungicidal activity of antibacterial antibiotic polymyxin B by allicin, an allyl sulfur compound from garlic. J Antibiot (Tokyo) 2007;60:511–8. doi: 10.1038/ja.2007.65. [DOI] [PubMed] [Google Scholar]
- 72.Mason AJ, Marquette A, Bechinger B. Zwitterionic phospholipids and sterols modulate antimicrobial peptide-induced membrane destabilization. Biophys J. 2007;93:4289–99. doi: 10.1529/biophysj.107.116681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ahmad I, Perkins WR, Lupan DM, et al. Liposomal entrapment of the neutrophil-derived peptide indolicidin endows it with in vivo antifungal activity. Biochim Biophys Acta. 1995;1237:109–14. doi: 10.1016/0005-2736(95)00087-j. [DOI] [PubMed] [Google Scholar]
- 74.Falla TJ, Hancock RE. Improved activity of a synthetic indolicidin analog. Antimicrob Agents Chemother. 1997;41:771–5. doi: 10.1128/aac.41.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garibotto FM, Garro AD, Masman MF, et al. New small-size peptides possessing antifungal activity. Bioorg Med Chem. 2010;18:158–67. doi: 10.1016/j.bmc.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 76.Perfect JR, Lang SD, Durack DT. Chronic cryptococcal meningitis: a new experimental model in rabbits. Am J Pathol. 1980;101:177–94. [PMC free article] [PubMed] [Google Scholar]
- 77.Winzeler EA, Shoemaker DD, Astromoff A, et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999;285:901–6. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- 78.Nierman WC, Pain A, Anderson MJ, et al. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature. 2005;438:1151–6. doi: 10.1038/nature04332. [DOI] [PubMed] [Google Scholar]
- 79.Ma LJ, Ibrahim AS, Skory C, et al. Genomic analysis of the basal lineage fungus Rhizopus oryzae reveals a whole-genome duplication. PLoS Genet. 2009;5:e1000549. doi: 10.1371/journal.pgen.1000549. [DOI] [PMC free article] [PubMed] [Google Scholar]