Abstract
Background
The intracellular and plasma concentrations of HIV protease inhibitors (HPIs) vary widely in vivo. It is unclear whether there is a concentration-dependent effect of HPIs such that at increasing concentration they may either block their own efflux (leading to ‘autoboosting’) or influx (leading to saturability/decreased intracellular accumulation).
Method
The effects of various concentrations (0–30 µM) of lopinavir, saquinavir, ritonavir and atazanavir on the accumulation of [14C]lopinavir, [3H]saquinavir, [3H]ritonavir and [3H]atazanavir, respectively, were investigated in CEMparental, CEMVBL [P-glycoprotein (ABCB1) overexpressing], CEME1000 (MRP1 overexpressing) and in peripheral blood mononuclear cells (PBMCs). We also investigated the effects of inhibitors of ABCB1/ABCG2 (tariquidar), ABCC (MK571) and ABCC1/2 (frusemide), singly and in combination with HPIs, on cellular accumulation.
Results
In all the cell lines, with increasing concentration of lopinavir, saquinavir and ritonavir, there was a significant increase in the cellular accumulation of [14C]lopinavir, [3H]saquinavir and [3H]ritonavir. Tariquidar, MK571 and frusemide (alone and in combination with lopinavir, saquinavir and ritonavir) significantly increased the accumulation of [14C]lopinavir, [3H]saquinavir and [3H]ritonavir. Ritonavir (alone or in combination with tariquidar) decreased the intracellular accumulation of [3H]ritonavir in PBMCs. Atazanavir decreased the accumulation of [3H]atazanavir in a concentration-dependent manner in all of the cells tested.
Conclusions
There are complex and variable drug-specific rather than class-specific effects of the HPIs on their own accumulation.
Keywords: lopinavir, saquinavir, ritonavir, atazanavir, transporters
Introduction
Highly active antiretroviral therapy (HAART) has markedly decreased the morbidity and mortality of HIV-infected individuals. However, despite the improvements associated with HAART, the virus continues to evolve in cellular reservoirs and anatomical sanctuary sites during therapy even when below detectable levels of HIV are achieved in plasma. The causes of persistent HIV infection despite undetectable plasma levels remain incompletely understood but probably include multiple factors such as persistence of virus in cellular reservoirs (e.g. CD4+ T cells and cells of the macrophage lineage) and anatomical sanctuary sites (brain and possibly testis),1–7 pharmacological and poor compliance. Collectively these sites represent a major impediment to the eradication of HIV. Viral sanctuary sites may result from the overexpression, in sites of HIV replication, of membrane-bound drug efflux transporters, e.g. P-glycoprotein (P-gp; ABCB1), multidrug resistance-associated proteins (MRPs; ABCCs) and breast cancer resistance protein (BCRP; ABCG2).
Some studies have provided evidence that HIV protease inhibitors (HPIs), e.g. saquinavir, ritonavir, lopinavir, atazanavir and darunavir, may be reduced by these drug efflux transporters,8–15 which may potentially promote the emergence of mutant viruses. Recent studies by others and us have also shown that organic anion transporting polypeptides (OATPs) may also influence the intracellular accumulation of some HPIs.16,17
HPIs exhibit complex interactions with drug transporters, drug metabolizing enzymes (CYPs) and serum proteins. These complex interactions lend support for the discrepancy between the intracellular concentrations of the HPIs measured in vivo and in vitro/ex vivo.18 Although data on association between genetic polymorphisms in drug metabolizing enzymes and transporter proteins are equivocal, they may explain, in part, the variable and complex plasma and cellular concentrations and treatment outcomes of HIV-infected patients. For example, despite some of the HPIs, e.g. lopinavir, being a substrate for ABCB1 and ABCC,12,19 an earlier retrospective study of HIV-infected patients under antiretroviral therapy found no influence of the ABCB1 C3435T polymorphism on the plasma and peripheral blood mononuclear cell (PBMC) levels of lopinavir (or the non-nucleoside reverse transcriptase inhibitor, efavirenz)20,21 even though polymorphisms at the ABCB1 C3435T and G2677T/A, MRP1 (ABCC1) C218T and G2168A and MRP2 (ABCC2) G1249A have been associated with alterations in ABCB1, ABCC1 and ABCC2 activity.22–26 However, some studies found no association between the concentrations of saquinavir (alone or when boosted with ritonavir), atazanavir or lopinavir and polymorphisms in ABCB1 C3435T and G2677T/A.27,28 Furthermore, recent studies on three common exonic ABCB1 polymorphisms, C1236T, G2677T/A and C3435T, showed that these are poor predictors of the concentrations of lopinavir and ritonavir in saliva, semen and plasma.29 However, there is evidence of some association between G4544A polymorphism in ABCC2 and higher accumulation of lopinavir in PBMCs of HIV-treated patients.21 Similar studies on 74 HIV-infected patients showed significantly higher plasma levels of atazanavir in patients with genotype CC than those with CT or TT for polymorphism at the ABCB1 C3435T.30 Studies in cultured cells showed that the permeability of amprenavir, indinavir, lopinavir and ritonavir was greater in ABCB1 (G1199A) cells than in ABCB1 wt cells, suggesting that ABCB1 G1199A polymorphism may impact on the systemic bioavailability of HPIs.12 Clearly if inter-individual differences in the bioavailability of HPIs is caused by genetic variants of ABCB1, ABCC1 and ABCC2, this may have a profound effect on the pharmacokinetics and pharmacodynamics of substrate drugs.
Inhibition of first-pass metabolism of the HPIs by cytochrome P450 (CYP) enzymes markedly increases the bioavailability of most HPIs and hence their therapeutic efficacy. Thus, ritonavir-boosted HPIs have become part of the standard of care for HIV-infected patients.31–38 Data on the interaction of HPIs are equivocal: we recently showed that amprenavir and atazanavir increased the intracellular accumulation of lopinavir in both cultured and primary human cells, suggesting a potential role of inhibiting ABCC and ABCB1 in boosting the intracellular concentration of some HPIs.19 Furthermore, combinations of HPIs with more potent efflux inhibitors have been shown to increase the brain penetration of HPIs.39–42 However, some combinations of HPIs may not efficiently increase their organ (e.g., brain) permeability.43–45 These complex interactions are accentuated as some HPIs are also known to up-regulate ABCB1, ABCC and CYP expression and function.46–51 Thus, optimum HIV treatment requires careful consideration of these parameters to avoid therapy-limiting drug–drug, drug–transporter and drug–enzyme interactions, and some important data necessary to fully understand the intracellular pharmacology of HPIs are still missing.
Inadequate plasma or intracellular concentrations of antiretrovirals may lead to treatment failure. In order to adequately manage this, dose modification, guided by therapeutic drug monitoring of plasma concentration, is sometimes used as a strategy to address this problem. As HAART involves the concomitant use of multiple drugs, it appears important to evaluate the concentration-dependent effects of these drugs on intracellular accumulation and this was the aim of the present study.
Materials and methods
Reagents
[14C]lopinavir, [3H]saquinavir, [3H]ritonavir and [3H]atazanavir (specific activities of 1.0 Ci/mmol, 1.0 Ci/mmol, 1.1 Ci/mmol and 3.1 Ci/mmol, respectively) were purchased from Moravek Biochemicals (Brea, CA, USA). Lopinavir and ritonavir were donated by Abbott Laboratories (North Chicago, IL, USA). Saquinavir was donated by Roche (Welwyn Garden City, UK) and atazanavir was a gift from Bristol Myers Squibb (Hounslow, UK). Tariquidar was a gift from Xenova Group Plc, (Slough, UK) and MK571 from Alexis Biochemicals (San Diego, CA, USA). CEM, CEMVBL and CEME1000 cell lines were from Dr R. Davey (Bill Walsh Cancer Research Laboratories, Royal North Shore Hospital, Sydney, Australia). PBMCs were from blood buffy coats obtained from the regional blood transfusion centre (Liverpool, UK). All other chemicals were supplied by Sigma Chemical Co. (Poole, UK).
Cell culture
The parental cell line was CEM (a CD4 T cell line). CEMVBL (VBL, P-gp overexpressing) cells were selected using vinblastine. CEME1000 (E1000, MRP1 overexpressing) cells were selected with epirubicin. Cell volume (range 0.8–1 pL) and cell density were measured using a CASY Cell Counter (Sedna Scientific Ltd, Dronfield, Derbyshire, UK). We have previously validated the expression of the transporters in our laboratory.10 The cells were maintained at 37°C and 5% CO2 in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS).
Isolation of PBMCs
PBMCs were isolated from blood buffy coats using Lymphoprep, following the manufacturer's instructions. Cell volume (0.3–0.4 pL) and cell density were measured using a CASY Cell Counter.
Ethics
No ethical approval was required in the collection and use of the blood products from the blood transfusion services.
Effects of various concentrations of unlabelled HPIs on the accumulation of radiolabelled equivalents
CEM and its variant cells (2.5 × 106 cells/mL) and isolated PBMCs (5 × 106 cells/mL) were pre-incubated with various concentrations (0–30 µM) of unlabelled lopinavir, ritonavir, saquinavir and atazanavir for 5 min before the addition of 0.5 µM [14C]lopinavir or 0.1 µM [3H]ritonavir or [3H]saquinavir or [3H]atazanavir in RPMI medium supplemented with 10% FCS. Given that HPIs are inhibitors of drug efflux transporters,9,46,47,52,53 we introduced the pre-incubation step to allow the inhibition of transporter activity by the unlabelled HPIs before the addition of their labelled equivalents. At the end of the initial pre-incubation step, the samples were incubated in 1.5 mL Eppendorf stubs for a further 15–20 min before the assay was terminated, samples processed and analysed as described previously.13 Briefly, after incubating the cells for 10–15 min at 37°C in a water bath, the samples were then centrifuged at 15 000 g for 1 min at 4°C. Then a 100 µL aliquot of the medium was taken from each sample for scintillation counting and the pellets were washed three times in ice-cold phosphate-buffered saline (PBS) before solubilization of the pellets in 100 µL of distilled water for radioactivity counting. Data from the radioactivity counts were expressed as cellular accumulation ratio (CAR), being the ratio of the amount of labelled HPI associated with the cell pellets to the amount in a similar volume of medium after incubation. The cell volumes for CEM and its variant cells and PBMCs ranged from 0.3 to 0.4 pL and 0.8 to 1 pL, respectively.
In order to investigate the mechanism of self-stimulation we also evaluated the effects of adding the radiolabelled HPIs before the unlabelled HPIs (termed co-incubation). Here we pre-incubated the cells with 0.5 µM [14C]lopinavir in RPMI medium supplemented with 10% FCS for 5 min, followed by a further incubation with various concentrations (0–30 µM) of unlabelled lopinavir for 5 min, before the samples were finally incubated for 15 min and later processed as described above. To further characterize the effects of lopinavir on transporter activity, we evaluated the accumulation of [3H]saquinavir and [3H]ritonavir (known substrates of P-gp and MRP) in CEM, CEMVBL and CEME1000 cells in the presence or absence of lopinavir. The cells (2.5 × 106 cells/mL) were incubated with various concentrations (0–30 µM) of unlabelled lopinavir in RPMI medium containing 0.1 µM [3H]saquinavir or 0.1 µM [3H]ritonavir before the samples were further incubated for ∼10–15 min and processed as described.
Effects of tariquidar, MK571 and frusemide (alone and in combination with unlabelled lopinavir) on the accumulation of [14C]lopinavir
As inhibitors of drug efflux transporters such as tariquidar (inhibits ABCB1/ABCG254–56), MK571 (inhibits ABCC10,17,57–59) and frusemide (inhibits ABCC1/213) increased the intracellular accumulation of HPIs,13,19 these agents were employed in the current studies, at the indicated concentrations, to characterize HPI-mediated activity against the cells. Here, CEM and its variant cells were initially pre-treated for 5 min with 1 µM tariquidar (CEM and CEMVBL cells) or 50 µM MK571 (CEM and CEME1000 cells) to inhibit ABCB1 and ABCC, respectively. Thereafter, the cells were incubated without or with 10 µM unlabelled lopinavir. In separate experiments, PBMCs were pre-treated for 5 min without or with 1 µM tariquidar or 50 µM frusemide (previously shown to increase the accumulation of lopinavir in PBMCs19), followed by a further incubation treatment of the cells in the absence or presence of 10 and 30 µM lopinavir for 5 min. Thereafter, 0.5 µM [14C]lopinavir in RPMI medium supplemented with 10% FCS was added and the samples incubated for 15 min before the assay samples were processed as described above.
Data and statistical analyses
Results are expressed as CAR, being the ratio of the amount of labelled HPI associated with the cell pellets to the amount in a similar volume of medium after incubation. Data from all of the experiments were expressed as mean ± SD. The Shapiro–Wilk test was used to assess the distribution of the data, followed by the Kruskal–Wallis test to allow multiple comparisons of drug-treated samples with respective controls. Analyses were performed using Statsdirect statistical software version 2.3.1, 2003 (StatsDirect Ltd, Altrincham, Cheshire, UK). In each case, significance between control and drug-treated means was assumed if P < 0.05.
Results
Unlabelled lopinavir increased the accumulation of [14C]lopinavir in both cultured cells and PBMCs
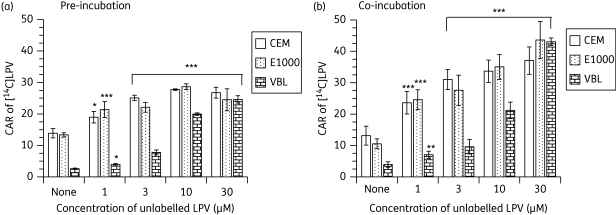
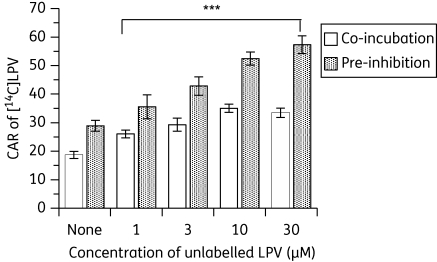
[14C]lopinavir accumulated differentially in CEM and its variant cells, with the following rank order: CEM ≥ CEME1000 ≫ CEMVBL (Figure 1a and b and see Figure 3a). Pre-incubation of the cultured cells with unlabelled lopinavir (1–30 µM) followed by the addition of 0.5 µM [14C]lopinavir significantly (P < 0.001) increased the CAR of [14C]lopinavir in CEM cells, with a concentration-dependent increase observed in CEMVBL cells (Figure 1a). This self-stimulation could be due to inhibition of efflux transporters by unlabelled compound or alternatively could be a true trans-acceleration phenomenon, in which the cycling of an exchange transporter is accelerated by outgoing pre-loaded unlabelled substrate, thus boosting the uptake of radiolabelled compound added later. To investigate the mechanism of self-stimulation we examined the effect of adding the radiolabel before the unlabelled compound. Pre-incubation of cells with [14C]lopinavir followed by various concentrations of unlabelled lopinavir also significantly (P < 0.001) increased the CAR of [14C]lopinavir in a concentration-dependent manner, suggesting that the mechanism is an inhibition of efflux transporters rather than true trans-acceleration (Figure 1b). We also observed that in PBMCs there was a significant increase (P < 0.001) in the CAR of [14C]lopinavir) in a concentration-dependent fashion (Figure 2).
Figure 1.
Effects of (a) pre-incubating CEM, CEME1000 (E1000) and CEMVBL (VBL) cells with various concentrations (0–30 µM) of unlabelled lopinavir (LPV) followed by the addition of [14C]LPV and (b) co-incubating the cells with [14C]LPV followed by the addition of various concentrations (0–30 µM) of unlabelled LPV on the accumulation of [14C]LPV. Bars indicate mean ± SD (n = 4, with four independent observations from cultured CEM and its variant cells). Results are expressed as the CAR, being the ratio of the amount of [14C]LPV associated with the cell pellets to the amount in a similar volume of medium after incubation. P values of *P < 0.05, **P < 0.01 and ***P < 0.001 indicate statistically significant differences in the CAR of [14C]LPV between control and drug-treated samples.
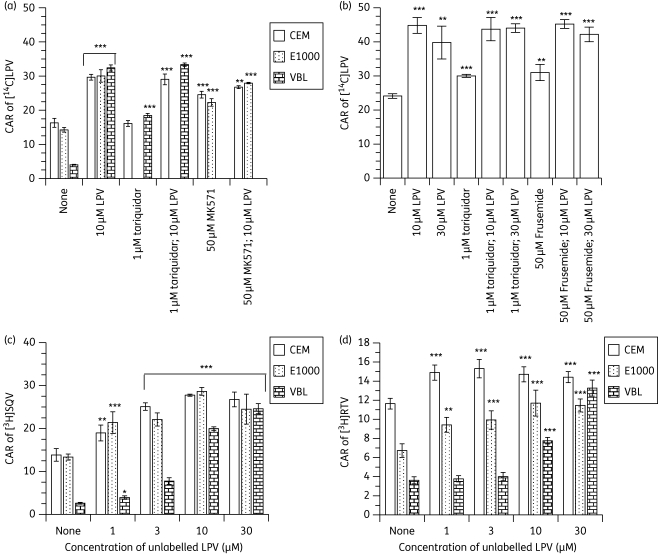
Figure 3.
Effects of (a) pre-incubating CEM, CEME1000 (E1000) and CEMVBL (VBL) cells with fixed concentrations (30 µM) of unlabelled lopinavir (LPV) (alone and in combination with 1 µM tariquidar or 50 µM MK571), (b) co-incubating the PBMCs with 10 and 30 µM unlabelled LPV (alone and in combination with 1 µM tariquidar or 50 µM frusemide) on the accumulation of [14C]LPV, (c) pre-incubating CEM, E1000 and VBL cells with various concentrations (0–30 µM) of unlabelled LPV followed by the addition of [3H]saquinavir (SQV) and (d) pre-incubating CEM, VBL and E1000 cells with various concentrations (0–30 µM) of unlabelled LPV on the accumulation of [3H] ritonavir (RTV). Bars indicate mean ± SD (n = 4, with four independent observations from cultured CEM and its variant cells and n = 4 with four independent observations from each buffy coat PBMC sample). P values of *P < 0.05, **P < 0.01 and ***P < 0.001 indicate statistically significant differences in the CAR of [14C]LPV or [3H]SQV between controls and drug-treated samples. Note that tariquidar/LPV combinations were compared with samples treated with tariquidar alone.
Figure 2.
Effects of pre-incubating PBMCs with various concentrations (0–30 µM) of unlabelled lopinavir (LPV) followed by the addition of [14C]LPV and co-incubating the cells with [14C]LPV followed by various concentrations (0–30 µM) of unlabelled LPV (trans-stimulation) on the accumulation of [14C]LPV. Bars indicate mean ± SD (n = 6, with four independent observations from each buffy coat PBMC sample). P values of *P < 0.05, **P < 0.01 and ***P < 0.001 indicate statistically significant differences in the CAR of [14C]LPV between control and LPV-treated samples.
Tariquidar, MK571 and frusemide (alone and in combination with unlabelled lopinavir) increased the accumulation of [14C]lopinavir
Given that 10 µM unlabelled lopinavir maximally increased the CAR of [14C]lopinavir in the CEM cells (Figure 1), we compared the inhibitory profile of 10 µM unlabelled lopinavir or in combination with known inhibitors of the two drug efflux transporters (tariquidar and MK571, inhibitors of ABCB1/ABCG2 and ABCC, respectively) expressed by the CEM cells. Tariquidar (at 1 µM) significantly (P < 0.001) increased the CAR of [14C]lopinavir in the ABCB1 overexpressing CEMVBL cells, but not in the parental CEM cells, indicating a lack of effect of tariquidar on or low levels of ABCB1 in these cells. In contrast, MK571 significantly (P < 0.001) increased the CAR of [14C]lopinavir in both CEM and CEME1000 cells. Although the CAR of [14C]lopinavir was significantly increased in cells treated with 1 µM tariquidar (P < 0.001) or 50 µM MK571 (P < 0.01) in combination with 10 µM unlabelled lopinavir, the observed increase was identical to that measured in samples treated with 10 µM unlabelled lopinavir alone (Figure 3a). Overall, co-incubation of MK571 or tariquidar with unlabelled lopinavir (at 10 µM) did not enhance the accumulation of [14C]lopinavir over that observed with unlabelled lopinavir alone.
In the PBMCs, the effects of fixed concentrations (10 and 30 µM) of lopinavir were investigated alone and in combination with 1 µM tariquidar or 50 µM frusemide (Figure 3b). Tariquidar and frusemide also significantly (P < 0.001) increased the CAR of [14C]lopinavir, but not as much as that observed with unlabelled lopinavir alone. Tariquidar (at 1 µM) or 50 µM frusemide in combination with unlabelled lopinavir (at 10 and 30 µM) significantly (P < 0.001) increased the accumulation of [14C]lopinavir over and above the increases measured for tariquidar and frusemide alone. However, the levels were identical to those observed in samples treated with unlabelled lopinavir alone (Figure 3b).
To further understand lopinavir-mediated increase in its own accumulation, we investigated its effects on other known substrates of ABCB1 and ABCC. To this end we evaluated the effects of various concentrations (0–30 µM) of unlabelled lopinavir on the accumulation of [3H]saquinavir and [3H]ritonavir in CEM, CEME1000 (ABCC1 overexpressing) and CEMVBL (ABCB1 overexpressing) cells. Data obtained from these manipulations showed that unlabelled lopinavir significantly (P ≤ 0.01) increased the accumulation of [3H]saquinavir and [3H]ritonavir in all of the cells in a concentration-dependent manner (Figure 3c and d).
Ritonavir increased the accumulation of [3H]ritonavir in cultured cells
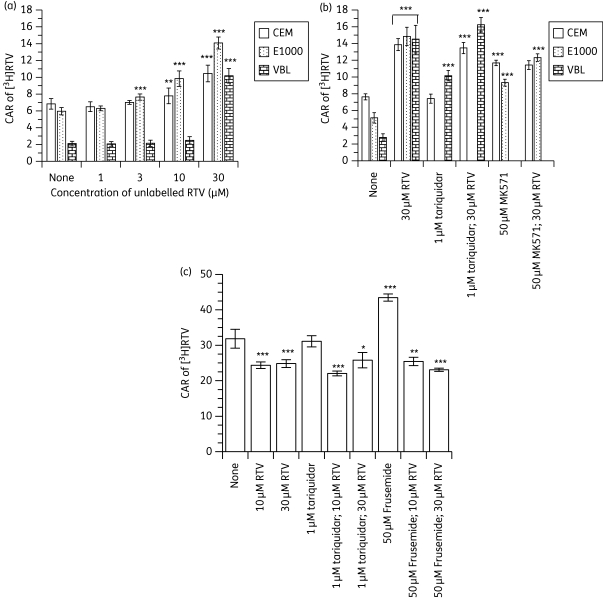
[3H]ritonavir accumulated differentially in CEM cells with the following rank order: CEM > CEME1000 ≫ CEMVBL (Figure 4a and b), indicating that ritonavir is a substrate for ABCB1 and ABCC1. Pre-treatment of the cultured cells with unlabelled ritonavir followed by the addition of [3H]ritonavir significantly (P < 0.001) increased the accumulation of [3H]ritonavir in CEME1000 cells in a concentration-dependent manner. In contrast, unlabelled ritonavir (at 10 and 30 µM) significantly (P ≤ 0.01) increased the CAR of [3H]ritonavir in parental CEM cells at 10 and 30 µM unlabelled ritonavir, whilst only ritonavir (at 30 µM) produced a significant (P < 0.001) increase in the accumulation of [3H]ritonavir in CEMVBL cells (Figure 4a). The differences between CEM cells and CEME1000 cells are significant at concentrations of ritonavir >3 µM (P ≤ 0.01).
Figure 4.
Effects of (a) pre-incubating CEM, CEME1000 (E1000) and CEMVBL (VBL) cells with various concentrations (0–30 µM) of unlabelled ritonavir (RTV), (b) fixed concentrations (30 µM) of RTV (alone and in combination with 1 µM tariquidar or 50 µM MK571) and (c) pre-incubating the PBMCs with 10 and 30 µM unlabelled RTV (alone and in combination with 1 µM tariquidar or 50 µM frusemide) on the accumulation of [3H]RTV. Bars indicate mean ± SD (n = 4, with four independent observations from cultured CEM and its variant cells and n = 4 with four independent observations from each buffy coat PBMC sample). P values of *P < 0.05, **P < 0.01 and ***P < 0.001 indicate statistically significant differences in the CAR of [3H]RTV between controls and drug-treated samples. Note that tariquidar/RTV combinations were compared with samples treated with tariquidar alone, and so on.
Tariquidar, MK571 and frusemide (alone and in combination with unlabelled ritonavir) demonstrated variable effects on the accumulation of [3H]ritonavir
Unlabelled ritonavir (at 30 µM) significantly (P < 0.001) increased the CAR of [3H]ritonavir in CEM, CEMVBL and CEME1000 cells compared with their respective controls (Figure 4b). Tariquidar (at 1 µM) significantly (P < 0.001) increased the accumulation of [3H]ritonavir in CEMVBL, but not in parental CEM cells. In contrast, 50 µM MK571 significantly (P < 0.001) increased the accumulation of [3H]ritonavir in both CEM and CEME1000 cells. Pre-treatment of the cells with 1 µM tariquidar followed by unlabelled ritonavir (at 30 µM) significantly (P < 0.001) increased the CAR of [3H]ritonavir compared with that observed for tariquidar-treated samples alone. However, the levels were identical to those measured for samples treated with unlabelled ritonavir (at 30 µM) alone. Compared with MK571-treated cells, pre-treatment of CEM and CEME1000 cells with 50 µM MK571 in the presence of 30 µM unlabelled ritonavir significantly (P < 0.001) increased the CAR of [3H]ritonavir in CEME1000 cells, but not in the parental CEM line—identical levels of accumulation of [3H]ritonavir were measured in MK571-treated CEM cells compared with samples treated with MK571 in the presence of 30 µM unlabelled ritonavir (Figure 4b).
Unexpectedly, unlabelled ritonavir (at 10 and 30 µM) significantly (P < 0.001) decreased the accumulation of [3H]ritonavir in the PBMCs tested (Figure 4c). Tariquidar did not alter the CAR of [3H]ritonavir, but frusemide significantly (P < 0.001) increased accumulation. Compared with cells treated with 10 or 30 µM unlabelled ritonavir alone, pre-treatment of the cells with 1 µM tariquidar in combination with 10 µM or 30 µM unlabelled ritonavir significantly decreased the CAR of [3H]ritonavir (P < 0.001 for 1 µM tariquidar + 10 µM ritonavir and P < 0.05 for 1 µM tariquidar + 30 µM ritonavir). Pre-treatment of the cells with frusemide in combination with unlabelled ritonavir significantly reduced the CAR of [3H]ritonavir; at both 10 (P < 0.01) and 30 µM (P < 0.001) of unlabelled ritonavir (Figure 4c). Both reductions were identical to that observed with unlabelled ritonavir alone.
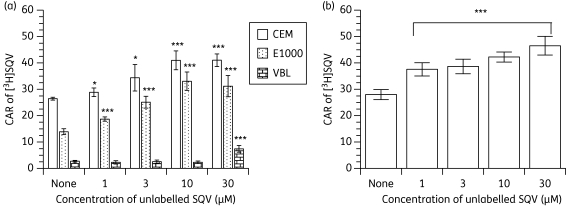
Unlabelled saquinavir increased the accumulation of [3H]saquinavir in both cultured cells and PBMCs
As can be seen in Figure 5(a), saquinavir accumulated differentially in the CEM and its variant cells with the following rank order: CEM≫CEME1000≫CEMVBL. Baseline levels of [3H]saquinavir were also variable in the PBMC samples tested (Figure 5b). Unlabelled saquinavir significantly (P < 0.001) increased the CAR of [3H]saquinavir in CEM, CEME1000 cells (Figure 5a) and in PBMCs (Figure 5b) in a concentration-dependent manner. However, the accumulation of [3H]saquinavir was only significantly (P < 0.001) increased in CEMVBL cells by 30 µM unlabelled saquinavir (Figure 5b).
Figure 5.
Effects of (a) pre-incubating CEM, CEME1000 (E1000) and CEMVBL (VBL) cells with various concentrations (0–30 µM) of unlabelled saquinavir (SQV) followed by the addition of [3H]SQV and (b) pre-incubating PBMCs with various concentrations (0–30 µM) of unlabelled SQV on the accumulation of [3H]SQV. Bars indicate mean ± SD (n = 4, with four independent observations from cultured CEM and its variant cells and n = 5 with four independent observations from each buffy coat PBMC sample). P values of *P < 0.05, **P < 0.01 and ***P < 0.001 indicate statistically significant differences in the CAR of [14C]LPV between control and drug-treated samples.
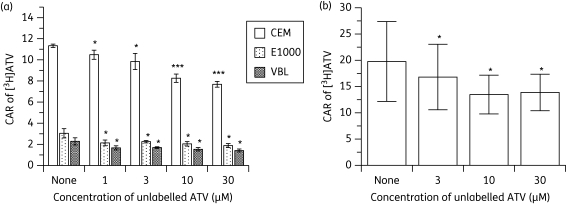
Atazanavir decreased the accumulation of [3H]atazanavir in both cultured cells and PBMCs
[3H]atazanavir accumulated differentially in CEM cells with the following rank order: CEM > CEME1000 ≥ CEMVBL (Figure 6a). Unlabelled atazanavir significantly (P ≤ 0.05) decreased the accumulation of [3H]atazanavir in a concentration-dependent manner in these cells, with the decreases being more marked in the parental CEM line than in its variants. Similarly, unlabelled atazanavir significantly (P < 0.05) decreased the CAR of [3H]atazanavir in PBMCs in a concentration-dependent manner.
Figure 6.
Effects of (a) pre-incubating CEM, CEME1000 (E1000) and CEMVBL (VBL) cells with various concentrations (0–30 µM) of unlabelled atazanavir (ATV) followed by the addition of [3H]ATV and (b) pre-incubating PBMCs with various concentrations (0–30 µM) of unlabelled ATV on the accumulation of [3H]ATV. Bars indicate mean ± SD (n = 4, with four independent observations from cultured CEM and its variant cells and n = 6 with four independent observations from each buffy coat PBMC sample). P values of *P < 0.05, **P < 0.01 and ***P < 0.001 indicate statistically significant differences in the CAR of [14C]LPV between control and drug-treated samples.
Discussion
Given the wide variability in plasma concentrations of HPIs achieved with standard dosing, it is important to understand how the variable plasma concentration impacts on intracellular drug accumulation. This is because being efficient inhibitors, substrates and inducers of some drug efflux proteins and drug metabolizing enzymes,9,46,47,51–53,60–65 there is a complex interaction between HPIs and drug efflux/influx transporters and enzymes, especially if the patients are on other medications.66–69 Indeed alterations in ABCB1 and ABCC2 activity have been associated with single nucleotide polymorphisms in ABCB1 (C3435T and G2677T/A), ABCC1 and ABCC2 (G1249A).22–26
While some studies showed no association between the exposure of HPIs and polymorphisms in ABCB1 C3435T, C1236T and G2677T/A,27–29 some in vitro and in vivo studies found some association between G4544A and G1199A polymorphisms in ABCC2 and ABCB1, respectively and higher accumulation of some HPIs,12,21,30 suggesting that these polymorphisms may impact on the systemic bioavailability of various HPIs that are substrates of ABCB1 and ABCC2.
Clearly, the effect of modifying extracellular drug concentrations on intracellular accumulation requires careful consideration. Here we demonstrate that unlabelled lopinavir pharmaco-enhances its own accumulation in both cultured and primary human cells. The ‘self-enhancement’ measured in CEM, CEME1000 (ABCC1 overexpressing) and CEMVBL (ABCB1 overexpressing) (Figure 1) suggests that lopinavir inhibits various efflux proteins, possibly including ABCB1 and ABCC1. However, since the effects are quite similar in CEM and CEME1000 it is possible that MRP1 is only minimally affected and that other efflux transporters might be involved. Given that PBMCs express ABCB1, ABCG2, ABCC1 and ABCC2,13,19 ‘the self-enhancement’ of the accumulation of [14C]lopinavir by unlabelled lopinavir in these cells (Figure 2) also suggests inhibition of one or more of these efflux proteins. However, there is also evidence that HPIs inhibit ABCG2, but are not substrates of this protein.52
If lopinavir is an inhibitor of ABCB1 and ABCC1 activity, how does its inhibitory profile compare with relatively specific inhibitors of these proteins (e.g. tariquidar and MK571)? To address this question, we compared (i) the inhibitory profiles of unlabelled lopinavir alone with those of tariquidar and MK571 and (ii) the inhibitory effects of unlabelled lopinavir (alone and in combination with tariquidar or MK571). As shown previously,19 we observed that tariquidar and MK571 significantly increased the CAR of [14C]lopinavir in cell lines and primary cells. However, the increase in the CAR of [14C]lopinavir by unlabelled lopinavir alone was markedly higher than that measured for tariquidar- or MK571-treated samples alone (Figure 3a). This is consistent with unlabelled lopinavir having a greater effect at increasing its own accumulation in cells overexpressing ABCB1 and ABCC than tariquidar and MK571, respectively, although this needs to be tested over a wider range of inhibitor concentrations.
Inhibition of efflux proteins leads to an increase in the intracellular accumulation of lopinavir.17,19,70 Here the CAR of [14C]lopinavir increased in cells pre-treated with tariquidar or MK571 in the presence of unlabelled lopinavir and the observed increases were identical to that measured in cells treated with unlabelled lopinavir alone (Figure 3a), suggesting no additional ‘boosting’ of the accumulation of [14C]lopinavir by tariquidar or MK571. In the PBMCs, pre-treatment of the cells with tariquidar or frusemide increased the accumulation of [14C]lopinavir. However, a similar profile of inhibition to that observed in CEM and its variant cells was measured when PBMCs were pre-treated with tariquidar or frusemide in the presence of unlabelled lopinavir (Figure 3a versus b). The observed effects of lopinavir on its own accumulation are consistent with inhibition of ABCB1 and ABCC activity, and supports previous observations that HPIs inhibit drug transporters.19,53 Indeed, the observation that unlabelled lopinavir increased the CAR of [3H]saquinavir and [3H]ritonavir in CEM, CEME1000 (ABCC1 overexpressing) and CEMVBL (ABCB1 overexpressing) cells (Figure 3c and d) provides additional evidence that the observed effects are mediated via ABCB1 and ABCC inhibition.
To extend these observations, we evaluated the effects of unlabelled ritonavir, saquinavir and atazanavir on the accumulation of [3H]ritonavir, [3H]saquinavir and [3H]atazanavir in CEM, its variant cells and PBMCs. The data presented in Figure 4 confirms that ritonavir is a substrate of both ABCB1 and ABCC1:10,71,72 (i) there is differential accumulation of the drug in the ABCC1 (CEME1000) and ABCB1 overexpressing (CEMVBL) cells compared with parental CEM cells (Figure 4a and b); and (ii) both tariquidar and MK571 increased the CAR of [3H]ritonavir in CEMVBL and CEME1000 cells, respectively (Figure 4b). It is also clear that ritonavir inhibits ABCB1 and ABCC1, with unlabelled ritonavir causing an enhancement in the intracellular accumulation of [3H]ritonavir in the cultured cells (Figure 4a). Although cells pre-treated with tariquidar, followed by the addition of unlabelled ritonavir (at 30 µM) to the bathing medium showed a significantly increased CAR of [3H]ritonavir compared with those treated with tariquidar alone, the observed increase was identical to that measured for unlabelled ritonavir alone, suggesting that pre-treatment with tariquidar did not cause a further enhancement in the accumulation of [3H]ritonavir. Similarly, although pre-treatment of the cells with MK571, followed by the addition of unlabelled ritonavir, significantly increased the CAR of [3H]ritonavir (in the CEME1000 cells above that observed for the MK571-treated cells), the overall effect of this manipulation, when compared with samples treated with unlabelled ritonavir alone, was that of an attenuated response (Figure 4b). Investigations using PBMCs showed that unlabelled ritonavir (at 10 and 30 µM) significantly decreased the CAR of [3H]ritonavir (Figure 4c). Overall, tariquidar did not alter the CAR of [3H]ritonavir in the samples tested, but frusemide significantly increased the CAR of [3H]ritonavir. However, co-incubation of the cells pre-treated with tariquidar or frusemide, followed by unlabelled ritonavir (at 10 and 30 µM) resulted in a decrease in the CAR of [3H]ritonavir. However, combination of unlabelled ritonavir (at 10 µM) with frusemide abrogated the increase in CAR of [3H]ritonavir previously measured with frusemide alone. This is possibly due to saturation of intracellular binding sites by high concentrations of unlabelled ritonavir such that inhibition of ABCC is unable to increase the intracellular concentration of radiolabelled ritonavir high enough to displace the unlabelled compound. We provide evidence for differential accumulation of atazanavir in the cultured cells and in PBMCs (Figure 6), supporting previous studies that atazanavir is a substrate of ABCB148,73 and ABCC. Although there is evidence that atazanavir inhibits ABCB1 activity,74 unlabelled atazanavir did not enhance the accumulation of [3H]atazanavir in cells overexpressing ABCB1 (CEMVBL and PBMCs) and ABCC1 (CEME1000 and PBMCs), but caused a concentration-dependent decrease in accumulation. Possible explanations are saturation of accumulation, inhibition/competition for intracellular influx, or activation of efflux. Differences between the HPIs indicate that influx/efflux mechanisms are drug specific rather than class specific.
In summary, we have demonstrated that unlabelled lopinavir and saquinavir enhanced the intracellular accumulation of [14C]lopinavir and [3H]saquinavir, respectively, in cultured cells and in PBMCs, but unlabelled atazanavir decreased the accumulation of [3H]atazanavir in both cell types. Unlabelled ritonavir increased the accumulation of [3H]ritonavir in cultured cells, but decreased accumulation in PBMCs. These observations clearly demonstrate a complex relationship between the extracellular and intracellular concentrations of the HPIs, which are drug specific rather than class specific. A better understanding of the relationship between plasma concentration and intracellular concentration in vivo is important when focusing on factors impacting on efficacy or toxicity of HPIs.
Funding
The Royal Irish Academy/Austrian Science Academy (O. J.), the National Institute of Health Research (NIHR; Department of Health), the Northwest Development Agency (NWDA) (S. H. K. and D. J. B.) and the Austrian Science Fund (SFB35) (P. C.) provided project support.
Transparency declarations
D. J. B. and S. H. K. have received grant/research support from Abbott, Bristol-Myers Squibb, GlaxoSmithKline and Roche Pharmaceuticals. They have also served as consultants for Gilead Sciences, GlaxoSmithKline and Vertex. All other authors have no conflicts of interest to declare.
Acknowledgements
We thank the National Institute of Health Research (NIHR; Department of Health) and the Northwest Development Agency (NWDA) for support. We are grateful to Dr Rabia I. Dabo, BM, MRCGP for helpful discussions during the preparation of this manuscript.
References
- 1.Kinman LM, Worlein JM, Leigh J, et al. HIV in central nervous system and behavioral development: an HIV-2287 macaque model of AIDS. AIDS. 2004;18:1363–70. doi: 10.1097/01.aids.0000131307.62828.a1. [DOI] [PubMed] [Google Scholar]
- 2.Cavert W, Notermans DW, Staskus K, et al. Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science. 1997;276:960–4. doi: 10.1126/science.276.5314.960. [DOI] [PubMed] [Google Scholar]
- 3.Gomez E. Hidden HIV reservoirs: will eradication of the virus be possible? Newsline People AIDS Coalit N Y. 1998:18–9. [PubMed] [Google Scholar]
- 4.Pomerantz RJ. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. Biomed Pharmacother. 2001;55:7–15. doi: 10.1016/s0753-3322(00)00016-0. [DOI] [PubMed] [Google Scholar]
- 5.Kinman L, Brodie SJ, Tsai CC, et al. Lipid-drug association enhanced HIV-1 protease inhibitor indinavir localization in lymphoid tissues and viral load reduction: a proof of concept study in HIV-2287-infected macaques. J Acquir Immune Defic Syndr. 2003;34:387–97. doi: 10.1097/00126334-200312010-00005. [DOI] [PubMed] [Google Scholar]
- 6.Saksena NK, Potter SJ. Reservoirs of HIV-1 in vivo: implications for antiretroviral therapy. AIDS Rev. 2003;5:3–18. [PubMed] [Google Scholar]
- 7.Bowman MC, Archin NM, Margolis DM. Pharmaceutical approaches to eradication of persistent HIV infection. Expert Rev Mol Med. 2009;11:e6. doi: 10.1017/S1462399409000970. [DOI] [PubMed] [Google Scholar]
- 8.Kim AE, Dintaman JM, Waddell DS, et al. Saquinavir, an HIV protease inhibitor, is transported by P-glycoprotein. J Pharmacol Exp Ther. 1998;286:1439–45. [PubMed] [Google Scholar]
- 9.Gutmann H, Fricker G, Drewe J, et al. Interactions of HIV protease inhibitors with ATP-dependent drug export proteins. Mol Pharmacol. 1999;56:383–9. doi: 10.1124/mol.56.2.383. [DOI] [PubMed] [Google Scholar]
- 10.Jones K, Bray PG, Khoo SH, et al. P-glycoprotein and transporter MRP1 reduce HIV protease inhibitor uptake in CD4 cells: potential for accelerated viral drug resistance? AIDS. 2001;15:1353–8. doi: 10.1097/00002030-200107270-00004. [DOI] [PubMed] [Google Scholar]
- 11.Huisman MT, Smit JW, Crommentuyn KM, et al. Multidrug resistance protein 2 (MRP2) transports HIV protease inhibitors, and transport can be enhanced by other drugs. AIDS. 2002;16:2295–301. doi: 10.1097/00002030-200211220-00009. [DOI] [PubMed] [Google Scholar]
- 12.Woodahl EL, Yang Z, Bui T, et al. MDR1 G1199A polymorphism alters permeability of HIV protease inhibitors across P-glycoprotein-expressing epithelial cells. AIDS. 2005;19:1617–25. doi: 10.1097/01.aids.0000183626.74299.77. [DOI] [PubMed] [Google Scholar]
- 13.Janneh O, Owen A, Chandler B, et al. Modulation of the intracellular accumulation of saquinavir in peripheral blood mononuclear cells by inhibitors of MRP1, MRP2, P-gp and BCRP. AIDS. 2005;19:2097–102. doi: 10.1097/01.aids.0000194793.36175.40. [DOI] [PubMed] [Google Scholar]
- 14.Janneh O, Chandler B, Hartkoorn R, et al. Intracellular accumulation of efavirenz and nevirapine is independent of P-glycoprotein activity in cultured CD4 T cells and primary human lymphocytes. J Antimicrob Chemother. 2009;64:1002–7. doi: 10.1093/jac/dkp335. [DOI] [PubMed] [Google Scholar]
- 15.Kwan WS, Janneh O, Hartkoorn R, et al. Intracellular ‘boosting’ of darunavir using known transport inhibitors in primary PBMC. Br J Clin Pharmacol. 2009;68:375–80. doi: 10.1111/j.1365-2125.2009.03462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su Y, Zhang X, Sinko PJ. Human organic anion-transporting polypeptide OATP-A (SLC21A3) acts in concert with P-glycoprotein and multidrug resistance protein 2 in the vectorial transport of saquinavir in Hep G2 cells. Mol Pharm. 2004;1:49–56. doi: 10.1021/mp0340136. [DOI] [PubMed] [Google Scholar]
- 17.Janneh O, Hartkoorn RC, Jones E, et al. Cultured CD4T cells and primary human lymphocytes express hOATPs: intracellular accumulation of saquinavir and lopinavir. Br J Pharmacol. 2008;155:875–83. doi: 10.1038/bjp.2008.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ford J, Khoo SH, Back DJ. The intracellular pharmacology of antiretroviral protease inhibitors. J Antimicrob Chemother. 2004;54:982–90. doi: 10.1093/jac/dkh487. [DOI] [PubMed] [Google Scholar]
- 19.Janneh O, Jones E, Chandler B, et al. Inhibition of P-glycoprotein and multidrug resistance-associated proteins modulates the intracellular concentration of lopinavir in cultured CD4 T cells and primary human lymphocytes. J Antimicrob Chemother. 2007;60:987–93. doi: 10.1093/jac/dkm353. [DOI] [PubMed] [Google Scholar]
- 20.Winzer R, Langmann P, Zilly M, et al. No influence of the P-glycoprotein genotype (MDR1 C3435T) on plasma levels of lopinavir and efavirenz during antiretroviral treatment. Eur J Med Res. 2003;8:531–4. [PubMed] [Google Scholar]
- 21.Elens L, Yombi JC, Lison D, et al. Association between ABCC2 polymorphism and lopinavir accumulation in peripheral blood mononuclear cells of HIV-infected patients. Pharmacogenomics. 2009;10:1589–97. doi: 10.2217/pgs.09.88. [DOI] [PubMed] [Google Scholar]
- 22.Mrozikiewicz PM, Seremak-Mrozikiewicz A, Semczuk A, et al. The significance of C3435T point mutation of the MDR1 gene in endometrial cancer. Int J Gynecol Cancer. 2007;17:728–31. doi: 10.1111/j.1525-1438.2007.00821.x. [DOI] [PubMed] [Google Scholar]
- 23.Vicente J, Sinues B, Fanlo A, et al. Polymorphism C3435T of the MDR1 gene in Central Americans and Spaniards. Mol Biol Rep. 2008;35:473–8. doi: 10.1007/s11033-007-9109-z. [DOI] [PubMed] [Google Scholar]
- 24.Hemauer SJ, Nanovskaya TN, Abdel-Rahman SZ, et al. Modulation of human placental P-glycoprotein expression and activity by MDR1 gene polymorphisms. Biochem Pharmacol. 2010;79:921–5. doi: 10.1016/j.bcp.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haenisch S, May K, Wegner D, et al. Influence of genetic polymorphisms on intestinal expression and rifampicin-type induction of ABCC2 and on bioavailability of talinolol. Pharmacogenet Genomics. 2008;18:357–65. doi: 10.1097/FPC.0b013e3282f974b7. [DOI] [PubMed] [Google Scholar]
- 26.Yin JY, Huang Q, Yang Y, et al. Characterization and analyses of multidrug resistance-associated protein 1 (MRP1/ABCC1) polymorphisms in Chinese population. Pharmacogenet Genomics. 2009;19:206–16. doi: 10.1097/FPC.0b013e328323f680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.la Porte CJ, Li Y, Beique L, et al. The effect of ABCB1 polymorphism on the pharmacokinetics of saquinavir alone and in combination with ritonavir. Clin Pharmacol Ther. 2007;82:389–95. doi: 10.1038/sj.clpt.6100157. [DOI] [PubMed] [Google Scholar]
- 28.Ma Q, Brazeau D, Zingman BS, et al. Multidrug resistance 1 polymorphisms and trough concentrations of atazanavir and lopinavir in patients with HIV. Pharmacogenomics. 2007;8:227–35. doi: 10.2217/14622416.8.3.227. [DOI] [PubMed] [Google Scholar]
- 29.Estrela Rde C, Ribeiro FS, Barroso PF, et al. ABCB1 polymorphisms and the concentrations of lopinavir and ritonavir in blood, semen and saliva of HIV-infected men under antiretroviral therapy. Pharmacogenomics. 2009;10:311–8. doi: 10.2217/14622416.10.2.311. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez Novoa S, Barreiro P, Rendon A, et al. Plasma levels of atazanavir and the risk of hyperbilirubinemia are predicted by the 3435C→T polymorphism at the multidrug resistance gene 1. Clin Infect Dis. 2006;42:291–5. doi: 10.1086/499056. [DOI] [PubMed] [Google Scholar]
- 31.Hsu A, Granneman GR, Bertz RJ. Ritonavir. Clinical pharmacokinetics and interactions with other anti-HIV agents. Clin Pharmacokinet. 1998;35:275–91. doi: 10.2165/00003088-199835040-00002. [DOI] [PubMed] [Google Scholar]
- 32.Sabbatani S, Legnani G, Fulgaro C. First evaluations of LPV/RTV (Kaletra) efficacy on HIV-positive patients treated with multiple drugs. Infez Med. 2003;11:18–24. [PubMed] [Google Scholar]
- 33.Johnson M, Grinsztejn B, Rodriguez C, et al. 96-week comparison of once-daily atazanavir/ritonavir and twice-daily lopinavir/ritonavir in patients with multiple virologic failures. AIDS. 2006;20:711–8. doi: 10.1097/01.aids.0000216371.76689.63. [DOI] [PubMed] [Google Scholar]
- 34.Avihingsanon A, van der Lugt J, Kerr SJ, et al. A low dose of ritonavir-boosted atazanavir provides adequate pharmacokinetic parameters in HIV-1-infected Thai adults. Clin Pharmacol Ther. 2009;85:402–8. doi: 10.1038/clpt.2008.244. [DOI] [PubMed] [Google Scholar]
- 35.Ribera E, Azuaje C, Lopez RM, et al. Atazanavir and lopinavir/ritonavir: pharmacokinetics, safety and efficacy of a promising double-boosted protease inhibitor regimen. AIDS. 2006;20:1131–9. doi: 10.1097/01.aids.0000226953.56976.ad. [DOI] [PubMed] [Google Scholar]
- 36.von Hentig N, Muller A, Rottmann C, et al. Pharmacokinetics of saquinavir, atazanavir, and ritonavir in a twice-daily boosted double-protease inhibitor regimen. Antimicrob Agents Chemother. 2007;51:1431–9. doi: 10.1128/AAC.00854-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi H, Jeong SJ, Lee HS, et al. Two cases of multidrug-resistant human immunodeficiency virus infection treated with atazanavir and lopinavir/ritonavir combination therapy. J Korean Med Sci. 2008;23:737–9. doi: 10.3346/jkms.2008.23.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Heeswijk RP, Veldkamp A, Mulder JW, et al. Combination of protease inhibitors for the treatment of HIV-1-infected patients: a review of pharmacokinetics and clinical experience. Antivir Ther. 2001;6:201–29. [PubMed] [Google Scholar]
- 39.Polli JW, Jarrett JL, Studenberg SD, et al. Role of P-glycoprotein on the CNS disposition of amprenavir (141W94), an HIV protease inhibitor. Pharm Res. 1999;16:1206–12. doi: 10.1023/a:1018941328702. [DOI] [PubMed] [Google Scholar]
- 40.Choo EF, Leake B, Wandel C, et al. Pharmacological inhibition of P-glycoprotein transport enhances the distribution of HIV-1 protease inhibitors into brain and testes. Drug Metab Dispos. 2000;28:655–60. [PubMed] [Google Scholar]
- 41.Edwards JE, Brouwer KR, McNamara PJ. GF120918, a P-glycoprotein modulator, increases the concentration of unbound amprenavir in the central nervous system in rats. Antimicrob Agents Chemother. 2002;46:2284–6. doi: 10.1128/AAC.46.7.2284-2286.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savolainen J, Edwards JE, Morgan ME, et al. Effects of a P-glycoprotein inhibitor on brain and plasma concentrations of anti-human immunodeficiency virus drugs administered in combination in rats. Drug Metab Dispos. 2002;30:479–82. doi: 10.1124/dmd.30.5.479. [DOI] [PubMed] [Google Scholar]
- 43.Huisman MT, Smit JW, Wiltshire HR, et al. P-glycoprotein limits oral availability, brain, and fetal penetration of saquinavir even with high doses of ritonavir. Mol Pharmacol. 2001;59:806–13. doi: 10.1124/mol.59.4.806. [DOI] [PubMed] [Google Scholar]
- 44.van Praag RM, Weverling GJ, Portegies P, et al. Enhanced penetration of indinavir in cerebrospinal fluid and semen after the addition of low-dose ritonavir. AIDS. 2000;14:1187–94. doi: 10.1097/00002030-200006160-00016. [DOI] [PubMed] [Google Scholar]
- 45.Tayrouz Y, Ganssmann B, Ding R, et al. Ritonavir increases loperamide plasma concentrations without evidence for P-glycoprotein involvement. Clin Pharmacol Ther. 2001;70:405–14. doi: 10.1067/mcp.2001.119212. [DOI] [PubMed] [Google Scholar]
- 46.Ford J, Meaden ER, Hoggard PG, et al. Effect of protease inhibitor-containing regimens on lymphocyte multidrug resistance transporter expression. J Antimicrob Chemother. 2003;52:354–8. doi: 10.1093/jac/dkg381. [DOI] [PubMed] [Google Scholar]
- 47.Chandler B, Almond L, Ford J, et al. The effects of protease inhibitors and nonnucleoside reverse transcriptase inhibitors on p-glycoprotein expression in peripheral blood mononuclear cells in vitro. J Acquir Immune Defic Syndr. 2003;33:551–6. doi: 10.1097/00126334-200308150-00001. [DOI] [PubMed] [Google Scholar]
- 48.Zastre JA, Chan GN, Ronaldson PT, et al. Up-regulation of P-glycoprotein by HIV protease inhibitors in a human brain microvessel endothelial cell line. J Neurosci Res. 2009;87:1023–36. doi: 10.1002/jnr.21898. [DOI] [PubMed] [Google Scholar]
- 49.Huang L, Wring SA, Woolley JL, et al. Induction of P-glycoprotein and cytochrome P450 3A by HIV protease inhibitors. Drug Metab Dispos. 2001;29:754–60. [PubMed] [Google Scholar]
- 50.Dixit V, Hariparsad N, Li F, et al. Cytochrome P450 enzymes and transporters induced by anti-human immunodeficiency virus protease inhibitors in human hepatocytes: implications for predicting clinical drug interactions. Drug Metab Dispos. 2007;35:1853–9. doi: 10.1124/dmd.107.016089. [DOI] [PubMed] [Google Scholar]
- 51.Perloff MD, Von Moltke LL, Marchand JE, et al. Ritonavir induces P-glycoprotein expression, multidrug resistance-associated protein (MRP1) expression, and drug transporter-mediated activity in a human intestinal cell line. J Pharm Sci. 2001;90:1829–37. doi: 10.1002/jps.1133. [DOI] [PubMed] [Google Scholar]
- 52.Gupta A, Zhang Y, Unadkat JD, et al. HIV protease inhibitors are inhibitors but not substrates of the human breast cancer resistance protein (BCRP/ABCG2) J Pharmacol Exp Ther. 2004;310:334–41. doi: 10.1124/jpet.104.065342. [DOI] [PubMed] [Google Scholar]
- 53.Storch CH, Theile D, Lindenmaier H, et al. Comparison of the inhibitory activity of anti-HIV drugs on P-glycoprotein. Biochem Pharmacol. 2007;73:1573–81. doi: 10.1016/j.bcp.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 54.Mistry P, Stewart AJ, Dangerfield W, et al. In vitro and in vivo reversal of P-glycoprotein-mediated multidrug resistance by a novel potent modulator, XR9576. Cancer Res. 2001;61:749–58. [PubMed] [Google Scholar]
- 55.Pick A, Muller H, Wiese M. Structure-activity relationships of new inhibitors of breast cancer resistance protein (ABCG2) Bioorg Med Chem. 2008;16:8224–36. doi: 10.1016/j.bmc.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 56.Kuhnle M, Egger M, Muller C, et al. Potent and selective inhibitors of breast cancer resistance protein (ABCG2) derived from the p-glycoprotein (ABCB1) modulator tariquidar. J Med Chem. 2009;52:1190–7. doi: 10.1021/jm8013822. [DOI] [PubMed] [Google Scholar]
- 57.Dallas S, Zhu X, Baruchel S, et al. Functional expression of the multidrug resistance protein 1 in microglia. J Pharmacol Exp Ther. 2003;307:282–90. doi: 10.1124/jpet.103.054304. [DOI] [PubMed] [Google Scholar]
- 58.Honda Y, Ushigome F, Koyabu N, et al. Effects of grapefruit juice and orange juice components on P-glycoprotein- and MRP2-mediated drug efflux. Br J Pharmacol. 2004;143:856–64. doi: 10.1038/sj.bjp.0706008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park S, Sinko PJ. P-glycoprotein and mutlidrug resistance-associated proteins limit the brain uptake of saquinavir in mice. J Pharmacol Exp Ther. 2005;312:1249–56. doi: 10.1124/jpet.104.076216. [DOI] [PubMed] [Google Scholar]
- 60.Perloff MD, von Moltke LL, Fahey JM, et al. Induction of P-glycoprotein expression by HIV protease inhibitors in cell culture. AIDS. 2000;14:1287–9. doi: 10.1097/00002030-200006160-00034. [DOI] [PubMed] [Google Scholar]
- 61.Hennessy M, Clarke S, Spiers JP, et al. Intracellular accumulation of nelfinavir and its relationship to P-glycoprotein expression and function in HIV-infected patients. Antivir Ther. 2004;9:115–22. [PubMed] [Google Scholar]
- 62.Perloff MD, von Moltke LL, Fahey JM, et al. Induction of P-glycoprotein expression and activity by ritonavir in bovine brain microvessel endothelial cells. J Pharm Pharmacol. 2007;59:947–53. doi: 10.1211/jpp.59.7.0006. [DOI] [PubMed] [Google Scholar]
- 63.Usansky HH, Hu P, Sinko PJ. Differential roles of P-glycoprotein, multidrug resistance-associated protein 2, and CYP3A on saquinavir oral absorption in Sprague-Dawley rats. Drug Metab Dispos. 2008;36:863–9. doi: 10.1124/dmd.107.017483. [DOI] [PubMed] [Google Scholar]
- 64.Knox TA, Oleson L, von Moltke LL, et al. Ritonavir greatly impairs CYP3A activity in HIV infection with chronic viral hepatitis. J Acquir Immune Defic Syndr. 2008 doi: 10.1097/qai.0b013e31818c7efe. [DOI] [PubMed] [Google Scholar]
- 65.Walubo A. The role of cytochrome P450 in antiretroviral drug interactions. Expert Opin Drug Metab Toxicol. 2007;3:583–98. doi: 10.1517/17425225.3.4.583. [DOI] [PubMed] [Google Scholar]
- 66.Hennessy M, Kelleher D, Spiers JP, et al. St Johns wort increases expression of P-glycoprotein: implications for drug interactions. Br J Clin Pharmacol. 2002;53:75–82. doi: 10.1046/j.0306-5251.2001.01516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patel J, Buddha B, Dey S, et al. In vitro interaction of the HIV protease inhibitor ritonavir with herbal constituents: changes in P-gp and CYP3A4 activity. Am J Ther. 2004;11:262–77. doi: 10.1097/01.mjt.0000101827.94820.22. [DOI] [PubMed] [Google Scholar]
- 68.Busti AJ, Hall RG, Margolis DM. Atazanavir for the treatment of human immunodeficiency virus infection. Pharmacotherapy. 2004;24:1732–47. doi: 10.1592/phco.24.17.1732.52347. [DOI] [PubMed] [Google Scholar]
- 69.Culm-Merdek KE, von Moltke LL, Gan L, et al. Effect of extended exposure to grapefruit juice on cytochrome P450 3A activity in humans: comparison with ritonavir. Clin Pharmacol Ther. 2006;79:243–54. doi: 10.1016/j.clpt.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 70.Agarwal S, Pal D, Mitra AK. Both P-gp and MRP2 mediate transport of Lopinavir, a protease inhibitor. Int J Pharm. 2007;339:139–47. doi: 10.1016/j.ijpharm.2007.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van der Sandt IC, Vos CM, Nabulsi L, et al. Assessment of active transport of HIV protease inhibitors in various cell lines and the in vitro blood–brain barrier. AIDS. 2001;15:483–91. doi: 10.1097/00002030-200103090-00007. [DOI] [PubMed] [Google Scholar]
- 72.Meaden ER, Hoggard PG, Newton P, et al. P-glycoprotein and MRP1 expression and reduced ritonavir and saquinavir accumulation in HIV-infected individuals. J Antimicrob Chemother. 2002;50:583–8. doi: 10.1093/jac/dkf161. [DOI] [PubMed] [Google Scholar]
- 73.Bousquet L, Roucairol C, Hembury A, et al. Comparison of ABC transporter modulation by atazanavir in lymphocytes and human brain endothelial cells: ABC transporters are involved in the atazanavir-limited passage across an in vitro human model of the blood-brain barrier. AIDS Res Hum Retroviruses. 2008;24:1147–54. doi: 10.1089/aid.2007.0022. [DOI] [PubMed] [Google Scholar]
- 74.Perloff ES, Duan SX, Skolnik PR, et al. Atazanavir: effects on P-glycoprotein transport and CYP3A metabolism in vitro. Drug Metab Dispos. 2005;33:764–70. doi: 10.1124/dmd.104.002931. [DOI] [PubMed] [Google Scholar]