Abstract
We confirmed circulation of human metapneumovirus (HMPV) among children with febrile and respiratory illness in an urban slum in Dhaka, Bangladesh, during active surveillance in 2001. HMPV was the most common single virus identified among febrile children and appears to contribute to the high rates of illness in this population.
Keywords: Human metapneumovirus, pneumonia, children, paramyxoviridae, Dhaka, Bangladesh, dispatch
Human metapneumovirus (HMPV) is the newest member of the family Paramyxoviridae, in the subfamily Pneumovirinae, shared with respiratory syncytial virus (RSV) (1). It appears to have 2 distinct genetic subgroups (2,3). HMPV was first described in a population of children in the Netherlands in 2001 (1) and has subsequently been linked with lower respiratory tract illness (LRTI) in children and adults (2,4). Although HMPV independently contributes to LRTI, some studies report more severe cases when HMPV is a coinfectant with RSV (5,6) or influenza (7); other studies have found no synergy (3).
The Study
As previously reported (8), we undertook fever surveillance in Kamalapur, an urban community in Dhaka used by the International Center for Diarrheal Disease Research, Bangladesh (ICDDR,B) as a field site since 1998. The site has 7 geographic strata and 379 clusters. We randomly selected clusters within strata and enrolled all households within those clusters for surveillance, after obtaining informed written consent.
Field research assistants (FRAs) screened for fever across all ages among 5,000 households once weekly using standardized calendar questionnaires. FRAs referred children <13 years of age who reported fever for any duration, or anyone >13 years who reported fever for >3 days, to our onsite clinic where study physicians conducted standardized history and physical examinations. If an axillary temperature of >38°C was confirmed, physicians collected 3–5 mL of blood from children <5 years and persons >5 years, respectively, as well as convalescent blood samples 14 days later. Blood samples were allowed to clot and then centrifuged to obtain serum.
We retrospectively selected serum samples to test for respiratory viruses from patients <13 years of age who had cough for 1–3 days and fever of >38.5°C; we also selected paired serum samples negative for dengue by immunoglobulin M antibody capture (MAC)–ELISA. These samples were sent to the Centers for Disease Control and Prevention (CDC; Atlanta, Georgia, USA) for testing by hemagglutination inhibition for influenza and enzyme immunoassay for RSV; parainfluenza types 1, 2, and 3; adenovirus; and HMPV by using standard methods (9,10). A positive acute HMPV infection was defined as a >4-fold rise in titer between acute-phase and convalescent-phase samples.
Statistical analysis was performed by using StataSE Release 9.2 (StataCorp, College Station, TX, USA). We compared continuous variables between groups by using analysis of variance. For univariate analysis of categorical variables, we used 2 × 2 tables; for multivariate analysis, we used conditional logistic regression to determine strength of association between HMPV infection and potential explanatory covariates to obtain relative odds (RO) and 95% confidence intervals (CIs); p values were obtained by using the Fisher exact test. This study was approved by the research review and ethical review committees of ICDDR,B and the Institutional Review Board of CDC.
From December 6, 2000, through December 5, 2001, 889 persons came to our clinic with fever, and blood samples were collected from 888 (99.9%). Of the 889, 775 (84.9%) were self-referred; 114 (93.4%) of 122 were referred by FRAs during the same period. Of the 888 sampled patients, we selected serum samples from 128 children <13 years of age who had paired samples, documented fever >38.5C, cough for 1–4 days before first blood collection, and negative test results for dengue antibodies by MAC-ELISA. These samples were tested by hemagglutination inhibition against influenza virus A (H1N1 and H3N2) and influenza type B. Among these, 107 paired samples had sufficient remaining serum to be tested for other respiratory viruses, including HMPV.
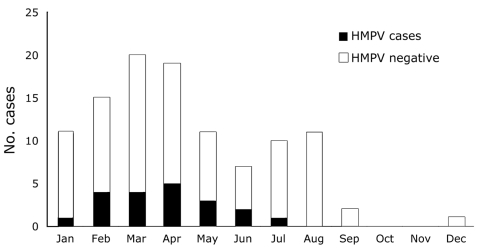
Table 1 shows the distribution of all virus infections detected by serologic testing of 107 paired specimens. Of 60 infections detected among these children, 20 (33.3%) were caused by HMPV, the largest single group after influenza (although more than either influenza A or B alone). HMPV was detected in the dry premonsoon season from January through the end of June (Figure).
Table 1. Viruses detected in children <13 years of age by serology, Kamalampur, Bangladesh, December 2000–December 2001.
| Virus | No. infections | % (N = 60) | Cumulative % |
|---|---|---|---|
| Human metapneumovirus | 20 | 33.3 | 33.3 |
| Respiratory syncytial virus | 3 | 5.0 | 38.3 |
| Adenovirus | 4 | 6.7 | 45.0 |
| Parainfluenza virus 3 | 9 | 15.0 | 60.0 |
| Influenza (H1N1) | 8 | 13.3 | 73.3 |
| Influenza (H3N2) | 2 | 3.3 | 76.6 |
| Influenza B | 14 | 23.3 | 99.9 |
Figure.
Human metapneumovirus (HMPV) infection in children <13 years of age, Kamalapur, Bangladesh, 2001.
We found no demographic differences in subgroup analysis by age group (<5 years and >5 years) or between children with acute HMPV infection and noninfected children (Table 2). Also, no differences were found in the reported history of fever duration or other complaints associated with febrile or respiratory illness in this population before treatment.
Table 2. Demographic and clinical characteristics of children <13 years of age presenting with fever, Kamalampur, Bangladesh, December 2000–December 2001*.
| Variables† | HMPV positive (N = 20) | HMPV negative (N = 87) | Relative odds (95% CI) | p value‡ |
|---|---|---|---|---|
| Mean age, y§ (SD, 95% CI) | 4.5 (2.6; 3.2–5.7) | 4.5 (3.1; 3.9–5.2) | – | 0.951 |
| Mean age for 0–4 age range, y§ (SD, 95% CI) | 2.9 (1.4; 2.1–3.8) | 2.3 (1.2; 2.0–2.7) | – | 0.113 |
| Mean age for 5–12 age range, y§ (SD, 95% CI) |
7.3 (1.9; 5.5–9.0) |
7.7 (1.8; 7.1–8.4) |
– |
0.556 |
| Children <5 y, no. (%) | 13 (65.0) | 52 (59.8) | 1.25 (0.41–4.08) | 0.801 |
| Male gender, no. (%) | 9 (45.0) | 49 (56.3) | 0.63 (0.21–1.89) | 0.457 |
| Duration of fever prior to clinic,§ d (SD, 95% CI) |
3.1 (0.8; 2.7–3.5) |
3.1 (1.7; 2.7–3.4) |
– |
0.983 |
| Symptoms | ||||
| Headache, no. (%) | 8 (40.0) | 34 (39.1) | 1.04 (0.33–3.10) | 1.000 |
| Body pain, no. (%) | 4 (20.0) | 25 (28.7) | 0.62 (0.14–2.20) | 0.580 |
| Rhinorrhea, no. (%) | 16 (80.0) | 60 (69.0) | 1.80 (0.51–8.06) | 0.419 |
| Difficulty breathing, no. (%) | 3 (15.0) | 4 (4.6) | 3.66 (0.48–23.48) | 0.119 |
| Normal activity/behavior, no. (%) | 15 (75.0) | 64 (73.6) | 0.73 (0.22–2.20) | 0.620 |
| Fever,§ °C (SD, 95% CI) | 39.2 (0.6; 39.0–39.5) | 39.1 (0.6; 39.0–39.2) | – | 0.899 |
| High fever (>39°C), no. (%) | 10 (50.0) | 49 (56.3) | 0.78 (0.26–2.32) | 0.627 |
| Respiratory rate§ (SD, 95% CI) | 42 (11; 36–47) | 41 (9; 39–43) | – | 0.808 |
| Crepitations (rales) or wheezing, no. (%) | 11 (55.0) | 28 (32.1) | 2.57 (0.85–7.86) | 0.072 |
| Altered mental status,¶ no. (%) | 5 (25.0) | 8 (9.2) | 3.29 (0.73–13.19) | 0.065 |
| Altered mental status¶ if <5 y#, no. (%) |
5 (38.5) |
6 (11.5) |
4.79 (0.90–23.86) |
0.035 |
| Pneumonia/LRTI diagnosis, no. (%) | 8 (40.0%) | 14 (16.1) | 3.48 (1.02–11.24) | 0.029 |
*HPMV, human metapneumovirus; CI, confidence interval; SD, standard deviation; LRTI, lower respiratory tract illness. †Categorical variables report N persons (percent of persons with characteristic in each group). Compared by 2 x 2 table, odds ratio with 95% CI. ‡2-tailed Fisher exact text. §Continuous variables. Means compared by using Student t test. ¶Irritability, lethargy. #N = 65 children <5 y.
Clinical findings (Table 2) showed no differences in mean fever or proportion of children with high fever (>39°C). However, compared with non-HMPV infection, acute HMPV infection was 3.5 times more likely (95% CI 1.02–11.24) to be associated with clinical pneumonia in all children and 4.8 times more likely (95% CI 0.90–23.86) to be associated with altered mental status (irritability/lethargy) in children <5 years old (Table 2). Only 1 child with acute HMPV infection had coinfection with another virus (influenza A).
Conclusions
To our knowledge, ours is the first reported finding of HMPV in Bangladesh demonstrating substantial contribution of HMPV to febrile and lower respiratory tract illness in children <13 years of age. Our report substantiates that of a study from India (11). In a hospital study conducted in Bangladesh, a virus was isolated in only 33.3% of children with LRTI (12). Although HPMV was unknown at that time, and thus would not have been reported, the contribution of viruses to LRTI in children is often underreported by studies that have focused on bacterial infection identification or that did not include collection of paired serum samples to detect viral infections (3). HMPV has likely been a major factor in pneumonia and bronchiolitis in this population, as it has in others (1,2,11). In this study, HMPV was not only significantly associated with pneumonia, but with lethargy, an indicator of severe pneumonia in young children. Given the high rates of illness and death from pneumonia in this population (8), this association has important implications for disease control strategies. HMPV was also found in the dry pre-Monsoon season, when incidence of pneumonia peaks in this population. Similarly, parainfluenza peaks from March–April. In contrast, influenza occurs before and during the Monsoon season (March–August).
Our pilot study to assess the possible effects of HMPV on LRTI in children in Bangladesh had the following limitations: 1) healthy control patients were not included in the study; 2) the study was not originally designed to look for respiratory viruses; 3) fever was a main selection criterion and may have biased selection of more severe illnesses (a previous pneumonia study indicated that <33% of children in this environment with severe pneumonia have fever (13), perhaps substantially underestimating HMPV prevalence); 4) the observation period of 1 year may not represent the typical seasonal pattern; 5) case identification was based on serologic test results, and some children may have had a subclinical immune response or acute-phase samples may have been collected too late to observe a significant rise in titer, thus underestimating prevalence of disease; and 6) the study included insufficient cases to analyze viral interaction. To more clearly define the role of HMPV and other respiratory viruses in this population, and to improve disease control strategies, fever surveillance targeting a broader range of clinical syndromes over a sustained period is needed.
Acknowledgments
This study was funded by the International Center for Tropical Disease Research of the National Institutes of Health, Bethesda, Maryland, USA; by a cooperative agreement from the US Agency for International Development (HRN-A-00-96-90005-00); and by core donors to ICDDR,B.
Biography
Dr Brooks is a specialist in pediatrics and preventive medicine, head of the Infectious Diseases Unit in the Division of Health Systems and Infectious Diseases at ICDDR,B in Dhaka, Bangladesh, and a faculty member of the Bloomberg School of Public Health at Johns Hopkins University in Baltimore, Maryland, USA. His interests involve surveillance and intervention studies on infectious diseases such as acute respiratory diseases, typhoid fever, and dengue.
Footnotes
Suggested citation for this article: Brooks WA, Erdman D, Terebuh P, Klimov A, Goswami D, Sharmeen AT, et al. Human metapneumovirus infection among children, Bangladesh. Emerg Infect Dis [serial on the Internet]. 2007 Oct [date cited]. Available from http://www.cdc.gov/EID/content/13/10/1611.htm
References
- 1.van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–24. 10.1038/89098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crowe JE Jr. Human metapneumovirus as a major cause of human respiratory tract disease. Pediatr Infect Dis J. 2004;23(Suppl):S215–21. 10.1097/01.inf.0000144668.81573.6d [DOI] [PubMed] [Google Scholar]
- 3.Williams JV, Harris PA, Tollefson SJ, Halburnt-Rush LL, Pingsterhaus JM, Edwards KM, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443–50. 10.1056/NEJMoa025472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams JV. Human metapneumovirus: an important cause of respiratory disease in children and adults. Curr Infect Dis Rep. 2005;7:204–10. 10.1007/s11908-005-0036-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greensill J, McNamara PS, Dove W, Flanagan B, Smyth RL, Hart CA. Human metapneumovirus in severe respiratory syncytial virus bronchiolitis. Emerg Infect Dis. 2003;9:372–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semple MG, Cowell A, Dove W, Greensill J, McNamara PS, Halfhide C, et al. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis. 2005;191:382–6. 10.1086/426457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosis S, Esposito S, Niesters HG, Crovari P, Osterhaus AD, Principi N. Impact of human metapneumovirus in childhood: comparison with respiratory syncytial virus and influenza viruses. J Med Virol. 2005;75:101–4. 10.1002/jmv.20243 [DOI] [PubMed] [Google Scholar]
- 8.Brooks WA, Santosham M, Naheed A, Goswami D, Wahed MA, Diener-West M, et al. Effect of weekly zinc supplements on incidence of pneumonia and diarrhea in children younger than 2 years in an urban, low-income population in Bangladesh: randomized controlled trial. Lancet. 2005;366:999–1004. 10.1016/S0140-6736(05)67109-7 [DOI] [PubMed] [Google Scholar]
- 9.Smith NM, Bresee JS, Shay DK, Uyeki TM, Cox NJ, Strikas RA. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(RR–10):1–42. [PubMed] [Google Scholar]
- 10.Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187:785–90. 10.1086/367901 [DOI] [PubMed] [Google Scholar]
- 11.Rao BL, Gandhe SS, Pawar SD, Arankalle VA, Shah SC, Kinikar AA. First detection of human metapneumovirus in children with acute respiratory infection in India: a preliminary report. J Clin Microbiol. 2004;42:5961–2. 10.1128/JCM.42.12.5961-5962.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huq F, Rahman M, Nahar N, Alam A, Haque M, Sack DA, et al. Acute lower respiratory tract infection due to virus among hospitalized children in Dhaka, Bangladesh. Rev Infect Dis. 1990;12(Suppl 8):S982–7. [DOI] [PubMed] [Google Scholar]
- 13.Brooks WA, Yunus M, Santosham M, Wahed MA, Nahar K, Yeasmin S, et al. Zinc for severe pneumonia in very young children: double-blind placebo-controlled trial. Lancet. 2004;363:1683–8. 10.1016/S0140-6736(04)16252-1 [DOI] [PubMed] [Google Scholar]