Abstract
We conducted a population-based survey to estimate the prevalence of Plasmodium falciparum infection among persons older than 1 month in the Artibonite Valley of Haiti during the high malaria transmission season in 2006. Results from PCR for 714 persons showed a prevalence of 3.1% for P. falciparum infection.
Keywords: Malaria, prevalence, Haiti, community survey, dispatch
Lying just 700 miles from the United States, Haiti is 1 of only 2 countries in the Caribbean with endemic transmission of Plasmodium falciparum malaria. Reportedly, 74%–80% of Haiti’s population live in malarious areas <300 m elevation (1,2). Anopheles albimanus has been identified as the vector responsible for nearly all malaria transmission in Haiti (3). However, reliable population-based estimates of the distribution and impact of malaria in Haiti are scarce (4); existing data are primarily from confirmed malaria cases reported through the health system. The seasonal peak in malaria transmission typically occurs from November through January, following the main rainy season (5). We conducted a population-based survey to estimate the prevalence of P. falciparum infection among persons older than 1 month in the Artibonite Valley of Haiti during the high transmission season in 2006.
The Study
This research was conducted in the Artibonite Valley. Urban areas were excluded. This site was chosen because of its low altitude and abundant rainfall, as well as the large number of malaria cases historically seen at hospitals in the area (5–7). The Artibonite Valley is heavily farmed; 80% of the area is irrigated for cultivation of rice and other crops.
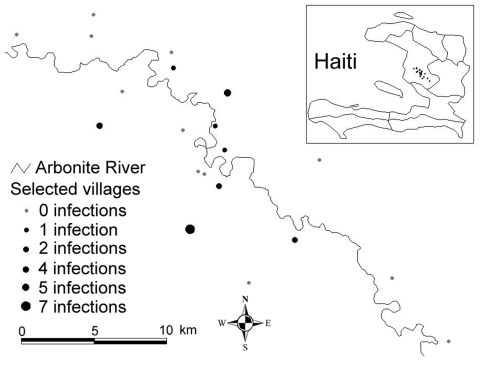
A 2-stage cluster design, probability proportional to cluster size, was used to generate a probability sample of 200 households within the study area; 20 primary sampling units were selected at the first stage and 10 households at the second stage (Figure 1). Fieldwork was conducted by trained data collectors from November 20 to December 10, 2006. Ethical approval was obtained from Tulane University and Hôpital Albert Schweitzer (HAS).
Figure 1.
Map of selected villages for estimating the prevalence of Plasmodium falciparum infection, including number of infections identified within each village, Artibonite Valley, Haiti, 2006.
After informed consent was obtained, blood samples were collected and axillary temperature determined for all persons older than 1 month within each selected household. Thick and thin blood films were prepared for each person, as well as 4 blots of blood on filter paper for PCR. Up to 3 return visits were made to each household to limit nonresponse. Basic personal and household demographic data were collected through an interview with the designated head of household; a standardized questionnaire was used for all 200 households eligible for inclusion in the survey.
Using standard methods (8), a trained laboratory technician at HAS interpreted the malaria blood slides. Positive persons were treated with chloroquine. Filter paper blots were transported back to the laboratory at Tulane University for PCR analysis to test for P. falciparum parasites. Blood samples on filter paper from microscopy-confirmed infections and respondents with temperatures >37.5°C were analyzed individually by PCR for P. falciparum. Pooled PCR analysis of 10 samples was used to detect P. falciparum infections in filter paper samples from respondents with negative microscopy results; positive pools were then analyzed individually.
Positive specimens were identified on the basis of PCR for conserved sequences in 18S small subunit RNA, with a single reverse primer for all Plasmodium species and a P. falciparum–specific forward primer (9); expected amplicon size was 276 bp for P. falciparum. The positive control contained DNA from a culture of the P. falciparum Haiti strain. The negative control contained water instead of DNA. The amplified electrophoresis products were visualized on a 1% agarose gel and stained with ethidium bromide.
Prevalence of P. falciparum infection was calculated as the proportion of sampled persons with a positive PCR result divided by the number of persons who provided blood samples. All point estimates were weighted, with empirically estimated standard errors used to account for clustering.
A total of 804 persons older than 1 month were eligible for inclusion in the survey; 714 agreed to provide a blood sample. This resulted in a nonresponse rate of 11.2% for estimating malaria parasite prevalence. Ages of persons in the sample ranged from <1 to 92 years; 46.1% were male. Ninety-one children <5 years of age (12.7%) were included in the sample. A total of 8.6% of the persons were considered febrile (axillary temperature >37.5°C).
Microscopy at HAS identified 7 malaria infections among the 714 persons who had provided a blood sample; all were confirmed by PCR. Diagnosis by individual and pooled PCR of the remaining blood samples on filter paper identified an additional 16 P. falciparum infections, totaling 23. Thus the total prevalence was estimated to be 3.1% (95% confidence interval 0.60%–5.7%) (Table). The resulting sensitivity and specificity of microscopy were 30.5% and 100%, respectively. Persons with infections ranged in age from 1 to 62 years; 65.2% were male. A total of 14.2% of febrile persons had positive malaria results, compared with only 2.1% of nonfebrile persons. Of the 20 villages included in the sample, all 23 persons with malaria infections came from 8 villages, which were 26–319 m above sea level.
Table. Malaria parasite prevalence by demographic characteristics, Artibonite Valley, Haiti, 2006.
| Characteristic | No. malaria infections identified* | No. respondents tested | Parasite prevalence, %† |
|---|---|---|---|
| Age, y | |||
| <5 | 2 | 91 | 2.2 |
| 5–9 | 5 | 100 | 4.8 |
| 10–19 | 2 | 186 | 1.5 |
| 20–29 | 6 | 95 | 4.7 |
| 30–39 | 2 | 59 | 3.8 |
| 40–49 | 3 | 69 | 4.0 |
| 50–59 | 1 | 42 | 5.1 |
| >60 | 1 | 50 | 1.6 |
| Unknown | 1 | 22 | 3.5 |
| Sex | |||
| Male | 15 | 329 | 3.9 |
| Female | 8 | 385 | 2.3 |
| Temperature | |||
| Febrile (>37.5°C)‡ | 9 | 61 | 14.2 |
| Nonfebrile | 14 | 647 | 2.1 |
| Total | 23 | 714 | 3.1§ |
*Plasmodium falciparum infections only. Results based on PCR, which includes all microscopy-confirmed infections. †Prevalence point estimates are weighted. ‡Sample size for febrile versus nonfebrile; 6 missing data records. §95% confidence interval 0.6–5.7.
Conclusions
Results from the survey show the prevalence of P. falciparum infection to be 3.1% in this area of Haiti. To our knowledge, this is the first population-based estimate of malaria parasite prevalence in Haiti that used PCR diagnosis. Among febrile persons, whose prevalence was 14.2%, our results are substantially higher than previous estimates from passive surveillance of suspected malaria case-patients (5,6,10).
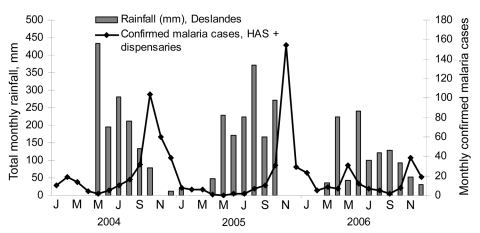
While moderate, a 3.1% prevalence represents a substantial level of illness, especially when one considers that the severity of the disease is likely high given the low level of acquired immunity among the Haitian population. Furthermore, based on passive case detection of confirmed malaria cases identified by HAS in the Artibonite Valley from 2004–2006 (Figure 2), transmission in 2006 appears to have been substantially lower than in previous years. Thus the population-based prevalence estimate of 3.1% likely represents the lower bound of the malaria impact in this area.
Figure 2.
Microscopy-confirmed malaria cases at Hôpital Albert Schweitzer (HAS) and total monthly rainfall, 2004–2006, Deslandes, Artibonite Valley, Haiti.
Malaria transmission was highly localized; all 23 infections were in persons from 8 villages (40%), which suggests that transmission is potentially based on a set of discrete ecologic determinants (Figure 1). Such clustering is consistent with the observed tendency for Anopheles mosquitoes to overdisperse (11). Surprisingly, 7 (30%) of the 23 infections were in persons from a village 319 m above sea level, although the exact location of inoculation cannot be confirmed. If transmission occurred at this elevation, it is above what has commonly been understood as the upper bound for transmission in Haiti.
The observed low level of sensitivity of microscopy compared with that of PCR for identifying P. falciparum infections is similar to findings observed elsewhere (12–14). We surmise that such a low level of sensitivity was attributable to 2 factors: 1) many of the infections likely occurred at low parasite densities, and 2) the laboratory technician was responsible for reading a large number of slides with low parasite prevalence over a relatively short period.
We argue that future malaria interventions in Haiti should be directed toward controlling malaria in the context of a moderate transmission setting; thus, large-scale distribution of insecticide-treated nets or widespread use of indoor residual spraying may be less cost-effective than enhanced surveillance with effective case management or focused larval control. A key aspect of future research in Haiti should therefore focus on understanding treatment-seeking behavior, barriers to accessing health services among febrile persons, and quantifying patterns of malaria transmission.
Acknowledgments
We are grateful to Matt Ward, the data collection team at Hôpital Albert Schweitzer, the population of the Artibonite Valley, and the Haitian Ministry of Health for allowing us to conduct this research in Haiti.
This research was funded in part by US Agency for International Development–Haiti, through a subcontract with RTI International.
Biography
Dr Eisele is an assistant professor in the Department of International Health and Development, Tulane University School of Public Health and Tropical Medicine. His areas of interest include epidemiology, community intervention strategies for controlling malaria, monitoring and evaluation, and sampling methodology.
Footnotes
Suggested citation for this article: Eisele TP, Keating J, Bennett A, Londono B, Johnson D, Lafontant C, et al. Prevalence of Plasmodium falciparum infection in rainy season, Artibonite Valley, Haiti, 2006. Emerg Infect Dis [serial on the Internet]. 2007 Oct [date cited]. Available from http://www.cdc.gov/eid/content/13/10/1494.htm
References
- 1.Garcia-Martin G. Status of malaria eradication in the Americas. Am J Trop Med Hyg. 1972;21:617–33. [DOI] [PubMed] [Google Scholar]
- 2.Duverseau YT, Magloire R, Zevallos-Ipenza A, Rogers HM, Nguyen-Dinh P. Monitoring of chloroquine sensitivity of Plasmodium falciparum in Haiti, 1981–1983. Am J Trop Med Hyg. 1986;35:459–64. [DOI] [PubMed] [Google Scholar]
- 3.Hobbs JH, Sexton JD, St Jean Y, Jacques JR. The biting and resting behavior of Anopheles albimanus in northern Haiti. J Am Mosq Control Assoc. 1986;2:150–3. [PubMed] [Google Scholar]
- 4.Malaria in the Americas. Epidemiol Bull. 1996;17:1–6, 11. [PubMed] [Google Scholar]
- 5.Bonnlander H, Rossignol AM, Rossignol PA. Malaria in central Haiti: a hospital-based retrospective study, 1982–1986 and 1988–1991. Bull Pan Am Health Organ. 1994;28:9–16. [PubMed] [Google Scholar]
- 6.Kachur SP, Nicolas E, Jean-Francois V, Benitez A, Bloland PB, Saint Jean Y, et al. Prevalence of malaria parasitemia and accuracy of microscopic diagnosis in Haiti, October 1995. Rev Panam Salud Publica. 1998;3:35–9. [DOI] [PubMed] [Google Scholar]
- 7.Pan American Health Organization. Health in the Americas: 1998 edition, Vol. II. Washington, DC: The Organization; 2001. [Google Scholar]
- 8.Centers for Disease Control and Prevention. Laboratory identification of parasites of public health concern. May 27, 2003. [cited 2007 Apr 19.] Available from http://www.dpd.cdc.gov/dpdx/html/diagnosticprocedures.htm
- 9.Padley D, Moody AH, Chiodini PL, Saldanha J. Use of a rapid, single-round, multiplex PCR to detect malarial parasites and identify the species present. Ann Trop Med Parasitol. 2003;97:131–7. 10.1179/000349803125002977 [DOI] [PubMed] [Google Scholar]
- 10.Beatty ME, Hunsperger E, Long E, Schurch J, Jain S, Colindres R, et al. Mosquitoborne infections after Hurricane Jeanne, Haiti, 2004. Emerg Infect Dis. 2007;13:308–10. 10.3201/eid1302.061132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keating J, Mbogo C, Mwangani J, Nzovu JG, Gu W, Regens JL, et al. Anopheles gambiae s.l. and Anopheles funestus mosquito distributions at 30 villages along the Kenyan Coast. J Med Entomol. 2005;42:241–6. 10.1603/0022-2585(2005)042[0241:AGSAAF]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.John CC, McHugh MM, Moormann AM, Sumba PO, Ofulla AV. Low prevalence of Plasmodium falciparum infection among asymptomatic individuals in a highland area of Kenya. Trans R Soc Trop Med Hyg. 2005;99:780–6. [DOI] [PubMed] [Google Scholar]
- 13.Coleman RE, Sattabongkot J, Promstaporm S, Maneechai N, Tippayachai B, Kengluecha A, et al. Comparison of PCR and microscopy for the detection of asymptomatic malaria in a Plasmodium falciparum/vivax endemic area in Thailand. Malar J. 2006;5:121. 10.1186/1475-2875-5-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasehagen LJ, Mueller I, McNamara DT, Bockarie MJ, Kiniboro B, Rare L, et al. Changing patterns of Plasmodium blood-stage infections in the Wosera region of Papua New Guinea monitored by light microscopy and high throughput PCR diagnosis. Am J Trop Med Hyg. 2006;75:588–96. [PMC free article] [PubMed] [Google Scholar]