Abstract
Macrophage activation often contributes to the strong immune response elicited upon infection. The ability of macrophages to become activated was discovered when sub-lethal primary infections of mice with the bacterium Listeria monocytogenes provided protection against secondary infections through nonhumoral immunity. L. monocytogenes infect and propagate in macrophages by escaping the phagosome into the cytosol, where they avoid humoral immune mediators. Activated macrophages kill L. monocytogenes by blocking phagosomal escape. The timing of the antimicrobial activities within the phagosome is crucial to the outcome. In non-activated macrophages, bacterial factors generally prevail, and L. monocytogenes can escape from the vacuoles and grow within cytoplasm. Activated macrophages generate reactive oxygen or nitrogen intermediates early after bacterial uptake, which prevent the bacteria from escaping vacuoles into cytoplasm. The heterogeneity in the interactions between L. monocytogenes and the macrophage indicate a complex relationship between the host and the pathogen governed by chemistries that promote and inhibit escape from vacuoles. This review examines the mechanisms used by activated and non-activated macrophages to kill microbes, and how those mechanisms are employed against L. monocytogenes.
Keywords: listeriolysin O, ROI, RNI, NOS2, phagosome maturation, cholesterol-dependent cytolysin, interferon-gamma, Rab5, lysosome
2. INTRODUCTION
2.1. Macrophages and phagocytosis
Macrophages are key mediators in eliciting both innate and adaptive immune responses. Monocytes produced in the bone marrow travel via the circulation to surrounding tissues, where they differentiate into macrophages. Macrophages perform multiple functions, including the phagocytosis and digestion of invading pathogens, presentation of antigen to T lymphocytes, and the production of cytokines that activate various other cell types. Activation of macrophages with soluble stimuli enhances all three of these activities.
Phagocytosis is a process by which macrophages, neutrophils and dendritic cells ingest particles, microbes or apoptotic cells (1). Ingestion and degradation of microbes by macrophages provides a first line of defense in the innate immune response to infection (2). Phagocytosis is also important for antigen processing and presentation, which helps to drive the adaptive immune response.
2.2. Phagosome maturation
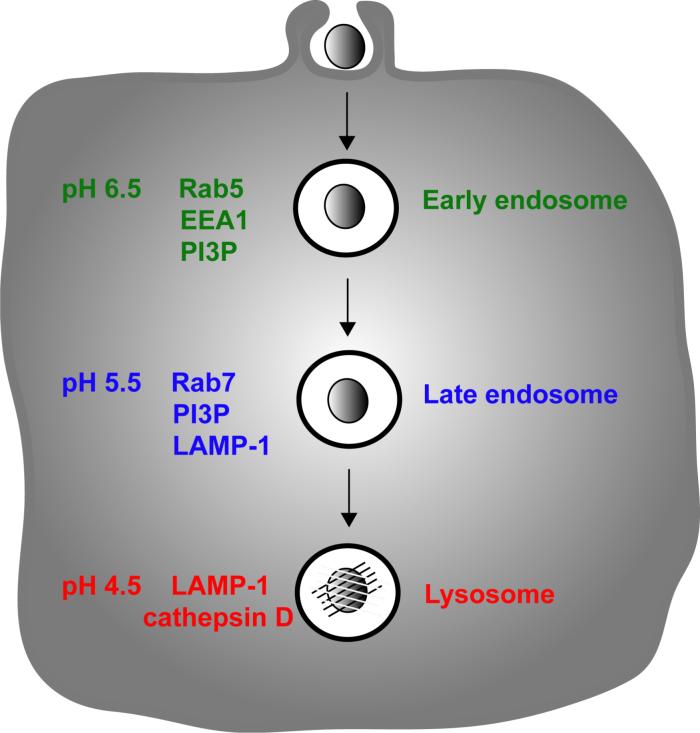
Although the mechanisms of entry are heterogeneous, the signals involved in phagosome maturation are similar for phagosomes containing a wide range of phagocytosed particles. Phagosome maturation in the endocytic pathway involves a series of fusion events (3), which are regulated in part by acidification of the endosome (4) (Figure 1). As vesicular compartments mature inside macrophages their luminal pH decreases, eventually reaching pH 4.5â5.0 after fusion with lysosomes (Figure 1). The acidic pH and the hydrolytic enzymes associated with the lysosomal compartment are toxic to most microorganisms (5, 6).
Figure 1.
Macrophages phagocytose foreign particles into membrane-bounded compartments that undergo fusion events guiding their maturation. After phagosome closure, the phagosome resembles an early endosome that transitions into a late endosome and fuses with the lysosomes.
A number of markers allow characterization of phagosome maturation inside macrophages. Rab GTPases regulate endocytic trafficking through molecular tethering events that precede fusion between endosomal membranous compartments (7, 8). Rab5a and Rab7 are GTPases that coordinate membrane fusion of early and late endosomes, respectively (7, 8) (Figure 1). During endosome or phagosome maturation, Rab7 is recruited to membranes as Rab5a leaves (9, 10). Early Endosome Antigen 1, EEA1, is another early endosomal protein that co-localizes with Rab5 but not Rab7 and is required for endosomal transport (11, 12). Other phagosomal and endosomal markers include phosphatidylinositol 3-phosphate (PI3P) and lysosome-associating membrane protein-1 (LAMP-1), a trans-membrane glycoprotein of late endosomes, trans-Golgi vesicles and lysosomes (Figure 1). Type III PI 3-kinases generate PI3P on phagosomal membranes (13, 14), which subsequently recruits effector proteins that mediate endocytic trafficking and microbial killing (15) (Figure 1). Phagosomes typically mature in a uniform sequence of marker arrival and departure (10).
2.3. Regulation of phagosome superoxide production
Phagosomes also recruit the reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex (phagocyte oxidase or Nox2), which generates the highly reactive superoxide into the phagosomal lumen (16, 17). Superoxide is produced from the electron donor NADPH and the one-electron reduction of oxygen (18). The inactive phagocyte oxidase complex consists of two membrane components, gp91phox and p22phox, and a set of cytosolic components, p47phox, p67phox, p40phox and Rac2 (19). Upon phagocytosis, vesicles containing gp91phox and p22phox fuse with the nascent phagosomal membranes and the cytosolic components associate with the gp91phox and p22phox to form active phagocyte oxidase complexes (19, 20). Luminal superoxide dismutates to various additional reactive oxygen intermediates (ROI) which degrade phagosomal contents and facilitate killing. Patients with chronic granulomatous disease exhibit increased susceptibility to bacterial or fungal infections, likely because their phagocytes are unable to produce superoxide due to deficiencies or mutations in components of the phagocyte oxidase. This indicates the importance of the phagocyte oxidase in the initial response to pathogens (20-23).
The macrophage must regulate the quantity and location of superoxide it produces to prevent damage to itself. Cytosolic superoxide dismutase converts superoxide to hydrogen peroxide (18). The macrophage also produces catalase which dismutates hydrogen peroxide into water and oxygen, a mechanism that limits self-inflicted damage (18). Once inside the phagosome, many pathogens subvert degradation by inhibiting lysosome fusion, or by countering the effects of ROI and related molecules such as reactive nitrogen intermediates (RNI) (24-27). Many bacteria express their own superoxide dismutase that can counteract superoxide generated inside phagosomes (28).
3. LISTERIOSIS
L. monocytogenes is a Gram-positive bacterium found in soil, water, sewage and decaying vegetation that can infect a wide range of animals, including humans (29). Listeriosis in people typically occurs after ingestion of L. monocytogenes in contaminated food, such as pre-packaged meat and cheese (30, 31). Because it can grow at food storage temperatures (4ÂC), L. monocytogenes is a serious concern in the food processing industry. L. monocytogenes affects primarily immuno-compromised individuals, neonates, and pregnant women (29). Infection by foods contaminated with L. monocytogenes can cause gastroenteritis, meningitis, meningoencephalitis, and abortions. Ingested L. monocytogenes reaches the large intestine where it infects intestinal epithelial cells, M cells, dendritic cells and macrophages (32-34). When systemic infection occurs, L. monocytogenes enter the circulation and transit to the liver and spleen, where they infect hepatocytes and macrophages (35, 36). Both innate and acquired immune responses work in concert to control L. monocytogenes infections (31).
4. CELL BIOLOGY OF L. MONOCYTOGENES INFECTION
L. monocytogenes infection of mice has provided a good model system to study innate and adaptive immune responses. Bacteria are internalized by the host cell into a phagosome, or vacuole, from which they escape into the cytosol, grow, and spread to neighboring cells without lysing the primary host cells (Figure 2). Upon entering the neighboring cell, the bacterium occupies a double membrane-bounded vacuole, which it lyses to enter the cytosol for continued growth. The distinct intracellular life-cycle of L. monocytogenes allows the bacterium both to escape the harsh environment of the phagosome and to evade humoral defenses in the extracellular milieu.
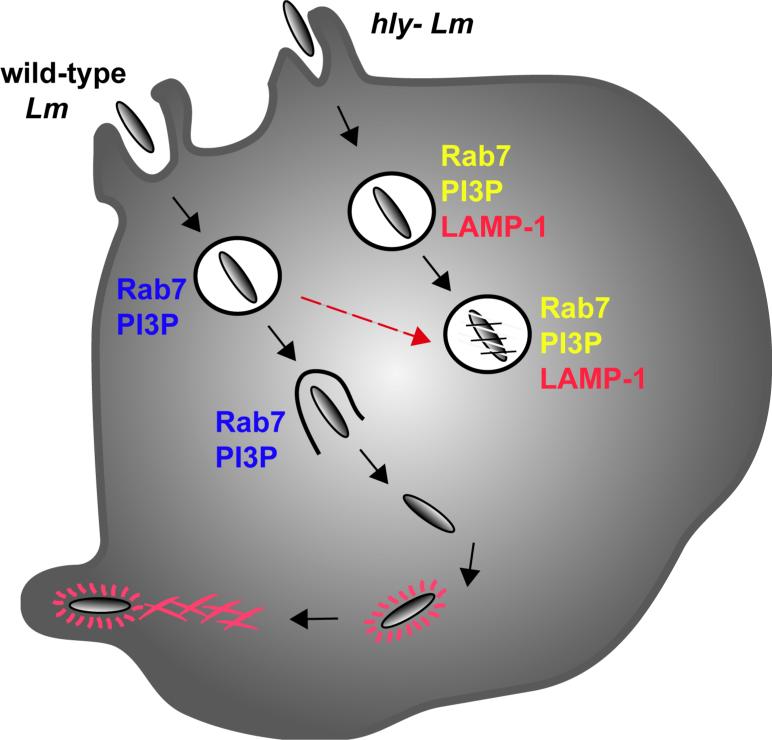
Figure 2.
In RAW 264.7 macrophages, wild-type L. monocytogenes vacuoles recruit and escape from Rab7-and PI3P- positive compartments. Wild-type L. monocytogenes also delay lysosome fusion, relative to hly-L. monocytogenes vacuoles. hly- L. monocytogenes vacuoles also label with Rab7 and PI3P. They eventually also acquire LAMP-1, an indication of their failure to escape.
L. monocytogenes secretes many virulence factors that contribute to its proliferation in epithelial cells and macrophages. Escape from the vacuole requires a pore-forming cytolysin, listeriolysin O (LLO), produced by the bacterium (37-39). LLO is a major determinant of L. monocytogenes pathogenesis, as mutants lacking LLO (hly-) are avirulent in mice due to their inability to escape from vacuoles. When Bacillus subtilis, a Gram-positive bacterium that normally cannot escape vacuoles, was engineered to secrete LLO; it acquired the capacity to escape (40). LLO belongs to a family of cholesterol-dependent cytolysins (CDCs) that are secreted by other Gram-positive bacteria (41). However, LLO is unique among CDCs because of its preferred activity in the vacuole (42). The pore-forming activity of LLO is optimal at pH 5.0 and the protein is unstable at neutral pH (43), which helps to explain its increased activity in acidic vacuolar compartments and the subsequent low cytotoxicity in the cytosol (44). In addition to LLO, L. monocytogenes secrete a phosphatidylinositol-specific phospholipase C (PI-PLC) and a broad range phospholipase C, which contribute to vacuolar escape and pathogenicity (45). L. monocytogenes also secrete ActA, a protein that mediates actin-based motility of bacteria inside host cells (46, 47).
5. MECHANISM OF L. MONOCYTOGENES ESCAPE FROM VACUOLES
Before L. monocytogenes escape into cytoplasm, they disrupt the maturation process of the phagosomal vacuoles that contain them, thereby inhibiting several macrophage antimicrobial processes (Figure 2). Escape from the phagosome occurs within 30 minutes following phagocytosis (48). Therefore, the chemistries of the anti-microbial attack by the macrophage and the response by L. monocytogenes must occur soon after phagocytosis.
5.1. Role of early endosomal GTPase, Rab5a, in L. monocytogenes infection
Early studies indicated that Rab5a, the small GTPase that regulates trafficking of early endosomes, contributes to macrophage resistance to L. monocytogenes. Alvarez-Dominguez and colleagues demonstrated that increased expression of Rab5a in macrophages increased lysosome fusion and subsequent degradation of intracellular L. monocytogenes (49). They also showed that down-modulation of Rab5a blocked lysosome fusion and extended the survival of L. monocytogenes, indicating that Rab5a was important in early endosome fusion events governing phagosome maturation. Later, Prada-Delgado et. al. demonstrated that Rab5a was important in mediating the interferon (IFN)âgamma-induced listericidal activities of macrophages (50). They identified a role for Rab5a in translocation of Rac2 (a component of the phagocyte oxidase) to the L. monocytogenes phagosome, which indicated phagocyte oxidase activation and superoxide production. However, recent studies examining the dynamics of Rab5a-yellow fluorescent protein (YFP) chimeras in RAW 264.7 macrophages found that L. monocytogenes vacuoles do not recruit Rab5a, indicating that even early in infection, L. monocytogenes may disrupt the maturation of the phagosome by excluding Rab5a (Figure 2) (51). Moreover, over-expression of a dominant-negative Rab5a did not affect L. monocytogenes escape from vacuoles (51). Nonetheless, Rab5a has been implicated in macrophage responses to L. monocytogenes (49, 50, 52, 53), so it remains likely that Rab5a contributes in some way to L. monocytogenes pathogenesis.
5.2. Avoidance of late endosomes and lysosomes by L. monocytogenes
L. monocytogenes vacuoles do contain Rab7 and PI3P in RAW 264.7 macrophages, and Rab 7 in J774 macrophages (50, 54) indicating partial maturation of the vacuole (Figure 2) (51). Compared to Fc-gamma receptor - mediated phagocytosis of IgG-opsonized red blood cells, which exhibit transient localization of endocytic markers, the L. monocytogenes vacuoles persist as Rab7- and PI3P- positive compartments, and L. monocytogenes escape from such vacuoles (Figure 2) (10, 51). Wild-type L. monocytogenes, but not hly- L. monocytogenes, delay vacuole fusion with LAMP-1-positive compartments; thus indicating a role for LLO in disrupting vacuole maturation (Figure 2) (51). Earlier studies showed that hly- Lm delay phagosome-lysosome fusion relative to heat-killed hly- Lm, indicating an additional LLO-independent mechanism for delaying lysosome fusion (55).
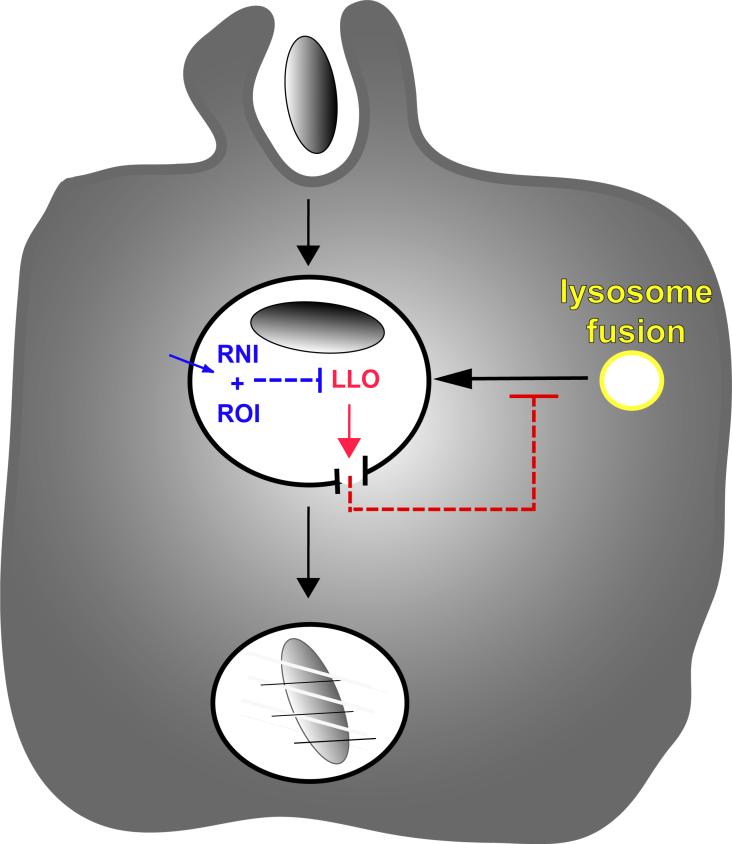
How does LLO delay vacuole maturation? One possible explanation is that LLO forms small holes in the vacuolar membrane which allow equilibration of vacuolar contents with cytoplasm (56). Vacuoles containing wild-type L. monocytogenes and fluorescent dye molecules show a transient size-selective loss of fluorescent molecules. Such vacuoles also frequently have higher pH and lower calcium concentrations than macropinosomes or vacuoles containing hly- L. monocytogenes, consistent with the presence of small pores formed by LLO that allow protons and calcium to equilibrate with the cytoplasm. Disruption of pH and calcium gradients in the endocytic pathway can inhibit fusion events and stall the maturation of endocytic compartments (4, 5, 57-59). Accordingly, the small perforations caused by LLO after some acidification of the L. monocytogenes vacuole (60) may delay its fusion with the lysosomes, thereby allowing L. monocytogenes to evade degradation (Figure 3).
Figure 3.
Macrophages activated with IFN-gamma and LPS generate reactive oxygen (ROI) and nitrogen (RNI) intermediates within or near the L. monocytogenes vacuole, thereby blocking the early perforations by LLO that otherwise allow L. monocytogenes to escape the vacuole.
Thus, the escape of L. monocytogenes from vacuoles in non-activated macrophages is preceded by two alterations of vacuolar trafficking. First, in some macrophages the newly formed L. monocytogenes vacuole lacks Rab5a, and this exclusion is independent of the presence of LLO (51). This may inhibit the macrophage's ability to launch an early attack by ROI or RNI. Second, phagosome maturation to a LAMP-1-positive stage is further delayed by the action of LLO in the phagosome, possibly via perforations that interrupt vacuole maturation. LLO-dependent inhibition of vacuole maturation may allow the bacterium more time to escape.
LLO-dependent delays of vacuole fusion with lysosomes may allow L. monocytogenes to avoid the inhibitory cathepsin D, thereby expanding the window of time available for escape. del Cerro-Vadillo et. al. demonstrated that the lysosomal aspartyl-protease cathepsin D inhibits L. monocytogenes propagation (61). By degrading LLO, cathepsin D inhibits L. monocytogenes growth in macrophages and fibroblasts, and escape from vacuoles (61).
The 30-minute window of opportunity to escape into cytoplasm may reflect the time L. monocytogenes takes to reach the relatively impenetrable late endocytic compartments (i.e. â those containing LAMP-1) (48). LLO works less efficiently from inside LAMP-1-positive compartments (51), and it may be that the bacteria can only escape early compartments. Only 30â80% of the wild-type L. monocytogenes internalized by macrophages escape the vacuole (48, 56, 62). The heterogeneity in both the timing and efficiency of escape reflects the balance between the mechanisms L. monocytogenes use to escape the vacuole and those that macrophages employ to block escape. The timing of those counteracting activities during the first 30 minutes after L. monocytogenes entry determines the fraction of bacteria that escape.
6. REGULATION OF ACTIVATED MACROPHAGES
6.1. Early evidence of macrophage activation
The concept of the activated macrophage was first described in early work by Mackaness showing that macrophages were capable of acquiring resistance and increased ability to inhibit L. monocytogenes infection (63, 64). In mice susceptible to L. monocytogenes infection, bacteria multiplied in macrophages. Macrophages isolated from mice after a sub-lethal infection with L. monocytogenes were more microbicidal in vitro. IFN-gamma was later shown to be the principle cytokine effector of macrophage activation that protected mice from both local and systemic infection with L. monocytogenes (65).
Death and degradation of phagocytosed bacteria and the processing of derived antigens are greatly increased following macrophage activation. Historically, IFN-gamma and lipopolysaccharide (LPS), derived from Gram-negative bacterial cell walls, were used as stimulating factors for the classical model of macrophage activation (66, 67). Together, they prime the macrophage for activation by binding to receptors that induce signal transduction cascades, specifically STAT1 and NF-kappaB activation, leading to the activation of pro-inflammatory genes and anti-microbial factors. Infected, non-activated macrophages produce and secrete IL-12, which stimulates T-cells to produce IFN-gamma, creating a feedback loop in which IFN-gamma then activates macrophages and other cells at the site of inflammation (68). Sensing of LPS by macrophages induces post-translational regulation of NF-kappaB and leads to the production of Tumor Necrosis Factor-alpha (TNF-alpha). TNF-alpha stimulates both pro-inflammatory and apoptotic responses.
6.2. Nitric oxide production in activated macrophages
Activated macrophages produce nitric oxide (NO) (69). Macrophage inducible nitric oxide synthase (iNOS) catalyzes two monooxygenase reactions, hydrolyzing L-arginine and producing NO. Of the three isoforms of nitric oxide synthase, iNOS is the most prevalent isoform expressed in murine macrophages (from the NOS2 gene) (70). Numerous cytokines (IFN-gamma) and bacterial products (LPS) stimulate macrophage expression of iNOS. NO is detrimental to intracellular pathogens, and the combination of NO and superoxide can make the highly reactive product peroxynitrite. Nitric oxide and the reactive nitrogen intermediates (RNI) that derive from it, including nitrite, nitrate and peroxynitrite, are all bactericidal and play a central role in the ability of the activated macrophage to kill ingested pathogens (71).
6.3. The role of interferons in macrophage activation
Type I (alpha/beta) and type II (gamma) interferons are important immunomodulatory cytokines during microbial infection (72-74). Interferons mediate a variety of functions through the transcriptional induction of IFN-stimulated genes (75, 76). IFN-gamma stimulates IFN-regulated transcription factors (IRFs) which activate genes for IFN-alpha/beta and inducible nitric oxide synthase (iNOS). Macrophages responding to IFN-gamma are capable of killing microorganisms more readily through increased production of NO. IFN-gamma stimulation also increases expression of major histocompatibility complex class two (MHC II) molecules essential for antigen presentation.
One set of IFN-gamma-stimulated genes up-regulated in response to pathogens includes six different p47 GTP-ases (Igtp, Lrg47, Irg47, Tgtp/Mg21, Iigp, and Gtpi) (77, 78). p47 GTPases are important immune mediators of intracellular pathogens (79). Mice lacking particular p47 GTPases are more susceptible to microbial infection, with each GTP-binding protein having a pathogen-specific response (80-82). LRG-47, which is induced by LPS, IFN-gamma, and IFN-alpha/beta (77), is up-regulated upon infection with L. monocytogenes and is important for resistance to L. monocytogenes infection (80, 83). These IFN-gamma-induced GTP-binding proteins are located in the ER and recruited to phagosomes, implicating their role in controlling phagosomal bacteria (78, 84-86). Infection of macrophages from LRG-47 â/â mice with Mycobacterium tuberculosis determined that phagosomal acidification and lysosome fusion was impaired compared to infection in wild-type macrophages (85).
Microbial products also induce a strong type I interferon response. Although type I interferons have been well studied as anti-viral cytokines, their role in bacterial infections is not yet clear. Interestingly, only L. monocytogenes capable of entering the cytosol (LLO+) induce a strong IFN-beta response (87). Mice lacking the type I IFN receptor have increased susceptibility to viral infections, demonstrating the anti-viral immune response elicited by type I interferons. However, analogous studies of L. monocytogenes infection showed increased resistance in mice lacking the type I IFN receptor (88-90). This indicates that cytosolic L. monocytogenes elicit the normally anti-microbial IFN-beta immune response, but somehow use it to their advantage. Induction of type I interferons also increase apoptosis in L. monocytogenes-infected macrophages (91).
6.4. Toll-like receptor signaling
Macrophages are also activated through cytokines elicited by microbial molecules, such as LPS (92). Macrophages recognize a number of molecules exhibiting pathogen-associated molecular patterns (PAMPs) which have limited variability and which are not shared by metazoan cells (93). PAMP recognition occurs through pattern recognition receptors (PRRs), primarily Toll-like receptors (TLRs) and Nod-like receptors (NLRs) (92, 94-96). For example, Toll-like receptor 4 (TLR4) and CD14 are PRR co-receptors that recognize the PAMP, LPS (97). LPS signaling through TLR4 in macrophages leads to synthesis of TNF-alpha, IL-1beta, and IL-12. The cytokines modulated by TLR signaling are important for the subsequent innate and adaptive immune response.
TLR-mediated signaling has been implicated in L. monocytogenes infections (31). The TLR signal adaptor protein MyD88 is necessary for resistance to L. monocytogenes infection and for activation of the innate immune response (98, 99). TLR2, which recognizes peptidoglycan and lipotechoic acid from Gram-positive bacteria, is not necessary for resistance to L. monocytogenes infection (98). Interestingly, neither MyD88 nor TLR2 are necessary for the listericidal activities by activated macrophages (98). LLO and other CDCs also stimulate TLR signaling. CDCs induce TNF-alpha and IL-6 in a TLR4-dependent manner and activate macrophages by inducing iNOS (100).
Another signaling protein that contributes to LPS-induced macrophage activation and defense against bacterial infection is protein kinase C (PKC) epsilon (101). PKC epsilon belongs to the novel subgroup in the PKC family of serine/threonine kinases which are specifically activated by diacylglycerol. Mice deficient in PKC epsilon have a decreased survival rate after bacterial infection (101), apparently as a result of deficient macrophage activation. Macrophages from PKC epsilon â/â mice produce less nitric oxide, TNF alpha, and IL-1 beta in response to LPS and IFN-gamma (101). PKC epsilon is also required for LPS-induced secretion of IL-12 in macrophages (102) and dendritic cells (103). This indicates a link between PKC epsilon and TLR signaling (104, 105). Vacuole perforation by LLO also activates PKC epsilon during L. monocytogenes infection (Shaughnessy, L.M., et. al., unpublished data).
7. THE ROLE OF THE ACTIVATED MACROPHAGE IN BLOCKING LISTERIA MONOCYTOGENES ESCAPE FROM VACUOLES
Peritoneal macrophages activated with IFN-gamma restrict the growth of L. monocytogenes by preventing its escape from the vacuole into the cytosol (62) (Figure 3). Bone marrow-derived macrophages (BMDM) activated with IFN-gamma, LPS, IL-6, and a neutralizing antibody against IL-10 inhibit escape and cytoplasmic growth of L. monocytogenes (48).
L. monocytogenes escape is inhibited in activated macrophages by both ROI and RNI. Activated BMDM from mice deficient in ROI (gp91phoxâ/â) or RNI (NOS2â/â) production block L. monocytogenes escape poorly compared to activated BMDM from wild-type mice (48). NOS2 â/â mice infected with L. monocytogenes do not survive as well as wild type mice (106). Additionally, macrophages from gp91phoxâ/â/NOS2â/â mice cannot kill L. monocytogenes as readily as wild-type mice (107). ROI are essential for inhibiting escape, and RNI augment those inhibitory effects (48). Reactive oxygen production is localized to L. monocytogenes vacuoles, indicating that the macrophage can direct its microbicidal activities into individual phagosomes (48).
It is not yet known how ROI and RNI inhibit escape in activated macrophages. ROI may be generated earlier after phagocytosis or at higher concentrations in vacuoles of activated macrophages (48). Rab5a activities on L. monocytogenes vacuoles of activated macrophages may accelerate maturation and allow earlier activation of the phagocyte oxidase or an enhancement of activities provided by localized synthesis of NO near the vacuole (49, 50, 108). LLO contains a single cysteine that must be reduced for hemolytic activity (109); ROI may inhibit LLO by affecting the vacuolar redox potential.
Macrophage activation does not always block L. monocytogenes escape (48, 62). Variable amounts of ROI are produced in the vacuoles of activated macrophages, either because of variable activity of the phagocyte oxidase or variable sizes of L. monocytogenes vacuoles (48). More spacious vacuoles may reduce the effective concentrations of ROI. Thus, although ROI and RNI are central to the ability of an activated macrophage to control L. monocytogenes infection, the mechanisms of their interference with L. monocytogenes escape are not known.
8. ADAPTIVE IMMUNITY CONTRIBUTES TO LISTERIA MONOCYTOGENES CLEARANCE BY MACROPHAGES
The intracellular life cycle of L. monocytogenes allows it to avoid humoral defenses such as antibodies and complement. Hence, innate immune responses are important during the initial stages of infection and for final clearance of L. monocytogenes (110). During primary infection, IL-12-producing macrophages elicit IFN-gamma secretion by NK cells. Both TLR-dependent secretion of TNF-alpha and the early IFN-gamma response are important for the activation of macrophages and clearance of bacteria (31, 111) as well as for the priming of the adaptive arm of the immune response.
A strong T-cell-mediated response to L. monocytogenes is required for clearance (112). L. monocytogenes invasion of the cytosol triggers an inflammatory response and gives rise to a protective immune response. LLO-expressing L. monocytogenes elicit CD8+ T cells, whereas CD4+ T cells are elicited by LLO+, LLO-, and heat-killed L. monocytogenes (113). Lysis of infected cells by cytotoxic T-cells is necessary for full clearance of L. monocytogenes (114). LLO is important for the generation of protective immunity, as LLO-derived peptides are dominant CD8+ T-cell epitopes (115).
9. PERSPECTIVES
Macrophages are important for eliciting both innate and adaptive immune responses. The activation of macrophages by IFN-gamma and the cytokines elicited by LPS or other microbial products prime macrophages to clear infections. Importantly, activated macrophages are the principle effectors of a strong immune response against L. monocytogenes. L. monocytogenes growth inside activated macrophages is restricted and bacteria are actively cleared. ROI and RNI, delivered into the L. monocytogenes-containing vacuoles, block both pore-forming activity and escape into the cytosol.
Thus, macrophage activation tips the balance of host-L. monocytogenes interactions in favor of the host, most likely by affecting the timing of ROI generation into L. monocytogenes vacuoles. In non-activated macrophages, L. monocytogenes perforate vacuoles quickly enough to inhibit fusion with lysosomes and the generation of high concentrations of ROI and RNI in the vacuole. This early perforation buys the bacterium time to finish its escape from the vacuole. Activated macrophages generate ROI earlier after L. monocytogenes phagocytosis, perhaps in combination with RNI, thereby preventing L. monocytogenes from generating the perforations that slow maturation and allow vacuolar escape. Future studies should reveal whether altered timing and magnitude of these chemistries are sufficient to kill L. monocytogenes in vacuole or if additional activities are needed beyond those that inhibit escape.
10. ACKNOWLEDGMENTS
The authors thank Drs. Daniel Portnoy, K.-D Lee, J.-D. Sauer and Rebecca Henry for helpful discussions. Supported by NIH # ROI AI 35950 to Joel Swanson.
Glossary
Abbreviations
- BMDM
bone marrow-derived macrophage
- CDC
cholesterol-dependent cytolysin
- EEA1
early endosome antigen 1
- IFN
interferon
- IFR
interferon-regulated transcription factor
- LLO
listeriolysin O
- LPS
lipopolysaccharide
- LAMP-1
lysosomal-associating membrane protein 1
- MHCII
major histocompatibility complex class II
- NADPH
reduced nicotinamide adenine dinucleotide phosphate
- NLR
Nod-like receptor
- NF-kappaB
nuclear factor-kappaB
- NO
nitric oxide
- NOS
nitric oxide synthase
- PAMP
pathogen-associated molecular pattern
- PI3P
phosphatidylinositol 3-phosphate
- ROI
reactive oxygen intermediate
- RNI
reactive nitrogen intermediate
- PRR
pattern recognition receptor
- PKC
protein kinase C
- TNF-alpha
tumor necrosis factor alpha
- TLR
toll-like receptor
- YFP
yellow fluorescent protein.
11. REFERENCES
- 1.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 2.Underhill DM, Ozinsky A. Phagocytosis of microbes: Complexity in action. Annu. Rev. Immunol. 2002;20:825–852. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- 3.Vieira OV, Botelho RJ, Grinstein S. Phagosome maturation: aging gracefully. Biochem. J. 2002;366:689–704. doi: 10.1042/BJ20020691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Ann. Rev. Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- 5.Kornfeld S, Mellman I. The biogenesis of lysosomes. Annu. Rev. Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- 6.Desjardins M. Biogenesis of phagolysosomes: the âkiss and runâ hypothesis. Trends Cell Biol. 1995;5:183–186. doi: 10.1016/s0962-8924(00)88989-8. [DOI] [PubMed] [Google Scholar]
- 7.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–17. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 8.Jordens I, Marsman M, Kuijl C, Neefjes J. Rab proteins, connecting transport and vesicle fusion. Traffic. 2005;6:1070–7. doi: 10.1111/j.1600-0854.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 9.Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–49. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 10.Henry RM, Hoppe AD, N. J, Swanson JA. The uniformity of phagosome maturation in macrophages. J. Cell Biol. 2004;164:185–194. doi: 10.1083/jcb.200307080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mu F-T, Callaghan JM, Steele-Mortimer O, Stenmark H, Parton RG, Campbell PL, McCluskey J, Yeo J-P, Tock EPC, Toh B-H. EEA1, an early endosome-associated protein. J. Biol. Chem. 1995;270:13503–13511. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- 12.Stenmark H, Aasland R, Toh B-H, D'Arrigo A. Endosomal localization of the autoantigen EEA1 in mediated by a zinc-binding FYVE finger. J. Biol. Chem. 1996;271 doi: 10.1074/jbc.271.39.24048. [DOI] [PubMed] [Google Scholar]
- 13.Vieira OV, Botelho RJ, Rameh L, Brachmann SM, Matsuo T, Davidson HW, Schreiber A, Backer JM, Cantley LC, Grinstein S. Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J. Cell Biol. 2001;155:19–25. doi: 10.1083/jcb.200107069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephens LR, Jackson TR, Hawkins PT. Agonist-stimulated synthesis of phosphatidylinositol(3,4,5)-trisphosphate: a new intracellular signalling system? Biochim Biophys Acta. 1993;1179:27–75. doi: 10.1016/0167-4889(93)90072-w. [DOI] [PubMed] [Google Scholar]
- 15.Lindmo K, Stenmark H. Regulation of membrane traffic by phosphoinositide 3-kinases. J Cell Sci. 2006;119:605–14. doi: 10.1242/jcs.02855. [DOI] [PubMed] [Google Scholar]
- 16.Babior BM, Kipnes RS, Curnutte JT. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. The Journal of Clinical Investigation. 1973;52:741–4. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park JB. Phagocytosis induces superoxide formation and apoptosis in macrophages. Exp Mol Med. 2003;35:325–35. doi: 10.1038/emm.2003.44. [DOI] [PubMed] [Google Scholar]
- 18.Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am J Respir Crit Care Med. 2002;166:S4–8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- 19.Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–76. [PubMed] [Google Scholar]
- 20.Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16:42–7. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Curnutte JT, Whitten DM, Babior BM. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974;290:593–7. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- 22.Curnutte JT, Scott PJ, Mayo LA. Cytosolic components of the respiratory burst oxidase: resolution of four components, two of which are missing in complementing types of chronic granulomatous disease. Proc Natl Acad Sci U S A. 1989;86:825–9. doi: 10.1073/pnas.86.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casimir C, Chetty M, Bohler MC, Garcia R, Fischer A, Griscelli C, Johnson B, Segal AW. Identification of the defective NADPH-oxidase component in chronic granulomatous disease: a study of 57 European families. Eur J Clin Invest. 1992;22:403–6. doi: 10.1111/j.1365-2362.1992.tb01481.x. [DOI] [PubMed] [Google Scholar]
- 24.Scott CC, Botelho RJ, Grinstein S. Phagosome maturation: a few bugs in the system. J Membr Biol. 2003;193:137–52. doi: 10.1007/s00232-002-2008-2. [DOI] [PubMed] [Google Scholar]
- 25.Duclos S, Desjardins M. Subversion of a young phagosome: the survival strategies of intracellular pathogens. Cell. Microbiol. 2000;2:365–377. doi: 10.1046/j.1462-5822.2000.00066.x. [DOI] [PubMed] [Google Scholar]
- 26.Sansonetti P. Phagocytosis of bacterial pathogens: implications in the host response. Sem. Immunol. 2001;13:381–390. doi: 10.1006/smim.2001.0335. [DOI] [PubMed] [Google Scholar]
- 27.Miller BH, Fratti RA, Poschet JF, Timmins GS, Master SS, Burgos M, Marletta MA, Deretic V. Mycobacteria inhibit nitric oxide synthase recruitment to phagosomes during macrophage infection. Infect Immun. 2004;72:2872–8. doi: 10.1128/IAI.72.5.2872-2878.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gellin BG, Broome CV. Listeriosis. Journal of American Medical Association. 1989;261:1313–1320. [PubMed] [Google Scholar]
- 30.Bibb WF, Gellin BG, Weaver R, Schwartz B, Plikaytis BD, Reeves MW, Pinner RW, Broome CV. Analysis of clinical and food-borne isolates of Listeria monocytogenes in the United States by multilocus enzyme electrophoresis and application of the method to epidemiologic investigations. Appl Environ Microbiol. 1990;56:2133–41. doi: 10.1128/aem.56.7.2133-2141.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–23. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 32.Czuprynski CJ, Haak-Frendscho M. Non-specific resistance mechanisms to listeriosis: implications for experimental and naturally occurring infection. Immunol Rev. 1997;158:47–56. doi: 10.1111/j.1600-065x.1997.tb00991.x. [DOI] [PubMed] [Google Scholar]
- 33.Havell EA, Beretich GR, Jr., Carter PB. The mucosal phase of Listeria infection. Immunobiology. 1999;201:164–77. doi: 10.1016/S0171-2985(99)80056-4. [DOI] [PubMed] [Google Scholar]
- 34.Pron B, Boumaila C, Jaubert F, Berche P, Milon G, Geissmann F, Gaillard JL. Dendritic cells are early cellular targets of Listeria monocytogenes after intestinal delivery and are involved in bacterial spread in the host. Cell Microbiol. 2001;3:331–40. doi: 10.1046/j.1462-5822.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 35.Gahan CG, Hill C. Gastrointestinal phase of Listeria monocytogenes infection. J Appl Microbiol. 2005;98:1345–53. doi: 10.1111/j.1365-2672.2005.02559.x. [DOI] [PubMed] [Google Scholar]
- 36.Vazquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Dominguez-Bernal G, Goebel W, Gonzalez-Zorn B, Wehland J, Kreft J. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 2001;14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Portnoy DA, Jacks PS, Hinrichs DJ. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cossart P, Vicente MF, Mengaud J, Baquero F, Perez-Diaz JC, Berche P. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect Immun. 1989;57:3629–36. doi: 10.1128/iai.57.11.3629-3636.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gedde MM, Higgins DE, Tilney LG, Portnoy DA. Role of listeriolysin O in cell-to-cell spread of Listeria monocytogenes. Infect Immun. 2000;68:999–1003. doi: 10.1128/iai.68.2.999-1003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bielecki J, Youngman P, Connelly P, Portnoy DA. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature. 1990;345:175–176. doi: 10.1038/345175a0. [DOI] [PubMed] [Google Scholar]
- 41.Alouf JE, Billington SJ, Jost BH. Repertoire and general features of the family of cholesterol-dependent cytolysins. In: Alouf JE, Popoff, Elsevier MR, editors. The Comprehensive Sourcebook of Bacterial Protein Toxins. Paris: 2006. [Google Scholar]
- 42.Vazquez-Boland JA, Stachowiak R, Lacharme L, Scortti M. Listeriolysin. In: Alouf JEP, Elsevier MR, editors. The Comprehensive Sourcebook of Bacterial Protein Toxins. Paris: 2006. [Google Scholar]
- 43.Schuerch DW, Wilson-Kubalek EM, Tweten RK. Molecular basis of listeriolysin O pH dependence. Proc Natl Acad Sci U S A. 2005;102:12537–42. doi: 10.1073/pnas.0500558102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Portnoy DA, Tweten RK, Kehoe M, Bielecki J. Capacity of Listeriolysin O, Streptolysin O, and Perfringolysin O to mediate growth of Bacillus subtilis within mammalian cells. Infect. Immun. 1992;60:2710–2717. doi: 10.1128/iai.60.7.2710-2717.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith GA, Marquis H, Jones S, Johnston NC, Portnoy DA, Goldfine H. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect. Immunity. 1995;63:4231–4237. doi: 10.1128/iai.63.11.4231-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tilney LT, Portnoy DA. Actin filaments and the growth, movement, and spread of intracellular bacterial parasite, Listeria monocytogenes. J. Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992;68:521–31. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- 48.Myers JT, Tsang AW, Swanson JA. Localized reactive oxygen and nitrogen intermediates inhibit escape of Listeria monocytogenes from vacuoles in activated macrophages. J. Immunol. 2003;171:5447–5453. doi: 10.4049/jimmunol.171.10.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alvarez-Dominguez C, Stahl PD. Increased expression of Rab5a correlates directly with accelerated maturation of Listeria monocytogenes phagosomes. J. Biol. Chem. 1999;274:11459–11462. doi: 10.1074/jbc.274.17.11459. [DOI] [PubMed] [Google Scholar]
- 50.Prada-Delgado A, Carrasco-Marin E, Bokoch GM, Alvarez-Dominguez C. Interferon-gamma listericidal action is mediated by novel Rab5a functions at the phagosomal environment. J Biol Chem. 2001;276:19059–65. doi: 10.1074/jbc.M101639200. [DOI] [PubMed] [Google Scholar]
- 51.Henry R, Shaughnessy L, Loessner MJ, Alberti-Segui C, Higgins DE, Swanson JA. Cytolysin-dependent delay of vacuole maturation in macrophages infected with Listeria monocytogenes. Cell Microbiol. 2006;8:107–19. doi: 10.1111/j.1462-5822.2005.00604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agaisse H, Burrack LS, Philips JA, Rubin EJ, Perrimon N, Higgins DE. Genome-wide RNAi screen for host factors required for intracellular bacterial infection. Science. 2005;309:1248–51. doi: 10.1126/science.1116008. [DOI] [PubMed] [Google Scholar]
- 53.Cheng LW, Viala JP, Stuurman N, Wiedemann U, Vale RD, Portnoy DA. Use of RNA interference in Drosophila S2 cells to identify host pathways controlling compartmentalization of an intracellular pathogen. Proc Natl Acad Sci U S A. 2005;102:13646–51. doi: 10.1073/pnas.0506461102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alvarez-Dominguez C, Barbieri AM, Beron W, Wandinger-Ness A, Stahl PD. Phagocytosed live Listeria monocytogenes influences Rab5-regulated in vitro phagosome-endosome fusion. J. Biol. Chem. 1996;271:13834–13843. doi: 10.1074/jbc.271.23.13834. [DOI] [PubMed] [Google Scholar]
- 55.Alvarez-Dominguez A, Roberts R, Stahl PD. Internalized Listeria monocytogenes modulates intracellular trafficking and delays maturation of the phagosome. J. Cell Sci. 1997;110:731–743. doi: 10.1242/jcs.110.6.731. [DOI] [PubMed] [Google Scholar]
- 56.Shaughnessy LM, Hoppe AD, Christensen KA, Swanson JA. Membrane perforations inhibit lysosome fusion by altering pH and calcium in Listeria monocytogenes vacuoles. Cell Microbiol. 2006;8:781–92. doi: 10.1111/j.1462-5822.2005.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peters C, Mayer A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 1998;396:575–580. doi: 10.1038/25133. [DOI] [PubMed] [Google Scholar]
- 58.Holroyd C, Kistner U, Annaert W, Jahn R. Fusion of endosomes involved in synaptic vesicle recycling. Mol. Biol. Cell. 1999;10:3035–3044. doi: 10.1091/mbc.10.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pryor PR, Mullock BM, Bright NA, Gray SR, Luzio JP. The role of intraorganellar Ca2+ in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J. Cell Biol. 2000;149:1053–1062. doi: 10.1083/jcb.149.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beauregard KE, Lee K-D, Collier RJ, Swanson JA. pH-dependent perforation of macrophage phagosomes by listeriolysin O from Listeria monocytogenes. J. Exp. Med. 1997;186:1159–1163. doi: 10.1084/jem.186.7.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.del Cerro-Vadillo E, Madrazo-Toca F, Carrasco-Marin E, Fernandez-Prieto L, Beck C, Leyva-Cobian F, Saftig P, Alvarez-Dominguez C. Cutting edge: a novel nonoxidative phagosomal mechanism exerted by cathepsin-D controls Listeria monocytogenes intracellular growth. J Immunol. 2006;176:1321–5. doi: 10.4049/jimmunol.176.3.1321. [DOI] [PubMed] [Google Scholar]
- 62.Portnoy DA, Schreiber RD, Connelly P, Tilney LG. Î-Interferon limits access of Listeria monocytogenes to the macrophage cytoplasm. J. Exp. Med. 1989;170:2141–2146. doi: 10.1084/jem.170.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mackaness GB. Cellular resistance to infection. J. Exp. Med. 1962;116:381–406. [PubMed] [Google Scholar]
- 64.Adams DO, Hamilton TA. The cell biology of macrophage activation. Annu. Rev. Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- 65.Kiderlen AF, Kaufmann SH, Lohmann-Matthes ML. Protection of mice against the intracellular bacterium Listeria monocytogenes by recombinant immune interferon. Eur J Immunol. 1984;14:964–7. doi: 10.1002/eji.1830141019. [DOI] [PubMed] [Google Scholar]
- 66.Adams DO, Hamilton TA. Molecular transductional mechanisms by which IFNÎ and other signals regulate macrophage development. Immunol. Rev. 1987;97:5–27. doi: 10.1111/j.1600-065x.1987.tb00514.x. [DOI] [PubMed] [Google Scholar]
- 67.Nacy CA, Meltzer MS. T-cell-mediated activation of macrophages. Curr. Opinion Immunol. 1991;3:330–335. doi: 10.1016/0952-7915(91)90033-w. [DOI] [PubMed] [Google Scholar]
- 68.Unanue ER. Inter-relationship among macrophages, natural killer cells and neutrophils in early stages of Listeria resistance. Curr Opin Immunol. 1997;9:35–43. doi: 10.1016/s0952-7915(97)80156-2. [DOI] [PubMed] [Google Scholar]
- 69.Stuehr DJ, Marletta MA. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1985;82:7738–42. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.MacMicking J, Xie Q.-w., Nathan C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 71.Nathan C. Inducible nitric oxide synthase: What difference does it make? J. Clin. Invest. 1997;100:2417–2423. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–64. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 73.Taki S. Type I interferons and autoimmunity: lessons from the clinic and from IRF-2-deficient mice. Cytokine Growth Factor Rev. 2002;13:379–91. doi: 10.1016/s1359-6101(02)00023-0. [DOI] [PubMed] [Google Scholar]
- 74.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–36. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 75.Der SD, Zhou A, Williams BR, Silverman RH. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc Natl Acad Sci U S A. 1998;95:15623–8. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Darnell JE, Jr., Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–21. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 77.Sorace JM, Johnson RJ, Howard DL, Drysdale BE. Identification of an endotoxin and IFN-inducible cDNA: possible identification of a novel protein family. J Leukoc Biol. 1995;58:477–84. doi: 10.1002/jlb.58.4.477. [DOI] [PubMed] [Google Scholar]
- 78.MacMicking JD. Immune control of phagosomal bacteria by p47 GTPases. Curr Opin Microbiol. 2005;8:74–82. doi: 10.1016/j.mib.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 79.Taylor GA, Feng CG, Sher A. p47 GTPases: regulators of immunity to intracellular pathogens. Nat Rev Immunol. 2004;4:100–9. doi: 10.1038/nri1270. [DOI] [PubMed] [Google Scholar]
- 80.Collazo CM, Yap GS, Semprowski GD, Lusby KC, Tessarollo L, VandeWoude GF, Sher A, Taylor GA. Inactivation of LRG-47 and IRG-47 reveals a family of interferon-Î-inducible genes with essential, pathogen-specific roles in resistance to infection. J. Exp. Med. 2001;194:181–187. doi: 10.1084/jem.194.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Feng CG, Collazo-Custodio CM, Eckhaus M, Hieny S, Belkaid Y, Elkins K, Jankovic D, Taylor GA, Sher A. Mice deficient in LRG-47 display increased susceptibility to mycobacterial infection associated with the induction of lymphopenia. J Immunol. 2004;172:1163–8. doi: 10.4049/jimmunol.172.2.1163. [DOI] [PubMed] [Google Scholar]
- 82.Taylor GA, Collazo CM, Yap GS, Nguyen K, Gregorio TA, Taylor LS, Eagleson B, Secrest L, Southon EA, Reid SW, Tessarollo L, Bray M, McVicar DW, Komschlies KL, Young HA, Biron CA, Sher A, Vande Woude GF. Pathogen-specific loss of host resistance in mice lacking the IFN-gamma-inducible gene IGTP. Proc Natl Acad Sci U S A. 2000;97:751–5. doi: 10.1073/pnas.97.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McCaffrey RL, Fawcett P, O'Riordan M, Lee KD, Havell EA, Brown PO, Portnoy DA. A specific gene expression program triggered by Gram-positive bacteria in the cytosol. Proc Natl Acad Sci U S A. 2004;101:11386–91. doi: 10.1073/pnas.0403215101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taylor GA, Stauber R, Rulong S, Hudson E, Pei V, Pavlakis GN, Resau JH, Vande Woude GF. The inducibly expressed GTPase localizes to the endoplasmic reticulum, independently of GTP binding. J Biol Chem. 1997;272:10639–45. doi: 10.1074/jbc.272.16.10639. [DOI] [PubMed] [Google Scholar]
- 85.MacMicking JD, Taylor GA, McKinney JD. Immune control of tuberculosis by IFNÎ-inducible LRG-47. Science. 2003;302:654–659. doi: 10.1126/science.1088063. [DOI] [PubMed] [Google Scholar]
- 86.Butcher BA, Greene RI, Henry SC, Annecharico KL, Weinberg JB, Denkers EY, Sher A, Taylor GA. p47 GTPases regulate Toxoplasma gondii survival in activated macrophages. Infect Immun. 2005;73:3278–86. doi: 10.1128/IAI.73.6.3278-3286.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O'Riordan M, Yi CH, Gonzales R, Lee K-D, Portnoy DA. Innate recognition of bacteria by a macrophage cytosolic surveillance pathway. Proc. Natl. Acad. Sci. USA. 2002;99:12861–13866. doi: 10.1073/pnas.202476699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carrero JA, Calderon B, Unanue ER. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J Exp Med. 2004;200:535–40. doi: 10.1084/jem.20040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O'Connell RM, Saha SK, Vaidya SA, Bruhn KW, Miranda GA, Zarnegar B, Perry AK, Nguyen BO, Lane TF, Taniguchi T, Miller JF, Cheng G. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J Exp Med. 2004;200:437–45. doi: 10.1084/jem.20040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Auerbuch V, Brockstedt DG, Meyer-Morse N, O'Riordan M, Portnoy DA. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J Exp Med. 2004;200:527–33. doi: 10.1084/jem.20040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stockinger S, Materna T, Stoiber D, Bayr L, Steinborn R, Kolbe T, Unger H, Chakraborty T, Levy DE, Muller M, Decker T. Production of type I IFN sensitizes macrophages to cell death induced by Listeria monocytogenes. J Immunol. 2002;169:6522–9. doi: 10.4049/jimmunol.169.11.6522. [DOI] [PubMed] [Google Scholar]
- 92.Janeway CA, Jr., Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 93.Takeda K, Akira S. Toll receptors and pathogen resistance. Cell. Microbiol. 2003;5:143–153. doi: 10.1046/j.1462-5822.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- 94.Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–44. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 95.Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005;26:447–54. doi: 10.1016/j.it.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 96.Creagh EM, O'Neill A, L TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 2006;27:352–7. doi: 10.1016/j.it.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 97.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 98.Edelson BT, Unanue ER. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: No role for either in macrophage Listericidal activity. J. Immunol. 2002;169:3869–3875. doi: 10.4049/jimmunol.169.7.3869. [DOI] [PubMed] [Google Scholar]
- 99.Seki E, Tsutsui H, Tsuji NM, Hayashi N, Adachi K, Nakano H, Futatsugi-Yumikura S, Takeuchi O, Hoshino K, Akira S, Fujimoto J, Nakanishi K. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J Immunol. 2002;169:3863–8. doi: 10.4049/jimmunol.169.7.3863. [DOI] [PubMed] [Google Scholar]
- 100.Park JM, Ng VH, Maeda S, Rest RF, Karin M. Anthrolysin O and other gram-positive cytolysins are toll-like receptor 4 agonists. J Exp Med. 2004;200:1647–55. doi: 10.1084/jem.20041215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Castrillo A, Pennington DJ, Otto F, Parker PJ, Owen MJ, Bosca L. Protein kinase CÎ is required for macrophage activation and defense against bacterial infection. J. Exp. Med. 2001;194:1231–1242. doi: 10.1084/jem.194.9.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fronhofer V, Lennartz MR, Loegering DJ. Role of PKC isoforms in the Fc(gamma)R-mediated inhibition of LPS-stimulated IL-12 secretion by macrophages. J Leukoc Biol. 2006;79:408–15. doi: 10.1189/jlb.0805438. [DOI] [PubMed] [Google Scholar]
- 103.Aksoy E, Amraoui Z, Goriely S, Goldman M, Willems F. Critical role of protein kinase C epsilon for lipopolysaccharide-induced IL-12 synthesis in monocyte-derived dendritic cells. Eur J Immunol. 2002;32:3040–9. doi: 10.1002/1521-4141(200211)32:11<3040::AID-IMMU3040>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 104.Aksoy E, Goldman M, Willems F. Protein kinase C epsilon: a new target to control inflammation and immune-mediated disorders. Int J Biochem Cell Biol. 2004;36:183–8. doi: 10.1016/s1357-2725(03)00210-3. [DOI] [PubMed] [Google Scholar]
- 105.McGettrick AF, Brint EK, Palsson-McDermott EM, Rowe DC, Golenbock DT, Gay NJ, Fitzgerald KA, O'Neill LA. Trif-related adapter molecule is phosphorylated by PKC{varepsilon} during Toll-like receptor 4 signaling. Proc Natl Acad Sci U S A. 2006;103:9196–201. doi: 10.1073/pnas.0600462103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie Q.-w., Sokol K, Hutchinson N, Chen H, Mudgett JS. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 107.Shiloh M, MacMicking JD, Nicholson S, Brause JE, Potter S, Marino M, Fang F, Dinauer M, Nathan C. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- 108.Vodovitz Y, Russell D, Xie Q.-w., Bogdan C, Nathan C. Vesicle membrane associations of nitric oxide synthase in primary mouse macrophages. J. Immunol. 1995;154:2914–2925. [PubMed] [Google Scholar]
- 109.Saito G, Amidon GL, Lee K-D. Enhanced cytosolic delivery of plasmid DNA by a sulfhydryl-activatable listeriolysin O/protamine conjugate utilizing cellular reducing potential. Gene Therapy. 2003;10:72–83. doi: 10.1038/sj.gt.3301859. [DOI] [PubMed] [Google Scholar]
- 110.Unanue ER. Studies in listeriosis show the strong symbiosis between the innate cellular system and the T-cell response. Immunol. Rev. 1997;158:11–25. doi: 10.1111/j.1600-065x.1997.tb00988.x. [DOI] [PubMed] [Google Scholar]
- 111.Tripp CS, Wolf SF, Unanue ER. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci U S A. 1993;90:3725–9. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lara-Tejero M, Pamer EG. T cell responses to Listeria monocytogenes. Curr Opin Microbiol. 2004;7:45–50. doi: 10.1016/j.mib.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 113.Brunt LM, Portnoy DA, Unanue ER. Presentation of Listeria monocytogenes to CD8+ T cells requires secretion of hemolysin and intracellular bacterial growth. J Immunol. 1990;145:3540–6. [PubMed] [Google Scholar]
- 114.Villanueva MS, Sijts AJ, Pamer EG. Listeriolysin is processed efficiently into an MHC class I-associated epitope in Listeria monocytogenes-infected cells. J Immunol. 1995;155:5227–33. [PubMed] [Google Scholar]
- 115.Berche P, Gaillard JL, Sansonetti PJ. Intracellular growth of Listeria monocytogenes as a prerequisite for in vivo induction of T cell-mediated immunity. J Immunol. 1987;138:2266–71. [PubMed] [Google Scholar]