Summary
The ability of phagocytes to discriminate between viable and foreign or abnormal cells is of fundamental importance; a recent study provides new molecular insights into CD47/SIRPÎ function in this discrimination.
On a daily basis, the human body turns over 100â200 billion cells. This includes unwanted cells that do not have the right developmental fitness such as during hematopoiesis, superfluous cells such as excess lymphocytes that remain after a pathogenic challenge has been removed, and damaged or aged cells, such as aged erythrocytes, that need to be removed as part of the cellular homeostasis in the body. The turnover of these cells begins with the induction of an apoptotic program or other cellular changes that mark them for removal, and the subsequent recognition of altered features by phagocytes that leads to a highly efficient and immunologically silent removal of these unwanted/dying cells [1]. The phagocytes that do such cleanup include macrophages, dendritic cells, Kupfer cells of the liver (e.g. for removal of aged erythrocytes) as well as many neighboring cells, though the relative contribution of each type of phagocyte is unknown. Inherent in this clean-up process is the need to specifically and selectively remove unwanted cells but spare neighboring healthy cells, within same tissue milieu.
The discrimination of the healthy from the unwanted/aged/dying cells appears to be achieved at two levels. First, the cells intended for removal display markers or ligands called âeat-meâ signals, i.e. âaltered selfâ, which can in turn be recognized by receptors on the phagocytes. Second, healthy cells appear to have markers called âdonât eat-meâ signals that actively inhibit phagocytosis [1â4]; these donât eat-me signals are either downregulated in the dying cells or present in an altered conformation. While significant strides in recent years have been made in our understanding of eat-me signals and their recognition (see refs [1, 5], for review), progress toward understanding how donât eat-me signals function has been slower. The cell surface protein CD47 on healthy cells and its engagement of a phagocyte receptor, SIRPÎ, appears to constitute a key donât eat-me signal. A recent study [6] now sheds light on the molecular events negatively affected by CD47/SIRPÎ with larger implications for our understanding of the phagocytic process (Figure 1).
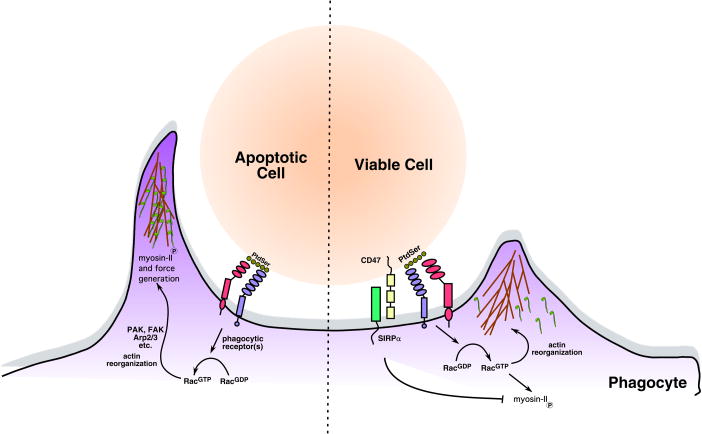
Figure 1. CD47/SIRPÎ block internalization of viable cells.
Recognition of the apoptotic cells, either through exposed phosphatidylserine (PtdSer) or other opsonins results in the activation of the Rac GTPase, extension of lamellipodia around the target, and ultimately internalization. CD47/SIRPÎ is down-modulated during apoptosis, and inhibits the inappropriate internalization of viable cells.
Phagocytes utilize a broad variety of receptors to recognize altered-self. Typically, the steps in phagocytosis involve the recognition of one or more ligands on the cells marked for removal by specific receptors on the phagocytes with formation of a âphagocytic synapseâ, the generation of an actin-rich phagocytic cup at the phagocytic synapse, extension of the phagocytic arms around the target through cytoskeletal rearrangements, and ultimately, the pulling of the target in to the phagocyte through force generated by motor proteins (Figure 1). While donât eat-me signals have been shown to prevent the inappropriate removal of living cells, how signaling negatively impacts particle internalization has not been fully elucidated. For example, blocking the CD47 mediated engagement of SIRPÎ on the phagocyte, or the loss of CD47 expression in knockout mice, can cause removal of live cells and non-aged erythrocytes [2, 7]. Alternatively, blocking SIRPÎ recognition also allows engulfment of targets that are not normally engulfed.
How does SIRPÎ signaling block the active process of engulfment? Based on studies in other contexts, SIRPÎ can be tyrosine phosphorylated when crosslinked and can recruit via its cytoplasmic tail the tyrosine phosphatase SHP-1 [8]. This led to the hypothesis that the CD47/SIRPÎ signaling may regulate the phosphorylation state of phagocytic receptors/effector proteins. However, features of this model have remained largely untested until recently.
A recent paper by Tsai and Discher [6] has addressed the molecular mechanism by which CD47/SIRPÎ module negatively regulates phagocytosis. They use human monocyte cell lines or primary human monocytes with two broad types of target to further address the role played by CD47/SIRPÎ. The first targets are sheep or human erythrocytes. Previous studies have shown that CD47 recognition of SIRPÎ is species specific [9]: the human SIRPÎ does not recognize CD47 expressed on sheep erythrocytes resulting in high level of basal uptake of the sheep erythrocytes by human macrophages, but little uptake of human erythrocytes. Adding soluble human CD47 along with the sheep erythrocytes inhibits uptake, demonstrating that human CD47/SIRPÎ engagement alone is sufficient to block internalization in this assay. The authors also elegantly use the purified human CD47 to obtain a quantitative estimate of the amount of phagocyte SIRPÎ engagement that is necessary for the inhibitory signal (10â20% of the normal CD47 density in erythrocytes is sufficient to inhibit phagocytosis). The second type of target that the authors use in their studies is a simplified one, where the uptake of IgG opsonized beads is mediated via the activating Fc receptor on human macrophages; coating these beads with varying amounts of purified human CD47 allows the simultaneous engagement of the FcR and SIRPÎ, in turn leading to inhibition of the uptake. The authors then take advantage of these two target systems coupled with microscopy to address the localization of various signaling proteins involved in uptake, and make several intriguing observations.
First, SIRPÎ ligation does not inhibit binding of phagocytic receptors to the target or receptor activation, but instead inhibits downstream signaling events. This is supported by observations that adhesion of opsonized RBCs, and actin enrichment adjacent to the target are unaffected by CD47/SIRPÎ. Similarly, phosphorylation of the scaffold protein paxillin (and its recruitment to the phagocytic cup) was unaffected by CD47/SIRPÎ engagement. The authors then demonstrate that the motor protein non-muscle myosin IIa isoform (NMM IIa), which can provide pulling forces for dragging the targets into the phagocyte, is enriched at the phagocytic cup during early stages of particle internalization (Figure 1). Surprisingly, NMM IIa recruitment is reduced and/or transient in the context of the CD47/SIRPÎ signaling. Since the function of SIRPÎ depends on its binding to the tyrosine phosphatase SHP-1, the authors used a proteomic approach to look for proteins with decreased phosphorylation following SIRPÎ ligation. Among the three proteins they identify, the one most relevant for this discussion is the non-muscle myosin II. The authors further narrow this to two evolutionarily conserved potential phosphorylation sites on the myosin head domain.
Phosphorylation has been shown to positively regulate assembly of myosin-II on actin fibers [10]. Consistent with this notion, decreased phosphorylation of NMM IIa achieved through engagement of SIRPÎ, or expression of a tyrosine phosphorylation deficient mutant protein, inhibits engulfment of sheep erythrocytes and opsonized beads. Moreover, siRNA-mediated targeting of myosin-II also decreased phagocytosis; actin was still enriched at the immune synapse, similar to what is seen when phagocytosis is blocked by CD47/SIRPÎ engagement. Taken together, this work identifies regulation of the phosphorylation status of NMM IIa as a potential point of regulation for CD47/SIRPÎ How does this work enhance our overall knowledge of phagocytosis and cell clearance in the body? First, it demonstrates that CD47 engagement of SIRPÎ can serve as a generic donât eat-me signal that can turn off engulfment mediated by multiple modalities, including apoptotic cell clearance (as has been demonstrated previously) and FcR mediated phagocytosis. This is particularly interesting, since inhibitory Fc receptors (such as FcÎRIIB) can also downmodulate activating types of FcR, although this is mediated through a lipid phosphatase and degradation of PI(3,4,5)P3 [11]. In contrast to FcR mediated uptake, apoptotic cell engulfment involves multiple ligands and variety of classes of receptors including tyrosine kinase receptors [12], lectins, integrins [13, 14], immunoglobulin-domain proteins [15], and 7-TM receptors [16]. How a single CD47/SIRPÎ module could inhibit signals downstream of multiple different receptors was of some concern, however the observation by Tsai and Discher that the early steps of recognition and actin polymerization are largely unaffected by CD47/SIRPÎ, may provide a clue.
This work also indirectly suggests a key role for the NMM IIa and non-muscle myosins in apoptotic cell clearance. To date, NMM IIa or its homologues has not been identified in the context of mammalian apoptotic cell engulfment or in model organisms, though other motor proteins (e.g. dynein) have been identified in unbiased screens [17]. How this protein may be recruited and regulated by signals generated during recognition of apoptotic cells, and how the pulling forces are generated could be of considerable interest. Other negative signals have also been identified for the removal of apoptotic cells: GTP-bound RhoA may serve as a âbrakeâ and limits the engulfment of apoptotic cells; downmodulation of RhoA activity can promote engulfment [18]. Similarly, CD31 has also been shown to promote the disengagement of live cells after they engage the phagocyte [4]. How CD47/SIRPÎ may integrate with other negative regulators such as RhoA and CD31 remains to be seen, though one possibility is that coordinated signaling of these players is required for disassembly/resolution of the âphagocytic synapse.â In this respect, it would be worthwhile to know whether CD47/SIRPÎ signaling is eventually overcome, or whether the phagocytic synapse will resolve itself over time.
Lastly, CD47â/â mice show inappropriate removal of erythrocytes [7], leading to the development of autoimmune conditions in certain genetic backgrounds [19]. Similarly, failed clearance of apoptotic cells has also been shown to lead to autoimmune phenotypes. Whether the signals derived from the CD47/SIRPÎ module also somehow contribute to the anti-inflammatory signaling response of phagocytes during apoptotic cell engulfment remains to be determined. Nonprofessional phagocytes, which typically do not express CD31 or SIRPÎ (but do express CD47, reviewed in [3]), have been shown to play key roles in apoptotic cell removal in vivo [20]. At this time, it is unknown whether a protein with similar function to SIRPÎ (utilizing CD47 as a self-ligand) or an entirely different system is employed to prevent inappropriate removal of cells by nonprofessional phagocytes.
References
- 1.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 2.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 3.van Beek EM, Cochrane F, Barclay AN, van den Berg TK. Signal regulatory proteins in the immune system. J Immunol. 2005;175:7781–7787. doi: 10.4049/jimmunol.175.12.7781. [DOI] [PubMed] [Google Scholar]
- 4.Brown S, Heinisch I, Ross E, Shaw K, Buckley CD, Savill J. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature. 2002;418:200–203. doi: 10.1038/nature00811. [DOI] [PubMed] [Google Scholar]
- 5.Kinchen JM, Ravichandran KS. Journey to the grave: signaling events regulating removal of apoptotic cells. J Cell Sci. 2007;120:2143–2149. doi: 10.1242/jcs.03463. [DOI] [PubMed] [Google Scholar]
- 6.Tsai RK, Discher DE. Inhibition of âselfâ engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J Cell Biol. 2008;180:989–1003. doi: 10.1083/jcb.200708043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 8.Oldenborg PA, Gresham HD, Lindberg FP. CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcgamma and complement receptor-mediated phagocytosis. J Exp Med. 2001;193:855–862. doi: 10.1084/jem.193.7.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subramanian S, Boder ET, Discher DE. Phylogenetic divergence of CD47 interactions with human signal regulatory protein alpha reveals locus of species specificity. Implications for the binding site. J Biol Chem. 2007;282:1805–1818. doi: 10.1074/jbc.M603923200. [DOI] [PubMed] [Google Scholar]
- 10.Castellano F, Chavrier P, Caron E. Actin dynamics during phagocytosis. Semin Immunol. 2001;13:347–355. doi: 10.1006/smim.2001.0331. [DOI] [PubMed] [Google Scholar]
- 11.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 12.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 13.Albert ML, Kim JI, Birge RB. alphavbeta5 integrin recruits the CrkII-Dock180-rac1 complex for phagocytosis of apoptotic cells. Nat Cell Biol. 2000;2:899–905. doi: 10.1038/35046549. [DOI] [PubMed] [Google Scholar]
- 14.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi N, Karisola P, Pena-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, Butte MJ, Nagumo H, Chernova I, Zhu B, Sharpe AH, Ito S, Dranoff G, Kaplan GG, Casasnovas JM, Umetsu DT, Dekruyff RH, Freeman GJ. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z, Klibanov AL, Mandell JW, Ravichandran KS. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 17.Kinchen JM, Doukoumetzidis K, Almendinger J, Stergiou L, Tosello-Trampont A, Sifri CD, Hengartner MO, Ravichandran KS. A pathway for phagosome maturation during engulfment of apoptotic cells. Nat Cell Biol. 2008 doi: 10.1038/ncb1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tosello-Trampont AC, Nakada-Tsukui K, Ravichandran KS. Engulfment of apoptotic cells is negatively regulated by Rho-mediated signaling. J Biol Chem. 2003;278:49911–49919. doi: 10.1074/jbc.M306079200. [DOI] [PubMed] [Google Scholar]
- 19.Oldenborg PA, Gresham HD, Chen Y, Izui S, Lindberg FP. Lethal autoimmune hemolytic anemia in CD47-deficient nonobese diabetic (NOD) mice. Blood. 2002;99:3500–3504. doi: 10.1182/blood.v99.10.3500. [DOI] [PubMed] [Google Scholar]
- 20.Monks J, Smith-Steinhart C, Kruk ER, Fadok VA, Henson PM. Epithelial cells remove apoptotic epithelial cells during post-lactation involution of the mouse mammary gland. Biol Reprod. 2008;78:586–594. doi: 10.1095/biolreprod.107.065045. [DOI] [PubMed] [Google Scholar]