Summary
The ingestion of particles or cells by phagocytosis and of fluids by macropinocytosis requires the formation of large endocytic vacuolar compartments inside cells by the organized movements of membranes and the actin cytoskeleton. Fc receptor-mediated phagocytosis is guided by zipper-like progression of local, receptor-initiated responses that conform to particle geometry. In contrast, macropinosomes and some phagosomes form with little or no guidance from receptors. The common organizing structure is the cup-shaped invagination of plasma membrane that becomes the phagosome or macropinosome. Recent studies indicate a feedback regulation of signal transduction based on the physical properties of forming cups.
Introduction
During the ingestion of particles or cells by phagocytosis and of extracellular fluids by macropinocytosis, cells form cup-shaped invaginations of their plasma membrane that subsequently close at their distal margins into intracellular, membrane-bounded organelles. Phagocytosis and macropinocytosis have significant roles in animal development, innate immunity, the initiation of specific immune responses and entry of pathogens into host cells, so the mechanisms of their regulation have broad implications1-5. Both processes create organelles that are orders of magnitude larger than the molecules used to build them. Professional phagocytic cells such as macrophages, dendritic cells and neutrophils ingest particles as small as single macromolecules and as large as filamentous bacteria many times their length, all through the organized assembly and movements of nanometer-scale proteins and lipids. Endocytosis of macromolecules and particles smaller than 0.2 Îm diameter occurs through small vesicles, which form by regulated curvature of membranes and self-assembly of ordered protein scaffolds, such as clathrin coats6. Distinct molecular mechanisms are enlisted for the phagocytosis of larger particles and for the wholesale gulping of extracellular fluids into macropinosomes, which can be more than 5 Îm in diameter. Such large organelles prompt questions of how molecules are organized over micrometer-sized regions of the plasma membrane.
This review compares phagocytosis and macropinocytosis by describing their molecular components and the arrangement of those components during the complex movements of ingestion, concentrating on the well studied processes of Fc receptor (FcR)-mediated phagocytosis and growth factor-stimulated macropinocytosis. It is organized around the common activities of building and closing cup-shaped invaginations of the plasma membrane, movements that are subject to positive and negative feedback control mechanisms at several levels of organization. Recent studies have shown that cell or particle morphology influences signal transduction mechanisms, indicating that receptor signaling is modulated by the context of its location at the cell surface. This review does not examine intracellular trafficking of phagosomes or macropinosomes or the contributions of phagocytosis to development or immunity. These important related subjects have been reviewed recently elsewhere1, 4, 7.
Different ways to enter cells
Although both phagocytosis and macropinocytosis use the actin cytoskeleton to construct a cup and both close the cup by contractile activities, very different models have been used to explain their formation. Phagosomes are often shaped by the particles they ingest, whereas macropinosomes can form in the absence of any particle. Many kinds of particle are ingested by a process that resembles macropinocytosis, in which a generally stimulated region of the cell surface internalizes fluid and particles together in macropinosome-like phagosomes.
The zipper model
In FcR-mediated phagocytosis, phagosomes form by a receptor-guided, zipper-like advance of membrane and the cytoskeleton over particle surfaces. Particles opsonized with immunoglobulin G (IgG) molecules are engaged by FcR on phagocyte membranes. Binding to IgG alters the cytoplasmic domains of FcR such that they recruit or activate proteins that signal cellular movements by actin polymerization and the extension of membrane over the opsonized surface8 (Fig. 1A). This advance engages other IgG molecules on the particle surface that initiate similar responses. The phagocytic cup extends over the particle by sequential local responses to the ligand-coated surface, eventually engulfing particles covered with IgG, but leaving hemispherically coated particles only half-eaten9. The locally controlled movements of the zipper model indicate that phagosomes are shaped by the surface they ingest. Consistent with a model in which phagocytosis is driven by local signals, macrophages and neutrophils engage IgG-coated coverslips in a frustrated phagocytic response, spreading out onto the planar surface and creating a tight seal against the particle surface in an apparent attempt to ingest an impossibly large particle10.
Figure 1. The movements of phagocytosis and macropinocytosis.
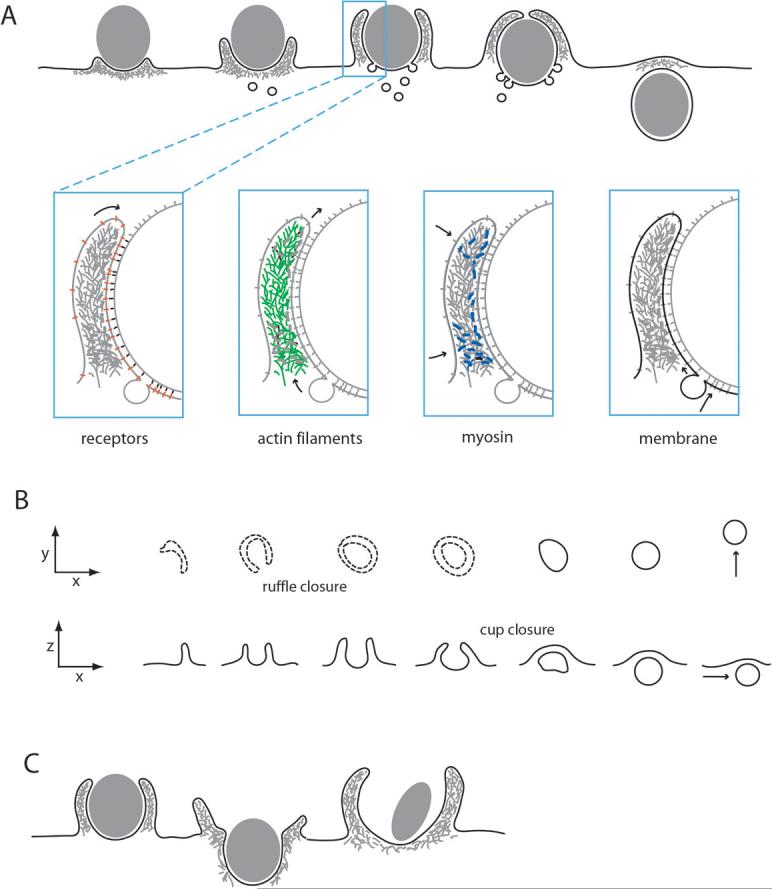
(A) During FcR-mediated phagocytosis, plasma membrane extends over particles as cup-shaped extensions of the cell surface, through progressive interactions of FcR (red) with particle-bound IgG (black). Actin filaments (green) and myosin (blue) are concentrated in the advancing cup, and membrane (black) from intracellular compartments is inserted into the base of the forming cup. Arrows indicate the net direction of receptor movement (receptors), the net displacement of actin filaments by polymerization, depolymerization and contraction (actin), the contraction of the actin-myosin network (myosin) and the net flow of membrane into cups (membrane). (B) Macropinosomes form from cell surface ruffles that close first into open cups (ruffle closure) and then into discrete intracellular vesicles (cup closure). Two aspects of macropinosome formation are presented: the x-y projection indicates the âtop downâ view typically seen in the light microscope and the x-z projection shows a side-view of membrane movements. Dotted lines indicate folds in the plasma membrane. Ruffle closure is the formation of a circular, open cup of plasma membrane. Cup closure is the separation of the macropinosome from the plasma membrane. Arrows indicate macropinosome displacement through cytoplasm. (C) Distinct movements of membranes and actin during various kinds of phagosome formation. (left) Extended, close-fitting cups are typical of the zipper model of FcR-mediated phagocytosis. (middle) During CR3-mediated phagocytosis, phagosomes appear to sink into cytoplasm, although ruffles may accompany the process. (right) In triggered phagocytosis, bacteria are internalized by stimulation of macropinocytosis and recruitment into forming macropinosomes.
Imaging of fluorescent actin molecules during phagocytosis shows the coordinated movement of the cytoskeleton in the cup. Actin is concentrated in the advancing cup and persists until closure of the phagosome, when it is lost from the phagosomal membrane. In many phagosomes, actin concentrations decrease at the base of the cup before closure, creating a belt-shaped band of actin that moves outward over the particle11, 12. The actin filament network and associated contractile proteins create circumferential contractile activities that visibly squeeze deformable particles, such that an IgG-coated erythrocyte engaged by two neighboring macrophages is actively constricted in two by the competing cells11. Contractile forces measured during phagocytosis by neutrophils indicate that contractions also flatten the phagocytic cup against the particle surface13. Several different classes of myosin have been implicated in FcR phagocytosis, including myosin 1C, II, IX and X14-16. The mechanism of cup closure is unknown, but presumably requires constriction of the distal margin to a small aperture, followed by a scission activity that separates the phagosome from the plasma membrane.
FcR-mediated phagocytosis of large particles requires the addition of membrane from intracellular organelles. This membrane, provided to varying degrees by recycling endosomes, late endosomes, lysosomes, specific granules and endoplasmic reticulum7, 17-19, is inserted into the base of the cup, and may facilitate remodeling of the cup membrane20, 21.
The coated vesicle protein clathrin can facilitate zipper-like ingestion of small IgG-coated particles and is present on phagosomes, but it does not contribute significantly to the uptake of larger particles22. However, FcR ligated by small soluble aggregates of IgG can be internalized into clathrin-coated vesicles22. Clathrin does participate in the invasion of epithelial cells by the pathogenic bacterium Listeria monocytogenes, which occurs through zipper-like assembly of close-fitting phagosomes23.
Macropinocytosis
Macropinosomes are self-organized structures which vary in size from 0.2 to 5.0 Îm or more in diameter. Macropinosome morphogenesis is not directly guided by ligand distribution. Instead, macropinosomes form spontaneously or in response to growth factor receptor stimulation from cell surface ruffles that close at their distal margins to engulf extracellular fluid (Fig. 1B). Ruffles are sheet-like extensions of cell surfaces that, like phagocytic cups, extend by localized assembly of actin filaments beneath the plasma membrane24. Ruffles usually recede into the cytoplasm without forming macropinosomes. However, ruffles sometimes curve into open, crater-like cups of the cell surface membrane25. This ruffle closure is followed by cup closure: the constriction of the cup distal margin and the combined membrane fusion and fission that separates the macropinosome from the plasma membrane into the cytoplasm. Although the morphology indicates that macropinosome formation is an occasional and incidental consequence of the ruffling, distinct signaling mechanisms regulate the two activities26.
Several kinds of phagocytosis resemble macropinocytosis in their movements. The bacterial pathogens Salmonella typhimurium and Legionella pneumophila enter cells by stimulating cell surface ruffling and macropinocytosis27 28. Bacteria bound to cell surfaces are internalized into spacious phagosomes as bystanders. Vaccinia virus and some adenoviruses also enter host cells by stimulating macropinocytosis29, 5. This relatively indiscriminate uptake of particles by macropinocytosis-like movements has been called triggered phagocytosis30 (Fig. 1C).
Intermediate Morphologies for Ingestion
A major function of phagocytosis in development and immunity is the clearance of apoptotic and necrotic cells. Disposal of apoptotic cells by macrophages contributes to organ morphogenesis, and the non-inflammatory clearance of apoptotic cells by macrophages and dendritic cells helps to suppress autoimmune responses. A number of different surface ligands have been identified as signals for ingestion of apoptotic cells by phagocytes and a large variety of receptors have been identified as well31-33. Signaling molecules that are essential to phagocytosis of apoptotic cells have been identified through genetic and molecular studies of Caenorhabditis elegans, Drosophila melanogaster and mammalian cells34. Engulfment of some apoptotic cells occurs by a triggered phagocytosis, in which one kind of receptor tethers apoptotic cells to macrophage surfaces, while a distinct receptor-triggered macropinocytic response engulfs the cell 35. However, phagocytosis of apoptotic cells often resembles the zipper mechanism, in that phagosomal membranes advance in close apposition to their apoptotic target cells36. The ingestion of apoptotic cells therefore exhibits features of both zippering and triggered phagocytosis.
Phagocytosis by receptors for the complement component C3bi (CR3) is morphologically distinct from FcR-mediated phagocytosis. CR3 is comprised of integrin chains ÎM and Î2, which when fully activated are capable of binding and internalizing C3bi-coated particles37. The morphology of CR3-mediated phagosome formation varies. In some cells, it resembles the zipper model38. In macrophages, the phagosome forms as a depression in the cell surface, with actin organized as discrete patches within the phagocytic cup39, 40 (Fig. 1C). However, other studies indicate that C3bi-opsonized particles are internalized within ruffles or loosely adherent phagocytic cups41.
Signaling components of ingestion
Although the morphologies of phagosomes and macropinosomes vary, the molecules that regulate the movements of membrane and the actin cytoskeleton have a number of shared features. Receptors and the cytoplasmic proteins that bind them initiate and amplify signaling. Essential plasma membrane lipids are synthesized by enzymes recruited to or activated by receptors. Small GTPases activate enzymes that regulate actin polymerization, myosin contractility and membrane fusion.
Receptors
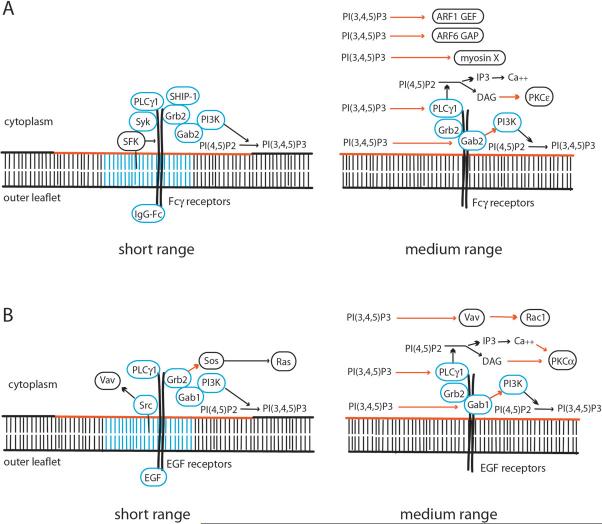
FcR are a family of transmembrane proteins and associated cytoplasmic proteins capable of organizing a complete phagocytic response. Signaling begins with ligand-induced receptor clustering. Cholesterol-rich microdomains associated with clustered FcR facilitate receptor phosphorylation by Src-family kinases (SFK) and increased particle binding42 (Fig. 2A). Phosphorylation of tyrosine residues in the receptor creates docking sites for the tyrosine kinase Syk, which in turn facilitates binding of the adapter proteins Grb2 and Gab243 and phosphatidylinositol 3-kinase type I (PI3K)8. The complex of FcR and recruited cytoplasmic proteins creates a small cluster of phosphoproteins and modified lipids, which either remain as part of the receptor complex or radiate from the receptor complex by diffusion in the membrane bilayer. Those signals modify the activities of other signaling proteins, which ultimately stimulate actin polymerization near the plasma membrane and protrusive extension of membrane over the particle. Some FcR inhibit phagocytosis by recruiting protein and lipid phosphatases that counteract the stimulatory kinases44.
Figure 2. Short- and medium-range signaling by activated FcR and EGF receptors.
The left panels show dimerized receptors and the proteins that bind (blue circles) or interact (black circles) with those receptors upon ligand-binding. The right panels show medium-range signals that are responsive to receptor-generated PI(3,4,5)P3 (red line in the inner leaflet of the bilayer). Black arrows indicate catalytic activation. Red arrows indicate allosteric activation. (A) FcÎ receptors dimerize in response to ligand binding, leading to conformational changes that favor phosphorylation by Src-family kinases (SFK). Lipid microdomains in the plasma membrane (blue lines) facilitate SFK recruitment to FcR. SFK phosphorylation increases binding and activity of the tyrosine kinase Syk, which stimulates recruitment of PI3K, PLCÎ1, Grb2 and Gab2. Gab2 recruits and activates PI3K in a PI(3,4,5)P3-dependent manner. Grb2 activates the Ras GEF Sos, and also binds the lipid phosphatase SHIP-1, which negatively affects PI(3,4,5)P3 signaling. PI(3,4,5)P3 also activates downstream activities including PLCÎ1 and myosin X. (B) Signaling by EGF receptor dimers is activated by ligand-dependent phosphorylation by Src, which also activates Rac1 via phosphorylation of the GEF Vav. Phosphorylated receptors recruit Grb2, which stimulates activation of the Ras GEF Sos, and recruits Gab1, which activates PI3K. PI(3,4,5)P3 generated by PI3K can activate Gab1, providing a positive-feedback amplification of signals.
Macropinocytosis occurs spontaneously in some cells45, or is stimulated by growth factors such as epidermal growth factor (EGF) and macrophage colony-stimulating factor (MCSF) in cells that express cognate receptors46. Receptor ligation is not required to initiate macropinocytosis, as oncogenic v-Src and K-Ras47, 48, phorbol esters25 and membrane-penetrating peptides49 can also stimulate macropinocytosis, presumably by activating chemical changes otherwise initiated by receptor ligation. EGF binding to receptors stimulates receptor autophosphorylation. Like FcR and other growth factor receptors, phosphorylated EGF receptors recruit kinases and adapter proteins that assemble as a complex of proteins near the plasma membrane (Fig. 2B) and stimulate both ubiquitylation leading to receptor endocytosis via clathrin-coated vesicles and phosphorylation of membrane lipids and proteins that activate cytoskeletal movements50. EGF receptor phosphorylation can also be stimulated in the absence of EGF by overexpressing the SFK c-Src51. This indicates that receptors can be activated by extracellular ligands as well as by signals generated inside the cell.
Membrane phospholipids
Lipid modification by receptor signaling creates the potential for radiating signals that can affect large areas of the plasma membrane. Phospholipid kinases, phosphatases and hydrolases are activated during phagocytosis and macropinocytosis, either by direct recruitment to receptor complexes or as a downstream consequence of receptor signaling. The predominant phospholipids of the inner leaflet of plasma membrane are phosphatidylcholine (PC) and phosphatidylserine (PS) and, to a lesser extent, phosphatidylethanolamine (PE) and phosphatidylinositol (PI)52. Phosphorylation and dephosphorylation of hydroxyls in the inositol group of PI generate phosphoinositides with important roles in the regulation of movement. In the plasma membrane, control of PI phosphorylation by phospholipid kinases and phosphatases generate phosphatidylinositol (4)-phosphate (PI(4)P), PI(5)P, PI(4,5)P2, PI(3,4)P2 and PI(3,4,5)P352. These phosphoinositides, especially PI(4,5)P2 and PI(3,4,5)P3, are capable of binding and increasing the activity of proteins that modify membrane chemistry and the actin cytoskeleton. As examples, PI(4,5)P2 increases the activity of WASP, a protein that stimulates actin polymerization via Arp2/353 whereas PI(3,4,5)P3 recruits myosin X to membranes of phagocytic cups14 (Fig. 2B).
Many essential phosphoinositides have been visualized during phagocytosis and macropinocytosis by fluorescence microscopy of cells expressing chimeric fluorescent proteins that bind selectively to those phosphoinositides. PI(4,5)P2 distributions and concentrations in membranes have been inferred using a green fluorescent protein (GFP)-tagged pleckstrin homology (PH) domain from phospholipase C (PLC)-Î1, which binds PI(4,5)P254. Similarly, GFP- or yellow fluorescent protein (YFP)-tagged PH domains from Akt or the Dictyostelium protein CRAC have been used to localize PI(3,4,5)P3 and PI(3,4)P2 55, 56 (Fig. 3A). Imaging with these and similar probes revealed that local levels of PI(4,5)P2 increase in membranes of ruffles and cups54 and that PI(3,4,5)P3 concentrations increase dramatically in phagocytic and macropinocytic cups12, 55-57.
Figure 3. Distinct patterns of signaling in phagocytic cups.
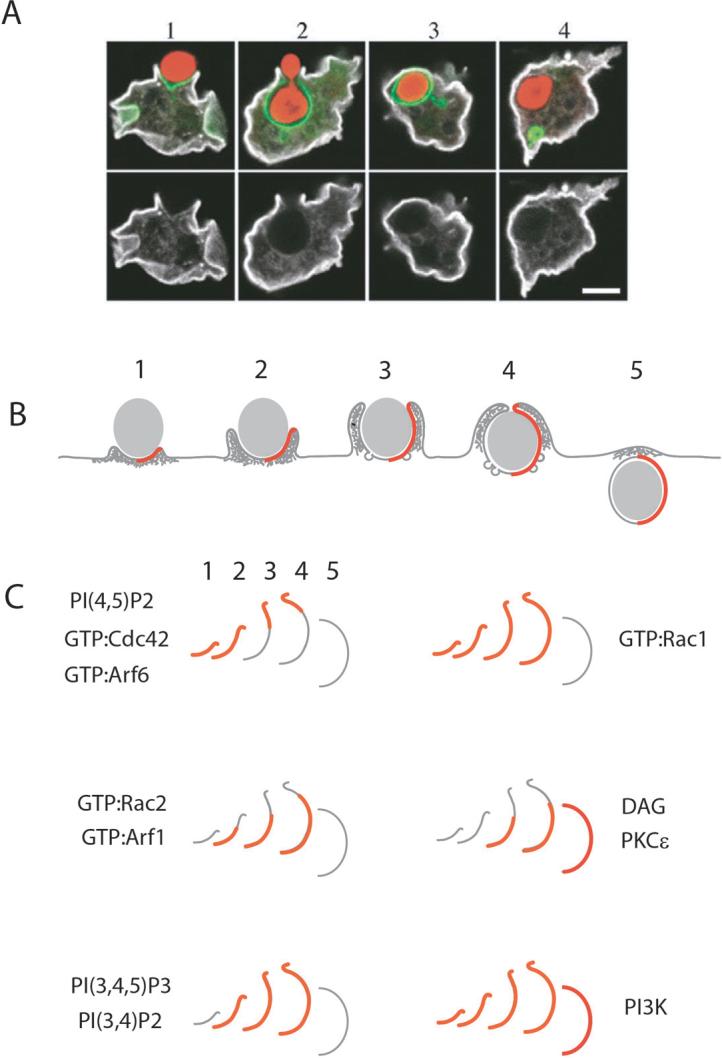
(A) Phagocytic cups in Dictyostelium discoideum show selective depletion of plasma membrane proteins. The panels show confocal fluorescence microscopy of fluorescent protein chimeras at various stages of phagosome formation. The top row shows distributions of GFP-CRAC (green), which reports the distributions of PI(3,4,5)P3, during phagocytosis of yeast cells (red) Both rows show the distribution of the plasma membrane protein H36 (white), which is selectively depleted from membranes of forming cups. (B) Signals for phagosome formation display distinct patterns during phagosome morphogenesis. The stages of phagocytic cup initiation (1), cup extension (2, 3), closure (4) and separation from the plasma membrane (5) are displayed as patterns in half-cups (red lines). (C) The membranes of half-cups at various stages of phagocytosis are indicated by gray lines. Signal distributions are overlaid as red lines. Early signals include increased concentrations of PI(4,5)P2 and activated Cdc42 and Arf6, which localize to advancing edges of cups. Rac1 is activated early and persists until just after cup closure. Late stages of signaling include activation of Rac2 and Arf1, the generation of DAG and the recruitment of PKCÎ, which are delayed relative to the advance of the cup over the particle. PI3K localizes to forming phagosomes, and its products PI(3,4,5)P3 and PI(3,4)P2 increase during cup formation.
Most forms of phagocytosis and macropinocytosis require PI3K, which generates PI(3,4,5)P3 and PI(3,4)P2 from PI(4,5)P2 and PI(4)P, respectively. Inhibitors of PI3K arrest phagocytosis and macropinocytosis after the assembly of actin-rich cups58. In macrophages treated with the PI3K inhibitors wortmannin or LY294002, phagosomes containing large particles remain stuck at the cup stage and macropinocytic cups that do form recede into the cytoplasm without closing. Thus, the early activities of actin polymerization and cup extension do not require PI3K, but later activities do, including contraction of the cup's distal margin and fusion of membrane vesicles with cup membranes58, 59. In some cells, early ruffling responses to growth factors are also PI3K-dependent60.
Phospholipases contribute significantly to phagocytosis and macropinocytosis. Phospholipase C-Î (PLCÎ) hydrolyzes PI(4,5)P2 to inositol trisphosphate (IP3) and diacylgycerol (DAG). IP3 stimulates release of calcium from endoplasmic reticulum. Calcium signaling is essential to some but not all kinds of phagocytosis61. DAG remains associated with the membrane of the phagocytic cup54, where it recruits and activates protein kinase C (PKC)-Î or PKCÎ 62. PLCÎ activity is required for v-Src-induced macropinocytosis, and is activated downstream of PI3K48. FcR-mediated phagocytosis requires PLCÎ162. Other phospholipases required for phagocytosis include phospholipase D (PLD)-1 and PLD2, which generate phosphatidic acid from PC63, 64, and phospholipase A2, which generates arachidonic acid from PC and PE65.
Thus, the lipids generated by receptor complexes can regulate the activities of proteins that are not part of the complex. This suggests two levels of spatial organization (Fig. 2). Short-range organization is that comprised of the receptors, the proteins that bind them and the cholesterol-rich domains of the proximal membrane. Medium-range organization consists of the proteins or lipids that are activated at some distance from the receptor by molecules that diffuse from the receptor complex. These may include proteins of other receptor complexes that are activated by these diffusing signals. Long-range organization would be that related to cell polarity or the regulation of cell dimensions.
Small GTPases
The organized movements of membranes and the actin cytoskeleton are coordinated in phagocytosis and macropinocytosis by small GTPases of the Ras superfamily. These GTPases associate with membranes by regulated exposure of covalently associated acyl chains and by non-covalent interactions with phospholipids or membrane proteins66, 67. The GTP-bound forms of these proteins bind and activate a variety of effector enzymes, which modify lipids or proteins that regulate membrane trafficking, actin polymerization, myosin contractility and other activities. GTPase activities are stimulated by guanine nucleotide exchange factors (GEFs) that facilitate binding of GTP, and are inhibited by GTPase-activating proteins (GAPs) that stimulate GTP hydrolysis and guanine nucleotide dissociation inhibitors (GDIs) that sequester GDP-bound proteins from membranes.
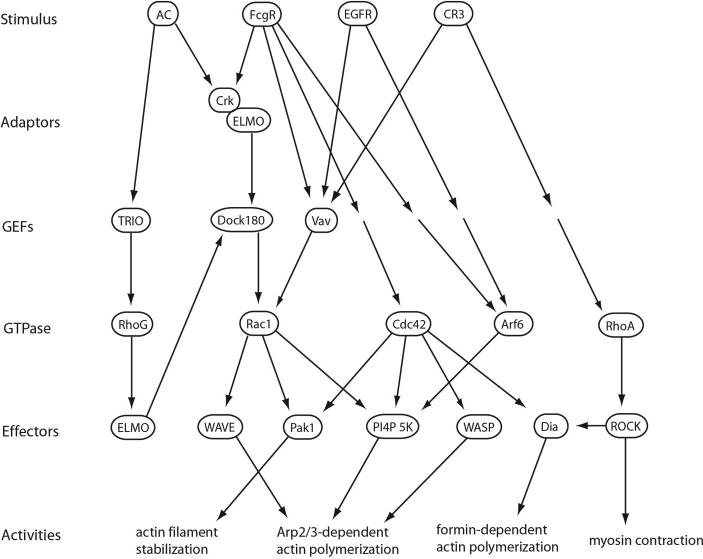
The GTPases control different cellular activities according to the various profiles of effector molecules they activate. Rac1 stimulates actin polymerization, principally through activation of WAVE68, but also by activating PI(4)P 5-kinase and Pak169, 70. WAVE stimulates actin polymerization by activating Arp2/371. PI(4)P 5-kinase synthesizes PI(4 ,5)P2, which activates a number of actin-modifying enzymes72. Pak1 phosphorylates LIM kinase, which phosphorylates and inactivates cofilin, with the net result of increasing actin filament turnover70. Pak1 also phosphorylates CtBP1/BARS, a protein localized to macropinocytic cups which mediates macropinosome closure73. RhoA stimulates myosin contractility through activation of Rho-kinase74 and actin polymerization through activation of formins75. Cdc42 binds and activates WASP, which stimulates actin polymerization through activation of the Arp2/3 complex53, 76. Arf6 stimulates the activation of PI(4)P 5-kinase and PLD2, with consequent effects on membrane curvature and membrane fusion77. Other GTPases regulate the activation of integrins (Rap1) or other GTPases (RhoG)34 78.
Various molecular and genetic methods have implicated these proteins, especially Rac1, in ruffling, phagocytosis and macropinocytosis. For many GTPases whose participation is established, the mechanistic details of their contributions remain to be established. Several GTPases, including Rac179, Cdc4280, Ras47, Rab581, Rab3482, Arf683 and RhoG84, contribute to ruffling and macropinocytosis. Circular ruffle formation and macropinocytosis induced by oncogenic Ras requires the activities of Rab5 and Rac181. Studies of apoptotic cell engulfment have demonstrated roles for the GTPases Rac, RhoG and Rab585. RhoA inhibits engulfment of apoptotic cells, but is required for CR3-mediated phagocytosis86. Thus, although a limited number of small GTPases have been implicated in the various forms of particle and fluid engulfment, their precise contributions to the component activities of ingestion are not understood.
A few of the GEFs that are essential for activation of these GTPases in phagocytosis and macropinocytosis have been identified, although most remain unknown. The GEFs are activated by phosphorylation, binding to other proteins, to phosphoinositides or to phosphatidic acid. Two isoforms of the GEF Vav activate Rac1 during CR3-mediated phagocytosis41 and sometimes also during FcR-mediated phagocytosis87. During phagocytosis of apoptotic cells, the GEF TRIO activates RhoG, which binds to the protein ELMO, activating the Rac1 GEF Dock18088. A similar activation of Rac has been demonstrated for FcR-mediated phagocytosis, involving CrkII, ELMO and Dock18089. SGEF activates RhoG in macropinocytosis by epithelial cells84. No GAPs or GDIs have been directly implicated in phagocytosis or macropinocytosis.
Spatio-temporal organization of signals
Although many of the essential ingredients have been identified, it is not yet clear how receptor complexes, GTPases and phospholipid chemistries are organized into the distinct activities of cup extension, contraction and membrane fusion. Imaging of molecular dynamics in living cells indicates that phagocytic and macropinocytic cups exhibit distinct patterns of signaling at various stages of their formation, and that phospholipids generated within the confines of a forming cup integrate and amplify signaling in that domain of membrane.
Distinct Features of Cup Membranes
The open cups of phagosomes and macropinosomes exhibit profiles of phospholipids, GTPases and enzyme activities that differ from those of the contiguous plasma membrane. Most striking are the depletion of PI(4,5)P2 from cup membranes and the increases in PI(3,4,5)P3 and DAG54, 57, the concentrations of which on membranes of cups remain high until after cup closure56 (Fig. 3B, C). Lipid mobility, as measured by fluorescence recovery after photobleaching, decreases in phagocytic cups during FcR phagocytosis90. The decreased mobility is independent of cholesterol in the membrane and the actin cytoskeleton, but dependent on signaling by SFK. FcR are depleted from the forming cups, possibly by insertion of new membrane from intracellular organelles. In D. discoideum, unclosed phagocytic and macropinocytic cups are enriched in PI(3,4,5)P3 and exclude some plasma membrane proteins55 (Fig. 3A). Similar high concentrations of PI(3,4,5)P3 or PI(3,4)P2 were observed on cups during phagocytosis of CR3-opsonized zymosan, with sharp concentration gradients at the edge of the cup38. During macropinosome formation in epithelial cells, concentrations of PI(3,4,5)P3 increase on cup membranes shortly after ruffle closure56, suggesting that ruffle closure either allows local inactivation of lipid phosphatases or creates a restricted membrane domain in which phospholipids of the membrane lining the open cup cannot diffuse freely into plasma membrane just outside the cup.
During FcR-mediated phagocytosis of large particles, the profile of activated GTPases and lipids varies along the length of the cup (Fig. 3B, C). The advancing edge contains increased concentrations of PI(4,5)P2, relative to the nearby plasma membrane, and high concentrations of PI(3,4,5)P3 and PI(3,4)P254, 57. PI3K localizes to forming cups, as well 57, 91. FRET-based microscopic studies have shown that the GTPases Rac1, Cdc42 and Arf6 are active at the leading edge, where they most likely regulate the advance of the cup over the particle by local stimulation of actin polymerization12. At the base of the cup, PI(4,5)P2 concentrations decrease, DAG levels increase, Cdc42 and Arf6 are inactive and Rac1, Rac2 and Arf1 show increased activity. Rac1 activity shows pronounced increases during phagocytic cup closure and immediately following ruffle closure (see supplementary video). Distinct patterns of signaling within macropinocytic cups have not yet been delineated as they have for phagocytic cups, although they may be expected from future quantitative microscopy.
The patterns of signaling during phagocytosis and macropinocytosis indicate the existence of a diffusion barrier at cup rims which restricts medium-range signaling to a limited region of plasma membrane. Receptor-generated phospholipids or peripheral proteins are confined to the domain of membrane delimited by the barriers, thereby facilitating signal amplification. The nature of this barrier is unknown. It may be created by the high negative curvature of the inner leaflet at the cup margin, or by the presence of as yet unidentified proteins or enzymes that restrict mobility across the cup rim.
Stages in Cup Construction
Imaging of cell movements during macropinosome formation indicates two kinds of behavior, one related to the extension of membranes as ruffles and another to the execution of cup formation and closure. Because phosphorylation and dephosphorylation of phosphoinositides are readily reversible reactions, they provide suitable chemistries for regulating reversible behaviors like ruffle extension and retraction. For example, generation of PI(4,5)P2 by PI(4)P 5-kinase stimulates actin polymerization, which is necessary for ruffling and pseudopod extension. PI(4,5)P2 serves as substrate for PI3K, and the PI(3,4,5)P3 generated by PI3K is subject to dephosphorylation by the 3â PI phosphatase PTEN or by the 5â PI phosphatase SHIP-152. PI(3,4,5)P3 visualized by fluorescent PH domains shows elevated but fluctuating concentrations in ruffles, likely due to the competing activities of lipid kinases and phosphatases and to diffusion of their substrates and products away from their sites of synthesis in the membrane92.
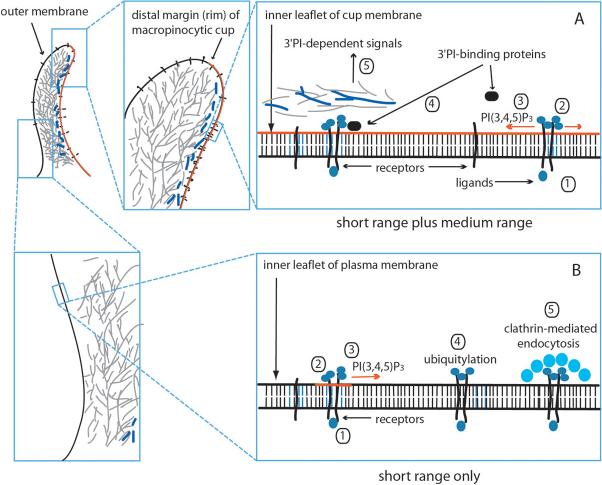
By contrast, PI(3,4,5)P3 levels increase abruptly in membranes of phagocytic and macropinocytic cups38, 56, indicating feedback activation of PI3K or feedback inhibition of PTEN or SHIP-1. In these restricted domains of the membrane, PI(3,4,5)P3 may reach sufficiently high concentrations to activate GEFs, GAPs or other enzymes essential to phagosome formation or the contractile activities of cup closure (Fig. 4A). For example, hydrolysis of PI(4,5)P2 to DAG by PLCÎ alters the net surface charge of the cytoplasmic leaflet of cup membranes such that the peripheral proteins Rac1 and K-Ras disengage93. Activation of PLCÎ1 for macropinocytosis requires prior activation of PI3K48, in part because PLCÎ1 contains a PI(3,4,5)P3-specific PH domain that facilitates enzyme activation94 (Fig. 2). Activation of PLCÎ1, which is necessary for recruitment of PKCÎ62, may require concentrations of PI(3,4,5)P3 that are only attained in the confined space of the cup due to limited diffusion of PI(3,4,5)P3 across the cup rim (Fig. 4A). In this way, the profile of phosphoinositides could define thresholds for commitment to later stages of phagocytosis or macropinocytosis.
Figure 4. Context-dependent signal transduction in cups.
Signaling in the membrane lining macropinocytic or phagocytic cups (red line) (A) is distinct from that in contiguous plasma membrane outside of the cup domain (B). (A) In the cup domain, growth factor or IgG binding to receptors (1) stimulates assembly of receptor complexes (short-range organization) (2). PI3K recruited to receptor complexes stimulates synthesis of PI(3,4,5)P3 (3)(red line), whose concentrations in the inner leaflet of the cup domain increase due to limited diffusion out of the cup and/or positive feedback amplification of its synthesis. Suprathreshold levels of PI(3,4,5)P3 facilitate recruitment or activation of 3â PI-binding proteins (4), which initiate late-stage signals associated with shaping or modifying the cup membrane (5). Such activities are enhanced in cup membranes, possibly due to barriers to diffusion across the distal margin of the cup. (B) Outside of the cup domain, receptor ligation (1) stimulates assembly of a receptor complex (2). PI3K is recruited and synthesizes PI(3,4,5)P3 (3)(red line). However, diffusion or the absence of a positive feedback amplification do not allow PI(3,4,5)P3 concentrations to reach levels that activate medium-range signals. Subsequent ubiquitylation of receptors (4) leads to their down-regulation by clathrin-mediated endocytosis (5).
The final stages of phagosome and macropinosome formation require closure of the cups into discrete intracellular organelles. This is mediated by a PI3K-dependent contractile activity11 and, for macropinocytosis, by Pak1-dependent phosphorylation of CtBP1/BARS73.
Signal transitions and thresholds for signaling
The interdependence of membrane lipid chemistry and the activities of small GTPases provides a basis for signal organization. Changes in the phospholipid profile of membranes in ruffles and cups effect coordinated changes in the profile of GTPase activities 95. This indicates that the signaling state in the cup, or in subdomains within cups, is determined by the predominant phospholipids.
The coordinated pattern of GTPases signaling along the length of the cup (Fig. 3C) is partly organized by the phospholipid transitions in the cup. The depletion of PI(4,5)P2 contributes to the loss of actin from the cup membrane96. When inhibition of PI3K arrests phagocytosis, the stable unclosed cups contain persistently active Arf6 and inactive Arf195. This suspended transition from active Arf6 to active Arf1 in the absence of PI3K activity indicates that the PI(3,4,5)P3 normally generated in cups regulates that transition, possibly by activating GAPs for the GTPases of the advancing edge or GEFs for the GTPases active during closure.
In macropinocytosis, receptors trigger the process, but the signals for macropinosome formation are organized independently. In epithelial cells, PI(3,4,5)P3 levels and Rac1 signals increase transiently early during macropinosome formation but at various times after addition of EGF56, 97, which indicates that signal amplification is unrelated to the timing of receptor ligation and depends instead on some morphological transition, perhaps the assembly of a completely closed cup (ie., ruffle closure, Fig. 1B). Barriers to diffusion at the distal margin of the cup could allow PI(3,4,5)P3 generated by receptor complexes within the cup to reach suprathreshold levels for late stages of signalling. Hence, in ruffling regions of membrane, PI(3,4,5)P3 levels will increase but remain subthreshold because of unrestricted diffusion in the plasma membrane bilayer and by lipid phosphatase activities. PI(3,4,5)P3 concentrations will increase greatly when the barriers to diffusion at distal margins of ruffles close into complete circles at ruffle closure.
Feedback Regulation at Different Scales
The protein and lipid activities that organize the formation of phagosomes and macropinosomes are regulated by positive and negative feedback mechanisms. Positive feedback amplifies receptor signaling or receptor-independent signals that follow cup assembly. Negative signals associated with inhibitory receptors, including the phosphatases SHP-1 and SHIP-1, are largely thought to prevent phagocytosis altogether44. Yet inhibitory signals may also be needed to shape the phagosome.
Short-range organization: receptors and microdomains
The complex of proteins and lipids that assemble around receptors is not a static structure. The recruited kinases, phosphatases and ubiquitin ligases alter the composition of the complex, the nature of the signals they generate and the fate of the complexes themselves. Many receptor complexes provide positive feedback amplification of signals. Amplification of signaling from FcR entails positive feedback via Gab2: the Gab2-dependent activation of PI3K is partly dependent on the PI3K product PI(3,4,5)P343 (Fig. 2A). EGF receptors show a similar feedback amplification via Gab1, PI3K and PI(3,4,5)P398 (Fig. 2B). Fluorescently tagged SHIP-1 associates with early phagocytic cups and the advancing edges of cups, but not with the base of the cup, indicating that SHIP-1 dissociates from FcR before PI3K does, potentially providing a transient suppression of PI(3,4,5)P3 signaling in the cup91.
Receptor signaling may have a default route to clathrin-mediated endocytosis and a regulated route that requires feedback amplification. FcR-mediated uptake of smaller particles and of soluble immune complexes is less dependent than phagocytosis of particles on signaling by Syk, PI3K and SFK99. An alternative pathway of c-Cbl-mediated ubiquitylation of FcR may stimulate receptor entry via clathrin-coated vesicles100 (Fig. 4B) or post-phagocytic sequestration of FcR into multivesicular bodies101. This indicates context-dependent states for the component molecules of the FcR complex.
Medium-range organization: cup domains
Signals that organize phagosome and macropinosome formation are integrated over relatively large domains of membrane. As indicated above, the amplification of PI(3,4,5)P3 and Rac1 signaling is commonly restricted to cups, which indicates the existence of isolated domains within the plasma membrane. These cup domains are enclosed subregions of plasma membrane in which medium-range signals can reach suprathreshold concentrations necessary for transitions to later stages of signaling.
Physical properties of particles may also modulate FcR signaling during phagocytosis. IgG-coated polyacrylamide particles stiffened by cross-linking are readily ingested by macrophages but softer particles, containing less cross-linker, are not, suggesting that FcR signaling is integrated in the phagosome and positively affected by mechanical resistance102.
In addition, a recent study showed that particle shape affects FcR-mediated phagocytosis103. A macrophage will readily ingest a rod-shaped particle coated uniformly with IgG if its highly curved tip contacts the macrophage surface, but not if its flat face contacts the cell. This indicates that some part of FcR signal transduction is inhibited by the flat surface of the particle. The physical or molecular basis of this shape-sensing is not known, but the phenomenon indicates integration of FcR signaling over large regions of the cell surface.
Finally, the signals required for phagocytosis differ for small and large particles. PI3K-dependent mechanisms that regulate contractile proteins and membrane fusion are necessary for the phagocytosis of particles larger than 3 Îm diameter, but not for phagocytosis of smaller particles59. The differential requirement for signaling elements during phagocytosis of large and small particles indicates spatial integration of signaling within forming phagosomes.
Concluding remarks
The zipper model for phagocytosis was based on the observations that particle uptake was restricted to opsonized particles â unopsonized neighboring particles bound to the same cell were not ingested104 â and that particles only half-covered with IgG became stuck in half-finished phagocytic cups9. These observations provide strong evidence for a locally controlled, receptor-guided mechanism of phagocytic cup assembly around particles. Newer findings demonstrate that additional positive signals are required for phagocytosis, including mechanical resistance from the particle itself and some uniform curvature of its surface. Imaging studies support the concept that successful phagosome formation requires organization of a cup-shaped domain of plasma membrane which is sufficiently isolated from the contiguous plasma membrane to allow qualitative changes in signal transduction. That is, complete receptor signaling requires positive feedback from the cup or local plasma membrane structure that allows signaling to exceed some threshold. Activated receptors in membranes that do not exceed these signaling thresholds (ie., those outside of cups) may be removed by clathrin-mediated endocytosis (Fig. 4B).
Despite the absence of a particle surface to guide morphogenesis, the signaling mechanisms and apparatus for macropinosome construction are similar to those for building phagosomes. Short-range signals for macropinocytosis are similar to those for phagocytosis; the activated receptors recruit a similar combination of proteins and generate similar signal intermediates. Early stages of receptor signaling increase ruffling at the cell surface, which is analogous to the extension of the actin-rich phagocytic cup. Late stages of signaling follow ruffle closure, which indicates that the assembly of a cup creates an isolated domain of plasma membrane that supports positive feedback amplification leading to cup closure. Receptors caught in cup domains may contribute to that feedback amplification by anchoring proteins that participate in that amplification (Fig. 2). In triggered phagocytosis, particle capture in forming macropinosomes may be favored by some level of receptor-dependent positive feedback to cup domain organization.
The mechanisms by which physical features of particles or cups feed back to signal transduction remain to be determined. We do not yet know what regulates local signal amplification in cups or the nature of the cup boundary that isolates cup domains. Molecular cell biology and quantitative microscopy should continue to light the way forward.
Highlighted terms
clathrin: a protein that facilitates endocytosis of receptors within small (> 0.1 Îm diameter) vesicles by forming and reorganizing a matrix on the cytoplasmic face of membranes.
Fc receptor: a class of cell surface receptors that bind to the Fc domain of immunoglobulin molecules such as immunoglobulin G (IgG).
opsonize: coating a particle with molecules that render it capable of binding and ingestion by phagocytic cells. From Greek, meaning, âto prepare a mealâ.
recycling endosomes: a subclass of endocytic vesicles that communicate by vesicle fusion with other endocytic compartments and the plasma membrane, including phagocytic cups.
ruffles: Thin, sheet-like protrusions of plasma membrane that extend from the cell surface through the formation and growth of a branched network of actin filaments, and that either retract into cytoplasm or close into macropinosomes.
microdomains: Small regions of membrane rich in cholesterol or sphingolipids to which certain classes of lipid-anchored peripheral membrane proteins, such as Src-family kinases, localize preferentially.
pleckstrin-homology domains: A structural domain common in many proteins, including pleckstrin, capable of binding specific phosphoinositol sugars, including those that comprise membrane phosphoinositides.
Ras superfamily: A large taxonomic group of small proteins that share structural features and the capabilities of regulated binding to GTP, GDP, membranes, proteins that regulate the species of bound nucleotide and various additional effector proteins.
multivesicular bodies: an intracellular membranous compartment that contains intracellular vesicles derived from its delimiting membrane and that communicates by vesicle fusion with endosomes, lysosomes and sometimes also the plasma membrane.
gelation: The formation of a cross-linked matrix of polymer. In the actin cytoskeleton, actin filaments are bridged by cross-linking proteins into a gel. The regulated dissolution of that matrix (solation) can be coupled with the activation of contractile proteins to effect motility.
lamellipodia: Sheet-like protrusions of the cell surface containing a branched network of actin filaments, which extends along surfaces during cell motility. Lamellipodia are structurally analogous to ruffles and phagocytic cups.
Box 1: Actin dynamics.
Phagocytosis and macropinocytosis both require a dynamic actin cytoskeleton. Actin is an intracellular ATP-binding protein essential for contractile motility. Its regulated assembly into helical polymers can generate forces that propel organelles or bacteria inside cytoplasm or alter the shape of the plasma membrane. Actin polymerization and its regulatory proteins have been implicated in both phagocytosis and macropinocytosis. Polymerization in phagocytosis is regulated by Arp2/3 and by formins105-107. Other proteins that affect actin polymerization during phagocytosis include WASP108, WAVE2109, amphiphysin-2110 and coronin111.
The actin cytoskeleton is maintained as a gel by cross-linking proteins, and is contracted by myosin molecules, mechanochemical ATPases that translocate along actin filaments, pulling membranes or other actin filaments. Several myosins have been implicated in phagocytosis.
The movements of cell crawling, phagocytosis and macropinocytosis are mediated by a combination of localized actin polymerization and depolymerization, together with the organized gelation, solation and contraction of actin filament networks. Migratory cells and many epithelial cells contain an actin-rich cytoskeleton just beneath the plasma membrane, which continually extends and retracts plasma membrane structures. Flat sheet-like protrusions are called lamellipodia or peripheral ruffles when extending along a substrate, dorsal ruffles when extending into the medium and phagocytic cups or lamellipodia when extending over a particle. Different cells and different signaling conditions produce different kinds of structures, and some cells display the entire variety.
The enzymes that control the actin cytoskeleton are regulated by small GTPases and the GEFs and GAPs that control their activities. Some GTPases affecting actin dynamics in phagocytosis and macropinocytosis are indicated here. Few, if any, of these elements bind directly to receptors; they therefore represent examples of medium-range signals. The illustration shows some of the GTPases which are activated during phagocytosis of apoptotic cells (AC), FcR-mediated phagocytosis, EGFR-stimulated macropinocytosis, and CR3-mediated phagocytosis.
Supplementary Material
Supplementary Video
Activation of Rac1 during M-CSF-induced macropinocytosis. Bone marrow-derived mouse macrophages expressing FRET probes for Rac1 activity (YFP-Rac1 and CFP-PBD) were stimulated with 200 ng/ml M-CSF just prior to the first frame of the video sequence. The left panel shows time-lapse, phase-contrast microscopy immediately following addition of M-CSF. Extensive ruffling is evident as the dark folds of plasma membrane that form and migrate from the cell periphery. The right panel shows the same phase-contrast sequence with an overlay showing GTP-bound Rac1, as detected by FRET microscopy. Rac1 was activated initially throughout the periphery of the cell, but its activation soon became limited to the forming macropinosomes. Images were collected every 20 sec and played back at 6 frames/sec (ie., 100x real time). Sequence courtesy of Dr. Sei Yoshida, University of Michigan Medical School, Ann Arbor, MI.
Acknowledgments
I am grateful for the suggestions of Drs. Sei Yoshida and Adam Hoppe, and for funding from the NIAID, NIH.
Biography
Joel Swanson, Ph.D., has studied the mechanisms of phagocytosis, macropinocytosis and organelle trafficking in macrophages as well as the cell biology of macrophage infections with bacterial pathogens. His lab presently uses quantitative microscopy of cells expressing fluorescent proteins to analyze relationships between cell morphology and the magnitudes of receptor-dependent signals.
Footnotes
Online summary
1. Phagocytosis is the ingestion by cells of particles or other cells. Receptors in membranes of phagocytic cells organize the advance of plasma membrane and the actin cytoskeleton around target particles, forming intracellular membrane-bounded compartments called phagosomes.
2. Macropinocytosis is the ingestion by cells of extracellular solutes and fluid into 0.2 to >5.0 Îm diameter vesicles, or macropinosomes. Macropinosomes can form spontaneously or in response to activation of cell surface receptors.
3. Common signaling mechanisms organize the construction of phagocytic or macropinocytic cup-shaped invaginations of plasma membrane. Receptors, GTPases of the Ras superfamily and membrane phospholipids regulate the component activities of actin filament assembly, disassembly and contraction as well as fusion of membrane vesicles with cup membranes.
4. Imaging of molecular dynamics in living cells indicates that phagocytic and macropinocytic cups exhibit distinct patterns of signaling that correspond to early and late stages of their formation.
5. Activated receptor complexes (short-range signals) generate diffusible signal molecules (medium-range signals) which are subject to feedback regulation and which, at suprathreshold concentrations, can activate transitions from early to later stages of signaling. Phospholipids generated within the confines of the forming cup integrate and amplify signaling in that domain of membrane.
6. Receptor-mediated signal transduction for phagocytosis and macropinocytosis is modulated by cell structure and is conditional on feedback regulation related to cup integrity, particle stiffness or particle shape.
References
- 1.Stuart LM, Ezekowitz RA. Phagocytosis and comparative innate immunity: learning on the fly. Nat Rev Immunol. 2008;8:131–141. doi: 10.1038/nri2240. [DOI] [PubMed] [Google Scholar]
- 2.Watts C, Amigorena S. Antigen traffic pathways in dendritic cells. Traffic. 2000;1:312–317. doi: 10.1034/j.1600-0854.2000.010404.x. [DOI] [PubMed] [Google Scholar]
- 3.Blander JM, Medzhitov R. On regulation of phagosome maturation and antigen presentation. Nat Immunol. 2006;7:1029–1035. doi: 10.1038/ni1006-1029. [DOI] [PubMed] [Google Scholar]
- 4.Reddien PW, Horvitz HR. The engulfment process of programmed cell death in caenorhabditis elegans. Annu Rev Cell Dev Biol. 2004;20:193–221. doi: 10.1146/annurev.cellbio.20.022003.114619. [DOI] [PubMed] [Google Scholar]
- 5.Amstutz B, et al. Subversion of CtBP1-controlled macropinocytosis by human adenovirus serotype 3. EMBO J. 2008;27:956–969. doi: 10.1038/emboj.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 7.Huynh KK, Kay JG, Stow JL, Grinstein S. Fusion, fission, and secretion during phagocytosis. Physiology (Bethesda) 2007;22:366–372. doi: 10.1152/physiol.00028.2007. [DOI] [PubMed] [Google Scholar]
- 8.Cox D, Greenberg S. Phagocytic signaling strategies: FcÎ receptor-mediated phagocytosis as a model system. Sem. Immunol. 2001;13:339–345. doi: 10.1006/smim.2001.0330. [DOI] [PubMed] [Google Scholar]
- 9.Griffin FM, Griffin JA, Silverstein SC. Studies on the mechanism of phagocytosis. II. The interaction of macrophages with anti-immunoglobulin IgG-coated bone marrow-derived lymphocytes. J. Exp. Med. 1976;144:788–809. doi: 10.1084/jem.144.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright SD, Silverstein SC. Phagocytosing macrophages exclude proteins from the zones of contact with opsonized targets. Nature. 1984;309:359–361. doi: 10.1038/309359a0. [DOI] [PubMed] [Google Scholar]
- 11.Swanson JA, et al. A contractile activity that closes phagosomes in macrophages. J. Cell Sci. 1999;112:307–316. doi: 10.1242/jcs.112.3.307. [DOI] [PubMed] [Google Scholar]
- 12.Hoppe AD, Swanson JA. Cdc42, Rac1 and Rac2 display distinct patterns of activation during phagocytosis. Mol. Biol. Cell. 2004;15:3509–3519. doi: 10.1091/mbc.E03-11-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herant M, Heinrich V, Dembo M. Mechanics of neutrophil phagocytosis: experiments and quantitative models. J Cell Sci. 2006;119:1903–1913. doi: 10.1242/jcs.02876. [DOI] [PubMed] [Google Scholar]
- 14.Cox D, et al. Myosin X is a downstream effector of PI(3)K during phagocytosis. Nature Cell Biol. 2002;4:469–477. doi: 10.1038/ncb805. [DOI] [PubMed] [Google Scholar]
- 15.Diakonova M, Bokoch G, Swanson JA. Dynamics of cytoskeletal proteins during FcÎ receptor-mediated phagocytosis in macrophages. Mol. Biol. Cell. 2002;13:402–411. doi: 10.1091/mbc.01-05-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araki N, Hatae T, Furukawa A, Swanson JA. Phosphoinositide-3-kinase-independent contractile activities associated with FcÎ-receptor-mediated phagocytosis and macropinocytosis in macrophages. J. Cell Sci. 2003;116:247–257. doi: 10.1242/jcs.00235. [DOI] [PubMed] [Google Scholar]
- 17.Braun V, et al. TI-VAMP/VAMP7 is required for optimal phagocytosis of opsonised particles in macrophages. Embo J. 2004;23:4166–4176. doi: 10.1038/sj.emboj.7600427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Czibener C, et al. Ca2+ and synaptotagmin VII-dependent delivery of lysosomal membrane to nascent phagosomes. J Cell Biol. 2006;174:997–1007. doi: 10.1083/jcb.200605004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gagnon E, et al. Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell. 2002;110:119–131. doi: 10.1016/s0092-8674(02)00797-3. [DOI] [PubMed] [Google Scholar]
- 20.Lee WL, Mason D, Schreiber AD, Grinstein S. Quantitative analysis of membrane remodeling at the phagocytic cup. Mol Biol Cell. 2007;18:2883–2892. doi: 10.1091/mbc.E06-05-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kay JG, Murray RZ, Pagan JK, Stow JL. Cytokine secretion via cholesterol-rich lipid raft-associated SNAREs at the phagocytic cup. J Biol Chem. 2006;281:11949–11954. doi: 10.1074/jbc.M600857200. [DOI] [PubMed] [Google Scholar]
- 22.Tse SML, et al. Differential role of actin, clathrin, and dynamin in FcÎ receptor-mediated endocytosis and phagocytosis. J. Biol. Chem. 2003;278:3331–3338. doi: 10.1074/jbc.M207966200. [DOI] [PubMed] [Google Scholar]
- 23.Veiga E, Cossart P. Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nat Cell Biol. 2005;7:894–900. doi: 10.1038/ncb1292. [DOI] [PubMed] [Google Scholar]
- 24.Weed SA, Parsons JT. Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene. 2001;20:6418–6434. doi: 10.1038/sj.onc.1204783. [DOI] [PubMed] [Google Scholar]
- 25.Swanson JA. Phorbol esters stimulate macropinocytosis and solute flow through macrophages. J. Cell Sci. 1989;94:135–142. doi: 10.1242/jcs.94.1.135. [DOI] [PubMed] [Google Scholar]
- 26.Li G, D'Souza-Schorey C, Barbieri MA, Cooper JA, Stahl PD. Uncoupling of membrane ruffling and pinocytosis during Ras signal transduction. J Biol Chem. 1997;272:10337–10340. [PubMed] [Google Scholar]
- 27.Alpuche-Aranda CM, Racoosin EL, Swanson JA, Miller SI. Salmonella stimulate macrophage macropinocytosis and persist within spacious phagosomes. J. Exp. Med. 1994;179:601–608. doi: 10.1084/jem.179.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watarai M, et al. Legionella pneumophila is internalized by a macropinocytotic uptake pathway controlled by the Dot/Icm system and the mouse Lgn1 locus. J. Exp. Med. 2001;194:1081–1095. doi: 10.1084/jem.194.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- 30.Griffin FM, Griffin JA, Leider JE, Silverstein SC. Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J. Exp. Med. 1975;142:1263–1282. doi: 10.1084/jem.142.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krysko DV, D'Herde K, Vandenabeele P. Clearance of apoptotic and necrotic cells and its immunological consequences. Apoptosis. 2006;11:1709–1726. doi: 10.1007/s10495-006-9527-8. [DOI] [PubMed] [Google Scholar]
- 32.Park D, et al. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 33.Miyanishi M, et al. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 34.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann PR, et al. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J. Cell Biol. 2001;155:649–659. doi: 10.1083/jcb.200108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krysko DV, et al. Macrophages use different internalization mechanisms to clear apoptotic and necrotic cells. Cell Death Differ. 2006;13:2011–2022. doi: 10.1038/sj.cdd.4401900. [DOI] [PubMed] [Google Scholar]
- 37.Caron E, Self AJ, Hall A. The GTPase Rap1 controls functional activation of macrophage integrin ÎMÎ2 by LPS and other inflammatory mediators. Current Biology. 2000;10:974–978. doi: 10.1016/s0960-9822(00)00641-2. [DOI] [PubMed] [Google Scholar]
- 38.Dewitt S, Tian W, Hallett MB. Localised PtdIns(3,4,5)P3 or PtdIns(3,4)P2 at the phagocytic cup is required for both phagosome closure and Ca2+ signalling in HL60 neutrophils. J Cell Sci. 2006;119:443–451. doi: 10.1242/jcs.02756. [DOI] [PubMed] [Google Scholar]
- 39.Kaplan G. Differences in the mode of phagocytosis with Fc and C3 receptors in macrophages. Scand. J. Immunol. 1977;6:797–807. doi: 10.1111/j.1365-3083.1977.tb02153.x. [DOI] [PubMed] [Google Scholar]
- 40.Allen L-AH, Aderem A. Molecular definition of distinct cytoskeletal structures involved in complement- and Fc receptor-mediated phagocytosis in macrophages. J. Exp. Med. 1996;184:627–637. doi: 10.1084/jem.184.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hall AB, et al. Requirements for Vav guanine nucleotide exchange factors and Rho GTPases in FcÎR- and complement-mediated phagocytosis. Immunity. 2006;24:305–316. doi: 10.1016/j.immuni.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Sobota A, et al. Binding of IgG-opsonized particles to Fc Î R is an active stage of phagocytosis that involves receptor clustering and phosphorylation. J Immunol. 2005;175:4450–4457. doi: 10.4049/jimmunol.175.7.4450. [DOI] [PubMed] [Google Scholar]
- 43.Gu H, Botelho RJ, Yu M, Grinstein S, Neel BG. Critical role for scaffolding adapter Gab2 in FcÎR-mediated phagocytosis. J. Cell Biol. 2003;161:1151–1161. doi: 10.1083/jcb.200212158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nimmerjahn F, Ravetch JV. FcÎ receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Racoosin EL, Swanson JA. Macrophage colony stimulating factor (rM-CSF) stimulates pinocytosis in bone marrow-derived macrophages. J. Exp. Med. 1989;170:1635–1648. doi: 10.1084/jem.170.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bar-Sagi D, Feramisco JR. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986;233:1061–1066. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- 48.Amyere M, et al. Constitutive macropinocytosis in oncogene-transformed fibroblasts depends on sequential permanent activation phosphoinositide 3-kinase and phospholipase C. Mol. Biol. Cell. 2000;11:3453–3467. doi: 10.1091/mbc.11.10.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Futaki S, Nakase I, Tadokoro A, Takeuchi T, Jones AT. Arginine-rich peptides and their internalization mechanisms. Biochem Soc Trans. 2007;35:784–787. doi: 10.1042/BST0350784. [DOI] [PubMed] [Google Scholar]
- 50.Schlessinger J. Common and distinct elements in cellular signaling via EGF and FGF receptors. Science. 2004;306:1506–1507. doi: 10.1126/science.1105396. [DOI] [PubMed] [Google Scholar]
- 51.Donepudi M, Resh MD. c-Src trafficking and co-localization with the EGF receptor promotes EGF ligand-independent EGF receptor activation and signaling. Cell Signal. 2008 doi: 10.1016/j.cellsig.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yeung T, Grinstein S. Lipid signaling and the modulation of surface charge during phagocytosis. Immunol Rev. 2007;219:17–36. doi: 10.1111/j.1600-065X.2007.00546.x. [DOI] [PubMed] [Google Scholar]
- 53.Miki H, Takenawa T. Regulation of actin dynamics by WASP family proteins. J Biochem. 2003;134:309–313. doi: 10.1093/jb/mvg146. [DOI] [PubMed] [Google Scholar]
- 54.Botelho RJ, et al. Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J. Cell Biol. 2000;151:1353–1367. doi: 10.1083/jcb.151.7.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mercanti V, et al. Selective membrane exclusion in phagocytic and macropinocytic cups. J Cell Sci. 2006;119:4079–4087. doi: 10.1242/jcs.03190. [DOI] [PubMed] [Google Scholar]
- 56.Araki N, Egami Y, Watanabe Y, Hatae T. Phosphoinositide metabolism during membrane ruffling and macropinosome formation in EGF-stimulated A431 cells. Exp Cell Res. 2007;313:1496–1507. doi: 10.1016/j.yexcr.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 57.Vieira OV, et al. Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J. Cell Biol. 2001;155:19–25. doi: 10.1083/jcb.200107069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis in macrophages. J. Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cox D, Tseng C-C, Bjekic G, Greenberg S. A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J. Biol. Chem. 1999;274:1240–1247. doi: 10.1074/jbc.274.3.1240. [DOI] [PubMed] [Google Scholar]
- 60.WennstrÃm S, et al. Activation of phosphoinositide 3-kinase is required for PDGF-stimulated membrane ruffling. Curr. Biol. 1994;4:385–393. doi: 10.1016/s0960-9822(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 61.Larsen EC, et al. Differential requirement for classic and novel PKC isoforms in respiratory burst and phagocytosis in RAW 264.7 cells. J. Immunol. 2000;165:2809–2817. doi: 10.4049/jimmunol.165.5.2809. [DOI] [PubMed] [Google Scholar]
- 62.Cheeseman KL, et al. Targeting of protein kinase C-Î during FcÎ receptor-dependent phagocytosis requires the Î-C1B domain and phospholipase C-Î1. Mol Biol Cell. 2006;17:799–813. doi: 10.1091/mbc.E04-12-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iyer SS, Barton JA, Bourgoin S, Kusner DJ. Phospholipases D1 and D2 Coordinately Regulate Macrophage Phagocytosis. J Immunol. 2004;173:2615–2623. doi: 10.4049/jimmunol.173.4.2615. [DOI] [PubMed] [Google Scholar]
- 64.Corrotte M, et al. Dynamics and function of phospholipase D and phosphatidic acid during phagocytosis. Traffic. 2006;7:365–377. doi: 10.1111/j.1600-0854.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- 65.Lennartz MR, et al. Phospholipase A2 inhibition results in sequestration of plasma membrane into electronlucent vesicles during IgG-mediated phagocytosis. J. Cell Sci. 1997;110:2041–2052. doi: 10.1242/jcs.110.17.2041. [DOI] [PubMed] [Google Scholar]
- 66.Hancock JF. Ras proteins: different signals from different locations. Nat Rev Mol Cell Biol. 2003;4:373–384. doi: 10.1038/nrm1105. [DOI] [PubMed] [Google Scholar]
- 67.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 68.Miki H, Suetsugu S, Takenawa T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. Embo J. 1998;17:6932–6941. doi: 10.1093/emboj/17.23.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tolias KF, et al. Type Ia phosphatidylinositol-4-phosphate 5-kinase mediates Rac-dependent actin assembly. Curr. Biol. 2000;10:153–156. doi: 10.1016/s0960-9822(00)00315-8. [DOI] [PubMed] [Google Scholar]
- 70.Edwards DC, Sanders LC, Bokoch GM, Gill GM. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nature Cell Biol. 1999;1:253–259. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- 71.Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- 72.Yin HL, Janmey PA. Phosphoinositide regulation of the actin cytoskeleton. Annu Rev Physiol. 2003;65:761–789. doi: 10.1146/annurev.physiol.65.092101.142517. [DOI] [PubMed] [Google Scholar]
- 73.Liberali P, et al. The closure of Pak1-dependent macropinosomes requires the phosphorylation of CtBP1/BARS. Embo J. 2008;27:970–981. doi: 10.1038/emboj.2008.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 75.Li F, Higgs HN. The mouse Formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr Biol. 2003;13:1335–1340. doi: 10.1016/s0960-9822(03)00540-2. [DOI] [PubMed] [Google Scholar]
- 76.Donaldson JG. Multiple roles for Arf6: Sorting, structuring, and signaling at the plasma membrane. J. Biol. Chem. 2003;278:41573–41576. doi: 10.1074/jbc.R300026200. [DOI] [PubMed] [Google Scholar]
- 77.Honda A, et al. Phosphatidylinositol 4-phosphate 5-kinase Î is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell. 1999;99:521–532. doi: 10.1016/s0092-8674(00)81540-8. [DOI] [PubMed] [Google Scholar]
- 78.Caron E. Rac signalling: a radical view. Nature Cell Biol. 2003;5:185–187. doi: 10.1038/ncb0303-185. [DOI] [PubMed] [Google Scholar]
- 79.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:410–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 80.Garrett WS, et al. Developmental control of endocytosis in dendritic cells by Cdc42. Cell. 2000;102:325–334. doi: 10.1016/s0092-8674(00)00038-6. [DOI] [PubMed] [Google Scholar]
- 81.Lanzetti L, Palamidessi A, Areces L, Scita G, Di Fiore PP. Rab5 is a signalling GTPase involved in actin remodelling by receptor tyrosine kinases. Nature. 2004;429:309–314. doi: 10.1038/nature02542. [DOI] [PubMed] [Google Scholar]
- 82.Sun P, et al. Small GTPase Rah/Rab34 is associated with membrane ruffles and macropinosomes and promotes macropinosome formation. J Biol Chem. 2003;278:4063–4071. doi: 10.1074/jbc.M208699200. [DOI] [PubMed] [Google Scholar]
- 83.Porat-Shliom N, Kloog Y, Donaldson JG. A Unique Platform for H-Ras Signaling Involving Clathrin-independent Endocytosis. Mol Biol Cell. 2008;19:765–775. doi: 10.1091/mbc.E07-08-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ellerbroek SM, et al. SGEF, a RhoG guanine nucleotide exchange factor that stimulates macropinocytosis. Mol Biol Cell. 2004;15:3309–3319. doi: 10.1091/mbc.E04-02-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakaya M, Tanaka M, Okabe Y, Hanayama R, Nagata S. Opposite effects of rho family GTPases on engulfment of apoptotic cells by macrophages. J Biol Chem. 2006;281:8836–8842. doi: 10.1074/jbc.M510972200. [DOI] [PubMed] [Google Scholar]
- 86.Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–1721. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- 87.Patel JC, Hall A, Caron E. Vav regulates activation of Rac but not Cdc42 during FcÎR-mediated phagocytosis. Mol. Biol. Cell. 2002;13:1215–1226. doi: 10.1091/mbc.02-01-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.deBakker CD, et al. Phagocytosis of apoptotic cells is regulated by a UNC-73/TRIO-MIG-2/RhoG signaling module and armadillo repeats of CED-12/ELMO. Curr Biol. 2004;14:2208–2216. doi: 10.1016/j.cub.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 89.Lee WL, Cosio G, Ireton K, Grinstein S. Role of CrkII in FcÎ receptor-mediated phagocytosis. J Biol Chem. 2007;282:11135–11143. doi: 10.1074/jbc.M700823200. [DOI] [PubMed] [Google Scholar]
- 90.Corbett-Nelson EF, Mason D, Marshall JG, Collette Y, Grinstein S. Signaling-dependent immobilization of acylated proteins in the inner monolayer of the plasma membrane. J Cell Biol. 2006;174:255–265. doi: 10.1083/jcb.200605044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kamen LA, Levinsohn J, Swanson JA. Differential Association of Phosphatidylinositol 3-Kinase, SHIP-1, and PTEN with Forming Phagosomes. Mol Biol Cell. 2007;18:2463–2475. doi: 10.1091/mbc.E07-01-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Seveau S, et al. A FRET analysis to unravel the role of cholesterol in Rac1 and PI 3-kinase activation in the InIB/Met signalling pathway. Cell Microbiol. 2007;9:790–803. doi: 10.1111/j.1462-5822.2006.00832.x. [DOI] [PubMed] [Google Scholar]
- 93.Yeung T, et al. Receptor activation alters inner surface potential during phagocytosis. Science. 2006;313:347–351. doi: 10.1126/science.1129551. [DOI] [PubMed] [Google Scholar]
- 94.Falasca M, et al. Activation of phospholipase C Î by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 1998;17:414–422. doi: 10.1093/emboj/17.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beemiller P, Hoppe AD, Swanson JA. A phosphatidylinositol-3-kinase-dependent signal transition regulates ARF1 and ARF6 during FcÎ receptor-mediated phagocytosis. PLoS Biol. 2006;4:e162. doi: 10.1371/journal.pbio.0040162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scott CC, et al. Phosphatidylinositol-4,5-bisphosphate hydrolysis directs actin remodeling during phagocytosis. J Cell Biol. 2005;169:139–149. doi: 10.1083/jcb.200412162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oberbanscheidt P, Balkow S, Kuhnl J, Grabbe S, Bahler M. SWAP-70 associates transiently with macropinosomes. Eur J Cell Biol. 2007;86:13–24. doi: 10.1016/j.ejcb.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 98.Rodrigues GA, Falasca M, Zhang Z, Ong SH, Schlessinger J. A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol Cell Biol. 2000;20:1448–1459. doi: 10.1128/mcb.20.4.1448-1459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang ZY, et al. Differential kinase requirements in human and mouse Fc-Î receptor phagocytosis and endocytosis. J Leukoc Biol. 2006;80:1553–1562. doi: 10.1189/jlb.0106019. [DOI] [PubMed] [Google Scholar]
- 100.Paolini R, et al. Activation of Syk tyrosine kinase is required for c-Cbl-mediated ubiquitination of FcÎ RI and Syk in RBL cells. J Biol Chem. 2002;277:36940–36947. doi: 10.1074/jbc.M204948200. [DOI] [PubMed] [Google Scholar]
- 101.Lee WL, Kim MK, Schreiber AD, Grinstein S. Role of ubiquitin and proteasomes in phagosome maturation. Mol Biol Cell. 2005;16:2077–2090. doi: 10.1091/mbc.E04-06-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Beningo KA, Wang Y-L. Fc-receptor-mediated phagocytosis is regulated by mechanical properties of the target. J. Cell Sci. 2002;115:849–856. doi: 10.1242/jcs.115.4.849. [DOI] [PubMed] [Google Scholar]
- 103.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci U S A. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Griffin FM, Bianco C, Silverstein SC. Characterization of the macrophage receptor for complement and demonstration of its functional independence from the receptor for the Fc portion of immunoglobulin G. J. Exp. Med. 1975;141:1269–1277. doi: 10.1084/jem.141.6.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.May RC, Caron E, Hall A, Machesky LM. Involvement of the Arp2/3 complex in phagocytosis mediated by FcÎR and CR3. Nature Cell Biol. 2000;2 doi: 10.1038/35008673. [DOI] [PubMed] [Google Scholar]
- 106.Seth A, Otomo C, Rosen MK. Autoinhibition regulates cellular localization and actin assembly activity of the diaphanous-related formins FRLalpha and mDia1. J Cell Biol. 2006;174:701–713. doi: 10.1083/jcb.200605006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Colucci-Guyon E, et al. A role for mammalian diaphanous-related formins in complement receptor (CR3)-mediated phagocytosis in macrophages. Curr Biol. 2005;15:2007–2012. doi: 10.1016/j.cub.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 108.Lorenzi R, Brickell PM, Katz DR, Kinnon C, Thrasher AJ. Wiskott-Aldrich syndrome protein is necessary for efficient IgG-mediated phagocytosis. Blood. 2000;95:2943–2946. [PubMed] [Google Scholar]
- 109.Abou-Kheir W, Isaac B, Yamaguchi H, Cox D. Membrane targeting of WAVE2 is not sufficient for WAVE2-dependent actin polymerization: a role for IRSp53 in mediating the interaction between Rac and WAVE2. J Cell Sci. 2008;121:379–390. doi: 10.1242/jcs.010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yamada H, et al. Amphiphysin 1 is important for actin polymerization during phagocytosis. Mol Biol Cell. 2007;18:4669–4680. doi: 10.1091/mbc.E07-04-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yan M, Collins RF, Grinstein S, Trimble WS. Coronin-1 function is required for phagosome formation. Mol Biol Cell. 2005;16:3077–3087. doi: 10.1091/mbc.E04-11-0989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Highlighted References
- 30.Park, et al. This paper identified a receptor phosphatidylserine presented on apoptotic cells and demonstrated a direct connection to Rac activation via ELMO and Dock180. 2007.
- 39.Hall, et al. Demonstrated that the Rac GEF Vav was necessary for CR3-mediated phagocytosis but not for FcR-mediated phagocytosis. This finding is at odds with studies using other cells, which indicated a role for Vav-activated Rac in FcR but not CR3 phagocytosis. 2006.
- 47.Amyere, et al. Demonstrated a role for PLCÎ downstream of PI3K during constitutive macropinocytosis in transformed cells. 2000.
- 52.Botelho, et al. First demonstration of localized changes in phosphoinositides and diacylglycerol concentrations in unclosed phagocytic cups. 2000.
- 53.Mercanti, et al. Demonstrated exclusion of membrane proteins from phagocytic and macropinocytic cups in Dictyostelium discoideum. 2006.
- 54.Araki, et al. Using quantitative fluorescence microscopy of phosphoinositide dynamics during macropinosome formation, this paper shows the abrupt increases in 3âPIs that precede macropinosome closure. 2007.
- 55.Vieira, et al. This paper provided the first images of 3âPI concentration in forming phagocytic cups, and of the transitions from one species of 3âPI to another that accompany phagosome maturation. 2001.
- 60.Cheeseman, et al. Demonstrated that PKCÎ recruitment to phagosomes required upstream activation of PLCÎ1. 2006.
- 80.Caron, Hall This paper demonstrated two distinct signal transduction pathways underlying CR3- and FcR-mediated phagocytosis. 1998.
- 85.Yeung, et al. A novel fluorescence microscopic method for measuring surface potential on surfaces of cytoplasmic membranes was described and applied to demonstrate a role for surface potential in retaining Ras and Rac at plasma membranes. 2006.
- 87.Beemiller, et al. Visualized distinct patterns of activation and deactivation for Arf1 and Arf6 during phagocytosis, and demonstrated a role for PI3K in organizing the signal transition. 2006.
- 94.Beningo, Wang Using opsonized polymer gels with variable crosslinker and stiffness, this work showed that FcR-mediated phagocytosis requires mechanical resistance to deformation. 2002.
- 95.Champion, Mitragotri This work showed that uniformly opsonized particles of various shapes were only ingested if the surface that contacted the macrophage membrane was less than a minimum tangent angle. This indicates a level of signal integration in forming phagocytic cups. 2006.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Video
Activation of Rac1 during M-CSF-induced macropinocytosis. Bone marrow-derived mouse macrophages expressing FRET probes for Rac1 activity (YFP-Rac1 and CFP-PBD) were stimulated with 200 ng/ml M-CSF just prior to the first frame of the video sequence. The left panel shows time-lapse, phase-contrast microscopy immediately following addition of M-CSF. Extensive ruffling is evident as the dark folds of plasma membrane that form and migrate from the cell periphery. The right panel shows the same phase-contrast sequence with an overlay showing GTP-bound Rac1, as detected by FRET microscopy. Rac1 was activated initially throughout the periphery of the cell, but its activation soon became limited to the forming macropinosomes. Images were collected every 20 sec and played back at 6 frames/sec (ie., 100x real time). Sequence courtesy of Dr. Sei Yoshida, University of Michigan Medical School, Ann Arbor, MI.