Abstract
Background:
Although cystic fibrosis (CF) is the most common inherited respiratory disease, the burden of influenza among individuals with CF is not well characterized.
Methods:
We used the CF Foundation Patient Registry to determine the relationship between pulmonary exacerbation incidence rate and influenza virus season from July 2003 through June 2007. The outcome of interest, pulmonary exacerbation, was defined as treatment of a respiratory illness with IV antibiotics. Each influenza season was defined as all months during which ≥ 15% of laboratory tests for influenza virus were positive in the US influenza virologic surveillance system. We calculated incidence rates of pulmonary exacerbation during the influenza and summertime seasons as well as relative rates with 95% CIs. A multivariate regression model adjusted for demographic and clinical predictors.
Results:
In 2003, the patient cohort size was 21,506 patients, and 7,727 patients experienced at least one pulmonary exacerbation. The overall pulmonary exacerbation incidence rate in the influenza season was 595.0 per 10,000 person-months compared with a summertime baseline of 549.6 per 10,000 person-months. The incidence rate ratio was 1.08 (95% CI: 1.06, 1.10). Multivariate analysis did not change our estimate of risk (adjusted odds ratio: 1.07; 95% CI: 1.05, 1.10). An estimated annual excess of 147.6 per 10,000 person-months or an excess 2.1% of total exacerbations occur during the influenza season.
Conclusion:
Our data demonstrate a substantial contribution of the influenza season to CF morbidity. Further studies to determine any causal link between influenza infection and CF pulmonary exacerbations are necessary.
An estimated 30,000 people in the United States have cystic fibrosis (CF).1 CF is an autosomal recessive disease that affects multiple organ systems; however, respiratory complications account for nearly 85% of CF mortality.1 Exacerbations of CF pulmonary disease are common and are characterized by cough, increased sputum production, dyspnea, decreased energy level and appetite, weight loss, and decreases in spirometric parameters.2 Over the last 2 decades, the quality of life and median survival among persons with CF have improved substantially.1 Nevertheless, increasing age in CF is associated with worsening nutritional status, declining lung function, and increased pulmonary exacerbations.1 Given that the rate of pulmonary exacerbations is associated with increased mortality,3-6 it is important to identify pulmonary exacerbations that may be preventable.
CF pulmonary exacerbations represent decreased host defenses within the lungs leading to alterations in airway microbiology, increased sputum production, and ventilatory obstruction.2 Pulmonary exacerbations are associated with the acquisition of new organisms and increased concentrations of airway flora.7-12 The presence of some organisms in the airways, including Pseudomonas aeruginosa, Burkholderia cepacia, and methicillin-resistant Staphylococcus aureus are associated with clinical deterioration.8-10,13-15 Furthermore, nearly two-thirds of all initial infections with P aeruginosa have been found to occur during winter months, suggesting that winter respiratory virus season may affect pulmonary host defenses.16 Although evidence of influenza and other respiratory virus infections has also been observed during pulmonary exacerbations,17-20 studies to date have not addressed the role of respiratory viruses in the etiology of the exacerbations.
We undertook this study to determine whether CF exacerbation rates were temporally associated with the influenza season and to determine the proportion of CF exacerbations that were attributable to the influenza season. We hypothesized that CF exacerbations would be seasonal and that a portion of these clinical events would be attributable to the influenza season.
Materials and Methods
Study Population
This was a cohort study of individuals followed in the CF Foundation Patient Registry (CF Registry) from January 1, 2003, through December 31, 2007. The CF Registry tracks the health of people with CF in the United States. Approximately 80% of an estimated 30,000 people with CF seen in > 115 CF Foundation-accredited care centers are included. Generally, patients enter the CF Registry at the time of diagnosis, which usually occurs during infancy. Little is known about persons with CF who are not included in the CF Registry. A full description of the registry is available.1 Demographic and clinical data are recorded at each clinic visit, during outpatient antibiotic therapy, during hospitalizations, and at year-end reviews. The reasons for individual hospitalizations and outpatient antibiotic therapy are recorded for each event. Baseline descriptive statistics were calculated for CF Registry patients from the 2003 year-end review.
Outcome of Interest
All persons in the CF Registry were included for analysis. The outcome of interest was a documented CF pulmonary exacerbation requiring hospitalization or home IV antibiotic therapy. Duplicate recorded pulmonary exacerbations in a single calendar month were excluded. Patients with a year-end review were considered present within the cohort for the entire calendar year for the purpose of patient-time follow-up. The institutional review board at the University of Washington approved this study.
Assessment of Exposure
The surveillance year was defined as July 1 through June 30 of the subsequent calendar year. As had been done in prior influenza modeling studies,21 the influenza season was defined as all months during which ≥ 15% of laboratory tests for influenza virus were positive in the US influenza virologic surveillance system.22 Three periods in the surveillance year were defined based on laboratory-confirmed influenza activity. The “influenza season” was October through December in 2003-2004, December through March in 2004-2005, January through March in 2005-2006, and January through March in 2006-2007. Because CF pulmonary exacerbation data were available by month, influenza epidemic activity was defined by month; however, epidemic influenza activity occurred in only partial months during these periods. The “peri-influenza season” was defined as the time from October through April in which nationwide laboratory tests for influenza were < 15%. The “summer season” was the remaining months in the surveillance year.
Statistical Analysis
Patients were categorized into two age groups for analyses (< 18 years and ≥ 18 years). Exacerbation incidence rates were calculated by dividing the number of exacerbations per selected period by the total person-time of follow-up during that period. To include an entire influenza season per 12-month period, incidence rate data were assessed by surveillance year. The influenza season-attributable risk was calculated by subtracting the rates during the summer period from rates during the influenza season. Analyses were based on incidence rate-difference models, which have been used to estimate influenza-associated deaths and hospitalizations.21,23-27
Multivariate logistic regression with robust standard errors was then used to assess the effect of demographic and clinical predictors on the odds of a pulmonary exacerbation during influenza activity periods. Covariates for the model were determined a priori based on a review of the literature for factors associated with pulmonary exacerbations. These covariates included age, gender, insurance status, race, median income of zip code in which patients lived, pancreatic insufficiency, FEV1, and whether height-weight measures met CF Foundation goals.1 To account for exacerbations by the same individual per surveillance year, marginal general estimation equations were used to calculate standard errors using the clustered sandwich estimator. P values < .05 were considered statistically significant. Analyses were performed with STATA statistical software (version 10.1; STATACorp; College Station, TX).
Results
Baseline Characteristics
In the 2003 calendar year, the CF Registry had a total of 21,506 patients (Table 1). Of these, 8,625 (40.1%) were adults and 12,881 (59.9%) were children. The majority of adults and children were non-Hispanic, white (92.8% and 86.1%), male (53.1% and 51.3%), and had private/health maintenance organization insurance (55.3% and 50.1%). The median household income by zip code of residence was $44,323.5 for adults and $42,998.0 for children.
Table 1.
—Baseline Characteristics of Patients Seen at Cystic Fibrosis Foundation-Accredited US Care Centers, 2003
| Characteristic | Adults (≥ 18 y) | Children (< 18 y) |
| Demographic data | ||
| Person-years | 8,625 | 12,881 |
| Sex, % female | 4,044 (46.9) | 6,275 (48.7) |
| Insurance status | ||
| Private/HMO | 4,767 (55.3) | 6,456 (50.1) |
| Public | 3,402 (39.4) | 6,161 (47.8) |
| No insurance | 287 (3.3) | 161 (1.3) |
| Other/unknown | 169 (2.0) | 103 (0.8) |
| Median zip code income (IQR) | 44,323.5 (35,609-56,393) | 42,998 (34,750-56,408) |
| Race/ethnicity | ||
| Non-Hispanic white | 8,000 (92.8) | 11,094 (86.1) |
| Non-Hispanic black | 224 (2.6) | 575 (4.5) |
| Hispanic | 313 (3.6) | 1,042 (8.1) |
| Other/unknown | 88 (1.0) | 170 (1.3) |
| Health care use | ||
| Total outpatient visits, median (IQR) | 4 (1-7) | 4 (2-6) |
| Total hospital admissions, median (IQR) | 0 (0-1) | 0 (0-1) |
| Clinical data | ||
| FEV1, L, mean (SD) | 2.3 (1.0) | 1.9 (0.8) |
| FEV1, % predicted, mean (SD) | 60.8 (23.9) | 86.8 (20.8) |
| FVC, L, mean (SD) | 3.3 (1.1) | 2.3 (1.0) |
| FVC, % predicted, mean (SD) | 74.6 (20.7) | 93.6 (18.2) |
| FEV1 > 50% predicted | 5,153 (63.7) | 8,242 (93.7) |
| Height-weight above US mean | 414 (5.0) | 5,242 (44.8) |
| Pancreatic insufficiency | 7,460 (86.5) | 12,018 (93.3) |
| Pseudomonas aeruginosa culture positive | 5,903 (77.8) | 5,455 (44.6) |
| Burkholderia cepacia culture positive | 398 (5.3) | 211 (1.7) |
| Staphylococcus aureus culture positive | 3,041 (40.1) | 7,106 (58.2) |
Baseline data are for the 2003 calendar year. Data are presented as No. (%) unless otherwise specified. CF = cystic fibrosis; HMO = health maintenance organization; IQR = interquartile range.
Baseline Clinical Description
Adults and children with CF had a median of four outpatient clinic visits and zero hospital admissions for any cause in the 2003 calendar year (Table 1). On average, adults had worse lung function than children (FEV1% predicted: 60.8% vs 86.8%), lower percent height-weight measures above US average (5.0% vs 44.8%), and lower rates of pancreatic insufficiency (86.5% vs 93.3%). Adults also had higher rates of P aeruginosa infection (77.8% vs 44.6%) and B cepacia infection (5.3% vs 1.7%), but lower rates of S aureus infection (40.1% vs 58.2%).
Pulmonary Exacerbations Seasonality
During the 2003-2004 surveillance year, CF Registry patients were followed for 264,234 person-months. A total of 7,727 unique patients experienced a pulmonary exacerbation requiring hospitalization or home IV therapy (Table 2). Pulmonary exacerbations included 6,748 unique patients who were hospitalized for treatment and 2,262 unique patients who received home IV therapy. There were 14,903 recorded pulmonary exacerbations, and the overall rate of pulmonary exacerbations in 2003-2004 was 564.0 per 10,000 person-months. Adults had nearly twice the exacerbation incidence rate of children (788.1 per 10,000 person-months vs 411.9 per 10,000 person-months).
Table 2.
—Number of Persons With CF Pulmonary Exacerbations and Site of Therapy, 2003-2004 Surveillance Year
| Exacerbations | Persons Hospitalizeda | Persons with Home IV Therapyb | Persons Hospitalized or Receiving Home IV Therapyb |
| 1 | 4,052 | 1,660 | 4,134 |
| 2 | 1,508 | 416 | 1,866 |
| 3 | 607 | 113 | 812 |
| 4 | 295 | 51 | 446 |
| 5 | 151 | 16 | 213 |
| 6 | 79 | 3 | 133 |
| 7 | 26 | 0 | 75 |
| 8 | 14 | 3 | 24 |
| 9 | 7 | 0 | 8 |
| 10 | 8 | 0 | 12 |
| 11 | 1 | 0 | 3 |
| 12 | 0 | 0 | 1 |
| Total persons with exacerbations | 6,748 | 2,262 | 7,727 |
| Mean (SD) | 0.5 (1.0) | 0.1 (0.5) | 0.6 (1.2) |
| Median (IQR) | 0 (0-1) | 0 (0-0) | 0 (0-1) |
The influenza surveillance year was defined as July 1 through June 30 of the subsequent calendar year. See Table 1 for expansion of abbreviations.
During the 2003-2004 surveillance year, persons in the Cystic Fibrosis Foundation Patient Registry were followed for 264,234 person-months.
The analysis was of monthly CF pulmonary exacerbations. Duplicate exacerbations in a single calendar month were excluded. Multiple exacerbations in one calendar month that included hospitalizations and home IV therapy were recorded as a single hospitalization. Because the same individual could be hospitalized and receive home IV therapy for multiple exacerbations in a year, the sum of persons hospitalized and persons receiving home IV therapy does not equal the total number of persons with exacerbations.
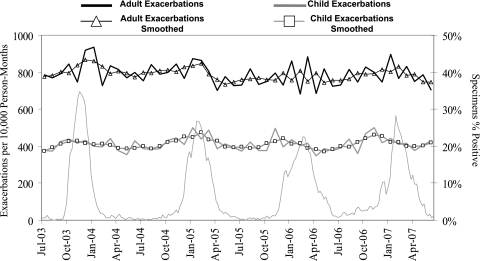
During the study period, there was an annual increase in the incidence rate of pulmonary exacerbations during the winter months (Fig 1). Further, incidence rates for adults and children were temporally associated with increases in influenza activity nationwide.
Figure 1.
Cystic fibrosis (CF) pulmonary exacerbation incidence rates per 10,000 person-months by age group and US influenzalike illness rates, July 2003 through June 2007. Weekly influenzalike illness rates are from US influenza surveillance data.21,22 Influenzalike illness rates are plotted on the Wednesday of each week. CF exacerbation incidence rates are plotted on the 15th day of each calendar month.
Pulmonary Exacerbations Attributable to the Influenza Season
The mean duration of influenza season during the study was 3.25 months per year. Over the entire influenza season, crude exacerbation incidence rates were 825.4 per 10,000 person-months among adults and 433.7 per 10,000 person-months among children. Exacerbations during the influenza season contributed to an excess of pulmonary exacerbations of 45.4 per 10,000 person-months (Table 3). Assuming a 3.25-month influenza season annually, we estimate that there are 147.6 pulmonary exacerbations per 10,000 people with CF that are attributable to influenza every year. Applied to the 2003 CF Registry census, this would equal 317 pulmonary exacerbations or 2.1% of the total number of pulmonary exacerbations observed in the registry that year. Estimated annual excess exacerbations attributable to the influenza season for adults and children are 55.9 per 10,000 people and 39.9 per 10,000 people.
Table 3.
—Seasonal Incidence Rates of CF Pulmonary Exacerbations and Estimated Annual Excess Events, July 2003 Through June 2007
| Age Group | Seasona | Incidence Rate per 10,000 Person-Mo | Event Frequency | Person-Mo | Influenza-Attributable Risk per 10,000 Person-Mob (95% CI) | Estimated Excess Annual Events per 10,000 Peoplec |
| Total | Influenza | 595.0 | 18,049.0 | 303,368.0 | 45.4 (34.3, 56.3) | 147.6 |
| Peri-influenza | 568.4 | 19,861.0 | 349,449.0 | … | … | |
| Summer | 549.6 | 25,473.0 | 463,453.0 | … | … | |
| Adults (181 y) | Influenza | 825.4 | 10,311.0 | 124,928.0 | 55.9 (35.6, 76.0) | 181.7 |
| Peri-influenza | 780.5 | 11,362.0 | 145,579.0 | … | … | |
| Summer | 769.5 | 14,792.0 | 192,220.0 | … | … | |
| Children (0-17 y) | Influenza | 433.7 | 7,738.0 | 178,440.0 | 39.9 (27.6, 52.1) | 129.7 |
| Peri-influenza | 416.9 | 8,499.0 | 203,870.0 | … | … | |
| Summer | 393.8 | 10,681.0 | 271,233.0 | … | … |
See Table 1 for an expansion of abbreviations.
Three influenza activity periods were defined by review of US influenza surveillance data.21,22 The influenza season was all months during which ≥ 15% of laboratory tests for influenza virus were positive in the US influenza virologic surveillance system. The peri-influenza season was all months during October through April during which influenzalike illness activity was below epidemic thresholds. The summer season was all months from April through September.
Rates during the summer season were used as the baseline rates for calculation of the influenza season-attributable risk.
Estimates are based on average influenza season of 3.25 mo.
Multivariate Model of Exacerbation
Crude incidence rates of pulmonary exacerbations were 7% to 10% greater during the influenza season compared with summertime baselines among both age categories (Table 4). A multivariate logistic regression model was constructed to estimate the probability of influenza season exacerbations when adjusted for patient demographic, socioeconomic, and clinical status. The multivariate risk estimates were comparable to the crude incidence rate ratios. Adjusted odds ratios (aORs) comparing exacerbations in the influenza season to the summer season baseline were 1.07 (95% CI: 1.05, 1.10) in adults and 1.08 (95% CI: 1.05, 1.12) in children.
Table 4.
—Multivariate Model of the Association Between CF Pulmonary Exacerbations and the Influenza Season, July 2003 Through June 2007
| Statistic | Influenza Season | Peri-influenza Season | Summer Season | |
| All ages | Incidence rate per 10,000 person-months | 595.0 | 568.4 | 549.6 |
| Incidence rate ratio (95% CI) | 1.08 (1.06, 1.10) | 1.03 (1.01, 1.05) | Referent | |
| Adjusted OR (95% CI) | 1.07 (1.05, 1.10) | 1.03 (1.00, 1.05) | Referent | |
| Adults (181 y) | Incidence rate per 10,000 person-months | 825.4 | 780.5 | 769.5 |
| Incidence rate ratio (95% CI) | 1.07 (1.05, 1.10) | 1.01 (0.99, 1.04) | Referent | |
| Adjusted OR (95% CI) | 1.07 (1.05, 1.10) | 1.02 (0.90, 1.04) | Referent | |
| Children (0-17 y) | Incidence rate per 10,000 person-months | 433.7 | 416.9 | 393.8 |
| Incidence rate ratio (95% CI) | 1.10 (1.07, 1.13) | 1.06 (1.03, 1.09) | Referent | |
| Adjusted OR (95% CI) | 1.08 (1.05, 1.12) | 1.04 (1.00, 1.07) | Referent |
Adjusted for age, gender, insurance status, race, median income of zip code, pancreatic insufficiency, FEV1 percent, whether height-weight measures met CF Foundation goals, and repeated outcomes for some individuals. OR = odds ratio. See Table 1 for expansion of other abbreviations.
Sensitivity Analyses
To account for some of the activity by other, cocirculating respiratory viruses in the winter, we conducted a sensitivity analysis comparing incidence rates from the influenza season and peri-influenza season. Relative risk estimates were lower for the entire cohort (1.05), adults (1.06), and children (1.04) when the influenza season was compared with the peri-influenza season. Estimates of excess annual CF pulmonary exacerbations attributable to the influenza season (the difference between influenza and peri-influenza incidence rates) were 26.6 per 10,000 persons among the entire cohort, 44.9 per 10,000 persons among adults, and 16.8 per 10,000 persons among children. Although the primary analysis has demonstrated that exacerbations during the influenza season are elevated when compared with a summer baseline with little to no respiratory virus activity, this sensitivity analysis demonstrates that influenza season exacerbations are also elevated when compared with other periods during the winter season when other respiratory viruses circulate.
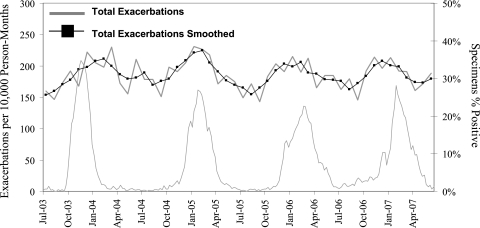
Because the distribution of individuals in the cohort with pulmonary exacerbations was so highly skewed, we investigated the effect outliers with multiple exacerbations had on our model. In another sensitivity analysis, we excluded all individuals with more than one pulmonary exacerbation (Fig 2). The overall exacerbation incidence rate during the influenza season was decreased to 205.8 per 10,000 person-months, whereas the summertime baseline was 169.7 per 10,000 person-months (Table 5). The relative risk of exacerbation between the two seasons was 1.21 (95% CI: 1.17, 1.26), which demonstrates a substantial increase in risk compared with the entire cohort. Of note, persons with < 2 pulmonary exacerbations per year represent roughly 84% of the entire CF Registry. When the multivariate logistic regression model was applied to this subset of patients, effect size was decreased (aOR, 1.12; 95% CI: 1.09, 1.16).
Figure 2.
CF pulmonary exacerbation incidence rates per 10,000 person-months and US influenzalike illness rates among patients with < 2 pulmonary exacerbations, July 2003 through June 2007. Y-axis changed from Figure 1 to demonstrate seasonal variation. Smoothed function is a moving average of five data points: the two previous, the current, and the two subsequent incidence rates. See Figure 1 legend for expansion of abbreviation.
Table 5.
—Seasonal Incidence Rates of CF Pulmonary Exacerbations Among Persons With Fewer Than Two Annual Exacerbations and More Than Four Annual Exacerbations, July 2003 Through June 2007
| Statistic | Influenza Season | Peri-influenza Season | Summer Season | |
| Persons with fewer than two exacerbations | ||||
| All ages | Incidence rate per 10,000 person-months | 205.8 | 191.1 | 169.7 |
| Incidence rate ratio (95% CI) | 1.21 (1.17, 1.26) | 1.13 (1.09, 1.17) | Referent | |
| Adults (181 y) | Incidence rate per 10,000 person-months | 266.1 | 240.6 | 217.4 |
| Incidence rate ratio (95% CI) | 1.22 (1.16, 1.29) | 1.11 (1.05, 1.17) | Referent | |
| Children (0-17 y) | Incidence rate per 10,000 person-months | 169.3 | 160.4 | 140.4 |
| Incidence rate ratio (95% CI) | 1.21 (1.15, 1.27) | 1.14 (1.09, 1.20) | Referent | |
| Persons with more than four exacerbations | ||||
| All ages | Incidence rate per 10,000 person-months | 4,329.2 | 4,156.3 | 4,089.7 |
| Incidence rate ratio (95% CI) | 1.06 (1.02, 1.10) | 1.02 (0.98, 1.05) | Referent | |
| Adults (181 y) | Incidence rate per 10,000 person-months | 4,337.4 | 4,179.0 | 4,159.7 |
| Incidence rate ratio (95% CI) | 1.04 (1.00, 1.09) | 1.00 (0.96, 1.05) | Referent | |
| Children (0-17 y) | Incidence rate per 10,000 person-months | 4,314.3 | 4,115.2 | 3,965.2 |
| Incidence rate ratio (95% CI) | 1.09 (1.03, 1.15) | 1.04 (0.99, 1.09) | Referent |
See Table 1 for expansion of abbreviations.
In a subsequent analysis, we investigated the effect of the influenza season on individuals with ≥ 4 pulmonary exacerbations. The overall influenza season incidence rate was 4,329.2 per 10,000 person-months, nearly an order of magnitude greater than the full cohort. Summertime baseline rates also increased, resulting in an overall relative risk of 1.06 (95% CI: 1.02, 1.10), similar to the estimate for the entire cohort. Although this subset represents only a small proportion of the overall patient registry, they experience an estimated annual excess of 778.4 exacerbations per 10,000 people that are attributable to the influenza season. The probability of exacerbation among this subset of patients was also similar when applied to a similar logistic regression model as described above (aOR, 1.08; 95% CI: 1.04, 1.13).
Discussion
Our analysis suggests that a substantial proportion of morbidity in CF is associated with the influenza season. Influenza is a substantial public health threat, and it is estimated to be associated with > 36,000 deaths and 220,000 hospitalizations in the United States annually.28,29 With the recent emergence of novel 2009 influenza A(H1N1) in the United States and Mexico,30 high rates of community transmission have been seen. However, rates of hospitalization are well below pandemic planning estimates.31,32 Nevertheless, early data indicate that individuals with comorbidities, including chronic lung disease, are at increased risk of influenza-associated hospitalization and death.31,33
Persons with chronic lung disease are also at increased risk of seasonal influenza-associated complications.34 A study of health care use by patients with chronic lung disease found an influenza-attributable excess of antibiotic courses (IV and po) were near 1,000 prescriptions per 10,000 patients.25 The study also determined the highest incidence of influenza-associated cardiopulmonary hospitalizations was approximately 6,500 per 10,000 person-months for persons > 65 years of age. Incidence rates of younger patients with chronic lung disease ranged from approximately 500 per 10,000 person-months for children 5 to 14 years of age and 3,500 per 10,000 person-months for adults 50 to 64 years of age. In our study, pulmonary exacerbation rates were found to be intermediate to these rates of outpatient and inpatient therapy during the influenza season. The outcome of interest in our study was pulmonary exacerbations treated with either home IV antibiotic therapy or inpatient hospitalization. The decision regarding the setting to treat the patient is based on disease severity and physician practice patterns not solely related to disease severity. Milder exacerbations are often treated with inhaled or oral antibiotics; these clinical events were not captured in our definition of pulmonary exacerbation, likely resulting in an underestimate of influenza-associated risk.
Although CF practitioners have long been aware of the seasonality of pulmonary exacerbations, this study is the first of which we are aware that has been able to demonstrate significant increases in event rates during the influenza season. Arguably the healthiest 84% of the patient registry have a 12% to 21% increased odds of an influenza season exacerbation, whereas the sickest minority with ≥ 4 exacerbations annually have an influenza season-attributable risk that is > 10 times the rest of the patient cohort. Although children generally have the highest influenza attack rates, individuals with chronic comorbid conditions and the elderly have the highest rates of death and hospitalizations. Given the progressive decline in lung function and overall health among patients with CF, it is possible that children experience higher influenza attack rates but experience more self-limited disease and less progression to pulmonary exacerbation.
CF pulmonary exacerbations are believed to be a result of decreased host defenses and colonizing bacterial overgrowth; however, moderate or severe, primary influenza infection may be indistinguishable from a pulmonary exacerbation in CF. Furthermore, influenza viral infection can alter host defense equilibrium, increase airway resistance and mucus production, and promote colonizing bacterial overgrowth and secondary bacterial infections.2,7-12 The relationship between influenza infection and subsequent bacterial pneumonia is long established.35 Other respiratory viruses can exacerbate chronic lung disease, and some, most notably respiratory syncytial virus (RSV), may also circulate during the winter months. Of note, RSV activity occurred 1 to 2 months after the 2003-2004 influenza peak, but coincided with subsequent influenza seasons. Nevertheless, both the primary analysis and sensitivity analysis demonstrate exacerbation increases during the influenza season when compared with peri-influenza baselines, periods during which other wintertime, respiratory viruses circulate. Further studies are underway to evaluate the risk of CF exacerbations in regions and times when influenza and RSV activity are not coincident, which may better distinguish their relative contribution to CF morbidity. Furthermore, although respiratory viruses have been isolated from individuals during pulmonary exacerbations,17-20 further studies are needed to determine the causal link between laboratory-confirmed influenza infection and CF morbidity.
Both trivalent inactivated influenza vaccine and intranasal live cold-adapted influenza vaccine are safe and immunogenic in persons with CF;36 however, there is some evidence that antibody response may be lower in children at high risk for influenza infection compared with healthy children.37-39 Nevertheless, influenza vaccine effectiveness has been demonstrated among children and adults with other chronic respiratory diseases.40-43 Additionally, a small, retrospective study among individuals with CF found significantly fewer laboratory-confirmed influenza virus infections in those who had received the influenza vaccine.44
Our study is subject to limitations. Laboratory test results for influenza were not available; therefore we could demonstrate that pulmonary exacerbations were associated with the influenza season but not influenza infection. We were unable to include influenza vaccination into our model for two reasons: first, the CF Registry began recording influenza vaccination three influenza seasons into the study period in 2006; and, second, date of receipt is not recorded in the registry so timing with regard to the influenza season is impossible. Nevertheless, it is likely that this is a highly vaccinated cohort. In 2006, among those for whom vaccine receipt/nonreceipt was documented, 91.0% of age-eligible children and 87.9% of adults received influenza vaccine. However, 26.9% of age-eligible children and 38.0% of adults did not have vaccine receipt/nonreceipt documented. One might expect higher morbidity in a cohort of patients with CF who had lower vaccination rates. Furthermore, our time-series analysis used only the month of pulmonary exacerbation and excluded duplicate exacerbations in the calendar month. This could lead to miscoded events as a hospitalization at the end of a month followed by discharge to home to complete a course of IV antibiotics at the beginning of the next month could be recorded as two separate events. This double counting of events, if they occur randomly throughout the course of the year, would bias our results toward a null given that the influenza season averaged less than one-third of the year.
This study demonstrated for the first time to our knowledge that CF pulmonary exacerbations increase significantly during the winter and that they are highly associated with the influenza season. These findings suggest that a substantial proportion of CF morbidity may be preventable by influenza vaccination or chemoprophylaxis. Although all patients with CF are recommended to receive influenza vaccination, CF Registry vaccination rates appear to be below these goals.1 Adults and persons with multiple annual pulmonary exacerbations have elevated rates of influenza season exacerbations, and they should be targeted for vaccination. Nevertheless, the risk of exacerbation during the influenza season as compared with summertime baselines was consistently elevated among all subgroups analyzed in the study and supports universal vaccination. Furthermore, vaccination against influenza of caregivers and close contacts of patients with CF is highly recommended, as it may decrease influenza transmission. Although further studies to determine any causal link between influenza and CF pulmonary exacerbations are necessary, influenza vaccination is the best way to avoid infection and its complications.
Acknowledgments
Author contributions: Dr. Ortiz had full access to all the data in the study, and he takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Ortiz: contributed to study conception and design, data analysis, data interpretation, and manuscript writing.
Dr Neuzil: contributed to study conception and design, data analysis, data interpretation, and manuscript writing.
Dr Victor: contributed to study conception and design, data analysis, data interpretation, and manuscript writing.
Dr Wald: contributed to study conception and design, data analysis, data interpretation, and manuscript writing.
Dr Aitken: contributed to data analysis, data interpretation, and manuscript writing.
Dr Goss: contributed to study conception and design, data analysis, data interpretation, and manuscript writing.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Wald has been a consultant to Immune Design. Drs Ortiz, Neuzil, Victor, Aitken, and Goss have reported that no potential conflicts of interest with any companies/organizations whose products or services may be discussed in this article.
Other contributions: The authors wish to acknowledge the following people for their help with this project: Ellen Caldwell, Lynette Brammer, Monica Brooks, Scott Epperson, Lyn Finelli, Amalia Magaret, Misty Saracino, David Shay, Nigel Thavasi, and the Cystic Fibrosis Foundation. This work was performed at the University of Washington.
Abbreviations
- aOR
adjusted odds ratio
- CF
cystic fibrosis
- CF Registry
Cystic Fibrosis Foundation Patient Registry
- RSV
respiratory syncytial virus
Funding/Support: This research was supported in part by National Heart, Lung and Blood Institute Respiratory Research Training Grant [HL007287] (Dr Ortiz); National Institute of Allergy and Infectious Diseases K24 Mentor Award [AI071113] (Dr Wald), and the Cystic Fibrosis Foundation (Dr Goss) [GOSS00L0].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Cystic Fibrosis Foundation . Patient Registry Annual Data Report 2007. Bethesda, MD: Cystic Fibrosis Foundation; 2008. [Google Scholar]
- 2.Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: epidemiology and pathogenesis. Thorax. 2007;62(4):360–367. doi: 10.1136/thx.2006.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liou TG, Adler FR, Cahill BC, et al. Survival effect of lung transplantation among patients with cystic fibrosis. JAMA. 2001;286(21):2683–2689. doi: 10.1001/jama.286.21.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001;153(4):345–352. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liou TG, Adler FR, Huang D. Use of lung transplantation survival models to refine patient selection in cystic fibrosis. Am J Respir Crit Care Med. 2005;171(9):1053–1059. doi: 10.1164/rccm.200407-900OC. [DOI] [PubMed] [Google Scholar]
- 6.Mayer-Hamblett N, Rosenfeld M, Emerson J, Goss CH, Aitken ML. Developing cystic fibrosis lung transplant referral criteria using predictors of 2-year mortality. Am J Respir Crit Care Med. 2002;166(12 Pt 1):1550–1555. doi: 10.1164/rccm.200202-087OC. [DOI] [PubMed] [Google Scholar]
- 7.Aaron SD, Ramotar K, Ferris W, et al. Adult cystic fibrosis exacerbations and new strains of Pseudomonas aeruginosa. Am J Respir Crit Care Med. 2004;169(7):811–815. doi: 10.1164/rccm.200309-1306OC. [DOI] [PubMed] [Google Scholar]
- 8.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34(2):91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 9.Kosorok MR, Zeng L, West SE, et al. Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatr Pulmonol. 2001;32(4):277–287. doi: 10.1002/ppul.2009.abs. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Kosorok MR, Farrell PM, et al. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA. 2005;293(5):581–588. doi: 10.1001/jama.293.5.581. [DOI] [PubMed] [Google Scholar]
- 11.Demko CA, Byard PJ, Davis PB. Gender differences in cystic fibrosis: Pseudomonas aeruginosa infection. J Clin Epidemiol. 1995;48(8):1041–1049. doi: 10.1016/0895-4356(94)00230-n. [DOI] [PubMed] [Google Scholar]
- 12.Nixon GM, Armstrong DS, Carzino R, et al. Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. J Pediatr. 2001;138(5):699–704. doi: 10.1067/mpd.2001.112897. [DOI] [PubMed] [Google Scholar]
- 13.Thomassen MJ, Demko CA, Klinger JD, Stern RC. Pseudomonas cepacia colonization among patients with cystic fibrosis. A new opportunist. Am Rev Respir Dis. 1985;131(5):791–796. doi: 10.1164/arrd.1985.131.5.791. [DOI] [PubMed] [Google Scholar]
- 14.Isles A, Maclusky I, Corey M, et al. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr. 1984;104(2):206–210. doi: 10.1016/s0022-3476(84)80993-2. [DOI] [PubMed] [Google Scholar]
- 15.Sawicki GS, Rasouliyan L, Pasta DJ, et al. Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis The impact of incident methicillin resistant Staphylococcus aureus detection on pulmonary function in cystic fibrosis. Pediatr Pulmonol. 2008;43(11):1117–1123. doi: 10.1002/ppul.20914. [DOI] [PubMed] [Google Scholar]
- 16.Johansen HK, Høiby N. Seasonal onset of initial colonisation and chronic infection with Pseudomonas aeruginosa in patients with cystic fibrosis in Denmark. Thorax. 1992;47(2):109–111. doi: 10.1136/thx.47.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wat D, Doull I. Respiratory virus infections in cystic fibrosis. Paediatr Respir Rev. 2003;4(3):172–177. doi: 10.1016/s1526-0542(03)00059-9. [DOI] [PubMed] [Google Scholar]
- 18.Wat D, Gelder C, Hibbitts S, et al. The role of respiratory viruses in cystic fibrosis. J Cyst Fibros. 2008;7(4):320–328. doi: 10.1016/j.jcf.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pribble CG, Black PG, Bosso JA, Turner RB. Clinical manifestations of exacerbations of cystic fibrosis associated with nonbacterial infections. J Pediatr. 1990;117(2 Pt 1):200–204. doi: 10.1016/S0022-3476(05)80530-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong EL, Ellis ME, Webb AK, et al. Infective respiratory exacerbations in young adults with cystic fibrosis: role of viruses and atypical microorganisms. Thorax. 1989;44(9):739–742. doi: 10.1136/thx.44.9.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson WW, Weintraub E, Dhankhar P, et al. Estimates of US influenza-associated deaths made using four different methods. Influenza Other Respi Viruses. 2009;3(1):37–49. doi: 10.1111/j.1750-2659.2009.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention . Overview of Influenza Surveillance in the United States. Atlanta, GA: Centers for Disease Control and Prevention; 2006. [Google Scholar]
- 23.Barker WH, Mullooly JP. Impact of epidemic type A influenza in a defined adult population. Am J Epidemiol. 1980;112(6):798–811. doi: 10.1093/oxfordjournals.aje.a113052. [DOI] [PubMed] [Google Scholar]
- 24.Barker WH, Mullooly JP. Pneumonia and influenza deaths during epidemics: implications for prevention. Arch Intern Med. 1982;142(1):85–89. [PubMed] [Google Scholar]
- 25.Griffin MR, Coffey CS, Neuzil KM, Mitchel EF, Jr, Wright PF, Edwards KM. Winter viruses: influenza- and respiratory syncytial virus-related morbidity in chronic lung disease. Arch Intern Med. 2002;162(11):1229–1236. doi: 10.1001/archinte.162.11.1229. [DOI] [PubMed] [Google Scholar]
- 26.Mullooly JP, Bridges CB, Thompson WW, et al. Vaccine Safety Datalink Adult Working Group Influenza- and RSV-associated hospitalizations among adults. Vaccine. 2007;25(5):846–855. doi: 10.1016/j.vaccine.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 27.O’Brien MA, Uyeki TM, Shay DK, et al. Incidence of outpatient visits and hospitalizations related to influenza in infants and young children. Pediatrics. 2004;113(3 Pt 1):585–593. doi: 10.1542/peds.113.3.585. [DOI] [PubMed] [Google Scholar]
- 28.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292(11):1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 29.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization . Influenza-like Illness in the United States and Mexico. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 31.Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team Emergence of a Novel Swine-Origin Influenza A (H1N1) Virus in Humans. N Engl J Med. 2009;360(25):2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 32.US Department of Health and Human Services . HHS Pandemic Influenza Plan. Washington, DC: US Dept of Health and Human Services; 2005. [Google Scholar]
- 33.Centers for Disease Control and Prevention (CDC) Hospitalized patients with novel influenza A (H1N1) virus infection - California, April-May, 2009. MMWR Morb Mortal Wkly Rep. 2009;58(19):536–541. [PubMed] [Google Scholar]
- 34.Fiore AE, Shay DK, Broder K, et al. Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008;57(RR-7):1–60. [PubMed] [Google Scholar]
- 35.Monto AS, Cavallaro JJ, Keller JB. Seasonal patterns of acute infection in Tecumseh, Mich. Arch Environ Health. 1970;21(3):408–417. doi: 10.1080/00039896.1970.10667259. [DOI] [PubMed] [Google Scholar]
- 36.Gruber WC, Campbell PW, Thompson JM, Reed GW, Roberts B, Wright PF. Comparison of live attenuated and inactivated influenza vaccines in cystic fibrosis patients and their families: results of a 3-year study. J Infect Dis. 1994;169(2):241–247. doi: 10.1093/infdis/169.2.241. [DOI] [PubMed] [Google Scholar]
- 37.Bell TD, Chai H, Berlow B, Daniels G. Immunization with killed influenza virus in children with chronic asthma. Chest. 1978;73(2):140–145. doi: 10.1378/chest.73.2.140. [DOI] [PubMed] [Google Scholar]
- 38.Groothuis JR, Lehr MV, Levin MJ. Safety and immunogenicity of a purified haemagglutinin antigen in very young high-risk children. Vaccine. 1994;12(2):139–141. doi: 10.1016/0264-410x(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 39.Herrera GA, Iwane MK, Cortese M, et al. Influenza vaccine effectiveness among 50-64-year-old persons during a season of poor antigenic match between vaccine and circulating influenza virus strains: Colorado, United States, 2003-2004. Vaccine. 2007;25(1):154–160. doi: 10.1016/j.vaccine.2006.05.129. [DOI] [PubMed] [Google Scholar]
- 40.Ritzwoller DP, Bridges CB, Shetterly S, Yamasaki K, Kolczak M, France EK. Effectiveness of the 2003-2004 influenza vaccine among children 6 months to 8 years of age, with 1 vs 2 doses. Pediatrics. 2005;116(1):153–159. doi: 10.1542/peds.2005-0049. [DOI] [PubMed] [Google Scholar]
- 41.Kramarz P, Destefano F, Gargiullo PM, et al. Vaccine Safety Datalink team Does influenza vaccination prevent asthma exacerbations in children? J Pediatr. 2001;138(3):306–310. doi: 10.1067/mpd.2001.112168. [DOI] [PubMed] [Google Scholar]
- 42.Wongsurakiat P, Maranetra KN, Wasi C, Kositanont U, Dejsomritrutai W, Charoenratanakul S. Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: a randomized controlled study. Chest. 2004;125(6):2011–2020. doi: 10.1378/chest.125.6.2011. [DOI] [PubMed] [Google Scholar]
- 43.Sugaya N, Nerome K, Ishida M, Matsumoto M, Mitamura K, Nirasawa M. Efficacy of inactivated vaccine in preventing antigenically drifted influenza type A and well-matched type B. JAMA. 1994;272(14):1122–1126. [PubMed] [Google Scholar]
- 44.Wat D, Gelder C, Hibbitts S, et al. Is there a role for influenza vaccination in cystic fibrosis? J Cyst Fibros. 2008;7(1):85–88. doi: 10.1016/j.jcf.2007.05.002. [DOI] [PubMed] [Google Scholar]