Abstract
Background:
Human rhinovirus (HRV) is the most frequent virus associated with COPD exacerbations. Viral infections increase exacerbation severity and likelihood of hospitalization. As ease of sampling blood makes serum a more practical marker than sputum, we investigated whether changes in serum interferon-γ-inducible protein 10 (IP-10) from baseline to exacerbation were higher in airway HRV-positive exacerbations and whether IP-10 levels related to HRV load.
Methods:
One hundred thirty-six patients with COPD and 70 controls were included over 2 years and 72 exacerbations sampled. HRV positivity and load were determined by reverse transcriptase-polymerase chain reaction in nasopharyngeal swabs and/or sputum at baseline and exacerbation. IP-10 was measured by enzyme-linked immunosorbent assay in serum and compared with HRV load.
Results:
At baseline, serum IP-10 was higher in patients with COPD than controls; medians were 149.4 pg/mL (103-215) and 111.7 pg/mL (82-178), P = .02. The presence of HRV at baseline did not increase IP-10: patients with COPD, 166.9 pg/mL (110-240) and 149.4 pg/mL (103-215), P = .30; controls, 136.4 pg/mL (77-204) and 111.7 pg/mL (82-178), P = .53. IP-10 increased significantly from baseline to exacerbation in HRV-positive exacerbations: 154.9 pg/mL (114.0-195.1) to 207.5 pg/mL (142.1-333.5), P = .009. There was no change in IP-10 between baseline and exacerbation in HRV-negative exacerbations: 168.3 pg/mL (94.3-249.8) and 175.6 pg/mL (107.2-290.4), P = .49. At exacerbation, IP-10 correlated with sputum viral load: rho = 0.48; P = .02. In receiver operating characteristics analysis, the combination of IP-10 and coryzal symptoms gave an area under the curve of 0.82 (95% CI, 0.74-0.90).
Conclusions:
IP-10 increases from baseline to exacerbation in HRV-positive exacerbations and correlates with sputum HRV load. Serum IP-10 may be useful as a novel marker for these events.
COPD is an increasing health problem worldwide.1 Exacerbations are episodes of acute symptomatic, physiologic, and functional deterioration that have important consequences for patients and health-care providers. They are predominantly triggered by infection and up to 64% of exacerbations are associated with colds.2 The most common respiratory virus detected in the airways at exacerbation is human rhinovirus (HRV), which using molecular techniques has been identified as present in up to 60% of respiratory virus-associated exacerbations.2,3
Cold symptoms at exacerbation increase the severity of the exacerbation, lengthen the recovery time,4 and when confirmed by polymerase chain reaction (PCR), the exacerbation is associated with higher levels of airway inflammatory markers, including interleukin (IL)-6,2 IL-8, and myeloperoxidase.3 Exacerbations associated with a virus are more likely to lead to hospitalization,5,6 and viral infection has been identified in up to 47% of patients with COPD with exacerbations requiring intubation and mechanical ventilation.7
Interferon-γ-inducible protein 10 (IP-10), a chemokine secreted by bronchial epithelial cells, monocytes, lymphocytes, and neutrophils in response to interferon-γ and tumor necrosis factor-α,8,9 has been shown to be elevated by HRV infection.10 HRV infects and replicates in bronchial epithelial cells.11 This active replication of HRV triggers production of cytokines and chemokines, including IP-10.10
To date, there is no specific biomarker to determine causation of COPD exacerbations as these are such heterogeneous events. Although there are airway and blood markers that increase from baseline to exacerbation, they are not specific. There is no systemic biomarker that can be used as a marker of airway HRV positivity in COPD. Therefore, the objectives of this study were to assess the use of serum IP-10 as a marker of airway HRV positivity at exacerbation and to assess the relationship between serum IP-10 and airway HRV load. We also investigated differences in serum IP-10 levels between patients with COPD at baseline and control subjects, both when positive and negative for HRV.
Materials and Methods
Patient Recruitment
One hundred thirty-six patients were studied between April 1, 2006, and May 31, 2008. Seventy smoking and nonsmoking control subjects of similar age but without COPD were recruited from a primary care practice. The recruitment and monitoring of patients in the London COPD study has previously been described2,4,12-14 and further details are available in the online supplement. This study was approved by the Royal Free Hospital Research Ethics Committee and patients gave written informed consent.
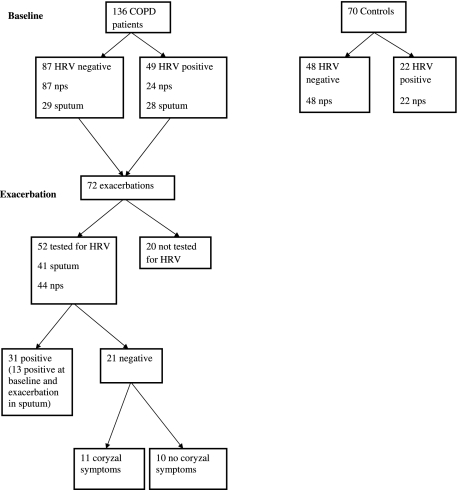
At the initial visit, daily respiratory symptoms, smoking history, drug history, and comorbidities were recorded for patients and controls. Height and weight were measured along with baseline lung function using a volumetric storage spirometer (Vitalograph 2160; Maids Moreton; Buckingham, England). Blood was collected for IP-10, IL-6, and C-reactive protein (CRP) assay. In each patient and control subject a nasopharyngeal swab (NPS) was taken if possible. Spontaneously produced sputum was collected when available in patients with COPD. All patients with COPD were at least > 42 days post-exacerbation and > 14 days pre-exacerbation onset. Sampling of patients is shown in Figure 1.
Figure 1.
Flow diagram showing patients with COPD and control subjects studied. HRV = human rhinovirus; NPS = nasopharyngeal swab.
Exacerbations
Patients with COPD completed daily diary cards, recording any increase in daily respiratory symptoms. Exacerbations were defined according to our previously validated criteria of two symptoms (at least one major) for 2 consecutive days, or if in the opinion of the attending clinician, the patient had an exacerbation.14 Major symptoms were increased dyspnea, sputum volume, or sputum purulence, and minor symptoms were increased cough, wheeze, sore throat, or coryzal symptoms. Our exacerbation definition has been validated against changes in quality of life,12 inflammatory markers,14 and FEV1 decline.13
At an exacerbation visit information was collected on symptom type and duration. Spirometry was performed and blood taken for cytokine assay. Sputum was collected if spontaneously produced, and an NPS was taken. All exacerbations were treated with bronchodilators, antibiotics and/or oral steroids. All samples were taken prior to the initiation of treatment. Only one exacerbation per patient was analyzed: the first reported and sampled exacerbation in the 2-year study period.
Patient Blood Sampling and Measurement of Inflammatory Markers
Venous blood (7 mL) was collected and centrifuged at 224 × g for 10 min at 4°C within 2 h of collection. The serum was separated and stored at −80°C for later analysis. Serum IL-6 and IP-10 were quantified using commercial sandwich enzyme-linked immunosorbent assay kits (R&D Systems; Abingdon, England). Serum CRP was measured in our hospital laboratory using an Olympus luminometric analyzer. The limit of detection for serum IL-6 was 0.7 pg/mL, IP-10 was 1.67 pg/mL, and CRP was 0.3 mg/L.
Virus Detection in NPS and Sputum
Sample Processing, Upper and Lower Airway:
A nasopharyngeal swab was rotated five to six times and allowed to remain in place for 5 s. The swab was then immediately placed in an Eppendorf containing 0.5 mL phosphate-buffered saline and stored at −80°C until RNA extraction. Sputum samples were examined within 2 h of collection. The sample was separated from contaminating saliva and processed using previously published methods.15
RNA Extraction, Reverse Transcription:
RNA was extracted from NPS using the High Pure Viral RNA kit (Roche; Burgess Hill, England) according to manufacturer instructions. cDNA was prepared using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems; Warrington, Cheshire, England) following the manufacturer instructions.
PCR:
Real-time PCR was performed using the ABI Prism 7500 Real Time PCR System (Applied Biosystems). Samples were initially run as singles and any positive samples were repeated in duplicates and data pooled. If the two runs varied, the sample was rerun in triplicate. Details of controls and standards used16,17 can be found in the online supplement.
Statistical Analysis
Data were analyzed using SPSS version 15 or STATA 8.2 (Stata Corporation; College Station, TX). The Kolmogorov-Smirnov test of normality was applied. Normally distributed data were expressed as mean and SD, and skewed data were expressed as median and interquartile range (IQR). Pearson correlation was used to assess parametric data, and Spearman rank was used to assess nonparametric correlations. Wilcoxon and Mann Whitney U tests were used for paired and unpaired nonparametric tests, respectively. For the regression analysis, IP-10 values were log transformed. P ≤ .05 was taken as significant for all statistical analysis. The use of IP-10 in confirming the presence of HRV at exacerbation was investigated by receiver operating characteristics (ROC) curve analysis. Results are expressed as an area under the curve (AUC) with 95% CI. The accepted standard of AUC is ≥ 0.8.18
Results
Baseline Patient Characteristics
One hundred thirty six patients with COPD were studied (83 men and 53 women). The baseline characteristics of the cohort are reported in Table 1. The patients had a mean FEV1 of 1.16 l or 53.9% predicted.
Table 1.
—Baseline Characteristics of 136 Patients with COPD
| Mean | SD | |
| Age, y | 72.6 | 8.4 |
| FEV1, L | 1.16 | 0.46 |
| FEV1, % predicted | 53.9 | 18.7 |
| FVC, L | 2.53 | 0.86 |
| BMI, kg/m2 | 26.3 | 5.8 |
| Pack-years smoking | 51.1 | 38.6 |
| Spo2 on air, % | 95 | 2 |
| Median | IQR | |
| IP-10, pg/mL | 154.4 | 107.0-220.6 |
| CRP, mg/L (n = 77) | 4.0 | 2.0-7.0 |
| IL-6, pg/mL (n = 72) | 3.2 | 2.4-5.9 |
Subjects included 83 men (61.0%), 41 current smokers (30.1%). CRP = C-reactive protein; IL = interleukin; IP-10 = interferon-γ-inducible protein 10; IQR = interquartile range; Spo2 = oxygen saturation measured by pulse oximetry.
Seventy control subjects were studied (28 men and 42 women). The baseline characteristics are reported in Table 2. The control subjects had a mean FEV1 of 2.63 L or 112.1% predicted. There were significant differences in age, smoking history, and oxygen saturations between the control subjects and patients with COPD (all P < .001) but not BMI (P = .7).
Table 2.
—Baseline Characteristics of 70 Control Subjects
| Mean | SD | |
| Age, y | 67.4 | 8.7 |
| FEV1, L | 2.63 | 0.80 |
| FEV1, % predicted | 112.1 | 28.3 |
| FVC, L | 3.43 | 1.08 |
| BMI, kg/m2 | 26.0 | 5.1 |
| Pack-years smoking | 18.4 | 20.9 |
| Spo2 on air, % | 96 | 1 |
| Median | IQR | |
| IP-10, pg/mL | 115.5 | 81.2- 181.6 |
| CRP, mg/L (n = 23) | 2.0 | 1.0- 3.3 |
| IL-6, pg/mL (n = 45) | 3.3 | 2.0- 5.4 |
Subjects included 28 men (40.0%), 12 current smokers (17.1%). See Table 1 for expansion of abbreviations.
Baseline Serum IP-10, IL-6, and CRP in Patients With COPD and Control Subjects When HRV Negative
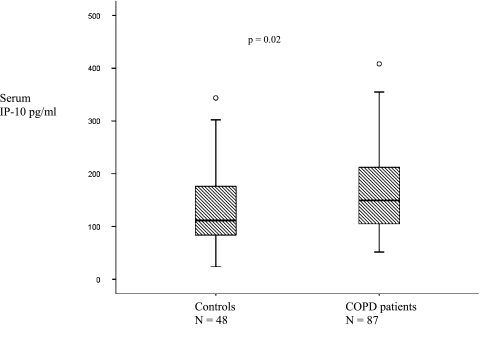
Eighty-seven patients with COPD (64.0%) and 48 of the control subjects (68.6%) were negative for HRV when sampled by NPS and/or sputum at a baseline visit and had serum IP-10 measured. Figure 2 shows that serum IP-10 levels were higher in patients with COPD than controls: medians of 149.4 pg/mL (IQR 103.4-215.2) and 111.7 pg/mL (82.6-178.5), respectively (P = .02). In a regression analysis accounting for age, smoking status, sex, and disease (COPD or control) there was still an effect of disease status on IP-10 (P = .01).
Figure 2.
Median serum IP-10 in 48 controls and 87 patients with COPD when HRV negative in the stable state. Circles represent extreme outliers. IP-10 = interferon-γ-inducible protein 10. See Figure 1 legend for expansion of other abbreviation.
CRP was measured in 77 patients with COPD and 23 controls, and IL-6 was measured in 72 patients and 45 controls. CRP was higher in the patients with COPD than the controls: medians of 4.0 mg/L (2.0-7.0) and 2.0 mg/L (1.0-3.3), respectively (P = .006), but there was no difference in serum IL-6: medians of 3.2 pg/mL (2.4-5.9) and of 3.3 pg/mL (2.0-5.4), respectively (P = .45).
Higher IP-10 correlated with increasing age both in patients with COPD and controls: rho = 0.24, P = .03 and rho = 0.34, P = .02, respectively. Serum IP-10 correlated with smoking pack-years in the patients with COPD: rho = −0.30, P = .005. Serum IP-10 did not correlate with increased disease severity (lower FEV1 % predicted) in the patients with COPD: rho = 0.17, P = .11.
Baseline IP-10 in Patients With COPD and Control Subjects When HRV Positive
Forty-nine patients with COPD and 22 control subjects when sampled in the stable state were found to be HRV positive at very low levels using a sensitive cutoff of 1 pfu/mL as positive.17 They were all asymptomatic at this time and the presence of HRV did not increase serum IP-10 levels in either the patients with COPD or control subjects: medians 166.9 pg/mL (110.1-240.3) and 149.4 pg/mL (IQR 103.4-215.2), P = .30, and medians 136.4 pg/mL (77.3-204.0) and 111.7 pg/mL (82.6-178.5), P = .53, respectively. There was no correlation between serum IP-10 levels and HRV load in NPS in the controls or patients with stable COPD: rho = 0.17, P = .42 and rho = 0.25, P = .27, respectively, or between serum IP-10 and sputum HRV load in patients with COPD at an HRV-positive baseline; rho = 0.002, P = .99.
Exacerbations and IP-10
All Exacerbations:
Seventy-two exacerbations were studied. In 63 exacerbations paired with a baseline sample, serum IP-10 increased from baseline to exacerbation: median baseline IP-10, 157.5 pg/mL (106.4-15.2) and exacerbation IP-10, 199.1 pg/mL (128.3-290.8), P =.004. Serum IL-6 and CRP also increased from baseline to exacerbation: medians 2.96 pg/mL (1.8-4.8) and 4.37 pg/mL (1.8-10.4), P =.049; medians 3.00 mg/L (1.3-7.0) and 7.00 mg/L (2.5-14.0), P < .001, respectively.
HRV-Positive Exacerbations:
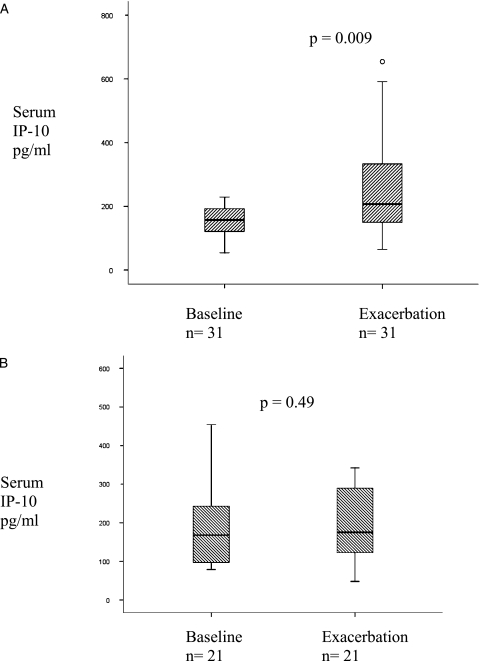
Fifty-two of the 72 exacerbations were tested for the presence of HRV in sputum (n = 41) and/or NPS (n = 44). Of those tested, 31 (59.6%) were positive. Figure 3A shows that serum IP-10 increased significantly from baseline to exacerbation in the HRV-positive exacerbations: medians 154.9 pg/mL (114.0-195.1) and 207.5 pg/mL (142.1-333.5), P = .009.
Figure 3.
Increase in IP-10 from baseline to exacerbation in 31 paired COPD exacerbations that were positive for HRV (A). Median serum IP-10 at baseline and exacerbation in 21 paired baseline-exacerbations that were negative for HRV (B). Circles represent extreme outliers. See Figures 1 and 2 legends for expansion of abbreviations.
HRV-Negative Exacerbations:
Figure 3B shows that there was no change in IP-10 levels between baseline and exacerbation in HRV-negative exacerbations: medians 168.3 pg/mL (94.3-249.8) and 175.6 pg/mL (107.2-290.4), P = .49, respectively.
IP-10 and Viral Load:
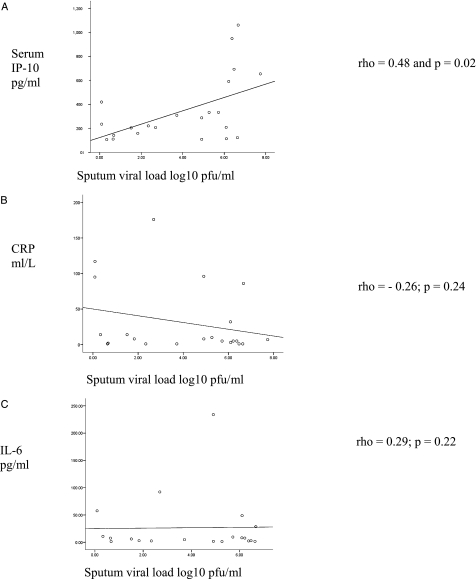
At exacerbation, serum IP-10 levels correlated with sputum viral load (Fig 4A): rho = 0.48 and P = .02, but not viral load measured from NPS: rho = 0.33, P = .14. NPS viral load did not correlate with sputum viral load: rho = 0.47, P = .12. HRV load was significantly higher in sputum than NPS: medians 81,409 pfu/mL (25.5-1,815,527) and 24 pfu/mL (2.4-2,379), P = .01. There was no relationship between CRP or IL-6 and viral loads in sputum (Figs 4B, 4C) or NPS: CRP rho = −0.26, P = .24 and rho = 0.26, P = .29; IL-6 rho = 0.29, P = .22 and rho = −0.12, P = .61. Furthermore, CRP and IL-6 did not correlate with IP-10: rho = 0.05, P = .72 and rho = 0.08, P = .54 respectively.
Figure 4.
At exacerbation IP-10 levels correlated with sputum viral load (A). IP-10 levels did not correlate with CRP (B) or IL-6 (C). CRP = C-reactive protein. See Figures 1 and 2 legends for expansion of other abbreviations.
Cold Symptoms at Exacerbation and IP-10:
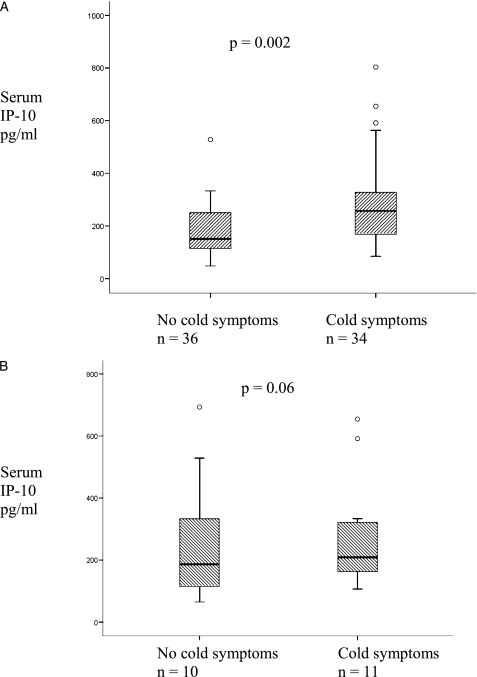
At exacerbation 36 patients reported coryzal symptoms, whereas 34 did not. In two patients data were not available. Figure 5A shows that patients reporting coryzal symptoms had significantly higher levels of serum IP-10 but not CRP or IL-6 at exacerbation: IP-10 medians 257.0 pg/mL (167.6-330.3) and 150.8 pg/mL (113.5-252.5), P = .002; CRP medians 7.0 mg/L (4.5-19.5) and 4.0 mg/L (1.8-11.0), P = .19 and IL-6 medians 5.7 pg/mL (2.3-9.5) and 3.3 pg/mL (1.6-14.0), P = .48, respectively.
Figure 5.
Thirty-four patients reporting coryzal symptoms had significantly higher levels of IP-10 than 36 patients not reporting coryzal symptoms. Circles represent extreme outliers (A). In 21 HRV-negative exacerbations in which 11 patients reported cold symptoms there was no difference in IP-10 (B). See Figures 1 and 2 for expansion of abbreviations.
Of the 21 patients who were tested for HRV and were negative, 11 reported coryzal symptoms. These patients did not have higher IP-10 levels than those who were HRV negative and did not report coryzal symptoms: medians 269.7 pg/mL (150.2-342.1) and 150.8 pg/mL (65.0-264.0), P = .06 (Fig 5B). There was no difference in CRP or IL-6 in the two groups: medians 7.5 mg/L (4.0-32.3) and 8.0 mg/L (2.0-43.0), P = .78 and medians 5.7 pg/mL (1.6-8.7) and 3.9 pg/mL (1.3-25.5), P = .96, respectively.
HRV Positivity at Baseline and Exacerbation
Thirteen patients were positive for HRV in sputum both at baseline and exacerbation. Figure 6 shows that sputum HRV loads were significantly higher at exacerbation than at an HRV-positive baseline: medians 1,236,514 pfu/mL (218.90-2,360,930) at exacerbation and 3.73 pfu/mL (1.43-23.41) at baseline; P = .007. IP-10 was significantly higher at HRV-positive exacerbation than HRV-positive baseline; medians 204.06 pg/mL (123.26-333.46) at exacerbation and 162.68 pg/mL (122.99-194.89) at baseline, P = .04.
Figure 6.
HRV load in 13 baseline-exacerbation pairs. The horizontal line represents the cutoff chosen for positivity at exacerbation. See Figure 1 legend for expansion of abbreviation.
Use of Serum IP-10 in the Diagnosis of HRV at Exacerbation in COPD
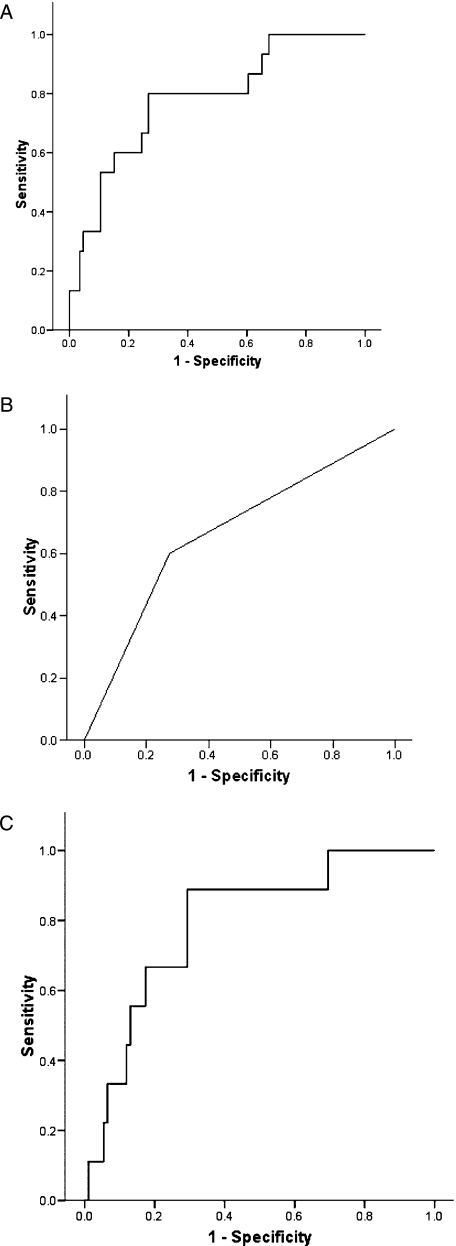
To apply these data to clinical practice, we set a cutoff of HRV load associated with COPD exacerbation based on the difference in HRV load between baseline and exacerbation (Fig 6). We used sputum samples only as HRV load in NPS did not correlate with sputum load or IP-10. We chose a log 10 HRV load value of 2.24, which corresponds to 175 pfu/mL as this was higher than the upper limit of the IQR for the baseline samples but less than that for the exacerbation samples. We would suggest that the presence of HRV at a load ≥ 175 pfu/mL at exacerbation indicates that HRV is the cause of that exacerbation and anything below this level, although HRV is present, does not cause symptoms of exacerbation or trigger much of an inflammatory response. We based our ROC analysis on this cutoff for HRV using all sputum samples (baseline and exacerbation, HRV positive and negative) and the presence of cold symptoms on the day of visit. The AUC for IP-10 alone was 0.78 (95% CI, 0.65-0.91). Using the presence of coryzal symptoms alone, the AUC was 0.66 (0.51-0.82). Using the combination of IP-10 and coryzal symptoms the AUC was 0.82 (0.74-0.90) (Figs 6, 7). Thus by measuring IP-10 in the blood in the presence of a coryzal symptom at exacerbation, the likelihood of HRV infection can be determined. Applying a cutoff of IP-10 in the blood of 260 pg/mL in the presence of cold symptoms would be 80% specific and 67% sensitive for a HRV exacerbation.
Figure 7.
ROC curves. Using IP-10 alone, AUC = 0.78 (95% CI, 0.65-0.91) (A). Using the presence of coryzal symptoms alone, AUC = 0.66 (0.51-0.82) (B). Using the combination of IP-10 and coryzal symptoms, AUC = 0.80 (0.66-0.94) (C). AUC = area under the curve; ROC = receiver operating characteristics. See Figure 2 legend for expansion of other abbreviations.
Discussion
We have shown for the first time that serum IP-10 increases from baseline to exacerbation in COPD specifically in exacerbations positive for HRV. Serum IP-10 has previously been shown to increase in virus-triggered acute asthma exacerbations,19 and we have now shown that in COPD exacerbations, sputum HRV load determined by quantitative RT-PCR correlates with IP-10 levels in the blood. Although HRV infection in most individuals is mild and self-limiting, in patients with COPD viral infection not only triggers exacerbations but increases the severity of the exacerbation, lengthens recovery time,4 and increases the likelihood of hospitalization.6 Thus serum IP-10 may be a useful biomarker to identify a patient for early intervention, either with standard exacerbation therapy20,21 or future novel22 antiviral agents.23,24
This study shows that sputum HRV load correlated with serum IP-10 at exacerbation, and thus IP-10 may be useful in detecting the presence of HRV and monitoring HRV exacerbation length and response to treatment. Currently there are no good markers of exacerbation severity and HRV causes more severe exacerbations than virus-negative exacerbations.25 Ease of sampling blood makes serum IP-10 a more practical marker than a sputum marker. Further work is required to examine the role of IP-10 as a marker of exacerbation recovery as there is increasing interest in this post-exacerbation period21,26 when morbidity is still significant.
In this study, neither serum CRP nor serum IL-6 correlated with HRV load with levels remaining low independent of high viral loads, thus indicating that they are poor markers of HRV-positive COPD exacerbations. Previous studies have shown that in exacerbations of asthma, neither serum IL-6 nor IL-8 significantly differed between viral and nonviral exacerbations19 and our data confirmed these findings in COPD. In response to experimental infection with HRV in COPD, nasal lavage IL-8 increases significantly, but not IL-6.27 The change in sputum IL-6 from baseline to exacerbation is greater if HRV is present,25 and absolute sputum IL-6 levels are higher at exacerbation if virus has been detected in nasal lavage.28 Thus although there are airway markers that may potentially indicate the presence of HRV at exacerbation, sputum is not readily available in all patients and serum IP-10 appears to be a novel and more appropriate marker.
The importance of the presence of HRV in the sputum at low concentrations in stable COPD is not fully understood, and was not associated with a heightened inflammatory response as measured by serum IP-10. Following extensive discussion in our group, we chose a very sensitive cutoff of 1 pfu/mL for HRV detection17 for this exploratory analysis. None of the negative water controls were positive at this level; however, there was some cross reactivity with RSV and the standard curve was extrapolated to reach this point. These low levels were only detected at baseline, predominantly in NPS samples, and were not used in the exacerbation ROC analysis. This very sensitive cutoff may account for the greater proportion of asymptomatic HRV positivity found at baseline in both the control population and patients with COPD compared with other studies.17 Other studies have used different primers and probes and have been designed to detect fewer strains of HRV. Both of these factors will lead to lower detection rates. Our HRV PCR was designed to detect as many different strains of HRV as possible and we sampled most of our baselines in the spring and autumn when HRV is most prevalent.
The relevance of low levels of HRV in COPD is not clear and we felt it important to investigate as this may play a role in exacerbation susceptibility. Viruses circulate all year round, and it is likely that patients with COPD acquire virus frequently, possibly only at low levels; however, only some strains of HRV demonstrate pathogenicity. With increasingly sensitive methods of viral detection available, the use of viral load in determining the significance of the presence of virus is increasingly common. We used these data to suggest a cutoff for viral load being significant at exacerbation (ie, most likely causing the exacerbation). As symptoms are integral to the definition of a COPD exacerbation,1 and coryzal symptoms are a putative marker of virus positivity, it is reasonable to combine the presence of coryzal symptoms with IP-10 as a predictor of HRV positivity. The current data from the ROC analysis presented indicate that serum IP-10 of ≥ 260 pg/mL and the presence of a cold symptom indicates an HRV exacerbation.
We have not tested whether IP-10 increases at exacerbation in the presence of other respiratory viruses. In tissue culture IP-10 has been shown to increase in the presence of influenza virus,29 and in mice IP-10 levels increase in response to RSV,30 but this has not been confirmed in vivo. Although it is possible other viruses not tested for may influence IP-10, we did not find an increase in IP-10 in the presence of coryzal symptoms (a marker of viral infection) when HRV was not detected. Cold illnesses are usually caused by respiratory viruses31 and we found that cold-associated exacerbations that were negative for HRV did not have increased serum IP-10 levels. This strengthens the case for IP-10 as a biomarker of HRV infection in the airway at exacerbation.
We have shown that patients with COPD have higher serum IP-10 levels in the stable state than controls. IP-10 may also play a role in the inflammatory process of COPD. Expression of IP-10 and its receptor CXCR3 is higher in the airway in smokers with COPD,32-35 and IP-10 is present at higher levels in induced sputum in patients with COPD compared with controls.35 Our finding that IP-10 correlated negatively with pack-years smoked indicates that local upregulation of IP-10 in the airways does not spill over into the systemic circulation.
This study focuses on the role of IP-10 as a marker of HRV infection and we have not defined the role of bacteria, although IP-10 is not known as a marker of bacterial infection. Previous studies of IP-10 have concentrated on HRV and it is possible that other viruses may also affect IP-10 levels, which deserves further study. It is interesting that NPS load was not related to serum IP-10. We know that colds do not always lead to exacerbation36 and the upper airway is difficult to sample.
In conclusion, we have shown that serum IP-10 can be used as a novel biomarker for HRV infection at exacerbation in COPD. Serum IP-10 is higher at baseline in patients with COPD than controls and increases with age. Measurement of serum IP-10 may enable more rational therapy for COPD exacerbations and reduce morbidity from this disabling disease.
Supplementary Material
Acknowledgments
Author contributions: Dr Quint: contributed to the conception and design of the study, the acquisition of samples, ELISA processing, data analysis and interpretation, writing the first draft of the manuscript, and editing and revising the manuscript.
Dr Donaldson: contributed to the conception and design of the study, data analysis and interpretation, and editing and revising the manuscript.
Dr Goldring: contributed to acquisition of samples and editing and revising the manuscript.
Dr Baghai-Ravary: contributed to acquisition of samples and editing and revising the manuscript.
Dr Hurst: contributed to the conception and design of the study, interpretation of data, and editing and revising the manuscript.
Dr Wedzicha: contributed to the conception and design of the study, interpretation of data, and editing and revising the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: We thank William Zermansky at Highgate Group Primary Care practice for his help in recruiting controls.
Abbreviations
- AUC
area under the curve
- CRP
C-reactive protein
- HRV
human rhinovirus
- IL
interleukin
- IP-10
interferon-γ-inducible protein 10
- IQR
interquartile range
- NPS
nasopharyngeal swab
- PCR
polymerase chain reaction
- ROC
receiver operating characteristics
Funding/Support: This work was supported by the National Institutes of Health [Grant RO1 HL082578].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Global Initiative for Chronic Obstructive Lung Disease Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. (GOLD) 2006. www.goldcopd.com. Accessed February 26, 2009.
- 2.Seemungal TAR, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson TMA, Hurst JR, Perera WR, Wilks M, Donaldson GC, Wedzicha JA. Effect of interactions between lower airway bacterial and rhinoviral infection in exacerbations of COPD. Chest. 2006;129(2):317–324. doi: 10.1378/chest.129.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seemungal TAR, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161(5):1608–1613. doi: 10.1164/ajrccm.161.5.9908022. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg SB, Allen M, Wilson J, Atmar RL. Respiratory viral infections in adults with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162(1):167–173. doi: 10.1164/ajrccm.162.1.9911019. [DOI] [PubMed] [Google Scholar]
- 6.Rohde G, Wiethege A, Borg I, et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58(1):37–42. doi: 10.1136/thorax.58.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu Y, Zhu J, Bandi V, et al. Biopsy neutrophilia, neutrophil chemokine and receptor gene expression in severe exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168(8):968–975. doi: 10.1164/rccm.200208-794OC. [DOI] [PubMed] [Google Scholar]
- 8.Cassatella MA, Gasperini S, Calzetti F, Bertagnin A, Luster AD, McDonald PP. Regulated production of the interferon-gamma-inducible protein-10 (IP-10) chemokine by human neutrophils. Eur J Immunol. 1997;27(1):111–115. doi: 10.1002/eji.1830270117. [DOI] [PubMed] [Google Scholar]
- 9.Sauty A, Dziejman M, Taha RA, et al. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J Immunol. 1999;162(6):3549–3558. [PubMed] [Google Scholar]
- 10.Spurrell JCL, Wiehler S, Zaheer RS, Sanders SP, Proud D. Human airway epithelial cells produce IP-10 (CXCL10) in vitro and in vivo upon rhinovirus infection. Am J Physiol Lung Cell Mol Physiol. 2005;289(1):L85–L95. doi: 10.1152/ajplung.00397.2004. [DOI] [PubMed] [Google Scholar]
- 11.Papi A, Johnston SL. Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule 1 (ICAM-1) via increased NF-kappaB-mediated transcription. J Biol Chem. 1999;274(14):9707–9720. doi: 10.1074/jbc.274.14.9707. [DOI] [PubMed] [Google Scholar]
- 12.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 pt 1):1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 13.Donaldson GC, Seemungal TAR, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhowmik A, Seemungal TAR, Sapsford RJ, Wedzicha JA. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax. 2000;55(2):114–120. doi: 10.1136/thorax.55.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhowmik A, Seemungal TAR, Sapsford RJ, Devalia JL, Wedzicha JA. Comparison of spontaneous and induced sputum for investigation of airway inflammation in chronic obstructive pulmonary disease. Thorax. 1998;53(11):953–956. doi: 10.1136/thx.53.11.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chapman J, Abbott E, Alber DG, et al. RSV604, a novel inhibitor of respiratory syncytial virus replication. Antimicrob Agents Chemother. 2007;51(9):3346–3353. doi: 10.1128/AAC.00211-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright PF, Deatly AM, Karron RA, et al. Comparison of results of detection of rhinovirus by PCR and viral culture in human nasal wash specimens from subjects with and without clinical symptoms of respiratory illness. J Clin Microbiol. 2007;45(7):2126–2129. doi: 10.1128/JCM.02553-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metz CE. Basic principles of ROC analysis. Semin Nucl Med. 1978;8(4):283–298. doi: 10.1016/s0001-2998(78)80014-2. [DOI] [PubMed] [Google Scholar]
- 19.Wark PAB, Bucchieri F, Johnston SL, et al. IFN-γ-induced protein 10 is a novel biomarker of rhinovirus-induced asthma exacerbations. J Allergy Clin Immunol. 2007;120(3):586–593. doi: 10.1016/j.jaci.2007.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Moussaoui R, Roede BM, Speelman P, Bresser P, Prins JM, Bossuyt PM. Short-course antibiotic treatment in acute exacerbations of chronic bronchitis and COPD: a meta-analysis of double-blind studies. Thorax. 2008;63(5):415–422. doi: 10.1136/thx.2007.090613. [DOI] [PubMed] [Google Scholar]
- 21.Roede BM, Bresser P, Bindels PJE, et al. Antibiotic treatment is associated with reduced risk of a subsequent exacerbation in obstructive lung disease: an historical population based cohort study. Thorax. 2008;63(11):968–973. doi: 10.1136/thx.2008.095349. [DOI] [PubMed] [Google Scholar]
- 22.Maugeri C, Alisi MA, Apicella C, et al. New anti-viral drugs for the treatment of the common cold. Bioorg Med Chem. 2008;16(6):3091–3107. doi: 10.1016/j.bmc.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 23.Pevear DC, Hayden FG, Demenczuk TM, Barone LR, McKinlay MA, Collett MS. Relationship of pleconaril susceptibility and clinical outcomes in treatment of common colds caused by rhinoviruses. Antimicrob Agents Chemother. 2005;49(11):4492–4499. doi: 10.1128/AAC.49.11.4492-4499.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinberger M. Respiratory infections and asthma: current treatment strategies. Drug Discov Today. 2004;9(19):831–837. doi: 10.1016/S1359-6446(04)03239-8. [DOI] [PubMed] [Google Scholar]
- 25.Seemungal TAR, Harper-Owen R, Bhowmik A, Jeffries DJ, Wedzicha JA. Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary disease. Eur Respir J. 2000;16(4):677–683. doi: 10.1034/j.1399-3003.2000.16d19.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hurst JR, Donaldson GC, Quint JK, Goldring JJ, Baghai-Ravary R, Wedzicha JA. Temporal clustering of exacerbations in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179(5):369–374. doi: 10.1164/rccm.200807-1067OC. [DOI] [PubMed] [Google Scholar]
- 27.Mallia P, Message SD, Kebadze T, Parker HL, Kon OM, Johnston SL. An experimental model of rhinovirus induced chronic obstructive pulmonary disease exacerbations: a pilot study. Respir Res. 2006;7:116. doi: 10.1186/1465-9921-7-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rohde G, Borg I, Wiethege A, et al. Inflammatory response in acute viral exacerbations of COPD. Infection. 2008;36(5):427–433. doi: 10.1007/s15010-008-7327-5. [DOI] [PubMed] [Google Scholar]
- 29.Veckman V, Osterlund P, Fagerlund R, et al. TNF-α and IFN-α enhance influenza-A-virus-induced chemokine gene expression in human A549 lung epithelial cells. Virology. 2006;345(1):96–104. doi: 10.1016/j.virol.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 30.Culley FJ, Pennycook AMJ, Tregoning JS, Hussell T, Openshaw PJ. Differential chemokine expression following respiratory virus infection reflects Th1- or Th2-biased immunopathology. J Virol. 2006;80(9):4521–4527. doi: 10.1128/JVI.80.9.4521-4527.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenberg SB. Rhinovirus and coronavirus infections. Semin Respir Crit Care Med. 2007;28(2):182–192. doi: 10.1055/s-2007-976490. [DOI] [PubMed] [Google Scholar]
- 32.Grumelli S, Corry DB, Song L-Z, et al. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med. 2004;1(1):75–83. doi: 10.1371/journal.pmed.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saetta M, Mariani M, Panina-Bordignon P, et al. Increased expression of the chemokine receptor CXCR3 and its ligand CXCL10 in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;165(10):1404–1409. doi: 10.1164/rccm.2107139. [DOI] [PubMed] [Google Scholar]
- 34.Hardaker EL, Bacon AM, Carlson K, et al. Regulation of TNF-alpha- and IFN-gamma-induced CXCL10 expression: participation of the airway smooth muscle in the pulmonary inflammatory response in COPD. FASEB J. 2004;18(1):191–193. doi: 10.1096/fj.03-0170fje. [DOI] [PubMed] [Google Scholar]
- 35.Costa C, Rufino R, Traves SL, et al. CXCR3 and CCR5 chemokines in induced sputum from patients with chronic obstructive pulmonary disease. Chest. 2008;133(1):26–33. doi: 10.1378/chest.07-0393. [DOI] [PubMed] [Google Scholar]
- 36.Hurst JR, Donaldson GC, Wilkinson TM, Perera WR, Wedzicha JA. Epidemiological relationships between the common cold and exacerbation frequency in COPD. Eur Respir J. 2005;26(5):846–852. doi: 10.1183/09031936.05.00043405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.