Abstract
Background:
The costimulatory molecule OX40 and its ligand, OX40L, mediate key aspects of allergic airway inflammation in animal models of asthma, including eosinophilic airway inflammation, airway hyperresponsiveness, and T helper 2 polarization. We sought to examine OX40/OX40L and interleukin (IL)-4 expression in asthma across severities.
Methods:
Bronchial biopsies were obtained from 27 subjects with asthma (mild Global Initiative for Asthma [GINA] 1 [n = 10], moderate GINA 2-3 [n = 7], and severe GINA 4-5 [n = 10]) and 13 healthy controls. The number of OX40+, OX40L+, IL-4+, and IL-4 receptor α (IL-4Rα)+ cells in the lamina propria and airway smooth muscle (ASM) bundle and the intensity of IL-4Rα+ expression by the ASM were assessed.
Results:
The number of OX40+, OX40L+, and IL-4+ cells in the lamina propria and OX40+ and IL-4+ cells in the ASM bundle was significantly increased in subjects with mild asthma, but not in those with moderate or severe asthma, compared with healthy controls. In the subjects with asthma, OX40/OX40L expression was positively correlated with the number of eosinophils and IL-4+ cells in the lamina propria. The number of IL-4Rα+ cells in the lamina propria was significantly increased in moderate-to-severe disease, but not in mild asthma, compared with controls. IL-4Rα expression by the ASM bundle was not different among groups.
Conclusions:
OX40/OX40L expression is increased in the bronchial submucosa in mild asthma, but not in moderate-to-severe disease, and is related to the degree of tissue eosinophilia and IL-4 expression. Whether these costimulatory molecules have a role as targets for asthma requires further investigation.
Asthma affects 5% of the adult population and is characterized by symptoms of variable airflow limitation, airway inflammation, and airway hyperresponsiveness. Polarization of the inflammatory response toward increased expression of T helper 2 (Th2) cytokines is considered an important component of the asthma paradigm.1 A major effector axis resulting in induction of Th2 polarization is the recognition of allergen presented by dendritic cells in local lymph nodes to CD4+ T cell. This axis is an optimal target for drug development because it orchestrates inflammation and the development of an allergen-specific humoral response and the development of T- and B-cell memory. The differentiation of naive T cells or reactivation of memory T cells depends on various costimulatory molecules expressed on the T-cell surface, and their cognate ligands.2 One of the most promising costimulatory targets is OX40 and its ligand, OX40L, which has relative T-cell specificity and is not expressed on naive T cells.3
OX40 is primarily induced on T cells in the effector phase and is predominantly expressed by Th2 cells.4 In mouse models of allergic inflammation, OX40 knockout mice have significantly reduced Th2 responses to allergen, accompanied by a reduction in airway eosinophilia and airway hyperreactivity.5 OX40L is expressed mainly by antigen-presenting cells, particularly dendritic cells,6 but also B cells, macrophages, and Langerhans cells.6 In the airway, dendritic cells express OX40L in response to stimulation by epithelial cell-derived thymic stromal lymphopoietin (TSLP).7 In murine and nonhuman primate models of asthma in vivo, blockade of OX40L inhibits TSLP-mediated Th2 inflammation and attenuates the number of OX40L+ dendritic cells in the lung.8 Whether OX40 and OX40L expression are increased in human airway tissue from subjects with asthma is unknown. We hypothesized that OX40 and OX40L expression is increased in the bronchial lamina propria and that this expression is related to disease severity and Th2 cytokine expression. To test our hypothesis, we enumerated OX40, OX40L, interleukin (IL)-4, and IL-4 receptor α (IL4-Rα) expression in bronchial biopsies from subjects with mild, moderate, and severe asthma, compared with healthy controls.
Materials and Methods
Subjects
Twenty-seven patients with asthma and 13 healthy controls were recruited from Glenfield Hospital, Leicester, England. All subjects were nonsmokers with a smoking history of < 10 pack-years, had been free of exacerbations, and were on stable treatment of 8 weeks prior to entry into the study. Asthma was defined by one or more of the following objective criteria: significant bronchodilator reversibility of > 200 mL, a provocation concentration of methacholine causing a 20% fall in FEV1 < 8 mg/mL, and/or a peak flow amplitude % mean over 2 weeks of > 20%. Asthma severity was defined according to the Global Initiative for Asthma (GINA) treatment steps.9 Normal subjects had no history of respiratory disease and normal spirometry. The study was approved by the Leicestershire Research Ethics Committee and informed consent was obtained from all subjects.
Protocol and Clinical Characterization
Subjects attended on two occasions. At the first visit, they underwent spirometry; allergen skin prick tests for Dermatophagoides pteronyssinus, dog, cat and grass pollen; a methacholine inhalation test;10 and sputum induction.11 At the second visit 1 week later, subjects underwent bronchoscopy.12 Mucosal biopsy specimens were processed into the water-soluble resin glycol methacrylate (Polysciences; Northampton, England) for embedding.13
Immunohistochemistry
Two-micrometer sections were cut and immunostained with the following mouse monoclonal antibodies: OX40 and OX40L (BD Biosciences; Oxford, England) and IL-4 (clones 4D9 and 3H4; AMS Biotechnology; Oxford, England) and IL-4Rα (R&D Systems; Abingdon, Oxfordshire, England) in quadruplicate; α-smooth muscle actin (Dako; Cambridge, England); and major basic protein (Caltag; Paiseley, England) with appropriate isotype controls (Dako). The number of positively stained nucleated cells was enumerated per square millimeter of the lamina propria or airway smooth muscle (ASM) by a blinded observer. A minimum ASM area of 0.1 mm2 was considered assessable, as described previously.14 The number of subjects with adequate smooth muscle was 10/13 controls, 10/10 GINA 1 asthma, 6/7 GINA 2-3 asthma, and 8/10 GINA 4-5 asthma. IL-4Rα expression by the ASM was also assessed using a semiquantitative intensity score of no staining = 0, low = 1, moderate = 2, and high = 3. Post hoc in three subjects with asthma, sequential sections were stained for OX40 and CD3 (T cells), and OX40L and CD1a (dendritic cells). The proportion of the OX40 or OX40L+ cells that were colocalized to T cells or dendritic cells, respectively, was determined as previously described.15
Analysis
Statistical analysis was performed using PRISM software, version 4 (GraphPad Software; La Jolla, CA). Parametric data were presented as mean (SEM) and nonparametric data as median (interquartile range [IQR]). Parametric data were analyzed with one-way analysis of variance (ANOVA) and Bonferroni’s posttest correction for intergroup comparison. Nonparametric data were analyzed using the Kruskal-Wallis tests and Dunn’s test for post hoc comparison. The Fisher exact test was used to assess the categorical differences in expression in ASM. Correlations between parametric data were assessed by Pearson’s correlation and nonparametric data by Spearman’s rank correlation. P < .05 was considered significant.
Results
The baseline clinical characteristics are as shown in Table 1. The subjects with asthma and the controls were matched for age and gender. Subjects with asthma had a higher BMI compared with controls, and evidence of airway hyperresponsiveness.
Table 1.
—Baseline Clinical Characteristics
| Control (n = 13) | Asthma |
|||
| GINA 1 (n = 10) | GINA 2-3 (n = 7) | GINA 4-5 (n = 10) | ||
| Age (y) | 40 (3) | 45 (5) | 55 (7) | 49 (5) |
| Sex, male (female) | 6 (7) | 5 (5) | 4 (3) | 6 (4) |
| Atopy, % | 33 | 50 | 57 | 67 |
| Disease duration, y | NA | 12 (4) | 12 (5) | 17 (5) |
| BDP equivalent/24 h, mcg | NA | 0 | 960 (194)a | 1,092 (136)a OCS (n = 2) |
| BMI, kg/m2 | 24 (1) | 32 (2) | 31 (2) | 30 (2) |
| Postbronchodilator FEV1% | 103 (4) | 95 (6) | 99 (4) | 83 (8) |
| Postbronchodilator FEV1/FVC, % | 81 (3) | 77 (4) | 78 (2) | 74 (3) |
| PC20,b mg/mL | > 16 | 0.5 (0.1-2.2) | 0.5 (0.2-1.9) | 0.4 (0.1-2.1) |
| Bronchodilator response FEV1% | 0.6 (1) | 12 (6) | 4 (3) | 16 (7) |
| Sputum eosinophils,b % | 0.3 (0.2-0.5) | 0.7 (0.2-2.8) | 1.2 (0.4-3.8) | 2.6 (0.4-17)a |
| Sputum neutrophils, % | 64 (20) | 51 (10) | 58 (9) | 52 (14) |
| Eosinophils/mm2 lamina propriac | 1.9 (3.7) | 21.3 (34.5)a | 12.1 (12.7) | 6.1 (38.4) |
Data expressed as mean (SEM) unless otherwise indicated. BDP = beclamethasone diproprionate equivalent dose; GINA = Global Initiative for Asthma; NA = not applicable; OCS = oral corticosteroid; PC20 = provocation concentration causing a 20% fall in FEV1.
P < .05.
Geometric mean (95% CI).
Median (interquartile range).
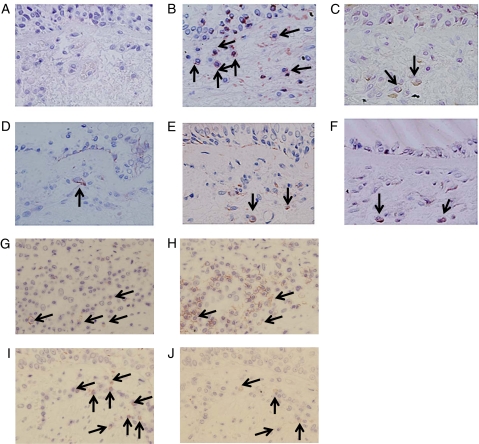
Photomicrographs illustrating OX40, OX40L, IL-4, IL-4Rα, and the colocalization of OX40, OX40 with T cells, and dendritic cells, respectively, in the lamina propria and their appropriate isotype controls are as shown (Fig 1). In a subset of asthmatics (n = 3), we determined that mean (SEM) 56 (6)% of the OX40L+ cells in the lamina propria were dendritic cells and 94 (6)% of the OX40+ cells were T cells.
Figure 1.
Examples of photomicrographs of immunohistochemical staining for isotype control (A), OX40 (B), OX40L (C), IL-4(3H4) (D), IL-4(4D9) (E), IL-4Rα (F), and sequential sections staining for OX40 (G), CD3 (H), CD1a (I) and OX40L (J) (original magnification × 400). Positive cells are highlighted by arrows in B-F. In G and I, positive cells are highlighted by arrows and the corresponding cell if positive in H and J. IL = interleukin; IL-4Rα = IL-4 receptor α; OX40L = OX40 ligand.
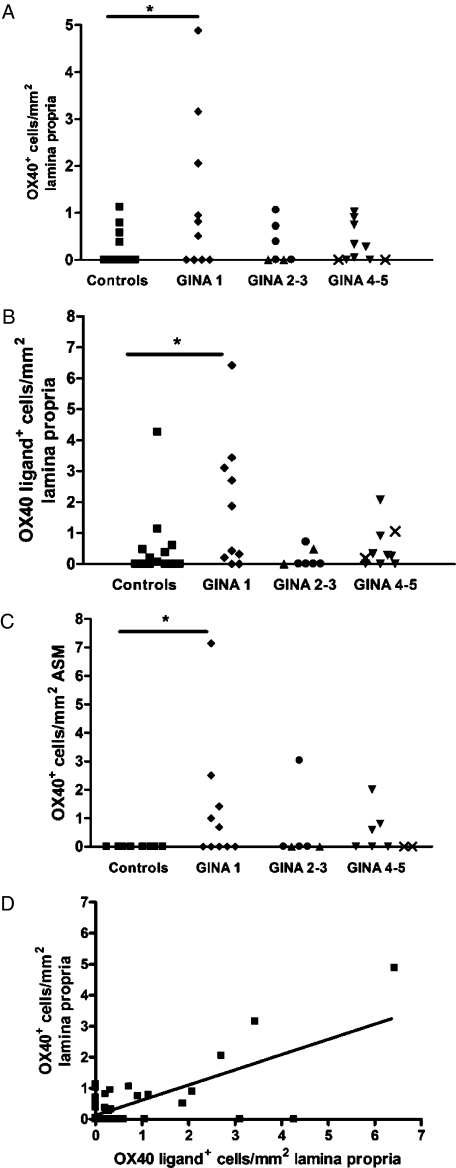
The mean (SEM) number of OX40+ cells in the lamina propria was significantly increased in subjects with mild corticosteroid-naive (GINA 1) asthma (1.2 [0.5]/mm2), but not in those with moderate asthma (GINA 2-3) (0.31 [0.16]/mm2) or severe asthma (GINA 4-5) (0.3 [0.16]/mm2), compared with healthy controls (0.2 [0.1]/mm2) (P = .048, ANOVA; P < .05, control vs GINA 1) (Fig 2A). Similarly, the number of OX40L+ cells in the lamina propria was significantly increased in subjects with mild asthma (1.9 [0.7]/mm2), but not in those with moderate asthma (0.17 [0.1]/mm2) or severe asthma (0.51 [0.21]/mm2), compared with healthy controls (0.55 [0.3]/mm2) (P = .04, ANOVA; P < .05, control vs GINA 1) (Fig 2B). The median (IQR) number of OX40+ cells in the ASM bundle was not significantly different across the groups (P = .08, Kruskal-Wallis), but the proportion of patients with OX40+ cells in the ASM was significantly increased in mild asthma (5/10), but not in moderate (1/6) or severe asthma (3/8), compared with the controls (0/10) (P = .03, Fisher exact test) (Fig 2 C). OX40L+ cells were not identified within the ASM bundle in health or asthma. There was a strong correlation between the number of OX40 and OX40L+ cells in the lamina propria (r = 0.83; P < .0001) (Fig 2D).
Figure 2.
The number of OX40+ (A) and OX40L+ cells (B) in the lamina propria and OX40+ cells (C) in the ASM bundle in subjects with asthma and healthy controls. The correlation between OX40 and OX40L expression in the lamina propria (D). □ = controls; ◆ = GINA 1; ● = GINA 2; ▲ = GINA 3; ▼ = GINA 4; X = GINA 5. *P < .05. ASM = airway smooth muscle; GINA = Global Initiative for Asthma. See Figure 1 for expansion of other abbreviations.
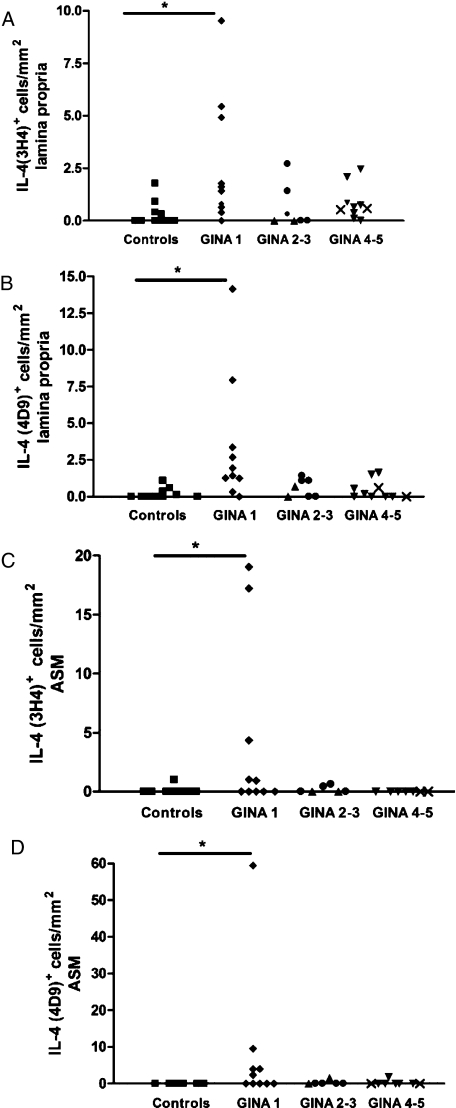
The mean (SEM) number of IL-4 (3H4)+ cells in the lamina propria was significantly increased in subjects with mild asthma (2.7 [1.0]/mm2), but not in those with moderate asthma (0.64 [0.4]/mm2) or severe asthma (0.84 [0.25]/mm2), compared with healthy controls (0.26 [0.15]/mm2) (P = .01, ANOVA; P < .01, control vs GINA 1) (Fig 3A). Similarly, the number of IL-4 (4D9)+ cells in the lamina propria was significantly increased in subjects with mild asthma (3.4 [1.4]/mm2), but not in those with moderate asthma (0.61 [0.23]/mm2) or severe asthma (0.45 [0.2]/mm2), compared with healthy controls (0.17 [0.09]/mm2) (P = .007, ANOVA; P < .01, control vs GINA 1) (Fig 3B). The median (IQR) number of IL-4+ (3H4 and 4D9) cells in the ASM bundle was significantly increased in subjects with mild asthma (0.5 [10.8] and 1.2 [6.8]/mm2), but not in those with moderate asthma (0 [0.5] and 0 [0.8]/mm2) or severe asthma (0 [0] and 0 [0]/mm2), compared with healthy controls (0 [0] and 0 [0]/mm2) (P = .04 and P = .027, Kruskal-Wallis) (Figs 3C, D).
Figure 3.
The number of IL-4 (3H4)+ cells (A) and IL-4 (4D9)+ cells (B) in the lamina propria and IL-4 (3H4)+ cells (C) and IL-4 (4D9)+ cells (D) in the ASM bundle in subjects with asthma and healthy controls. □ = controls; ◆ = GINA 1; ● = GINA 2; ▲ = GINA 3; ▼ = GINA 4; X = GINA 5. *P < .05. See Figure 1 and 2 legends for expansion of abbreviations.
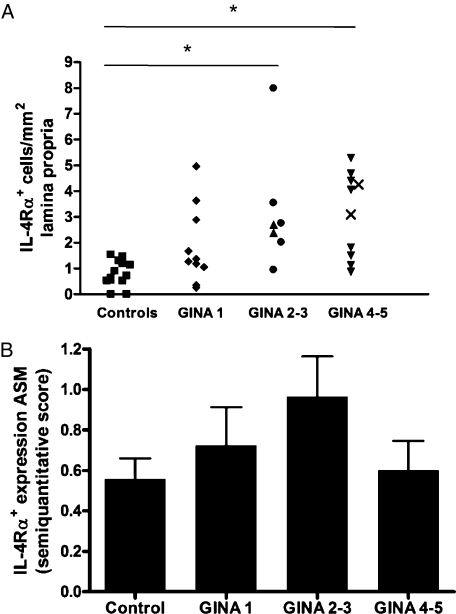
In contrast, the mean (SEM) number of IL-4Rα+ cells in the lamina propria was increased in moderate asthma (3.2 [0.9] cells/mm2) and severe asthma (3.1 [0.5] cells/mm2), but not in mild disease (1.9 [0.5] cells/mm2), compared with healthy controls (P = .002, ANOVA) (Fig 4A). The intensity of IL-4Rα expression by ASM was not significantly different between asthma and healthy controls (P = .3, ANOVA) (Fig 4B).
Figure 4.
The number of IL-4Rα+ cells in the lamina propria (A) and IL-4Rα expression by the ASM bundle (B) in subjects with asthma and healthy controls. See Figures 1 and 2 legends for expansion of abbreviations. □ = controls; ◆ = GINA 1; ● = GINA 2; ▲ = GINA 3; ▼ = GINA 4; X = GINA 5. *P < .05.
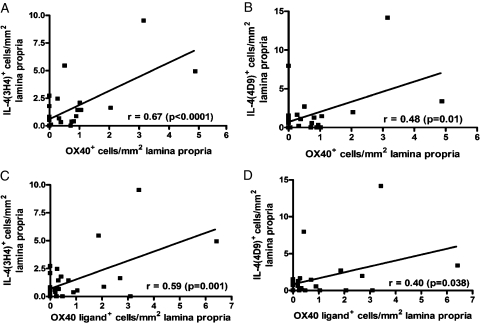
In the subjects with asthma, OX40 and OX40L expression in the lamina propria was strongly correlated with the IL-4 (3H4) (r = 0.67, P < .0001 and r = 0.59, P = .001) and (4D9) (r = 0.48, P = .01 and r = 0.4, P = .038) expression, respectively (Fig 5). The number of OX40+ cells in the ASM bundle was not significantly correlated with the number of IL-4+ (3H4 or 4D9) cells (data not shown). We also observed a strong correlation between the number of OX40L+ cells and eosinophils in the lamina propria (rs = 0.64, P < .0001), but not with OX40/IL-4 expression (data not shown). There were no significant correlations among OX40, OX40L, or IL-4 expression in the lamina propria or ASM bundle and FEV1% predicted, bronchodilator response, or airway hyperresponsiveness (data not shown).
Figure 5.
The correlations between OX40/OX40L and IL-4 expression in the lamina propria. See Figure 1 legend for expansion of abbreviations.
Discussion
We report here, to our knowledge for the first time, that the number of OX40+ and OX40L+ cells are increased in the lamina propria and OX40+ cells in the ASM bundle in mild asthma, but not in moderate-to-severe disease. OX40L+ cells were not observed in the ASM bundle in health or disease. The number of OX40/OX40L cells in the lamina propria was associated with IL-4 expression and eosinophilic inflammation.
To date, there have been no reports of OX40 or OX40L expression in bronchial submucosal tissue in asthma. Therefore, our observation that OX40 and OX40L expression is increased in mild asthma provides further evidence of a potential role of these molecules in asthma. We identified that the majority of OX40+ cells were T cells and OX40L+ cells were dendritic cells. Previously, animal studies have implicated these costimulatory molecules in the immunopathogenesis of asthma. In mouse models of allergic inflammation, OX40 knockout mice have significantly reduced Th2 responses to allergen with a reduction in airway eosinophilia and airway hyperreactivity.5,16,17 Dendritic cell-derived OX40L is induced by TSLP, a IL-7-like cytokine produced by epithelial cells, which provides an important interaction between the epithelial-dendritic cell.7 Critically, OX40L neutralization in mouse and nonhuman primate models of asthma inhibited TSLP-induced immune responses, including Th2 inflammatory cell infiltration, cytokine secretion, and IgE production8. Consistent with these observations, our findings showed a close association between the number of eosinophils and IL-4 expression in the lamina propria and the OX40/OX40L axis. However, in contrast to the animal models, a relationship was not indicated between OX40/OX40L expression and disordered airway physiology, including airway hyperresponsiveness. This dissociation between airway function and Th2-mediated eosinophilic inflammation is consistent with observations made in noneosinophilic bronchitis18,19 and the response to highly specific anti-IL-5 therapy.20,21 These observations in human disease may challenge a potential role for the OX40/OX40L axis in airway dysfunction in asthma and need to be examined further.
Interestingly, OX40L expression by dendritic cells is also increased by environmental fungal22 and endotoxin23 exposure, which have both been implicated in asthma and asthma exacerbations.24,25 OX40L production is also not restricted to dendritic cells, because other cells such as ASM may be important potential sources.26,27 Ex vivo data in human ASM cells have shown that OX40/OX40L expression was upregulated by tumor necrosis factor-α, and, interestingly, was significantly greater in asthmatic vs nonasthmatic ASM cells. We did not observe OX40/OX40L expression by ASM, but mast cells located within the ASM bundle14,28 are an important source of tumor necrosis factor-α29 and following activation may induce ASM-derived OX40L. ASM cells have established contractile and synthetic function and to this may be added a possible immunomodulatory role. Therefore, the role of mesenchymal cells and their interactions with inflammatory cells in the OX40/OX40L axis need to be investigated further.
Importantly, we were unable to demonstrate increased OX40/OX40L expression in moderate-to-severe disease, in contrast to mild asthma. This may reflect real differences among disease severities, perhaps as a consequence of treatment. Importantly, the number of subjects included in this study was relatively small and therefore our inability to demonstrate an increase in moderate-to-severe disease may be because of inadequate powering of the study. Furthermore, we may have underestimated OX40 and OX40L expression in asthma, because it may be transient and may increase in response to activation such as allergen challenge. Similarly, the potential benefits of therapeutic blockade of OX40/OX40L may depend on sensitization and the duration of post-allergen challenge. In mouse models of asthma, conflicting data exist regarding the most efficacious time point to neutralize OX40L, with one report suggesting that presensitization alone, and not subsequent challenge, led to a reduction in the asthmatic response,17 and another finding that airway inflammation was attenuated in postsensitized animals.30 Therefore, although our inability to demonstrate increased expression in severe asthma does question its potential role as a therapeutic target for this group of patients, our findings do not exclude the possibility that the OX40/OX40L axis may be important across all severities of asthma.
In keeping with the OX40/OX40L expression, IL-4 upregulation was restricted to those subjects with mild disease. This is consistent with an earlier report,31 but is in stark contrast to IL-13 expression, which resulted in an increase in the sputum and bronchial mucosa of severe asthmatics.32,33 Interestingly, the number of IL-4Rα+ cells was increased in the lamina propria in moderate-to-severe disease, with a small, nonsignificant increase in mild asthmatics. This differential expression of IL-4 and IL-13 in severe disease has important implications for potential therapeutic strategies in severe asthma directed toward either cytokine neutralization34,35 or receptor antagonism.36 In addition, the balance between the expression of cytokines and their receptors in severe disease is likely to be clinically important and requires further consideration.
Conclusions
In conclusion, we have shown that the OX40/OX40L axis is upregulated in mild asthma. but not in moderate-tosevere disease, and is related to the intensity of IL-4 expression and eosinophilic inflammation. Whether these costimulatory molecules present new therapeutic targets for asthma, and how we can determine which severity of asthma and what stage of the natural history of disease may benefit, require further investigation.
Acknowledgments
Author contributions: Dr Siddiqui: contributed to patient recruitment, bronchoscopies, manuscript preparation, and data analysis.
Mr Mistry: contributed to tissue processing and immunohistochemistry.
Ms Doe: contributed to tissue processing and immunohistochemistry.
Ms Stinson: contributed to technical advice provision and coauthorship of the manuscript.
Dr Foster: contributed to technical advice provision and coauthorship of the manuscript.
Dr Brightling: contributed to patient recruitment, bronchoscopies, manuscript preparation, and data analysis.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Brightling has received consultancy fees from MedImmune, AstraZeneca, GlaxoSmithKline, Roche, and Genentech and research grants from AZ, MedImmune, and GSK. Dr Foster and Ms Stinson are employees of AstraZeneca. Dr Siddiqui, Mr Mistry, and Ms Doe have reported that no potential conflicts exist with any companies/organizations whose products or services may be discussed in this article.
Abbreviations
- ASM
airway smooth muscle
- GINA
Global Initiative for Asthma
- IL
interleukin
- IL-4Rα
IL-4 receptor α
- IQR
interquartile range
- OX40L
OX40 ligand
- Th2
T helper 2
- TSLP
thymic stromal lymphopoietin
Footnotes
Funding/Support: This study was supported by AstraZeneca, a Department of Health Clinician Scientist award, and a Wellcome Senior Clinical Fellowship.
References
- 1.Brightling CE, Symon FA, Birring SS, Bradding P, Pavord RD, Wardlaw AJ. Th2 cytokine expression by bronchoalveolar lavage fluid T lymphocytes and bronchial submucosa is a feature of asthma and eosinophilic bronchitis. J Allergy Clin Immunol. 2002;110(6):899–905. doi: 10.1067/mai.2002.129698. [DOI] [PubMed] [Google Scholar]
- 2.Beier KC, Kallinich T, Hamelmann E. Master switches of T-cell activation and differentiation. Eur Respir J. 2007;29(4):804–812. doi: 10.1183/09031936.00094506. [DOI] [PubMed] [Google Scholar]
- 3.Gramaglia I, Weinberg AD, Lemon M, Croft M. Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol. 1998;161(12):6510–6517. [PubMed] [Google Scholar]
- 4.Lane P. Role of OX40 signals in coordinating CD4 T cell selection, migration, and cytokine differentiation in T helper (Th)1 and Th2 cells. J Exp Med. 2000;191(2):201–206. doi: 10.1084/jem.191.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jember AG, Zuberi R, Liu FT, Croft M. Development of allergic inflammation in a murine model of asthma is dependent on the costimulatory receptor OX40. J Exp Med. 2001;193(3):387–392. doi: 10.1084/jem.193.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang YH, Liu YJ. Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses. Clin Exp Allergy. 2009;39(6):798–806. doi: 10.1111/j.1365-2222.2009.03241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu YJ. Thymic stromal lymphopoietin and OX40 ligand pathway in the initiation of dendritic cell-mediated allergic inflammation. J Allergy Clin Immunol. 2007;120(2):238–244. doi: 10.1016/j.jaci.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Seshasayee D, Lee WP, Zhou M, et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J Clin Invest. 2007;117(12):3868–3878. doi: 10.1172/JCI33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Global strategy for asthma management and prevention. 2008. http://www.ginasthma.com Accessed July 30, 2009.
- 10.Juniper EF, Cockcroft DW, Hargreave FE. Histamine and Methacholine Inhalation Tests: A Laboratory Tidal Breathing Protocol. 2nd ed. Lund, Sweden: Astra Draco AB; 1994. [Google Scholar]
- 11.Pavord ID, Pizzichini MM, Pizzichini E, Hargreave FE. The use of induced sputum to investigate airway inflammation. Thorax. 1997;52(6):498–501. doi: 10.1136/thx.52.6.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.British Thoracic Society. British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax. 2001;56(suppl 1):i1–i21. doi: 10.1136/thorax.56.suppl_1.i1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Britten KM, Howarth PH, Roche WR. Immunohistochemistry on resin sections: a comparison of resin embedding techniques for small mucosal biopsies. Biotech Histochem. 1993;68(5):271–280. doi: 10.3109/10520299309105629. [DOI] [PubMed] [Google Scholar]
- 14.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346(22):1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 15.Saunders R, Siddiqui S, Kaur D, et al. Fibrocyte localization to the airway smooth muscle is a feature of asthma. J Allergy Clin Immunol. 2009;123(2):376–384. doi: 10.1016/j.jaci.2008.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arestides RS, He H, Westlake RM, et al. Costimulatory molecule OX40L is critical for both Th1 and Th2 responses in allergic inflammation. Eur J Immunol. 2002;32(10):2874–2880. doi: 10.1002/1521-4141(2002010)32:10<2874::AID-IMMU2874>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Hoshino A, Tanaka Y, Akiba H, et al. Critical role for OX40 ligand in the development of pathogenic Th2 cells in a murine model of asthma. Eur J Immunol. 2003;33(4):861–869. doi: 10.1002/eji.200323455. [DOI] [PubMed] [Google Scholar]
- 18.Brightling CE. Chronic cough due to nonasthmatic eosinophilic bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1) suppl:116S–121S. doi: 10.1378/chest.129.1_suppl.116S. [DOI] [PubMed] [Google Scholar]
- 19.Gibson PG, Fujimura M, Niimi A. Eosinophilic bronchitis: clinical manifestations and implications for treatment. Thorax. 2002;57(2):178–182. doi: 10.1136/thorax.57.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leckie MJ, ten Brinke A, Khan J, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356(9248):2144–2148. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 21.Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi T, Iijima K, Radhakrishnan S, et al. Asthma-related environmental fungus, Alternaria, activates dendritic cells and produces potent Th2 adjuvant activity. J Immunol. 2009;182(4):2502–2510. doi: 10.4049/jimmunol.0802773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan W, So T, Croft M. Antagonism of airway tolerance by endotoxin/lipopolysaccharide through promoting OX40L and suppressing antigen-specific Foxp31 T regulatory cells. J Immunol. 2008;181(12):8650–8659. doi: 10.4049/jimmunol.181.12.8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denning DW, O’Driscoll BR, Powell G, et al. Randomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitization: The Fungal Asthma Sensitization Trial (FAST) study. Am J Respir Crit Care Med. 2009;179(1):11–18. doi: 10.1164/rccm.200805-737OC. [DOI] [PubMed] [Google Scholar]
- 25.Simpson JL, Grissell TV, Douwes J, Scott RJ, Boyle MJ, Gibson PG. Innate immune activation in neutrophilic asthma and bronchiectasis. Thorax. 2007;62(3):211–218. doi: 10.1136/thx.2006.061358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgess JK, Blake AE, Boustany S, et al. CD40 and OX40 ligand are increased on stimulated asthmatic airway smooth muscle. J Allergy Clin Immunol. 2005;115(2):302–308. doi: 10.1016/j.jaci.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Burgess JK, Carlin S, Pack RA, et al. Detection and characterization of OX40 ligand expression in human airway smooth muscle cells: a possible role in asthma? J Allergy Clin Immunol. 2004;113(4):683–689. doi: 10.1016/j.jaci.2003.12.311. [DOI] [PubMed] [Google Scholar]
- 28.Siddiqui S, Hollins F, Saha S, Brightling CE. Inflammatory cell microlocalisation and airway dysfunction: cause and effect? Eur Respir J. 2007;30(6):1043–1056. doi: 10.1183/09031936.00162506. [DOI] [PubMed] [Google Scholar]
- 29.Howarth PH, Babu KS, Arshad HS, et al. Tumour necrosis factor (TNFalpha) as a novel therapeutic target in symptomatic corticosteroid dependent asthma. Thorax. 2005;60(12):1012–1018. doi: 10.1136/thx.2005.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salek-Ardakani S, Song J, Halteman BS, et al. OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J Exp Med. 2003;198(2):315–324. doi: 10.1084/jem.20021937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vrugt B, Wilson S, Underwood J, et al. Mucosal inflammation in severe glucocorticoid-dependent asthma. Eur Respir J. 1999;13(6):1245–1252. doi: 10.1183/09031936.99.13612539. [DOI] [PubMed] [Google Scholar]
- 32.Berry MA, Parker D, Neale N, et al. Sputum and bronchial submucosal IL-13 expression in asthma and eosinophilic bronchitis. J Allergy Clin Immunol. 2004;114(5):1106–1109. doi: 10.1016/j.jaci.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 33.Saha SK, Berry MA, Parker D, et al. Increased sputum and bronchial biopsy IL-13 expression in severe asthma. J Allergy Clin Immunol. 2008;121(3):685–691. doi: 10.1016/j.jaci.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borish LC, Nelson HS, Corren J, et al. IL-4R Asthma Study Group. Efficacy of soluble IL-4 receptor for the treatment of adults with asthma. J Allergy Clin Immunol. 2001;107(6):963–970. doi: 10.1067/mai.2001.115624. [DOI] [PubMed] [Google Scholar]
- 35.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev. 2004;202:175–190. doi: 10.1111/j.0105-2896.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 36.Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet. 2007;370(9596):1422–1431. doi: 10.1016/S0140-6736(07)61600-6. [DOI] [PubMed] [Google Scholar]