Abstract
The polarization of nascent embryonic fields and the endowment of cells with organizer properties are key to initiation of vertebrate organogenesis. One such event is antero-posterior (AP) polarization of early limb buds and activation of morphogenetic Sonic Hedgehog (SHH) signaling in the posterior mesenchyme, which in turn promotes outgrowth and specifies the pentadactylous autopod. Inactivation of the Hand2 transcriptional regulator from the onset of mouse forelimb bud development disrupts establishment of posterior identity and Shh expression, which results in a skeletal phenotype identical to Shh deficient limb buds. In wild-type limb buds, Hand2 is part of the protein complexes containing Hoxd13, another essential regulator of Shh activation in limb buds. Chromatin immunoprecipitation shows that Hand2-containing chromatin complexes are bound to the far upstream cis-regulatory region (ZRS), which is specifically required for Shh expression in the limb bud. Cell-biochemical studies indicate that Hand2 and Hoxd13 can efficiently transactivate gene expression via the ZRS, while the Gli3 repressor isoform interferes with this positive transcriptional regulation. Indeed, analysis of mouse forelimb buds lacking both Hand2 and Gli3 reveals the complete absence of antero-posterior (AP) polarity along the entire proximo-distal axis and extreme digit polydactyly without AP identities. Our study uncovers essential components of the transcriptional machinery and key interactions that set-up limb bud asymmetry upstream of establishing the SHH signaling limb bud organizer.
Author Summary
During early limb bud development, posterior mesenchymal cells are selected to express Sonic Hedgehog (Shh), which controls antero-posterior (AP) limb axis formation (axis from thumb to little finger). We generated a conditional loss-of-function Hand2 allele to inactivate Hand2 specifically in mouse limb buds. This genetic analysis reveals the pivotal role of Hand2 in setting up limb bud asymmetry as initiation of posterior identity and establishment of the Shh expression domain are completely disrupted in Hand2 deficient limb buds. The resulting loss of the ulna and digits mirror the skeletal malformations observed in Shh-deficient limbs. We show that Hand2 is part of the chromatin complexes that are bound to the cis-regulatory region that controls Shh expression specifically in limb buds. In addition, we show that Hand2 is part of a protein complex containing Hoxd13, which also participates in limb bud mesenchymal activation of Shh expression. Indeed, Hand2 and Hoxd13 stimulate ZRS–mediated transactivation in cells, while the Gli3 repressor form (Gli3R) interferes with this up-regulation. Interestingly, limb buds lacking both Hand2 and Gli3 lack AP asymmetry and are severely polydactylous. Molecular analysis reveals some of the key interactions and hierarchies that govern establishment of AP limb asymmetries upstream of SHH.
Introduction
An important step during the initiation of vertebrate organogenesis is the setting-up of morphogenetic signaling centers that coordinately control cell specification and proliferation. One paradigm model to study these processes is the developing limb bud and recent studies have revealed how morphogenetic Sonic hedgehog (SHH) signaling from the zone of polarizing activity (ZPA) and Fibroblast growth factor (FGF) signaling from the apical ectodermal ridge (AER) coordinate cell specification with proliferation along both major limb bud axes [1]. AER-FGF signaling mainly controls the establishment of the proximo-distal (PD) limb bud axis (sequence: stylopod-zeugopod-autopod) [2], while SHH signaling by the polarizing region controls antero-posterior (AP) axis formation (radius and ulna, thumb to little finger) [3],[4]. Cells receiving the SHH signal inhibit the constitutive processing of Gli3 to its repressor form (Gli3R) and upregulate the expression of the Gli1 transcriptional activator, which results in positive regulation of SHH target genes [5]–[7]. In limb buds of mouse embryos lacking Gli3, the expression of initially posteriorly restricted genes such as Hand2, 5′HoxD genes and the BMP antagonist Gremlin1 (Grem1) expands anteriorly from early stages onwards and an anterior ectopic Shh expression domain is established at late stages [8]. However, the resulting digit polydactyly arises in a SHH-independent manner, as limbs of embryos lacking both Shh and Gli3 are morphologically and molecularly identical to Gli3 deficient mouse embryos [9],[10]. These and other studies indicate that Gli3 acts initially up-stream of SHH signaling to restrict the expression of genes activated prior to Shh to the posterior limb bud [11] and that SHH-mediated inhibition of Gli3R production is subsequently required to enable distal progression of limb bud development [9].
The molecular interactions that polarize the nascent limb bud along its AP axis and activate SHH signaling in the posterior limb bud mesenchyme have only been partially identified. Previous studies implicated the basic helix-loop-helix (bHLH) transcription factor Hand2 (dHand) in these early determinative processes upstream of SHH signaling [1]. In particular, the development of fin and limb buds of Hand2 deficient mouse and zebrafish embryos arrests at an early stage and no Shh expression is detected [12],[13]. This early developmental arrest in conjunction with massive generalized apoptosis of Hand2 deficient mouse limb buds precluded an in depth analysis of the molecular circuits and signaling systems that control initiation and progression of limb bud development. Furthermore, transgene-mediated over-expression of Hand2 induces digit duplications in mouse limb buds [14]. The functional importance of Hand2 as a transcriptional regulator in these processes was further corroborated by an engineered mutation that inactivates the Hand2 DNA binding domain in mouse embryos, which results in limb bud defects resembling the Hand2 null phenotype [15]. Cell-biochemical analysis showed that Hand2 interacts with so-called Ebox DNA sequence elements most likely as a heterodimer with other bHLH transcription factors such as E12 [16],[17] and Twist1, which is also required for early limb bud development [18],[19].
Genetic analysis in mouse embryos showed that Gli3 is required to restrict Hand2 expression to the posterior limb bud mesenchyme as part of a mutually antagonistic interaction [11]. This interaction was proposed to pre-pattern the limb bud mesenchyme along its AP axis prior to activation of SHH signaling. However, the functional importance of this pre-patterning mechanism for normal progression of limb development remained unknown. Additional pathways are also required for establishment of the Shh expression domain in the posterior limb bud mesenchyme such as retinoic acid signaling from the flank and AER-FGF8 signaling [20],[21]. During the onset of limb bud development, the expression of the 5′ most members of the HoxD gene cluster is restricted to the posterior mesenchyme by Gli3 [22],[23]. During these early stages, the 5′HoxA and 5′HoxD transcriptional regulators are required to activate Shh expression in the posterior limb bud mesenchyme [24]–[26]. Consistent with this genetic analysis, the Hoxd10 and Hoxd13 proteins interact directly with the cis-regulatory region that controls Shh expression in limb buds [27]. This evolutionary conserved cis-regulatory region is called ZPA regulatory sequence (ZRS) and is located about 800 Kb up-stream of the Shh gene [28]. Genetic inactivation of the highly conserved core region of the ZRS (termed MFCS1) results in limb bud-specific loss of Shh expression and a Shh loss-of-function limb skeletal phenotype [29]. Interestingly, this limb bud specific cis-regulatory region is absent from vertebrate species that have lost their limbs during evolution [30]. Transgenic analysis in mouse embryos revealed that ZRS-LacZ transgenes recapitulate major aspects of Shh expression in limb buds [28]. However, this study did not reveal specific cis-regulatory elements or sub-regions within the ZRS that regulate transcription, but rather indicated that the entire ZRS is required for correct Shh expression. A recent study shows that the ZRS interacts directly with the Shh transcription unit in both the anterior and posterior limb bud mesenchyme [31]. However, the Shh locus loops out of its chromosomal territory only in the posterior mesenchyme, which results in initiation of transcription. The evolutionary conserved function of the ZRS is underscored by an ever increasing large number of point mutations that are scattered through large parts of ZRS region and cause congenital preaxial polydactylies (PPD) in humans and many other mammals [32]. In summary, these studies establish that the far upstream ZRS cis-regulatory region controls Shh expression in different tetrapod species and that point mutations cause PPD, while deletion of the central part of the ZRS results in limbless phenotypes.
We have generated a conditional Hand2 mouse loss-of-function allele and use it to study the requirement of Hand2 during limb bud initiation. Inactivation of Hand2 in the forelimb field mesenchyme using the Prx1-Cre transgenic mouse strain disrupts the development of posterior skeletal elements. Complete and early inactivation results in a limb skeletal phenotype identical to limbs lacking Shh. Indeed, establishment of the Shh expression domain in the posterior limb bud is disrupted and early molecular markers of posterior identity are lost, while anterior markers expand posteriorly. This reveals the early requirement of Hand2 for establishing posterior identity and activation of Shh expression. Using specific antibodies, we identify protein complexes containing both Hand2 and Hoxd13 transcriptional regulators in wild-type limb buds. Chromatin immunoprecipitation using Hand2 antibodies reveals the specific enrichment of the ZRS in comparison to adjacent non-ZRS DNA sequences in wild-type limb buds. Functional analysis of the DNA-protein interactions in cultured fibroblasts reveals that Hand2 and Hoxd13 transactivate expression of a ZRS-luciferase reporter construct, while this is partially inhibited by Gli3R, which has been previously shown to interact with 5′Hoxd proteins [33]. Indeed, mouse limb buds deficient for both Gli3 and Hand2 lack AP asymmetry along the entire PD limb axis and display severe digit polydactyly with complete loss of identities. Our study uncovers the interactions of Hand2 with the Gli3 and Hoxd13 transcriptional regulators and the far-upstream ZRS cis-regulatory region that are required to polarize the nascent limb bud mesenchyme and establish Shh expression in the posterior limb bud.
Results
Limb bud–specific inactivation of Hand2 results in skeletal defects identical to Shh deficient limbs
Mouse embryos lacking Hand2 die during mid-gestation due to cardiovascular defects and limb bud development arrests prior to formation of limb skeletal elements [12],[34]. Therefore, we generated a conditional Hand2 loss-of-function allele by inserting two loxP sites into the locus (“floxed” allele: Hand2 f or H2 f), which enables Cre-recombinase mediated deletion of the Hand2 transcription unit (Figure S1). Hand2 was inactivated in the limb bud mesenchyme (H2 Δ ˜Δc; Δc: conditional inactivation of the Hand2 f allele) using the Prx1-Cre transgene, which is expressed in the forelimb field mesenchyme from about E8.5 onwards (14 somites) [35],[36]. The inactivation of Hand2 was verified by monitoring the clearance of Hand2 transcripts and proteins in forelimb buds and mesenchymal cells (Figure 1A and Figure S2A, S2B, S2C). Limb bud specific inactivation of Hand2 (H2 Δ ˜Δc; Figure 1A) causes distal truncations of the forelimb skeleton and loss of the autopod (Figure 1B). The skeletal phenotypes of Hand2 deficient forelimbs are variable, but the most severely affected cases (39% of all limbs, n = 80; Figure S3A, S3D) are identical to Shh deficient limbs (Figure 1B). Indeed, Shh expression and SHH signal transduction are lacking from a similar fraction of all H2 Δ ˜Δc limb buds (Figure 1C and Figure S3C). Therefore, the most severely affected H2 Δ ˜Δc limb buds correspond to the limb-specific complete Hand2 loss-of-function phenotype (Figure 1A–1C and Figure S3). Between two and four digits form in hypomorphic H2 Δ ˜Δc limbs (Figure S3A, S3D) as a likely consequence of residual Hand2 expression, which triggers SHH signal transduction (Figure S3B, S3C).
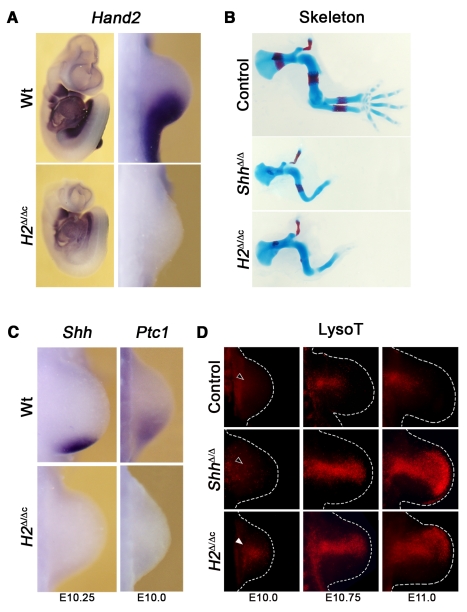
Figure 1. Early deletion of Hand2 in mouse forelimb buds phenocopies the Shh loss-of-function skeletal phenotype.
(A) Whole mount in situ hybridization detects Hand2 transcripts in wild-type (Wt) and mouse embryos that lack the Hand2 gene in their forelimb bud mesenchyme (H2 Δ/Δc) at E9.75 (28 somites). Hand2 transcripts are absent from forelimb buds of H2 Δ/Δc mouse embryos. (B) Skeletons of mouse forelimbs at E14.5, stained with alcian blue (cartilage) and alizarin red (bone). Prx1-Cre mediated inactivation of Hand2 (H2 Δ/Δc) phenocopies the Shh Δ/Δ limb skeletal phenotype. Control: Prx1-Cre tg/+. (C) Shh and Ptc1 transcripts are absent from H2 Δ/Δc limb buds at E10.25 (32 somites for Shh) and E10.0 (29 somites for Ptc1). (D) Detection of apoptotic cells by LysoTracker Red (LysoT). Hand2 deficient limb buds are compared to control (Prx1-Cre tg/+ and H2 +/f) and Shh Δ/Δ limb buds at E10.0 (30 somites), E10.75 (37 somites), and E11.0. The white arrowhead points to the precocious initiation of cell death in H2 Δ/Δc forelimb buds (compare white to open arrowheads; n = 2/4). In all panels, limb buds are oriented with the anterior to the top and the posterior to the bottom.
In the most severely affected forelimb buds, cells along the entire PD axis, but in particular in the distal-anterior mesenchyme are eliminated by apoptosis (Figure 1D), which is distinct from the generalized apoptosis and developmental arrest of mouse embryos lacking Hand2 constitutively (Figure S1D, S1E) [12]. In H2 Δ ˜Δc forelimb buds, cell death is limited to the core mesenchyme at embryonic day E10.0 (Figure 1D, white arrowhead). In contrast, no significant apoptosis is detected in forelimb buds of wild-type and Shh deficient limb buds at these early stages (Figure 1D, open arrowhead). Therefore, Hand2 is required for cell survival upstream of its role in activation of SHH signaling (Figure 1D, left panels). During progression of limb bud development, the apoptotic domain expands distal-anterior in H2 Δ ˜Δc limb buds and becomes similar to the cell death domain observed in Shh deficient limb buds (Figure 1D, middle and right panels).
In mouse embryos, hindlimb development is delayed by ∼12 hrs and activation of the Prx1-Cre transgene in the posterior mesenchyme is delayed by ∼24 hrs in comparison to forelimb buds [35],[36]. The resulting ∼12 hrs delay in Hand2 inactivation at equivalent stages in the posterior hindlimb bud allows formation of an autopod with 4–5 digits, while the tarsal bones are always fused (Figure 2A). Furthermore, inactivation of Hand2 specifically in the distal forelimb bud mesenchyme from E10.5 onwards no longer alters skeletal development (data not shown). In agreement with the subtle skeletal alterations following Prx1-Cre-mediated Hand2 inactivation in hindlimb buds (Figure 2A) Shh remains expressed, albeit at slightly lower levels than in wild-types (Figure 2B). Taken together, these studies show that Hand2 is essential to establish Shh expression in the posterior mesenchyme during initiation of limb bud development. Subsequently, it contributes to transcriptional up-regulation of Shh expression.
Figure 2. Delayed inactivation of Hand2 in hindlimb buds results in rather normal Shh expression and development.
(A) Hindlimb buds skeletons at E14.5, stained with alcian blue (cartilage) and alizarin red (bone). Prx1-Cre mediated inactivation of Hand2 (H2 Δ/Δc) in hindlimb buds results in all cases in fusion of the tarsals (arrowheads) and formation of 5 (n = 11/24) or 4 (n = 13/24) digits. Please note that in latter case the formation of digit 2 and/or 3 (not shown), which depend mostly on long-range SHH signaling [7] is always affected. (B) Shh expression in wild-type and Hand2 deficient hindlimb buds at E10.75 (37 somites). Note that the expression domain is correctly positioned in H2 Δ/Δc hindlimb buds, but expression levels are reduced.
Hand2 is essential for establishment of posterior identity upstream of SHH signaling
Our further analysis focused on the most severe, complete Hand2 loss-of-function phenotypes in forelimb buds (Figure 1). The early essential requirement of Hand2 upstream of SHH in forelimb buds (for cell survival, Figure 1D) is further substantiated by molecular analysis, which reveals the lack of Tbx3 and Tbx2 expression [37] in the posterior mesenchyme of H2 Δ ˜Δc forelimb buds. In contrast, their posterior expression is initiated but not up-regulated in Shh Δ ˜Δ forelimb buds (Figure 3A and 3B). The expression of 5′HoxD genes is activated but not propagated in Hand2 deficient limb buds (Figure S4A, S4B), likely due to the disruption of SHH signaling (Figure 1C). Concurrently, the expression of anterior genes such as Cry-μ, Alx4 and Gli3 is ectopically activated or expands to the posterior margin in H2 Δ ˜Δc forelimb buds earlier and/or more prominently than in Shh Δ ˜Δ limb buds (Figure 3C–3E and Figure S4C). This loss of posterior and gain of anterior molecular markers reveal the early essential requirement of Hand2 for establishing posterior limb bud identity.
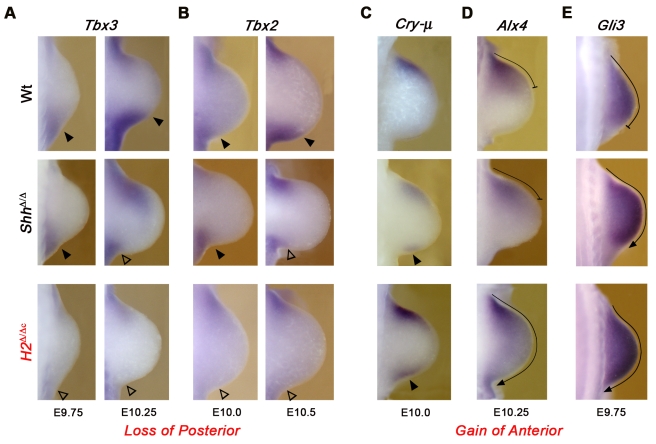
Figure 3. Establishment of posterior forelimb bud identity requires Hand2.
(A,B) The loss of the posterior Tbx3 and Tbx2 expression domains in early Hand2 deficient (H2 Δ/Δc) limb buds (from E9.75: 27 somites to E10.5: 35 somites) points to a failure in establishing posterior identity upstream of Shh activation. Open arrowheads: loss of expression in Hand2 deficient forelimb buds; solid arrowheads: normal expression in wild-type and Shh deficient limb buds. By E10.25–10.5 the posterior expression of Tbx2 and Tbx3 is also down-regulated in Shh Δ/Δ limb buds. (C–E) Posterior expansion of anterior markers in H2 Δ/Δc limb buds. (C) Crystallin-μ (Cry-μ) is expressed ectopically in the posterior mesenchyme of H2 Δ/Δc limb buds at E10.0 (30 somites; indicated by solid arrowheads). The ectopic posterior Cry-μ expression is detected earlier than in Hand2 than Shh deficient limb buds (not shown). The Alx4 (D) and Gli3 (E) expression domains are posteriorly expanded (indicated by arrows) in Hand2 deficient limb buds at E9.75 (27 somites) and E10.25 (32 somites), respectively. Note that the posterior expansion of the Gli3 expression domain is less pronounced in Shh Δ/Δc than in H2 Δ/Δc limb buds. In all panels, limb buds are oriented with the anterior to the top and the posterior to the bottom.
In wild-type limb buds, Hand2-containing chromatin complexes are bound to the ZRS cis-regulatory region that controls Shh expression
This analysis (Figure 1, Figure 2, Figure 3) led us to consider the possibility that Hand2 might directly transactivate Shh expression, possibly in conjunction with 5′Hox genes, which are essential for Shh activation in mouse limb buds [24],[26]. Chromatin immunoprecipitation (ChIP) studies showed previously that Hoxd13 containing chromatin complexes are bound to the far up-stream ZRS cis-regulatory region that controls Shh expression in limb buds [27]. In addition, Hoxd13 is able to transactivate a ZRS-luciferase reporter construct in transfected cells [27]. Therefore, the potential direct interactions of Hand2 with Hoxd13 proteins and the ZRS were assessed by luciferase transactivation assays in NIH3T3 cells, which are mouse fibroblasts commonly used to analyze the SHH pathway [38]. A luciferase reporter construct encoding the entire ZRS (ZRS-Luc) was generated by inserting the ∼1.7 kb mouse ZRS region (Figure 4A and Figure S5) [28] upstream of an adenovirus minimal promoter (for details see Text S1). The basal activity of this ZRS-Luc reporter construct was set to 1 and transfection of either Hand2 (∼3-fold) or Hoxd13 (∼6.5-fold) induced luciferase activity and their co-transfection resulted in an ∼10.5-fold increase (Figure 4B). In silico analysis revealed 6 bona fide Ebox sequence elements within the ZRS (Figure 4A and Figure S5). Inactivating point mutations in either individual or several of these Ebox elements reduce the activity of the ZRS, but not in a strictly Hand2-dependent manner as the transactivation by Hoxd13 alone is also affected (data not shown). As Hand2 and Gli3R act in a mutually antagonistic manner during initiation of limb bud development [11], the potential effects of Gli3R on transactivation were assessed. As neither the Gli3 nor Gli1 activator forms are able to activate the ZRS-Luc reporter on their own (data not shown), the ZRS likely lacks functional Gli binding sites [39], suggesting that any effects of Gli3R would be indirect. Indeed, co-expression of Gli3R results in significant inhibition of transactivation in the presence of Hoxd13 (Figure 4B), in agreement with the proposal that Gli3R can bind to and potentially antagonize Hoxd13 function [33]. In particular, Gli3R represses Hand2-Hoxd13 mediated transactivation of the ZRS-Luc reporter by ∼50% (Figure 4B).
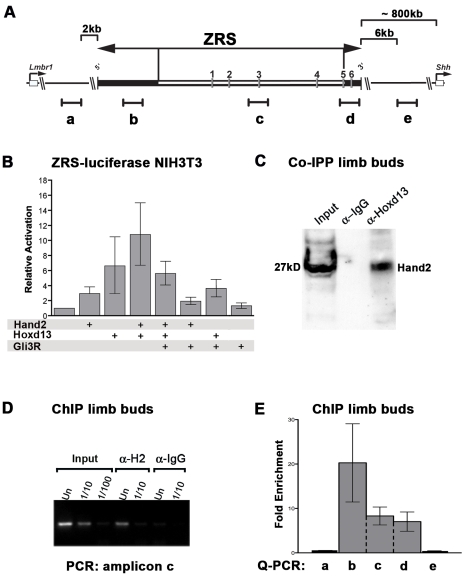
Figure 4. Hand2 interacts with Hoxd13 and is part of the chromatin complexes bound to the ZRS in limb buds.
(A) Scheme of the ∼1.7 kb mouse ZRS cis-regulatory and the flanking genomic regions. The ZRS is located within an intron of the mouse Lmbr1 gene (indicated on the left) and located ∼800 kb upstream of the Shh proximal promoter and coding exons (indicated on the right, see also Figure S5). The evolutionary conserved ZRS region drives expression of a LacZ reporter gene in Shh-like pattern in mouse limb buds [28], while deletion of the MFCS1 core region (indicated in white) disrupts Shh activation in limb buds [29]. Six Ebox sequences in the ZRS, which could potentially interact with Hand2 proteins are numbered “1” to “6”. Black lines indicate the approximate positions and sizes of the PCR amplicons for ChIP analysis. Note that amplicons “b” to “d” reside within the mouse ZRS, while amplicons “a” and “e” are located ∼2 kb upstream and ∼6 kb downstream of the ZRS and serve as non–ZRS controls. (B) Luciferase transactivation assay in NIH3T3 fibroblasts. Cells were co-transfected with ZRS-Luc and the expression plasmids indicated. Bars represent standard deviations. P<0.0001 for all samples except Gli3R alone: P = 0,0519. (C) Co-immunoprecipitation of Hand2 and Hoxd13 from wild-type limb buds (E10.5) using anti-Hoxd13 antibodies (α-Hoxd13) or IgGs (control). Hand2 proteins associated to Hoxd13 protein complexes were detected by Western blotting. (D,E) ChIP from wild-type limb buds (E11.0) to detect Hand2-containing chromatin complexes bound to the ZRS. (D) Analysis of amplicon “c” by conventional PCR (186 bp). Input: DNA isolated from cross-linked chromatin of E11.0 limb buds prior to ChIP was used as a positive control for PCR amplification. α-H2: ChIP using Hand2 antibodies. α-IgG: ChIP using non-specific goat IgGs as a control. Un: undiluted sample; dilutions as indicated. (E) Q–PCR analysis of three completely independent ChIP experiments using freshly cross-linked chromatin and α-Hand2 antibodies. The average values ± standard error are shown. Values obtained by amplifying a particular region from ChIP experiments using non-specific goat IgGs were arbitrarily set at 1 and used to calculate the values for the α-Hand2 ChIP experiments. Statistical evaluation by the Mann-Whitney test shows that the amplicons within the ZRS (“b” to “d”) are enriched in a statistically highly significant manner in comparison to the adjacent non-ZRS amplicons (“a” and “e”; p = 0.0018).
The relevance of these interactions for limb bud development was determined by co-immunoprecipitation (Figure 4C and Figure S6) and ChIP analysis (Figure 4D and 4E). Immunoprecipitation of Hoxd13 proteins in combination with Western blotting reveals the existence of protein complexes containing both Hoxd13 and Hand2 protein in wild-type limb buds (Figure 4C). The likely direct nature of these interactions is supported by efficient co-precipitation of epitope-tagged Hand2 and Hoxd13 proteins from transfected cells (Figure S6). These experiments establish that Hand2 interacts directly with Hoxd13 but not with Gli3R (Figure S6), which is relevant with respect to their genetic interaction (see below). As the available polyclonal Hand2 antibodies specifically recognize and immunoprecipitate Hand2 proteins (Figure S2B, S2C, S2D), ChIP on wild-type mouse limb buds was performed [40] to enrich Hand2 containing chromatin complexes and the analysis of three independent, fresh chromatin preparations is shown in Figure 4D and 4E. Conventional PCR using the amplicon “c” (Figure 4A) detected this ZRS region in chromatin precipitated with anti-Hand2 antibodies (lanes α-H2, Figure 4D), while no such amplification was detected when non-specific IgGs were used (lanes α-IgG; Figure 4D). To further analyze this apparent association of Hand2 containing chromatin complexes with the ZRS, three amplicons (“b”, “c”, “d”) probing different regions of the ∼1.7 kb mouse ZRS (Figure 4A) were used for real-time PCR (Q-PCR) analysis. In addition, two amplicons located outside the mouse ZRS were chosen as likely negative controls (non-ZRS amplicons “a” and “e” in Figure 4A and 4E and Figure S5). Indeed, Q-PCR analysis revealed a minimally 14-fold enrichment of the amplicons located within the ZRS in comparison to the adjacent non-ZRS regions (Figure 4E). This enrichment is specific as ChIP using non-specific IgGs resulted in much lower Q-PCR amplification of all five regions. In particular, the enrichment of the ZRS in comparison to flanking non-ZRS regions is highly significant (amplicons “b” to “d” versus “a” and “e”; p = 0.0018), while the variability among the three ZRS amplicons is not significantly different. Interestingly, the ZRS region encompassing amplicon “b”, whose enrichment is most variable, does not encode any bona fide Ebox elements (Figure 4A and 4E). This provides additional evidence for the fact that the interaction of Hand2-containing chromatin complexes with the ZRS may not depend only on Ebox sequences. This ChIP analysis (Figure 4D and 4E) provides good evidence that the Hand2-containing chromatin complexes bind to the ZRS cis-regulatory region, but not to adjacent non-ZRS sequences.
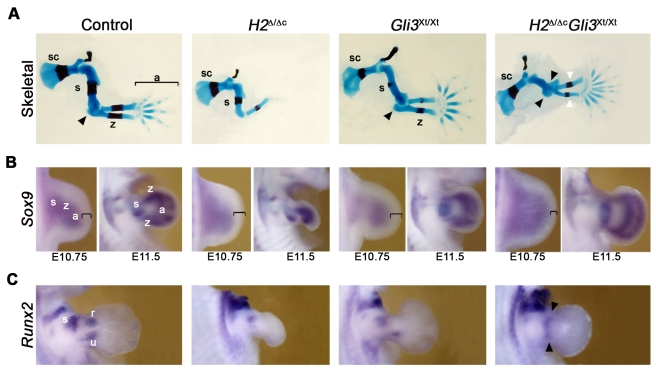
Mouse limb buds deficient for both Hand2 and Gli3 lack AP asymmetry along the entire PD axis and are severely polydactylous
As embryos lacking Hand2 in limb buds survive to advanced stages (Figure 1B), the functional relevance of the pre-patterning mechanism [11] can now be genetically investigated in Hand2 and Gli3 compound mutant (H2 Δ/Δc Gli3 Xt/Xt) embryos (Figure 5, Figure 6, Figure 7). In contrast to the Hand2 deficiency, H2 Δ/Δc Gli3 Xt/Xt limbs are severely polydactylous and display little phenotypic variability (Figure 5A and Figure S7A). In addition, the zeugopodal bones and elbow joints appear strikingly symmetrical (Figure 5A, white and black arrowheads in panel H2 Δ ˜Δc Gli3 Xt/Xt). These limb skeletal abnormalities are much more severe than the ones of Gli3 Xt/Xt and Shh Δ ˜Δ Gli3 Xt/Xt limbs (Figure 4A, panel Gli3 Xt/Xt; see also [9],[10]). While the skeletal elements of H2 Δ ˜Δc Gli3 Xt/Xt limbs seem to lack AP asymmetry, survival of the zeugopod and autopod progenitors is restored and the primordia are expanded in contrast to H2 Δ ˜Δc limbs (Figure S7B and data not shown). Moreover, the Sox9 expression domain, which marks the pre-chondrogenic lineage [41], is expanded in H2 Δ ˜Δc Gli3 Xt/Xt limb buds that tend to be larger than normal (Figure 5B, panel H2 Δ ˜Δc Gli3 Xt/Xt). However, no significant changes in proliferation were observed in H2 Δ ˜Δc Gli3 Xt/Xt limb buds (data not shown). While the pre-chondrogenic condensations of all major skeletal elements are discernible by E10.75 in wild-type and Gli3 deficient limb buds, Sox9 expression remains diffuse and non-polarized in H2 Δ ˜Δc Gli3 Xt/Xt limb buds (Figure 5B). During autopod development, the pool of Sox9 expressing digit progenitors is significantly expanded in H2 Δ ˜Δc Gli3 Xt/Xt limb buds in comparison to Gli3 mutants and wild-types (Figure 5B; compare limb buds at E11.5). The apparent symmetry of in particular the zeugopod in the H2 Δ ˜Δc Gli3 Xt/Xt limbs contrasts with the normal AP asymmetry in Gli3 Xt/Xt and Shh Δ ˜Δ Gli3 Xt/Xt limbs (Figure 5A) [9]. This observation indicates that Hand2 and Gli3 participate in establishment of the AP asymmetry of the proximal limb skeleton independent of SHH signaling. Indeed, the expression of Runx2, which marks proximal skeletal primordia [42], is altered in double mutant limb buds (Figure 5C). By E12.0, Runx2 is expressed in the presumptive stylopod and zeugopodal domains of wild-type limb buds, while few Runx2 positive cells are detected in Hand2 deficient limb buds (Figure 5C). In contrast, the Runx2 expression domain is expanded and lacks polarity in the proximal part of double mutant limb buds (Figure 5C, black arrowheads). Taken together, these results indicate that the skeletal phenotypes and the severe polydactyly of H2 Δ ˜Δc Gli3 Xt/Xt limbs arise as a consequence of disrupting AP asymmetry (proximally as indicated by Runx2) and aberrant expansion of the skeletal progenitor pools (distally as indicated by Sox9).
Figure 5. Forelimb buds lacking Hand2 and Gli3 lack AP polarity along the entire PD axis.
(A) Skeletal preparations of H2 Δ/Δc Gli3 Xt/Xt, H2 Δ/Δc, and Gli3 Xt/Xt single mutant and control (H2 Δ./) forelimbs at E14.5. The black arrowheads point to the duplicated elbow-like structure while the white arrowheads point to the symmetrical zeugopodal skeletal elements in H2 Δ/Δc Gli3 Xt/Xt limbs. Note the shortening of the stylopod in double mutant limbs. (B) Expression of Sox9 in limb buds at E10.75 (38 somites) and E11.5. Black brackets indicate the non-expressing distal mesenchyme that is reduced in H2 Δ/Δc Gli3 Xt/Xt limb buds. (C) Runx2 expression in wild-type limb buds marks the presumptive stylopod (s) and zeugopodal domains (r/u) at E12.0. Note that establishment of anterior expression domain is delayed in Gli3 Xt/Xt mutant limbs as it becomes visible by E12.5 (data not shown). Black arrowheads point to the apolar proximal expression of Runx2 in H2 Δ/Δc Gli3 Xt/Xt mutant limb buds. In wild-type limb buds, the presumptive expression domains for Sox9 and Runx2 are indicated as previously defined [42],[63]. sc: scapula; s: stylopod; z: zeugopod; a: autopod; u: ulna; r: radius. All limb buds are oriented with the anterior to the top and the posterior to the bottom.
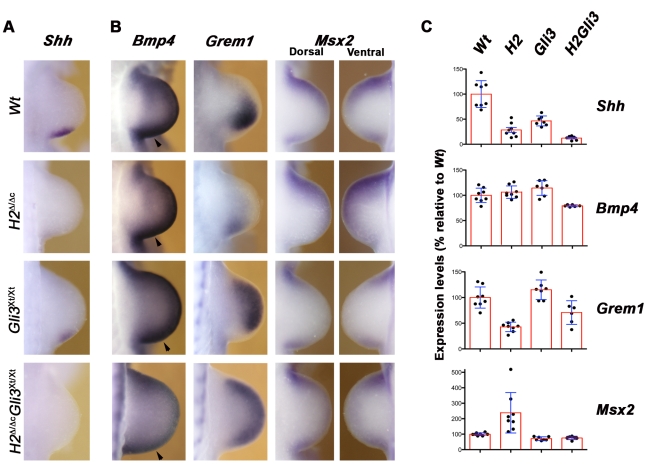
Figure 6. Shh expression and BMP pathway activity in H2 Δ/Δc and H2 Δ/Δc Gli3 Xt/Xt forelimb buds.
(A) No Shh expression is detected in the posterior mesenchyme of H2 Δ/Δc and H2 Δ/Δc Gli3 Xt/Xt limb buds at E10.25 (32–33 somites). (B) Bmp4, Grem1, and Msx2 expression at E10.5 (34–35 somites). Note that Grem1 expression is activated, but not up-regulated and expanded distal-anterior in H2 Δ/Δc limb buds. In contrast, the Grem1 expression domain appears rather uniform in the majority of all H2 Δ/Δc Gli3 Xt/Xt limb buds. (C) Q–PCR quantitation of Shh, Bmp4, Grem1 and Msx2 expression in single limb buds of mouse embryos of the indicated genotypes at ∼E10.5 (34–37 somites). Boxes show the average (± standard deviation), dots indicate levels in individual limb bud determined by triplicate analysis. The vertical axis indicates expression levels in percentages of wild-type levels (wild-type average set at 100%). Wt: wild-type (n = 8 single limb buds analyzed); H2: H2 Δ/Δc (n = 8); Gli3: Gli3 Xt/Xt (n = 7); H2Gli3: H2 Δ/Δc Gli3 Xt/Xt (n = 6). All differences discussed in the text are statistically highly significant (p-values between p<0.001 and p<0.05 using Mann-Whitney tests).
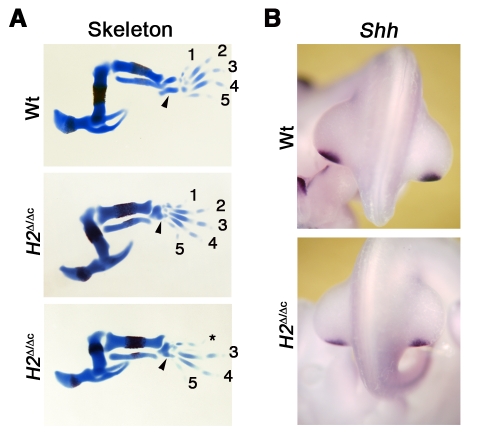
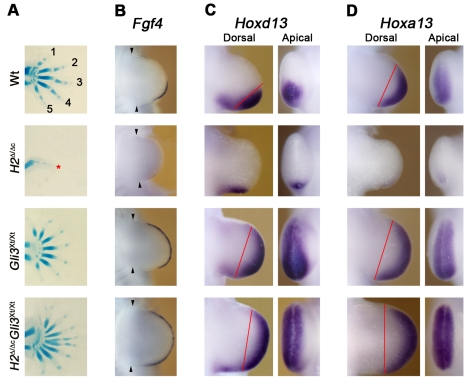
Figure 7. Apolar expression of Fgf4, Hoxd13, and Hoxa13 in the autopod primordia of H2 Δ/Δc Gli3 Xt/Xt forelimb buds.
(A) Skeletal preparations of the autopod (E14.5) of H2 Δ/Δc Gli3 Xt/Xt, H2 Δ/Δc, and Gli3 Xt/Xt single mutant and wild-type forelimbs. Digit identities are indicated by numbers 1 (thumb, anterior) to 5 (little finger, posterior). Black asterisks indicate digits with undetermined identities; red asterisk indicates the rudimentary digit formed in H2 Δ/Δc forelimbs. (B) Fgf4 expression in the AER of wild-type and mutant limb buds at E10.5 (36 somites). Fgf4 is expressed at very low levels in the posterior of in H2 Δ/Δc limb buds, but expands throughout the AER of H2 Δ/Δc Gli3 Xt/Xt forelimb buds. Arrowheads indicate the anterior and posterior margins of limb buds. (C) Hoxd13 expression at E10.75 (40 somites). The late Hoxd13 expression domain in H2 Δ/Δc Gli3 Xt/Xt limb buds appears symmetrical in contrast to e.g. Gli3 deficient limb buds (expression borders are indicated by red lines). This is best seen by comparing apical views. (D) Hoxa13 expression at E10.75 (41 somites). The Hoxa13 expression domain appears also symmetrical in H2 Δ/Δc Gli3 Xt/Xt limb buds, while some asymmetry is retained in Gli3 deficient limb buds (red lines in dorsal views; best seen by comparing the apical views).
Disruption of the self-regulatory system that interlinks the SHH, BMP, and FGF signaling pathways in limb buds
In H2 Δ ˜Δc Gli3 Xt/Xt limb buds, Shh expression is not detected by in situ hybridization (Figure 6A) and its expression is ∼10-fold lower than in wild-types (Figure 6C). Interestingly, the variability in Shh expression following Prx1-Cre mediated inactivation of Hand2 (Figure 1C, Figure S3B, S3C, S3D, and Figure 6C) is no longer observed in H2 Δ ˜Δc Gli3 Xt/Xt limb buds (Figure 6A and 6C), which agrees with the lack of significant variability in the resulting skeletal phenotypes (Figure 5A). This could be linked to the fact that posterior Shh expression is already reduced by ∼50% in Gli3 Xt/Xt limb buds (Figure 6A and 6C). The low Shh transcript levels detected in the most severely affected H2 Δ ˜Δc and H2 Δ ˜Δc Gli3 Xt/Xt limb buds (between 8% and 20%, Figure 6C) likely reflect basal expression not detected by in situ hybridization (Figure 1D, Figure 6A; see Discussion). BMP4-mediated up-regulation of its antagonist Grem1 in the posterior mesenchyme is essential to initiate the self-regulatory signaling system that promotes distal limb bud development [43],[44]. In H2 Δ ˜Δc limb buds, Bmp4 expression appears not significantly altered, while its expression is slightly reduced in H2 Δ ˜Δc Gli3 Xt/Xt limb buds (panels Bmp4 in Figure 6B and 6C). In particular, the posterior expression domain in double mutant limb buds appears smaller (arrowheads, panels Bmp4 in Figure 6B), which results in rather symmetrical Bmp4 expression along the AP limb bud axis. Furthermore, Grem1 expression is activated, but not up-regulated and distal-anteriorly expanded in Hand2 deficient limb buds (panel Grem1 in Figure 6B), similar to Shh deficient limb buds [44]. In double mutant limb buds, the Grem1 expression domain appears symmetrical due to its anterior expansion. However, the rather variable Grem1 transcript levels are overall reduced in H2 Δ ˜Δc Gli3 Xt/Xt limb buds in comparison to wild-type and Gli3 deficient limb buds (panels Grem1 in Figure 6C). Finally, the expression of the direct BMP transcriptional target Msx2 [43] is expanded in H2 Δ ˜Δc limb buds, while its expression is significantly reduced in Gli3 deficient and double mutant limb buds as a likely consequence of the alterations in Grem1 (panels Msx2 in Figure 6B and 6C). Taken together, these results corroborate the proposal that the initial phase of Grem1 expression in the posterior mesenchyme depends on BMP4 activity [43]. The rather symmetrical Grem1 expression in H2 Δ ˜Δc Gli3 Xt/Xt limb buds indicates that the second phase of SHH-dependent distal-anterior expansion of its expression in wild-type limb buds is a likely consequence of SHH-mediated inhibition of Gli3R activity [6].
Loss of AP asymmetry in the autopod of H2 Δ ˜Δc Gli3 Xt/Xt limb buds
The lack of discernible AP identities in the autopod of H2 Δ ˜Δc Gli3 Xt/Xt limb buds (Figure 7A) is confirmed by molecular analysis. In agreement with the rather symmetric distribution of Bmp4 and Grem1 in the distal limb bud mesenchyme (Figure 6B), Fgf4 is expressed uniformly by the AER in double mutant limb buds (Figure 7B). The distal expression domains of the Hoxd13 and Hoxa13 genes mark the presumptive autopod territory and are required for specification and expansion of the digit progenitors [45],[46]. Within the distal mesenchyme of H2 Δ ˜Δc Gli3 Xt/Xt forelimb buds, the expression of Hoxd13 is anteriorly expanded and appears apolar in comparison to wild-type and Gli3 mutant limb buds (Figure 7C; best seen in the apical views). In addition, the AP asymmetry of the distal Hoxa13 domain is also lost in double mutant limb buds (Figure 7D; best seen in the apical views). The expanded and apolar expression of these genes (Figure 7B–7D) together with the alterations in Sox9, Runx2 (Figure 5B and 5C), Bmp4 and Grem1 (Figure 6B) reveal the striking loss of the asymmetrical expression of molecular and cellular markers of the AP axis along the entire PD axis in limb buds lacking both Hand2 and Gli3.
Discussion
In this study, we uncover the key regulatory interactions involving Hand2 that control establishment of posterior limb bud identity upstream of SHH signaling, in particular the genetic interactions with Gli3 that initiate AP axis polarity. Secondly, we reveal that Hand2, which like 5′Hox genes is essential for establishment of the Shh expressing limb bud organizer in the posterior-proximal mesenchyme, is part of the chromatin complexes bound to ZRS cis-regulatory region. The striking loss of posterior and gain of anterior molecular markers in Hand2 deficient limb buds indicates that limb field symmetry may normally be broken by Gli3R-mediated posterior restriction of Hand2 expression. This most likely parallels activation of 5′HoxD genes in the posterior mesenchyme [45]. In Hand2 deficient limb buds, the SHH dependent establishment of the late 5′HoxD expression domains is disrupted, while in limb buds lacking both Hand2 and Gli3, the late 5′HoxD expression domains expand uniformly throughout the distal autopod. Therefore, the down-regulation of 5′HoxD genes in Hand2 deficient limb buds is a likely consequence of increased Gli3R activity due to lack of SHH signaling [23]. Furthermore, Hand2 participates in transcriptional activation and/or upregulation of Tbx2/3 and Shh expression in the posterior mesenchyme and is required for anterior restriction of Gli3 and Alx4 expression. In Hand2 deficient limb buds, expression of the BMP antagonist Grem1 is activated in the posterior mesenchyme under the influence of BMP signaling (ref. 43 and this study). This previous analysis and the observed anterior expansion of Grem1 expression in H2 Δ ˜Δc Gli3 Xt/Xt limb buds reveals that the transcriptional activation and positioning of the Grem1 expression domain is controlled by interaction of BMP4 (positive) with GLI3R (negative). In wild-type limb buds, the Grem1 expression domain is always located distal-anterior to the Shh expressing cells and their descendents [47],[48], while it remains proximal and low due to the lack of SHH signaling in H2 Δ ˜Δ limb buds (this study). Taken together, these results provide further insights into the molecular mechanism controlling spatial and temporal aspects of BMP4-mediated initiation and SHH-dependent progression of Grem1 expression, which acts as an essential node in the self-regulatory signaling system that controls limb development [1].
Hand2, the ZRS, and establishment of the Shh expression domain in the posterior limb bud mesenchyme
Our biochemical analysis of chromatin isolated from wild-type mouse limb buds reveals that Hand2-containing chromatin complexes are bound to the ZRS, which is the far upstream cis-regulatory region required for Shh expression in limb buds [28],[29]. In particular, ZRS sequences are specifically and significantly enriched in Hand2 containing chromatin complexes in contrast to flanking regions. Furthermore, Hand2 is part of Hoxd13 protein complexes in limb buds and in transfected cells, the two proteins transactivate the expression of a luciferase reporter gene in a ZRS-dependent manner. Albeit the fact that such transactivation studies are of somewhat artificial nature, the conclusions reached by this analysis completely agree with the results of our genetic analysis of Hand2 functions during mouse limb bud development. Early and complete genetic inactivation of Hand2 in limb buds disrupts establishment of the Shh expression domain in the posterior limb bud, while either incomplete or temporally delayed inactivation does no longer disrupt initiation of Shh expression (this study). This reveals the early essential requirement of Hand2 for establishment of the posterior Shh expression domain, while subsequently Hand2 appears to contribute to transcriptional up-regulation of Shh expression. This may happen as part of an auto-regulatory loop because SHH signaling in turn up-regulates Hand2 expression most likely via repressing production of the Gli3R isoform [9],[11],[49]. The low levels of Shh expression detected by Q-PCR even in the most affected H2 Δ ˜Δc and H2 Δ ˜Δc Gli3 Xt/Xt limb buds, but not in Shh deficient limb buds (JDB and RZ, unpublished) are indicative of basal transcription of the Shh locus in the absence of Hand2, which is not detectable by in situ hybridization (this study). This basal expression may depend on Hox transcription factors [24],[26] or other regulators of Shh expression in limb buds (see below). However, our study shows that Hand2 is essential to establish and upregulate Shh expression in the posterior mesenchyme, which defines the SHH signaling limb bud organizer [1]. This Hand2-mediated transactivation of Shh expression is a likely consequence of its direct interaction with the ZRS cis-regulatory region and is possibly enhanced by formation of transcriptional complexes with Hoxd13 protein in limb buds.
Genetic and experimental manipulation of paired appendage buds in mouse, chicken and zebrafish embryos have begun to reveal the factors required in addition to Hand2 and 5′HoxD genes for Shh activation. In particular, AER-FGF and retinoic acid signaling have also been implicated in the activation of Shh expression [21],[50]. Deletion of both the HoxA and HoxD clusters in mouse embryos disrupts Shh activation and causes early arrest of limb bud development such that the limb skeleton is truncated at the level of the stylopod [24],[26]. But in contrast to Hand2, loss-of-function mutations in these genes alone or in combination do not phenocopy the Shh loss-of-function limb skeletal phenotypes [51],[52]. The Hand2 protein interacts with Hoxd13 and is part of the chromatin complexes bound to the ZRS in limb buds (this study). However, other transacting factors will likely contribute to ZRS dependent activation of Shh transcription. In fact, the overlap of the Hand2 and Hoxd13 expression domains in the posterior limb bud mesenchyme is much bigger than the initial Shh expression domain. During limb bud initiation stages, the Hand2 and Gli3 expression domains overlap significantly, but then become rapidly mutually exclusive [11]. Therefore, these early dynamic changes in the expression domains of the Hand2, Gli3 and Hoxd13 transcriptional regulators may well alter their interactions and spatially restrict the formation of transcription initiating/enhancing Hand2-Hoxd13 chromatin complexes at the ZRS to the posterior limb bud (this study). These direct interactions would restrict the up-regulation of Shh expression to the posterior limb bud mesenchyme, thereby establishing the SHH signaling limb bud organizer. A recent study shows that the distant ZRS is in close proximity to the Shh transcription unit in both the anterior and posterior limb bud mesenchyme, but only loops out of its chromosomal territory in the posterior mesenchyme [31]. Interestingly, Shh is apparently transcribed by only a fraction of all ZPA cells at one particular time point, which indicates that the chromosomal conformation dynamics control Shh expression at the cellular level [31].
It is known that Hand2 binds DNA primarily as a heterodimer with E12 and/or the bHLH transcription factor Twist1 [16],[19]. Interestingly, Twist1 is also required during early limb bud development [18] and point mutations in the human Twist1 gene alter its dimerization with Hand2, which causes congenital limb malformations [19]. Therefore, these additional factors may also participate in regulation of Shh expression. The expression of Hand2 and 5′HoxD genes is activated in parallel, but then they converge functionally on the ZRS to establish the Shh expression domain in the posterior limb bud (this study and ref. 24). Furthermore, the establishment of the posterior Tbx2 and Tbx3 expression domains is disrupted in Hand2 deficient limb buds. The cis-regulatory elements controlling their expression are currently unknown, but it has been shown that Tbx2 expression requires the overlying non-AER ectoderm [53]. Additional experimental and genetic evidence indicates that Tbx2 and Tbx3 act likely upstream of Shh to restrict its transcriptional activation to the posterior limb bud margin [53],[54]. In particular, ectopic expression of Tbx3 in early chicken limb buds induces an anterior shift of the entire limb bud together with transient anterior expansion of Hand2 expression [55]. These studies indicate that Tbx genes are part of the molecular circuits that position the limb bud, specify posterior identity and restrict activation of Shh to its posterior margin.
Breaking limb bud symmetry
The genetic inactivation of the pre-patterning mechanism in H2 Δ ˜Δc Gli3 Xt/Xt limb buds disrupts establishment of AP asymmetry and self-regulatory limb bud signaling [43], while PD axis outgrowth and formation of all three major limb skeletal segments are the likely consequence of uniform AER-FGF signaling [2]. This results in a shortened and symmetric stylopod, zeugopod and a polydactylous autopod with highly dysmorphic digits. Similar to H2 Δ ˜Δc Gli3 Xt/Xt limb buds, limbs lacking 5′HoxD genes and Gli3 are also severely polydactylous but retain some polarity [56],[57]. Therefore, the loss of AP polarity along the entire proximo-distal axis is more severe than the phenotypes observed in limb buds lacking Gli3 alone or in combination with genes such as Shh, Alx4 or 5′HoxD genes [9], [56]–[58]. Over-expression of Hand2 in the entire limb bud mesenchyme results in a duplication of the anterior zeugopod (ulna) and posterior autopod (digits) [12], which indicates that disturbing the balance between Hand2 and Gli3 either by gene inactivation or over-expression alters AP polarity. Therefore, the balance of the opposing activities of Hand2 and Gli3R in concert with 5′HoxD genes may control specification of the AP limb axis independent and up-stream of SHH signaling. In mouse limb buds lacking the Plzf zinc finger protein, 5′HoxD genes are uniformly expressed from early stages onwards and AP polarity is partially lost in combination hindlimb digit polydactyly [59].
It remains unclear why the digit polydactyly in H2 Δ ˜Δc Gli3 Xt/Xt forelimbs is more severe than the one of Gli3 Xt/Xt (and Shh Δ ˜Δ Gli3 Xt/Xt [9]) forelimbs. However, in H2 Δ ˜Δc Gli3 Xt/Xt forelimb buds, the distal expression domains of Hoxa13 and Hoxd13, which delineate the autopod territory and function in digit development (see [refs. 24],[26] for further detail) are anteriorly expanded in comparison to Gli3 deficient limb buds. Such anterior expansion may point to an enlarged pool of autopod/digit progenitors, which could underlie the more severe digit polydactyly. As discussed before, this expansion of the Hoxa/d13 expression domains and the presumptive autopod territory are a likely consequence of the early loss of AP polarity along the entire PD axis in double mutant forelimb buds in contrast to Gli3 Xt/Xt mutants. In particular, the H2 Δ ˜Δc Gli3 Xt/Xt forelimb skeletons bear some resemblance to the primitive paired appendages of Devonian fish and the polydactylous limbs of early tetrapods [60]. We shows that these rather “primitive” limb structures develop in the absence of pre-patterning (Hand2, Gli3) and the self-regulatory signaling system that interlinks the SHH, BMP and FGF signaling pathways, which are both key to normal limb skeletal development [1]. During tetrapod evolution, the symmetry of primitive polydactylous autopods from the Devonian period [61] was likely broken by beginning to set-up the regulatory interactions described in this study as they initiate posterior polarity up-stream or in parallel to their requirement for establishment of the SHH signaling limb bud organizer. The establishment of these transcriptional regulatory network acting upstream of SHH signaling might have enabled the development of the more refined and better functional pentadactylous limbs of modern tetrapods.
Materials and Methods
All animal experiments were performed in accordance with Swiss law and have been approved by the regional veterinary and ethics authorities.
Mice and embryos
The generation of Hand2 conditional mutant mice is shown in Figure S1. Hand2 mouse strains were kept in a mixed 129SvJ/C57BL6 genetic background. For details of the generation and analysis of Hand2 mice and embryos see Text S1.
Immunoprecipitation (IP) and co-IP experiments
For IP, fore- and hind-limb buds from E11.0 embryos were collected in PBS and lysed in lysis buffer (Tris-HCl 10 mM pH 8.0; EDTA 1 mM; NaCl 140 mM; Triton 1%; SDS 0.1%; NaDeoxycholate 0.1%). Protein lysates (about 300 mg) were incubated overnight at 4°C with the anti-Hand2 (M-19, Santa Cruz; 1 mg) and protein G beads were added the next morning for about 5 hours at 4°C. After several washes in lysis buffer, beads were resuspended in Laemmli loading buffer and SDS-PAGE was performed under non-reducing conditions. Goat IgG antibodies were used as control. For Co-IP of endogenous embryonic proteins, 50 limb buds at E10.5 were dissected in PBS and processed as described [33]. The Hoxd13 or control rabbit IgG antibodies used for co-IPs were covalently cross-linked to G protein beads and bound proteins were detected with Hand2 antibodies (AF3876, R&D System).
Chromatin Immunoprecipitation (ChIP)
ChIP was performed using wild-type fore- and hindlimb buds at E11.0 (38–42 somites). For each experiment, 85 limb buds were dissected, pooled and the freshly cross-linked chromatin divided among the starting samples. The average size of the DNA fragments in the cross-linked and sonicated chromatin was ∼500–2000 bp. Samples were processed as described [62] with the following modifications: protein G magnetic beads (Dynabeads, Invitrogen) were pre-absorbed with goat IgG (1–2 mg for 30 ml of beads for each sample) for minimally 1 hour at 4°C. After washing them with BSA-PBS (5 mg/ml), the beads were added to the chromatin extracts and gently rocked for 1 hour at 4°C. Afterwards, beads were spun down and the chromatin in the supernatant transferred to a new tube and incubated overnight with Hand2 antibodies (M-19, Santa Cruz; 1 mg) or goat IgG antibodies as control (1 mg). The following day, 25 ml of beads were added and the DNA-immunocomplexes were precipitated for 4 hours at 4°C. ChIP-enriched DNA samples were amplified by Q-PCR and conventional PCR. To compute the enrichment for a particular amplicon, its values were compared with the ones of a completely unrelated amplicon within the mouse β-actin gene that provides an additional negative control. The β-actin gene is located ∼114 Mb downstream of the ZRS on mouse chromosome 5. The fold of enrichment was then calculated as the fold of increase in the specific signal in relation to the values obtained when using non-specific goat IgGs for ChIP (values set arbitrarily at 1). All oligos used are listed in Table S1. Three ChIP experiments were performed using completely independent and fresh (i.e. non-frozen) chromatin preparations. The values obtained were analyzed and the graphs shown in Figure 4D (means ± standard error) were drawn using the Prism Graphpad Software (La Jolla, USA). The statistical significance of all results was assessed using the Mann-Whitney test as part of the Prism software package.
Luciferase assays
Mouse NIH3T3 fibroblasts were plated on 24-well plates and transfected using Lipofectamine LTX (Invitrogen) including a total of 500 ng of DNA. Reporter constructs were co-transfected with 100 ng of Hand2 and/or Hoxd13 and/or Gli3 expression constructs in combination with a Renilla luciferase vector. A detailed description of the generation of the expression constructs is available in Text S1. Cells were collected 28–30 hours post-transfection and luciferase reporter assays were performed using the Dual Luciferase Kit (Promega). Each assay was repeated at least 10 times. It is important to note that NIH3T3 cells do not express the endogenous Hand2, Hoxd13 and Gli3 genes (data not shown). For the co-immuno-precipitation assays in cells see Text S1.
Supporting Information
Generation and validation of the Hand2 conditional allele. (A) Scheme depicting the Hand2 gene targeting strategy. A targeting vector was constructed in order to flank both Hand2 coding exons with loxP sites (blue triangles). An EcoRV (ERV) restriction site was inserted to enable screening of ES-clones by Southern blot analysis. The PGK-Neo-pA cassette was inserted into the construct 3′ to the loxP site for positive selection. This selection cassette is flanked with two FRT sites (green triangles) to enable excision by the flipase (FLPe) recombinase. For genomic Southern blot analysis, the 5′ probe (violet box) and the 3′ probe (orange box) were used. The PCR oligos and sizes of amplified bands are indicated. Arrows indicate the direction of transcription. To induce FRT and loxP mediated recombination at the Hand2 locus, mice carrying the Hand2 floxed-neo allele (H2 fneo) were intercrossed with FLPe and with CMV-Cre transgenic mice. (B) Southern blot analysis showing wild-type, the correctly recombined 4D7 ES-cell clone and DNA biopsies from mice heterozygous for the H2 fneo and the Hand2 floxed (H2 f) allele. The 5′ probe detects a 15 kb ERV fragment for the wild-type (Wt) locus, while an 8 kb ERV fragment is detected when the locus is correctly recombined. The 3′ probe detects a 7.3 kb wild-type PacI fragment and a 9.3 kb fragment in the correctly targeted allele. Following excision of the PGK-Neo-pA cassette, the 9.3 kb is reduced to a 7.5 kb fragment in the H2 f allele. (C) PCR genotyping. (D) Morphology of mouse embryos at embryonic day E9.5–9.75 (25–27 somites). Hand2 deficient embryos are growth retarded, the aortic and pericardial sac are dilated and branchial arches are malformed [13]. The heart (h), first (I) and second branchial arches (II) are indicated. Asterisks indicate the outgrowing forelimb buds. (E) LysoTracker Red (LysoT) analysis reveals the massive and generalized cell death in Hand2 deficient embryos and limb buds at E9.5 (25 somites). a: anterior; d: dorsal; p: posterior; v: ventral.
(6.79 MB TIF)
Clearance of Hand2 transcripts from mutant forelimb buds and specificity of α-Hand2 antibodies. (A) Q-PCR analysis to determine Hand2 transcript levels in wild-type, Hand2 floxed (H2 f), Hand2 heterozygous and Hand2 deficient limb buds at E10.25–10.5 (33–35 somites; n = 6–8). Note that no Hand2 transcripts are detected in Hand2 deficient limb buds. Bars: ±standard deviation. asterisk: P = 0.0009. (B) Immunofluorescense using α-Hand2 antibodies (M-19, Santa Cruz) reveals the specific nuclear localization of Hand2 proteins in posterior (Wt-p) but not anterior (Wt-a) limb buds mesenchymal cells. No specific fluorescence is detected in mesenchymal cells isolated from Hand2 deficient limb buds. (C) Hand2 proteins are cleared from Hand2 deficient limb buds by embryonic day E10.5. Protein extracts were normalized for their vinculin content. (D) Immunoprecipitation (IPP) of Hand2 proteins from E11.0 limb buds. Hand2 proteins are detected by Western blotting. Control: α-IgG. Asterisks indicate the cross-reactivity with the light chains of the IgGs (control and α-Hand2) used for IPP.
(2.42 MB TIF)
Incomplete/delayed inactivation of Hand2 in forelimb buds results in a hypomorphic phenotype. (A) Skeletal preparations of control (Prx1-Cre heterozygous) and Hand2 deficient forelimbs at E14.5. Due to slight variability in Prx1-Cre mediated inactivation of the conditional Hand2 allele in forelimb buds, three classes of skeletal phenotypes are observed. The most hypomorphic phenotype (Weak) results in formation of two misplaced zeugopodal bones, three anterior digits and a hypoplastic digit that resembles digit 4 (indicated by an asterisk). The arrowhead points to the twisted bones of the zeugopod. The less hypomorphic phenotype (Intermediate) results in formation of one zeugopodal bone and two digits. The null phenotype (Strong) is identical to the skeletal phenotypes observed in Shh deficient limb buds (Figure 1A). Asterisks indicate digits with unclear identities. (B) Analysis of Hand2 expression reveals the variable nature of Prx1-Cre mediated inactivation of Hand2 at E9.75 (28 somites). (C) This variability is also apparent when levels of SHH signal transduction are monitored by Gli1 expression at E9.75 (27 somites). Complete absence of Hand2 (B) and Gli1 transcripts (C) was observed in 50% of all Prx1-Cre1, Hand2 deficient limb buds (n = 4/8). The others display varying degrees of Hand2 and Gli1 expression. All limb buds are oriented with the anterior to the top and the posterior to the bottom. (D) Table summarizing the frequencies of the three classes of limb skeletal phenotypes observed in Hand2 mutant forelimbs. This variability is in agreement with the fact, that developmentally slightly later Hand2 inactivation in hindlimb buds results in almost normal Shh expression and limb skeletal development (Figure 2). Taken together, these results indicate that Hand2 needs to be inactivated very early and rapidly during the onset of limb bud development to disrupt establishment of the posterior Shh expression domain.
(3.68 MB TIF)
Activation of 5′HoxD genes and posterior expansion of Gli3 expression in Hand2 deficient limb buds. Hoxd11 expression at E9.75 (27 somites) and E10.75 (36 somites). Expression of Hoxd11 is initiated in limb buds lacking Hand2 (arrowheads), but its up-regulation is disrupted. (B) Hoxd13 expression is initiated, but rapidly down-regulated in Hand2 deficient limb buds (arrowheads E10.5, 33 somites). (C) Gli3 expression is expanded posteriorly in Hand2 deficient limb buds at E10.0 (32 somites; compare white to black arrowhead). In Shh deficient limb buds, Gli3 is not expanded to the posterior margin (compare white to open arrowheads). All limb buds are oriented with the anterior to the top and the posterior to the bottom. (D) Inactivation of Hand2 alters Gli3 protein processing. Protein extracts prepared from limb buds of the indicated genotypes at E10.5 (35 somites) were analyzed by immunoblotting using α-Gli3 antibodies. The full-length Gli3 protein is about 190 kD, while the processed Gli3R isoform is about 83 kD. Note that Gli3R form is more abundant in Hand2 and Shh deficient than in wild-type limb buds. Samples are normalized for their vinculin contents. The asterisk points to an unrelated cross-reacting protein.
(5.95 MB TIF)
The genomic landscape encompassing the mouse ZRS. Scheme depicting part of mouse chromosome 5 (Ensemble: Mus musculus genomic region from position 29621310 to 29662806) analyzed in the ChIP experiments by Q-PCR. The Lmbr1 locus encodes the mouse ZRS (1.67 kb) within intron 4, which is about 800 kb away from the Shh locus. The 6 Ebox elements (1 to 6) located in the ZRS are indicated. The framed orange and blue boxes indicate the 20 kb downstream and upstream flanking regions. These two regions are shown in the enlargements and potential Ebox elements are indicated. Coding exons are represented by filled boxes. Amplicon a is located about 2 kb downstream and amplicon e about 6 kb upstream of the ZRS (the primers used for Q-PCR amplification are indicated by green arrows).
(0.32 MB PDF)
Evidence that Hand2 interacts directly with the Hoxd13 but not Gli3R protein. Co-immunoprecipitation reveals the direct interaction of Hand2 with Hoxd13 in HEK293T cells (Hand2: Flag-epitope tagged; Gli3R: Myc-epitope tagged). In contrast, Gli3R is unable to directly interact with Hand2, but binds to Hoxd13 [12]. Protein extracts were immunoprecipitated (IP) using the following antibodies: α-Flag for Hand2, α-Hoxd13 for Hoxd13, α-Myc for Gli3R and immunoblotted (IB) using the appropriate antibodies.
(2.52 MB TIF)
Morphological defects in limb buds lacking Hand2 and Gli3. (A) The forelimb morphology of double mutant mouse embryos at E14.5. Note the stunted forelimbs and the extreme pre- and post-axial polydactyly in comparison to Gli3 Xt/Xt limb buds. White brackets indicate forelimb length. Asterisks indicate digits with undetermined identities. (B) The massive apoptosis of mesenchymal cells in Hand2 deficient limb buds is suppressed in limb buds lacking both Hand2 and Gli3. Apoptotic cells were detected by TUNEL fluorescence on limb bud sections at E10.25 (33 somites). Sections are oriented with the anterior to the top and posterior to the bottom.
(3.99 MB TIF)
Oligos used for the study. All primers used for genotyping of mice and embryos, Q-PCR analysis of Hand2 transcripts, Q-PCR analysis of the ChIP experiments are listed. Conditions for use are available upon request.
(0.04 MB DOC)
Supporting materials and methods.
(0.08 MB DOC)
Acknowledgments
The Gli3R, Hoxd13 and other protein expression constructs were kindly provided by J. Briscoe, J. Innis, and M. Williams. We are thankful to A. Zuniga and J.A. Cobb for providing the Cry-μ and Runx2 in situ probes. Chimeric mice were generated by D. Klewe-Nebenius, and C. Saegesser is acknowledged for animal care. The authors wish to thank M. Kmita, A. Zuniga, and all group members for helpful comments on the manuscript.
Footnotes
The authors have declared that no competing interests exist.
This research was supported by the Swiss National Science Foundation (grants 3100A0-100240 and -113866) and the University of Basel (to RZ); the Center for Cancer Research, NCI and NIH (to SM); the EU Epigenome Network of Excellence; and the ETH Zurich (to RP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zeller R, Lopez-Rios J, Zuniga A. Vertebrate limb bud development: moving towards integrative analysis of organogenesis. Nat Rev Genet. 2009;10:845–858. doi: 10.1038/nrg2681. [DOI] [PubMed] [Google Scholar]
- 2.Mariani FV, Ahn CP, Martin GR. Genetic evidence that FGFs have an instructive role in limb proximal-distal patterning. Nature. 2008;453:401–405. doi: 10.1038/nature06876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Towers M, Mahood R, Yin Y, Tickle C. Integration of growth and specification in chick wing digit-patterning. Nature. 2008;452:882–886. doi: 10.1038/nature06718. [DOI] [PubMed] [Google Scholar]
- 4.Zhu J, Nakamura E, Nguyen MT, Bao X, Akiyama H, et al. Uncoupling Sonic hedgehog control of pattern and expansion of the developing limb bud. Dev Cell. 2008;14:624–632. doi: 10.1016/j.devcel.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn S, Joyner AL. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118:505–516. doi: 10.1016/j.cell.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 6.Wang B, Fallon JF, Beachy PA. Hedgehog-Regulated Processing of Gli3 Produces an Anterior/Posterior Repressor gradient in the Developing Vertebrate Limb. Cell. 2000;100:423–434. doi: 10.1016/s0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 7.Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, et al. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Hui C, Joyner A. A mouse model of greig cephalopolysyndactyly syndrome: the extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nat-Genet. 1993;3:241–246. doi: 10.1038/ng0393-241. [DOI] [PubMed] [Google Scholar]
- 9.te Welscher P, Zuniga A, Kuijper S, Drenth T, Goedemans HJ, et al. Progression of Vertebrate Limb Development through SHH-Mediated Counteraction of GLI3. Science. 2002;298:827–830. doi: 10.1126/science.1075620. [DOI] [PubMed] [Google Scholar]
- 10.Litingtung Y, Dahn RD, Li Y, Fallon JF, Chiang C. Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature. 2002;418:979–983. doi: 10.1038/nature01033. [DOI] [PubMed] [Google Scholar]
- 11.te Welscher P, Fernandez-Teran M, Ros MA, Zeller R. Mutual genetic antagonism involving GLI3 and dHAND prepatterns the vertebrate limb bud mesenchyme prior to SHH signaling. Genes Dev. 2002;16:421–426. doi: 10.1101/gad.219202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charité J, McFadden DG, Olson EN. The bHLH transcription factor dHAND controls Sonic hedgehog expression and establishment of the zone of polarizing activity during limb development. Development. 2000;127:2461–2470. doi: 10.1242/dev.127.11.2461. [DOI] [PubMed] [Google Scholar]
- 13.Yelon D, Baruch T, Halpern ME, Ruvinsky I, Ho RK, et al. The bHLH transcription factor Hand2 plays parallel roles in zebrafish heart and pectoral fin development. Development. 2000;127:2573–2582. doi: 10.1242/dev.127.12.2573. [DOI] [PubMed] [Google Scholar]
- 14.McFadden DG, McAnally J, Richardson JA, Charite J, Olson EN. Misexpression of dHAND induces ectopic digits in the developing limb bud in the absence of direct DNA binding. Development. 2002;129:3077–3088. doi: 10.1242/dev.129.13.3077. [DOI] [PubMed] [Google Scholar]
- 15.Liu N, Barbosa AC, Chapman SL, Bezprozvannaya S, Qi X, et al. DNA binding-dependent and -independent functions of the Hand2 transcription factor during mouse embryogenesis. Development. 2009;136:933–942. doi: 10.1242/dev.034025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai YS, Cserjesi P. The basic helix-loop-helix factor, HAND2, functions as a transcriptional activator by binding to E-boxes as a heterodimer. J Biol Chem. 2002;277:12604–12612. doi: 10.1074/jbc.M200283200. [DOI] [PubMed] [Google Scholar]
- 17.Markus M, Benezra R. Two isoforms of protein disulfide isomerase alter the dimerization status of E2A proteins by a redox mechanism. J Biol Chem. 1999;274:1040–1049. doi: 10.1074/jbc.274.2.1040. [DOI] [PubMed] [Google Scholar]
- 18.Zuniga A, Quillet R, Perrin-Schmidt F, Zeller R. Mouse Twist is required for FGF-mediated epithelial-mesenchymal signaling and cell survival during limb morphogenesis. Mech Dev. 2002;114:51–59. doi: 10.1016/s0925-4773(02)00048-5. [DOI] [PubMed] [Google Scholar]
- 19.Firulli BA, Krawchuk D, Centonze VE, Vargesson N, Virshup DM, et al. Altered Twist1 and Hand2 dimerization is associated with Saethre-Chotzen syndrome and limb abnormalities. Nat Genet. 2005;37:373–381. doi: 10.1038/ng1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tickle C, Alberts BM, Wolpert L, Lee J. Local application of retinoic acid in the limb bud mimics the action of the polarizing region. Nature. 1982;296:564–565. doi: 10.1038/296564a0. [DOI] [PubMed] [Google Scholar]
- 21.Niederreither K, Vermot J, Schuhbaur B, Chambon P, Dolle P. Embryonic retinoic acid synthesis is required for forelimb growth and anteroposterior patterning in the mouse. Development. 2002;129:3563–3574. doi: 10.1242/dev.129.15.3563. [DOI] [PubMed] [Google Scholar]
- 22.Buscher D, Bosse B, Heymer J, Ruther U. Evidence for genetic control of Sonic hedgehog by Gli3 in mouse limb development. Mech Dev. 1997;62:175–182. doi: 10.1016/s0925-4773(97)00656-4. [DOI] [PubMed] [Google Scholar]
- 23.Zuniga A, Zeller R. Gli3 (Xt) and formin (ld) participate in the positioning of the polarising region and control of posterior limb-bud identity. Development. 1999;126:13–21. doi: 10.1242/dev.126.1.13. [DOI] [PubMed] [Google Scholar]
- 24.Tarchini B, Duboule D, Kmita M. Regulatory constraints in the evolution of the tetrapod limb anterior-posterior polarity. Nature. 2006;443:985–988. doi: 10.1038/nature05247. [DOI] [PubMed] [Google Scholar]
- 25.Knezevic V, De Santo R, Schughart K, Huffstadt U, Chiang C, et al. Hoxd-12 differentially affects preaxial and postaxial chondrogenic branches in the limb and regulates Sonic hedgehog in a positive feedback loop. Development. 1997;124:4523–4536. doi: 10.1242/dev.124.22.4523. [DOI] [PubMed] [Google Scholar]
- 26.Kmita M, Tarchini B, Zakany J, Logan M, Tabin CJ, et al. Early developmental arrest of mammalian limbs lacking HoxA/HoxD gene function. Nature. 2005;435:1113–1116. doi: 10.1038/nature03648. [DOI] [PubMed] [Google Scholar]
- 27.Capellini TD, Di Giacomo G, Salsi V, Brendolan A, Ferretti E, et al. Pbx1/Pbx2 requirement for distal limb patterning is mediated by the hierarchical control of Hox gene spatial distribution and Shh expression. Development. 2006;133:2263–2273. doi: 10.1242/dev.02395. [DOI] [PubMed] [Google Scholar]
- 28.Lettice LA, Heaney SJ, Purdie LA, Li L, de Beer P, et al. A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum Mol Genet. 2003;12:1725–1735. doi: 10.1093/hmg/ddg180. [DOI] [PubMed] [Google Scholar]
- 29.Sagai T, Hosoya M, Mizushina Y, Tamura M, Shiroishi T. Elimination of a long-range cis-regulatory module causes complete loss of limb-specific Shh expression and truncation of the mouse limb. Development. 2005;132:797–803. doi: 10.1242/dev.01613. [DOI] [PubMed] [Google Scholar]
- 30.Sagai T, Masuya H, Tamura M, Shimizu K, Yada Y, et al. Phylogenetic conservation of a limb-specific, cis-acting regulator of Sonic hedgehog (Shh). Mamm Genome. 2004;15:23–34. doi: 10.1007/s00335-033-2317-5. [DOI] [PubMed] [Google Scholar]
- 31.Amano T, Sagai T, Tanabe H, Mizushina Y, Nakazawa H, et al. Chromosomal dynamics at the Shh locus: limb bud-specific differential regulation of competence and active transcription. Dev Cell. 2009;16:47–57. doi: 10.1016/j.devcel.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Hill RE. How to make a zone of polarizing activity: insights into limb development via the abnormality preaxial polydactyly. Dev Growth Differ. 2007;49:439–448. doi: 10.1111/j.1440-169X.2007.00943.x. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Knezevic V, Ervin V, Hutson R, Ward Y, et al. Direct interaction with Hoxd proteins reverses Gli3-repressor function to promote digit formation downstream of Shh. Development. 2004;131:2339–2347. doi: 10.1242/dev.01115. [DOI] [PubMed] [Google Scholar]
- 34.Srivastava D, Thomas T, Lin Q, Kirby ML, Brown D, et al. Regulation of cardiac mesodermal and neural crest development by the bHLH transcription factor dHAND. Nature Genetics. 1997;16:154–160. doi: 10.1038/ng0697-154. [DOI] [PubMed] [Google Scholar]
- 35.Logan M, Martin JF, Nagy A, Lobe C, Olson EN, et al. Expression of Cre recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33:77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- 36.Hasson P, Del Buono J, Logan MP. Tbx5 is dispensable for forelimb outgrowth. Development. 2007;134:85–92. doi: 10.1242/dev.02622. [DOI] [PubMed] [Google Scholar]
- 37.King M, Arnold JS, Shanske A, Morrow BE. T-genes and limb bud development. Am J Med Genet A. 2006;140:1407–1413. doi: 10.1002/ajmg.a.31250. [DOI] [PubMed] [Google Scholar]
- 38.Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 39.Vokes SA, Ji H, Wong WH, McMahon AP. A genome-scale analysis of the cis-regulatory circuitry underlying sonic hedgehog-mediated patterning of the mammalian limb. Genes Dev. 2008;22:2651–2663. doi: 10.1101/gad.1693008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orlando V, Strutt H, Paro R. Analysis of chromatin structure by in vivo formaldehyde cross-linking. Methods. 1997;11:205–214. doi: 10.1006/meth.1996.0407. [DOI] [PubMed] [Google Scholar]
- 41.Kawakami Y, Rodriguez-Leon J, Belmonte JC. The role of TGFbetas and Sox9 during limb chondrogenesis. Curr Opin Cell Biol. 2006;18:723–729. doi: 10.1016/j.ceb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Cobb J, Dierich A, Huss-Garcia Y, Duboule D. A mouse model for human short-stature syndromes identifies Shox2 as an upstream regulator of Runx2 during long-bone development. Proc Natl Acad Sci U S A. 2006;103:4511–4515. doi: 10.1073/pnas.0510544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benazet JD, Bischofberger M, Tiecke E, Goncalves A, Martin JF, et al. A self-regulatory system of interlinked signaling feedback loops controls mouse limb patterning. Science. 2009;323:1050–1053. doi: 10.1126/science.1168755. [DOI] [PubMed] [Google Scholar]
- 44.Zuniga A, Haramis AP, McMahon AP, Zeller R. Signal relay by BMP antagonism controls the SHH/FGF4 feedback loop in vertebrate limb buds. Nature. 1999;401:598–602. doi: 10.1038/44157. [DOI] [PubMed] [Google Scholar]
- 45.Tarchini B, Duboule D. Control of Hoxd genes' collinearity during early limb development. Dev Cell. 2006;10:93–103. doi: 10.1016/j.devcel.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 46.Fromental-Ramain C, Warot X, Messadecq N, LeMeur M, Dollé P, et al. Hox-a13 and Hox-d13 play a crucial role in patterning of the limb autopod. Development. 1996;122:2997–3011. doi: 10.1242/dev.122.10.2997. [DOI] [PubMed] [Google Scholar]
- 47.Scherz PJ, Harfe BD, McMahon AP, Tabin CJ. The limb bud Shh-Fgf feedback loop is terminated by expansion of former ZPA cells. Science. 2004;305:396–399. doi: 10.1126/science.1096966. [DOI] [PubMed] [Google Scholar]
- 48.Panman L, Galli A, Lagarde N, Michos O, Soete G, et al. Differential regulation of gene expression in the digit forming area of the mouse limb bud by SHH and gremlin 1/FGF-mediated epithelial-mesenchymal signaling. Development. 2006;133:3419–3428. doi: 10.1242/dev.02529. [DOI] [PubMed] [Google Scholar]
- 49.Fernandez-Teran M, Piedra ME, Kathiriya IS, Srivastava D, Rodriguez-Rey JC, et al. Role of dHAND in the anterior-posterior polarization of the limb bud: implications for the Sonic hedgehog pathway. Development. 2000;127:2133–2142. doi: 10.1242/dev.127.10.2133. [DOI] [PubMed] [Google Scholar]
- 50.Lewandoski M, Sun X, Martin GR. Fgf8 signaling from the AER is essential for normal limb development. Nat Genet. 2000;26:460–463. doi: 10.1038/82609. [DOI] [PubMed] [Google Scholar]
- 51.Chiang C, Litingtung Y, Harris MP, Simandl BK, Li Y, et al. Manifestation of the Limb Prepattern: Limb Development in the Absence of Sonic Hedgehog Function. Developmental Biology. 2001;236:421–435. doi: 10.1006/dbio.2001.0346. [DOI] [PubMed] [Google Scholar]
- 52.Kraus P, Fraidenraich D, Loomis CA. Some distal limb structures develop in mice lacking Sonic hedgehog signaling. Mech Dev. 2001;100:45–58. doi: 10.1016/s0925-4773(00)00492-5. [DOI] [PubMed] [Google Scholar]
- 53.Nissim S, Allard P, Bandyopadhyay A, Harfe BD, Tabin CJ. Characterization of a novel ectodermal signaling center regulating Tbx2 and Shh in the vertebrate limb. Dev Biol. 2007;304:9–21. doi: 10.1016/j.ydbio.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davenport TG, Jerome-Majewska LA, Papaioannou VE. Mammary gland, limb and yolk sac defects in mice lacking Tbx3, the gene mutated in human ulnar mammary syndrome. Development. 2003;130:2263–2273. doi: 10.1242/dev.00431. [DOI] [PubMed] [Google Scholar]
- 55.Rallis C, Del Buono J, Logan MP. Tbx3 can alter limb position along the rostrocaudal axis of the developing embryo. Development. 2005;132:1961–1970. doi: 10.1242/dev.01787. [DOI] [PubMed] [Google Scholar]
- 56.Sheth R, Bastida MF, Ros M. Hoxd and Gli3 interactions modulate digit number in the amniote limb. Dev Biol. 2007;310:430–441. doi: 10.1016/j.ydbio.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 57.Zakany J, Zacchetti G, Duboule D. Interactions between HOXD and Gli3 genes control the limb apical ectodermal ridge via Fgf10. Dev Biol. 2007;306:883–893. doi: 10.1016/j.ydbio.2007.03.517. [DOI] [PubMed] [Google Scholar]
- 58.Panman L, Drenth T, Tewelscher P, Zuniga A, Zeller R. Genetic interaction of Gli3 and Alx4 during limb development. Int J Dev Biol. 2005;49:443–448. doi: 10.1387/ijdb.051984lp. [DOI] [PubMed] [Google Scholar]
- 59.Barna M, Pandolfi PP, Niswander L. Gli3 and Plzf cooperate in proximal limb patterning at early stages of limb development. Nature. 2005;436:277–281. doi: 10.1038/nature03801. [DOI] [PubMed] [Google Scholar]
- 60.Shubin N, Tabin C, Carroll S. Fossils, genes and the evolution of animal limbs. Nature. 1997;388:639–647. doi: 10.1038/41710. [DOI] [PubMed] [Google Scholar]
- 61.Shubin N, Tabin C, Carroll S. Deep homology and the origins of evolutionary novelty. Nature. 2009;457:818–823. doi: 10.1038/nature07891. [DOI] [PubMed] [Google Scholar]
- 62.Vokes SA, Ji H, McCuine S, Tenzen T, Giles S, et al. Genomic characterization of Gli-activator targets in sonic hedgehog-mediated neural patterning. Development. 2007;134:1977–1989. doi: 10.1242/dev.001966. [DOI] [PubMed] [Google Scholar]
- 63.Sun X, Mariani FV, Martin GR. Functions of FGF signaling from the apical ectodermal ridge in limb development. Nature. 2002;418:501–508. doi: 10.1038/nature00902. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Generation and validation of the Hand2 conditional allele. (A) Scheme depicting the Hand2 gene targeting strategy. A targeting vector was constructed in order to flank both Hand2 coding exons with loxP sites (blue triangles). An EcoRV (ERV) restriction site was inserted to enable screening of ES-clones by Southern blot analysis. The PGK-Neo-pA cassette was inserted into the construct 3′ to the loxP site for positive selection. This selection cassette is flanked with two FRT sites (green triangles) to enable excision by the flipase (FLPe) recombinase. For genomic Southern blot analysis, the 5′ probe (violet box) and the 3′ probe (orange box) were used. The PCR oligos and sizes of amplified bands are indicated. Arrows indicate the direction of transcription. To induce FRT and loxP mediated recombination at the Hand2 locus, mice carrying the Hand2 floxed-neo allele (H2 fneo) were intercrossed with FLPe and with CMV-Cre transgenic mice. (B) Southern blot analysis showing wild-type, the correctly recombined 4D7 ES-cell clone and DNA biopsies from mice heterozygous for the H2 fneo and the Hand2 floxed (H2 f) allele. The 5′ probe detects a 15 kb ERV fragment for the wild-type (Wt) locus, while an 8 kb ERV fragment is detected when the locus is correctly recombined. The 3′ probe detects a 7.3 kb wild-type PacI fragment and a 9.3 kb fragment in the correctly targeted allele. Following excision of the PGK-Neo-pA cassette, the 9.3 kb is reduced to a 7.5 kb fragment in the H2 f allele. (C) PCR genotyping. (D) Morphology of mouse embryos at embryonic day E9.5–9.75 (25–27 somites). Hand2 deficient embryos are growth retarded, the aortic and pericardial sac are dilated and branchial arches are malformed [13]. The heart (h), first (I) and second branchial arches (II) are indicated. Asterisks indicate the outgrowing forelimb buds. (E) LysoTracker Red (LysoT) analysis reveals the massive and generalized cell death in Hand2 deficient embryos and limb buds at E9.5 (25 somites). a: anterior; d: dorsal; p: posterior; v: ventral.
(6.79 MB TIF)
Clearance of Hand2 transcripts from mutant forelimb buds and specificity of α-Hand2 antibodies. (A) Q-PCR analysis to determine Hand2 transcript levels in wild-type, Hand2 floxed (H2 f), Hand2 heterozygous and Hand2 deficient limb buds at E10.25–10.5 (33–35 somites; n = 6–8). Note that no Hand2 transcripts are detected in Hand2 deficient limb buds. Bars: ±standard deviation. asterisk: P = 0.0009. (B) Immunofluorescense using α-Hand2 antibodies (M-19, Santa Cruz) reveals the specific nuclear localization of Hand2 proteins in posterior (Wt-p) but not anterior (Wt-a) limb buds mesenchymal cells. No specific fluorescence is detected in mesenchymal cells isolated from Hand2 deficient limb buds. (C) Hand2 proteins are cleared from Hand2 deficient limb buds by embryonic day E10.5. Protein extracts were normalized for their vinculin content. (D) Immunoprecipitation (IPP) of Hand2 proteins from E11.0 limb buds. Hand2 proteins are detected by Western blotting. Control: α-IgG. Asterisks indicate the cross-reactivity with the light chains of the IgGs (control and α-Hand2) used for IPP.
(2.42 MB TIF)
Incomplete/delayed inactivation of Hand2 in forelimb buds results in a hypomorphic phenotype. (A) Skeletal preparations of control (Prx1-Cre heterozygous) and Hand2 deficient forelimbs at E14.5. Due to slight variability in Prx1-Cre mediated inactivation of the conditional Hand2 allele in forelimb buds, three classes of skeletal phenotypes are observed. The most hypomorphic phenotype (Weak) results in formation of two misplaced zeugopodal bones, three anterior digits and a hypoplastic digit that resembles digit 4 (indicated by an asterisk). The arrowhead points to the twisted bones of the zeugopod. The less hypomorphic phenotype (Intermediate) results in formation of one zeugopodal bone and two digits. The null phenotype (Strong) is identical to the skeletal phenotypes observed in Shh deficient limb buds (Figure 1A). Asterisks indicate digits with unclear identities. (B) Analysis of Hand2 expression reveals the variable nature of Prx1-Cre mediated inactivation of Hand2 at E9.75 (28 somites). (C) This variability is also apparent when levels of SHH signal transduction are monitored by Gli1 expression at E9.75 (27 somites). Complete absence of Hand2 (B) and Gli1 transcripts (C) was observed in 50% of all Prx1-Cre1, Hand2 deficient limb buds (n = 4/8). The others display varying degrees of Hand2 and Gli1 expression. All limb buds are oriented with the anterior to the top and the posterior to the bottom. (D) Table summarizing the frequencies of the three classes of limb skeletal phenotypes observed in Hand2 mutant forelimbs. This variability is in agreement with the fact, that developmentally slightly later Hand2 inactivation in hindlimb buds results in almost normal Shh expression and limb skeletal development (Figure 2). Taken together, these results indicate that Hand2 needs to be inactivated very early and rapidly during the onset of limb bud development to disrupt establishment of the posterior Shh expression domain.
(3.68 MB TIF)
Activation of 5′HoxD genes and posterior expansion of Gli3 expression in Hand2 deficient limb buds. Hoxd11 expression at E9.75 (27 somites) and E10.75 (36 somites). Expression of Hoxd11 is initiated in limb buds lacking Hand2 (arrowheads), but its up-regulation is disrupted. (B) Hoxd13 expression is initiated, but rapidly down-regulated in Hand2 deficient limb buds (arrowheads E10.5, 33 somites). (C) Gli3 expression is expanded posteriorly in Hand2 deficient limb buds at E10.0 (32 somites; compare white to black arrowhead). In Shh deficient limb buds, Gli3 is not expanded to the posterior margin (compare white to open arrowheads). All limb buds are oriented with the anterior to the top and the posterior to the bottom. (D) Inactivation of Hand2 alters Gli3 protein processing. Protein extracts prepared from limb buds of the indicated genotypes at E10.5 (35 somites) were analyzed by immunoblotting using α-Gli3 antibodies. The full-length Gli3 protein is about 190 kD, while the processed Gli3R isoform is about 83 kD. Note that Gli3R form is more abundant in Hand2 and Shh deficient than in wild-type limb buds. Samples are normalized for their vinculin contents. The asterisk points to an unrelated cross-reacting protein.
(5.95 MB TIF)
The genomic landscape encompassing the mouse ZRS. Scheme depicting part of mouse chromosome 5 (Ensemble: Mus musculus genomic region from position 29621310 to 29662806) analyzed in the ChIP experiments by Q-PCR. The Lmbr1 locus encodes the mouse ZRS (1.67 kb) within intron 4, which is about 800 kb away from the Shh locus. The 6 Ebox elements (1 to 6) located in the ZRS are indicated. The framed orange and blue boxes indicate the 20 kb downstream and upstream flanking regions. These two regions are shown in the enlargements and potential Ebox elements are indicated. Coding exons are represented by filled boxes. Amplicon a is located about 2 kb downstream and amplicon e about 6 kb upstream of the ZRS (the primers used for Q-PCR amplification are indicated by green arrows).
(0.32 MB PDF)
Evidence that Hand2 interacts directly with the Hoxd13 but not Gli3R protein. Co-immunoprecipitation reveals the direct interaction of Hand2 with Hoxd13 in HEK293T cells (Hand2: Flag-epitope tagged; Gli3R: Myc-epitope tagged). In contrast, Gli3R is unable to directly interact with Hand2, but binds to Hoxd13 [12]. Protein extracts were immunoprecipitated (IP) using the following antibodies: α-Flag for Hand2, α-Hoxd13 for Hoxd13, α-Myc for Gli3R and immunoblotted (IB) using the appropriate antibodies.
(2.52 MB TIF)
Morphological defects in limb buds lacking Hand2 and Gli3. (A) The forelimb morphology of double mutant mouse embryos at E14.5. Note the stunted forelimbs and the extreme pre- and post-axial polydactyly in comparison to Gli3 Xt/Xt limb buds. White brackets indicate forelimb length. Asterisks indicate digits with undetermined identities. (B) The massive apoptosis of mesenchymal cells in Hand2 deficient limb buds is suppressed in limb buds lacking both Hand2 and Gli3. Apoptotic cells were detected by TUNEL fluorescence on limb bud sections at E10.25 (33 somites). Sections are oriented with the anterior to the top and posterior to the bottom.
(3.99 MB TIF)
Oligos used for the study. All primers used for genotyping of mice and embryos, Q-PCR analysis of Hand2 transcripts, Q-PCR analysis of the ChIP experiments are listed. Conditions for use are available upon request.
(0.04 MB DOC)
Supporting materials and methods.
(0.08 MB DOC)