Summary
The first step toward light perception is 11-cis to all-trans photoisomerization of the retinaldehyde chromophore in a rod or cone opsin-pigment molecule. Light sensitivity of the opsin pigment is restored through a multistep pathway called the visual cycle, which effects all-trans to 11-cis re-isomerization of the retinoid chromophore. The maximum throughput of the known visual cycle, however, is too slow to explain sustained photosensitivity in bright light. Here, we demonstrate three novel enzymatic activities in cone-dominant ground-squirrel and chicken retinas: an all-trans-retinol isomerase, an 11-cis-retinyl-ester synthase, and an 11-cis-retinol dehydrogenase. Together these activities comprise a novel pathway that regenerates opsin photopigments at a rate 20-fold faster than the known visual cycle. We suggest that this pathway is responsible for sustained daylight vision in vertebrates.
Introduction
Light perception in vertebrates is mediated by two types of photoreceptors, rods and cones. Rods are specialized for vision at night while cones provide high-resolution color vision in daylight. Although cones comprise only about 5% of photoreceptors in the human retina, they are far more important than rods for vision in daylight. With the advent of artificial lighting, people spend most of the time under light conditions where the rod response is saturated and sight is mediated entirely by cones.
Rod and cone photoreceptors contain a light-sensitive structure called the outer segment, comprising a stack of membranous discs (Figure 1A). These discs are loaded with rhodopsin (in rods) or cone opsin visual pigments. The light-absorbing chromophore for both pigment types is 11-cis-retinaldehyde. Absorption of a single photon by a visual pigment molecule results in 11-cis to all-trans isomerization of the chromophore. This induces a conformational change in the opsin protein that activates the visual transduction cascade. Before light sensitivity can be restored to the apo-opsin, all-trans-retinaldehyde must be converted back to 11-cis-retinaldehyde through a multistep pathway called the visual cycle (Figure 1B). Most of what is known about the visual cycle has come from the study of rod-dominant species such as cattle and rodents.
Figure 1. Organization of the Retina and the Known Visual Cycle.
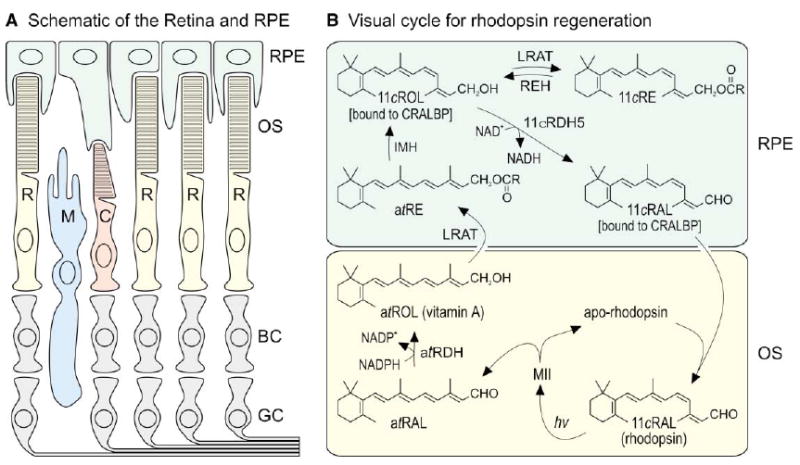
(A) Schematic drawing of a typical vertebrate retina. Rod (R) and cone (C) photoreceptors each contain an outer segment (OS), which is the site of photon capture and the reactions of visual transduction. Adjacent to the outer segments is the retinal pigment epithelium (RPE), which plays a role in the processing of retinoids but is not considered part of the retina. The Müller glial cell (M) may also play a role in retinoid processing. Second-order bipolar cells (BC) and third-order ganglion cells (GC) are shown but not discussed. (B) Visual cycle in rod-dominant retinas. Absorption of a single photon (hv) by a rhodopsin pigment molecule in the rod outer segment induces 11-cis to all-trans isomerization of the 11-cis-retinaldehyde (11cRAL) chromophore to form active metarhodopsin II (MII). MII rapidly decays to yield apo-rhodopsin and free all-trans-retinaldehyde (atRAL). The all-trans-retinaldehyde is reduced to all-trans-retinol (atROL) by all-trans-retinol dehydrogenase (atRDH), which uses NADPH as a cofactor (Lion et al., 1975; Palczewski et al., 1994; Rattner et al., 2000). The all-trans-retinol diffuses across the narrow extracellular space and is taken up by the RPE. Lecithin retinol acyl-transferase (LRAT) catalyzes trans-esterification of a fatty acid from phosphatidylcholine to all-trans-retinol, resulting in formation of an all-trans-retinyl ester (atRE) (Ruiz et al., 1999; Saari and Bredberg, 1989; Shi et al., 1993). Isomerohydrolase (IMH) is postulated to catalyze coupled hydrolysis and isomerization of all-trans-retinyl esters into 11-cis-retinol (11cROL) (Deigner et al., 1989). 11-cis-retinol is oxidized to 11-cis-retinaldehyde by 11-cis-retinol dehydrogenase type-5 (11cRDH5), which uses NAD+ as a cofactor (Lion et al., 1975; Simon et al., 1995). In the dark, under conditions of low 11-cis-retinaldehyde utilization, 11-cis-retinol can also be esterified by LRAT to form 11-cis-retinyl esters (11cRE). Cellular retinaldehyde binding protein (CRALBP) binds 11-cis-retinol and 11-cis-retinaldehyde with high affinity, but not all-trans-retinol (Bunt-Milam and Saari, 1983; Saari and Bredberg, 1987). With chromophore depletion, apo-CRALBP stimulates hydrolysis of 11-cis-retinyl esters by retinyl ester hydrolase (REH) (Stecher et al., 1999). 11-cis-retinaldehyde diffuses across the extracellular space, is taken up by the outer segment, and recombines with apo-rhodopsin to regenerate rhodopsin.
Several lines of evidence suggest that rod and cone photopigments regenerate by different mechanisms. In frog retinas separated from the retinal pigment epithelium (RPE), cone opsin, but not rhodopsin, regenerates spontaneously (Goldstein and Wolf, 1973; Hood and Hock, 1973). After bleaching, isolated salamander cones, but not rods, recover sensitivity with addition of 11-cis-retinol (Jones et al., 1989). Cultured Müller cells isomerize all-trans-retinol to 11-cis-retinol, which they secrete into the medium (Das et al., 1992). Müller cells, in addition to RPE cells, contain cellular retinaldehyde binding protein (CRALBP), which specifically binds 11-cis-retinoids (Bunt-Milam and Saari, 1983; Saari and Bredberg, 1987). These observations imply involvement of Müller cells in the recycling of visual chromophore, and suggest an interaction between Müller cells and cones.
The response kinetics of cones are very different from rods. Cones are several-hundred-fold less sensitive than rods (Pugh and Lamb, 2000). Following light flashes that generate similar membrane currents, cones recover sensitivity approximately 10-fold faster than do rods (Baylor et al., 1979; Perry and McNaughton, 1991). The rod photoresponse is saturated at photoisomerization rates above 500 per second (Baylor et al., 1984). Cones, on the other hand, remain responsive to light at photoisomerization rates up to 1,000,000 per second (Schnapf et al., 1990). Thus, the maximal rate of visual-pigment regeneration is 2000-fold higher in cones than rods.
In the current study, we investigated the mechanism of retinoid recycling in two cone-dominant species, chicken and ground squirrel. We demonstrate three novel catalytic activities in retinas from these species that mediate the processing of visual retinoids. We propose that these activities represent enzymatic steps in a novel visual cycle that provides high-throughput pigment regeneration in cone photoreceptors.
Results
Cone-Dominant Retinas Contain Retinyl Esters
We determined the content of fatty-acyl retinyl esters in light-adapted bovine, mouse, chicken, and ground-squirrel retina and RPE membranes by high-performance liquid chromatography (HPLC) (Weng et al., 1999). 11-cis- and all-trans-retinyl esters were present in chicken (Nicotra et al., 1994; Rodriguez and Tsin, 1989) and ground-squirrel retinas, but were virtually undetectable in retinas from cattle and mice (Figure 2A). In contrast, retinyl esters were higher in RPE from cattle and mice compared to chicken and ground squirrels (Figure 2B). We determined the fatty acid composition of each ester sample by saponification (hydrolysis in alkali) and derivitization of the free fatty acids. Retinyl esters from the retinal tissues contained approximately 25% unsaturated fatty acids while those from the RPE tissues contained predominantly saturated palmitic and stearic acids. These differences reflect the distinct fatty acid compositions of phospholipids from each tissue (Gulcan et al., 1993), and offer evidence that the retinas and RPE were cleanly separated during dissection. Representative chromatograms for retinyl esters from ground-squirrel retinas and authentic retinyl ester standards are shown in Figures 2C and 2D. To confirm our identification of retinyl esters in this and all subsequent experiments, we saponified esters from each tissue and analyzed the resulting retinols in parallel with saponified ester standards by HPLC. As shown for ground squirrel in Figures 2E and 2F, the retinol products of ester hydrolysis were in agreement with the identified intact esters.
Figure 2. Retinyl Esters in Retina and RPE.
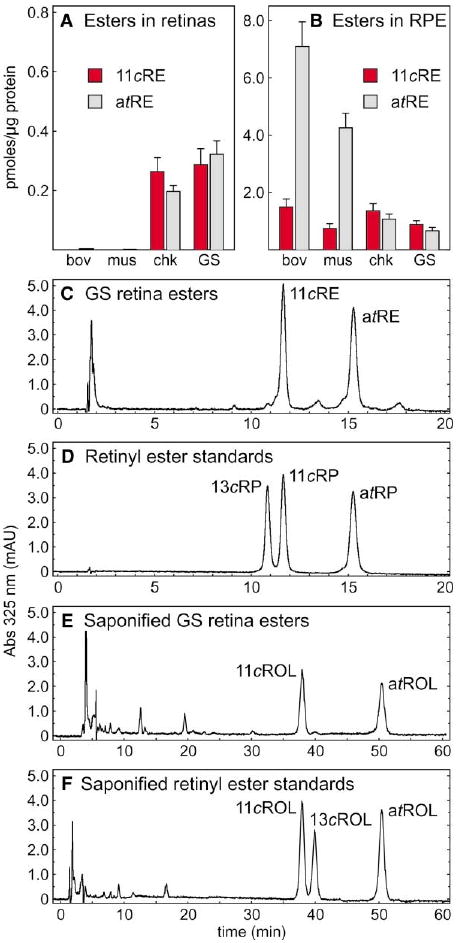
(A) Levels of 11-cis-retinyl esters (11cRE, red bars) and all-trans-retinyl esters (atRE, gray bars) in bovine (bov), mouse (mus), chicken (chk), and ground squirrel (GS) retinas. (B) Levels of 11-cis-retinyl esters and all-trans-retinyl esters in RPE from the four species. Values are shown as pmols per μg protein in microsomal membranes. Error bars show standard deviations (n = 4). (C) Representative HPLC chromatogram of retinyl esters from ground squirrel retina. (D) Representative HPLC chromatogram of the retinyl-ester standards: 13-cis-retinyl palmitate (13cRP), 11-cis-retinyl palmitate (11cRP), and all-trans-retinyl palmitate (atRP). (E) Representative chromatogram of saponified retinyl esters from GS retina showing 11-cis-retinol (11cROL) and all-trans-retinol (atROL). (F) Representative chromatogram of saponified retinyl-ester standards showing 11-cis-retinol, 13-cis-retinol (13cROL), and all-trans-retinol. UV absorption at 325 nm is shown in milliabsorption units (mAU).
Novel Retinyl-Ester Synthase in Chicken and Ground-Squirrel Retinas
We began our investigation into the sources of all-trans-and 11-cis-retinyl esters in ocular tissues by measuring LRAT activity in bovine and mouse RPE membranes. First, RPE microsomes were irradiated with UV light to eliminate endogenous retinoids. Incubation with all-trans-retinol resulted in the rapid formation of all-trans-retinyl esters (Figures 3A and 3B). Retinyl-ester production plateaued after several minutes incubation due to depletion of usable phosphatidylcholine (Saari et al., 1993), which is the fatty-acyl donor for LRAT. No 11-cis-retinyl esters (or 11-cis-retinol) were produced by bovine or mouse RPE membranes from all-trans-retinol substrate during the time course of these reactions. All-trans-retinyl α-bromoacetate (tRBA) is a potent competitive inhibitor of LRAT (Trehan et al., 1990). As expected, addition of tRBA to bovine or mouse RPE membranes blocked formation of all-trans-retinyl esters from all-trans-retinol (Figures 3A and 3B). These data are consistent with the observation that the dominant retinyl-ester synthase in RPE is LRAT. We observed no detectable formation of retinyl esters by bovine or mouse retinal membranes from either all-trans-retinol or 11-cis-retinol substrates.
Figure 3. Retinyl Esters Synthesized by RPE and Retinal Membranes from All-trans-Retinol.
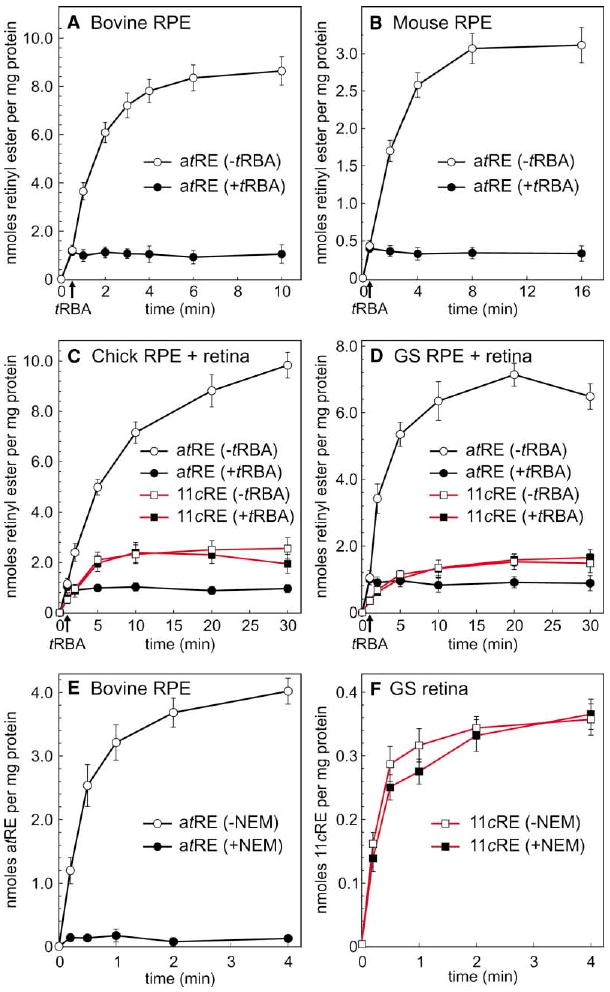
(A) Time course of all-trans-retinyl ester (atRE) synthesis by bovine RPE membranes in the absence (open circles) or presence (filled circles) of tRBA inhibitor. Arrow on the baseline indicates the time of tRBA addition. (B) Time course of all-trans-retinyl ester synthesis by mouse RPE membranes in the absence or presence of tRBA inhibitor. (C) Time course of all-trans-retinyl ester (circles, black lines) or 11-cis-retinyl ester (11cRE, squares, red lines) synthesis by chicken combined RPE + retinal membranes in the absence (open figures) or presence (filled figures) of tRBA inhibitor. (D) Time course of all-trans-retinyl ester or 11-cis-retinyl ester synthesis by ground-squirrel combined RPE + retinal membranes in the absence or presence of tRBA inhibitor. (E) Time course of all-trans-retinyl ester synthesis by bovine RPE membranes plus (open circles) or minus (filled circles) pre-treatment with NEM. (F) Time course of 11-cis-retinyl ester synthesis by ground-squirrel retinal membranes plus (open squares) or minus (filled squares) pre-treatment with NEM. For each experiment, the indicated all-trans- and 11-cis-retinyl esters were the only retinyl esters formed, shown as nmols per mg protein. Error bars show standard deviations (n = 4).
Strikingly different results were obtained with RPE + retinal membranes from chicken or ground squirrel. Membranes from these combined tissues synthesized both all-trans- and 11-cis-retinyl esters from all-trans-retinol substrate (Figures 3C and 3D). Addition of tRBA had no effect on the formation of 11-cis-retinyl esters from all-trans-retinol by these chicken or ground-squirrel membranes. The rate of 11-cis-retinyl-ester synthesis from all-trans-retinol by ground-squirrel RPE + retinal membranes was ∼10-fold slower than the rate of all-trans-retinyl ester synthesis by an equivalent sample of bovine RPE membranes. Prior heating of ground-squirrel or chicken RPE + retinal membranes at 100°C for 2 min abolished the synthesis of 11-cis-retinyl esters, confirming an enzymatic process. These data suggest that chicken and ground-squirrel retinas contain an additional retinyl-ester synthase distinct from LRAT.
Another potent inhibitor of LRAT is N-ethylmaleimide (NEM) (MacDonald and Ong, 1988), which presumably reacts with a cysteine residue in the LRAT catalytic site (Mondal et al., 2000). Preincubation of bovine RPE membranes with NEM completely suppressed synthesis of all-trans-retinyl esters from all-trans-retinol (Figure 3E). Preincubating ground-squirrel retinal membranes with NEM, however, had no effect on the synthesis of 11-cis-retinyl esters from all-trans-retinol (Figure 3F). Synthesis of 11-cis-retinyl esters from all-trans-retinol by chicken retinal membranes was similarly insensitive to NEM (not shown). No all-trans-retinyl esters were synthesized by ground-squirrel or chicken retinal membranes due to the absence of LRAT, which is expressed in RPE but not retina (Fulton and Rando, 1987). These studies provide further evidence for a retinyl-ester synthase in ground-squirrel and chicken retinas, distinct from LRAT, that catalyzes formation of 11-cis- but not all-trans-retinyl esters from all-trans-retinol substrate.
Synthesis of 11-cis-Retinyl Esters by Ground-Squirrel Retinas Is Stimulated by Palmitoyl Coenzyme A
Conversion of all-trans-retinol to 11-cis-retinyl esters involves two chemical transformations: all-trans to 11-cis isomerization, and fatty-acyl esterification. All-trans to 11-cis isomerization of retinol is an endothermic reaction. A possible source of the isomerization energy is ATP hydrolysis. To test this possibility, we added exogenous ATP to reaction mixtures containing all-trans-retinol and chicken or ground-squirrel retinal membranes. No increase in the rate of isomerization was observed with addition of ATP at concentrations up to 10 mM, which rules out ATP as the energy source.
Another potential source of energy is hydrolysis of an activated fatty acid. We assayed membranes from chicken and ground-squirrel retinas for endogenous fatty-acyl esters of coenzyme A (CoA). Palmitoyl (16:0), myristoleoyl (14:1), and stearoyl (18:0) CoA's were present at 940 ± 70, 790 ± 50, and 670 ± 60 pmols per mg protein, respectively. To test for dependence of retinyl-ester synthesis on the presence of fatty-acyl CoA's, we performed a dual-label experiment in which [3H]-all-trans-retinol and [14C]-palmitoyl CoA (palm-CoA) were added to ground-squirrel RPE + retinal membranes. Levels of 11-cis- and all-trans-retinyl esters synthesized during the incubation were determined by UV absorption and [3H]- and [14C]-incorporation following HPLC (Figure 4). Synthesis of 11-cis-retinyl esters increased more than 7-fold with addition of 100 μM palm-CoA (Figures 4A, 4B, and 4G). This increase was accompanied by a 25% reduction in the synthesis of all-trans-retinyl esters, presumably representing competition of the 11-cis-retinyl ester synthase with LRAT for all-trans-retinol substrate. Similar stimulation of 11-cis-retinyl ester synthesis was observed with addition of 100 μM myristoleoyl and stearoyl CoA. No stimulation was observed with addition of acetyl CoA, however. As before, we confirmed our identification of each ester species by saponifying the eluted esters and analyzing the released retinols by HPLC. The radiograms generated by online analysis of [3H]-decays per minute (dpm) were similar to the UV chromatograms. Online analysis of [14C]-dpm showed labeling of 11-cis- but not all-trans-retinyl esters upon addition of [14C]-palm-CoA (Figure 4F). We repeated this experiment using chicken RPE + retina membranes with similar results (not shown). Three conclusions can be drawn from these observations: (1) all-trans to 11-cis isomerization of retinol is dependent on palm-CoA; (2) palm-CoA serves as a fatty-acyl donor for the 11-cis-retinyl ester synthase in chicken and ground-squirrel retinas; and (3) palm-CoA is not an acyl donor for LRAT-mediated synthesis of all-trans-retinyl esters, as previously reported (Saari and Bredberg, 1988). The latter two observations represent further evidence to distinguish the 11-cis-retinyl-ester synthase in cone-dominant retinas from LRAT. Together, the data presented in Figure 4 suggest that all-trans to 11-cis isomerization of retinol is energetically coupled to palm-CoA-dependent synthesis of 11-cis-retinyl esters.
Figure 4. Synthesis of 11-cis-Retinyl Esters by Ground-Squirrel RPE + Retinal Membranes Is Stimulated by Palm-CoA.
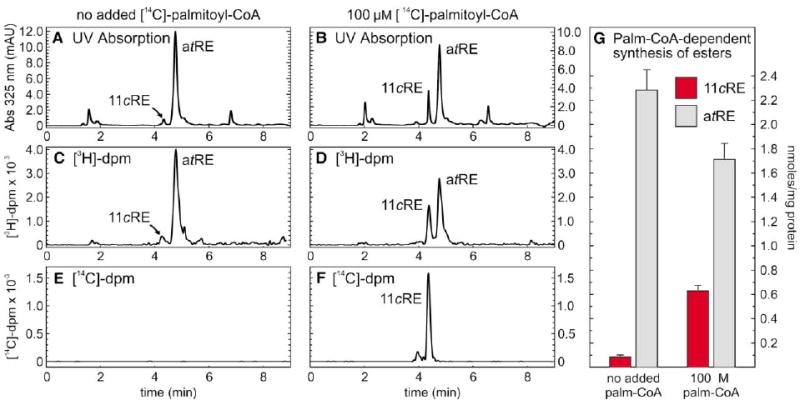
[3H]-all-trans-retinol, plus or minus [14C]-palm-CoA, were added to UV-treated, combined RPE + retinal membranes from ground squirrel. Following incubation for 40 min, extracted retinoids were resolved by HPLC with simultaneous UV and online-radiometric analysis. Chromatograms are shown for 325 nm absorption (A and B), [3H]-decays per minute (dpm) (C and D), and [14C]-dpm (E and F). Note that [14C]-palmitate is incorporated into 11-cis-retinyl esters (11cRE) but not all-trans-retinyl esters (atRE). (G) Histogram showing levels of 11-cis-retinyl esters (red bars) and all-trans-retinyl esters (gray bars) synthesized during a 5 min incubation in the absence or presence of palm-CoA, in nmols per mg protein. Error bars show standard deviations (n = 4).
The Isomerase in Cone-Dominant Retinas Catalyzes Passive Interconversion of All-trans- and 11-cis-Retinol
The data presented in Figures 3 and 4 are consistent with two possible mechanisms for the coupling of retinol isomerization to palm-CoA-dependent ester synthesis. According to the first mechanism, all-trans-retinol is “activated” by palm-CoA to form a high-energy intermediate, which decays to yield 11-cis-retinyl esters in a single catalytic step. According to the second mechanism, passive isomerization of all-trans- to 11-cis-retinol is driven by mass action through subsequent palm-CoA-dependent synthesis of 11-cis-retinyl esters. Both mechanisms are consistent with the observations that no all-trans-retinyl esters and only low levels of 11-cis-retinol are formed during the time course of the reaction. To distinguish between these two possibilities, we tested the effects of apo-CRALBP, with or without added palm-CoA, on the conversion of [3H]-all-trans-retinol to [3H]-11-cis-retinyl esters and [3H]-11-cis-retinol by chicken and ground-squirrel retinal membranes. As discussed, apo-CRALBP binds 11-cis-retinol (and 11-cis-retinaldehyde) but not all-trans-retinol (Bunt-Milam and Saari, 1983; Saari and Bredberg, 1987). If all-trans-retinol is converted to 11-cis-retinyl esters in a single enzymatic step per the first mechanism, we would predict no effect upon addition of apo-CRALBP. However, if passive isomerization of retinol is a distinct enzymatic step from synthesis of 11-cis-retinyl esters, per the second mechanism, we would predict a shift in the equilibrium to favor formation of 11-cis-retinol with addition of apo-CRALBP. The highest levels of 11-cis-retinyl esters were formed with addition of both apo-CRALBP and palm-CoA (Figure 5A). Omission of apo-CRALBP caused a modest reduction in 11-cis-retinyl ester synthesis. Omission of palm-CoA dramatically reduced synthesis of 11-cis-retinyl esters, with our without added apo-CRALBP. Very little 11-cis-retinol was produced in the absence of apo-CRALBP, with or without added palm-CoA (Figure 5B). Addition of apo-CRALBP dramatically increased production of 11-cis-retinol. The highest levels of 11-cis-retinol were produced in the presence of apo-CRALBP and the absence of palm-CoA. We performed a parallel study on retinal membranes from ground squirrel with similar results (not shown). These data indicate that 11-cis-retinol is an intermediate in the conversion of all-trans-retinol to 11-cis-retinyl esters, and suggest that the new isomerase catalyzes the equilibrium between all-trans-retinol and 11-cis-retinol. Retinol isomerization and 11-cis-retinyl-ester synthesis appear to represent distinct enzymatic steps.
Figure 5. Effects of apo-CRALBP on the Synthesis of 11-cis-Retinyl Esters and 11-cis-Retinol by Chicken Retinal Membranes.
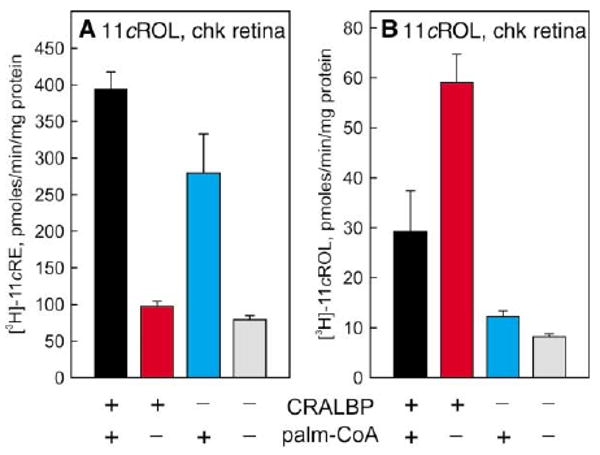
(A) Synthesis of [3H]-11-cis-retinyl esters (11cRE) from [3H]-all-trans-retinol by chicken retinal membranes in the presence of 30 μM apo-CRALBP (black and red bars), 100 μM palm-CoA (black and cyan bars), or absence of both (gray bar). Shown as specific activities (pmols [3H]-11-cis-retinyl esters per min per mg protein). (B) Synthesis of [3H]-11-cis-retinol (11cROL) from [3H]-all-trans-retinol by ground-squirrel retinal membranes in the presence of apo-CRALBP, palm-CoA, or absence of both. Shown as specific activities (pmols [3H]-11-cis-retinol per min per mg protein). Error bars show standard deviations (n = 4). Note the palm-CoA dependence of 11-cis-retinyl ester synthesis and the apo-CRALBP dependence of 11-cis-retinol synthesis.
Isomerohydrolase Activity in Chicken RPE
To compare the maximum turnover rates of all-trans-retinol isomerase in cone-dominant retinas and isomerohydrolase in RPE, we performed kinetic analysis of isomerohydrolase activity in microsomes from chicken RPE at increasing concentrations of all-trans-retinyl-ester substrate. Vmax for chicken isomerohydrolase was 45 pmol/min/mg protein, which is in good agreement with the published Vmax of 44.3 pmol/min/mg for isomerohydrolase in bovine RPE microsomes (Winston and Rando, 1998). The KM's were 3.91 μM for chicken and 3.66 μM all-trans-retinyl ester for bovine isomerohydrolase in RPE microsomes. We also performed kinetic analysis of isomerohydrolase activity in total chicken RPE membranes. Here, Vmax was 18.5 pmol/min/mg. The concentration of protein in total membranes was 1.5 mg per chicken RPE.
Novel 11-cis-Retinol Dehydrogenase Activity in Cone-Dominant Retinas
Isolated cones regenerate opsin pigment upon addition of 11-cis-retinol (Jones et al., 1989), suggesting that these photoreceptors can oxidize 11-cis-retinol to 11-cis-retinaldehyde. To test this possibility, we assayed microsomes prepared from ground-squirrel and chicken retinas for 11-cis-retinol dehydrogenase activity. Microsomes from both cone-dominant species contained an 11-cis-retinol dehydrogenase with a preference for NADP+ cofactor (Figures 6A and 6B). In contrast, the major 11-cis-retinol dehydrogenase in RPE (11-cis-retinol dehydrogenase type-5) uses NAD+ as a cofactor (Jang et al., 2000). To characterize further the 11-cis-retinol dehydrogenase in cone-dominant retinas, we assayed the activities with stereospecifically labeled [3H]-11-cis-retinol or [3H]-NADPH. The enzymes showed high specificity for pro-S-11-cis-retinol (Figure 6C) and pro-S-NADPH substrates (Figure 6D). To confirm the dinucleotide specificity, we also assayed the 11-cis-retinol dehydrogenase from ground-squirrel and chicken retinal membranes in the “forward” direction (conversion of 11-cis-retinol to 11-cis-retinaldehyde). Here, we observed similar specificity for NADP+/NADPH (not shown).
Figure 6. 11-cis-Retinol Dehydrogenase Activity in Ground-Squirrel and Chicken Retinas.
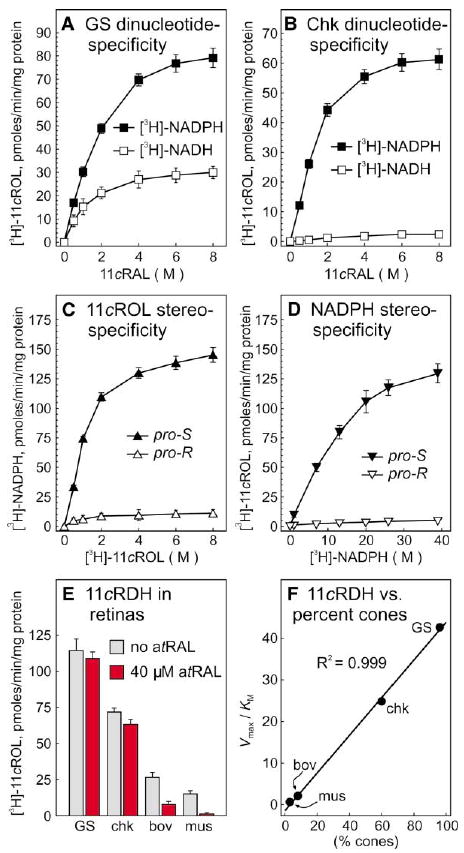
Microsomal membranes from ground squirrel (A) and chicken (B) retinas were assayed for the capacity to reduce 11-cis-retinaldehyde (11cRAL) to [3H]-11-cis-retinol (11cROL) in the presence of [3H]-NADH (open squares) or [3H]-NADPH (filled squares). 11-cis-retinol dehydrogenase (11cRDH) activity is expressed in pmols [3H]-11-cis-retinol per min per mg protein. (C) Ground-squirrel retinal microsomes were assayed for the capacity to synthesize [3H]-NADPH from pro-R- or pro-S-[3H]-11-cis-retinol plus unlabeled NADP+. (D) Ground-squirrel retinal microsomes were assayed for the capacity to synthesize [3H]-11-cis-retinol from pro-R- or pro-S-[3H]-NADPH. (E) Microsomes from ground squirrel (GS), chicken (chk), bovine (bov), and mouse (mus) retinas were assayed for the capacity to synthesize [3H]-11-cis-retinol from 11-cis-retinaldehyde and [3H]-NADPH in the absence (gray bars) or presence (red bars) of 40 μM all-trans-retinaldehyde (atRAL). Error bars show standard deviations (n = 4). (F) Derived Vmax/KM (catalytic efficiencies) for 11-cis-retinol dehydrogenase in mouse, bovine, chicken, and ground-squirrel retinas are plotted against the percentage of cone photoreceptors in each species. Linear regression analysis revealed a correlation coefficient (R2) of 0.999.
Members of the short-chain dehydrogenase (SDR) family, which includes the retinol dehydrogenases, exhibit low specificity with regards to alcohol substrate. To exclude the possibility that the 11-cis-retinol dehydrogenase activity observed in retinas is due to the promiscuous use of 11-cis-retinaldehyde substrate by another SDR, we added all-trans-retinaldehyde to the reaction mixtures at 40 μM, approximately ten times the Km for 11-cis-retinaldehyde. Under these assay conditions, nonspecific utilization of 11-cis-retinaldehyde would be inhibited by the excess all-trans-retinaldehyde. No inhibition would be expected, however, of a dehydrogenase with true 11-cis-retinol/11-cis-retinaldehyde specificity. The 11-cis-retinol dehydrogenase activities in bovine and mouse retinal microsomes were significantly suppressed under these stringent assay conditions (Figure 6E). Only slight reductions were seen with ground squirrel and chicken. Activities measured under these stringent conditions identify an 11-cis-specific retinol dehydrogenase present in all four retinas.
If this 11-cis-retinol dehydrogenase is present in cones, we would expect the activity in retinal membranes to vary proportionally with the percentage of cone photoreceptors in each animal species. The ratio of cones to rods is 3:97 in mouse (Carter-Dawson and LaVail, 1979), 8:92 in bovine (Krebs and Krebs, 1982), 60:40 in chicken (Meyer and May, 1973), and 96:4 in ground-squirrel (West and Dowling, 1975) retinas. We plotted the “catalytic efficiency” (Vmax/KM) of 11-cis-retinol dehydrogenase assayed under stringent conditions against the percentage of cone photoreceptors in each retinal tissue (Figure 6F). Linear-regression analysis of the data points revealed a correlation coefficient (R2) of 0.999. The tight correlation of enzymatic activity with the percentage of cone photoreceptors in each species suggests that the new 11-cis-retinol dehydrogenase is present in cones, or another cell type that varies proportionally with cone abundance.
We also assayed retinal microsomes for all-trans-retinol dehydrogenase activity. Table 1 shows the kinetic parameters, Vmax and KM, for 11-cis-retinol dehydrogenase and all-trans-retinol dehydrogenase in retinal microsomes from the four species. Higher levels of all-trans-retinol dehydrogenase activity were observed in ground squirrel and chicken compared to bovine and mouse. Interestingly, the Vmax values for the new 11-cis-retinol dehydrogenase and all-trans-retinol dehydrogenase were comparable in the two cone-dominant species. This implies similar rates for the reduction of all-trans-retinaldehyde and oxidation of 11-cis-retinol in cones.
Table 1. Kinetic Parameters for Retinol Dehydrogenases in Retina.
| 11-cis-Retinol Dehydrogenase | All-trans-Retinol Dehydrogenase | |||
|---|---|---|---|---|
| Vmax | KM | Vmax | KM | |
| Ground squirrel | 138 ± 6.3 | 3.1 | 148 ± 8.1 | 6.1 |
| Chicken | 95 ± 4.5 | 2.9 | 104 ± 4.2 | 4.6 |
| Bovine | 9.0 ± 1.7 | 2.2 | 66 ± 3.4 | 2.6 |
| Mouse | 3.0 ± 0.7 | 1.5 | 27 ± 2.5 | 1.0 |
Vmax in pmol/min/mg protein ± standard deviations; KM in μmol/l.
Discussion
In the current study, we have demonstrated three novel catalytic activities in membrane fractions of cone-dominant chicken and ground-squirrel retinas. These include: (1) an isomerase that catalyzes direct interconversion of all-trans-retinol and 11-cis-retinol; (2) a specific 11-cis-retinyl-ester synthase that uses palm-CoA as an acyl donor; and (3) an 11-cis-specific retinol dehydrogenase that uses NADP+ as a cofactor. We propose that these activities represent catalytic steps in a novel visual cycle that serves cones.
An All-trans-Retinol Isomerase in Cone-Dominant Retinas
The isomerase activity observed in ground-squirrel and chicken retinas is distinct from the previously described isomerohydrolase in RPE. While isomerohydrolase uses all-trans-retinyl esters as a substrate (Deigner et al., 1989), the new isomerase uses all-trans-retinol. Isomerization of all-trans-retinol to 11-cis-retinol is accompanied by a free-energy change of +4.1 kcal/mol (Rando and Chang, 1983). In RPE membranes, this energy is thought to come from coupled hydrolysis of the carboxylate ester in all-trans-retinyl esters (ΔG = −5 kcal/mol) (Deigner et al., 1989). Alternatively, it has been suggested that retinol isomerization may be driven by mass action using the exothermic regeneration of rhodopsin as an energy source (Stecher et al., 1999). Neither explanation is valid, however, for the isomerization of retinol described here in cone-dominant retinas. As discussed, the all-trans-retinol isomerase in retinal membranes does not use all-trans-retinyl esters as a substrate and hence cannot utilize the energy of ester hydrolysis to drive the reaction. Recombination of 11-cis-retinaldehyde with apo-rhodopsin to form rhodopsin is virtually irreversible (Defoe and Bok, 1983), which is in contrast to the readily reversible association of 11-cis-retinaldehyde with apo-cone opsin (Fukada et al., 1990; Kefalov et al., 2001). Thus, while regeneration of rhodopsin may be a plausible driving force for all-trans to 11-cis isomerization of rod chromophore, the same cannot be said for the regeneration of cone photopigment. What then is the energy source for all-trans to 11-cis isomerization of retinol in cone-dominant retinal membranes?
Synthesis of 11-cis-retinyl esters from all-trans-retinol by ground-squirrel membranes was strongly stimulated by addition of palm-CoA, which served as the acyl donor for retinyl-ester synthesis (Figure 4). Fatty-acyl esters of CoA were present endogenously in ground-squirrel and chicken retinal membranes at micromolar concentrations, which explains the basal synthesis of 11-cis-retinyl esters with no added palm-CoA. Addition of apo-CRALBP to the reaction mixture strongly stimulated synthesis of 11-cis-retinol (Figure 5). Thus, all-trans to 11-cis isomerization was only observed under conditions where the 11-cis-retinol product was removed from the equilibrium reaction through esterification or binding to apo-CRALBP. The most parsimonious explanation for these data is that the new all-trans-retinol isomerase catalyzes passive isomerization, and that the production of 11-cis-retinoids is driven by mass action.
An 11-cis-Retinyl-Ester Synthase in Retinas
The 11-cis-retinyl-ester synthase described here in chicken and ground-squirrel retinas has catalytic properties that are distinct from the two previously described retinyl-ester synthases. One is LRAT, which has been cloned (Ruiz et al., 1999). LRAT is strongly inhibited by both tRBA and NEM. LRAT generates all-trans-retinyl esters from all-trans-retinol substrate (Saari and Bredberg, 1988). LRAT is present in RPE but has never been observed in retinal tissues. Finally, LRAT uses phosphatidylcholine as a fatty-acyl donor (Shi et al., 1993). In contrast, the new 11-cis-retinyl-ester synthase was not inhibited by tRBA or NEM (Figure 3), synthesized only 11-cis-retinyl esters from all-trans-retinol substrate, was present in retinal membranes, and used palm-CoA as an acyl donor (Figure 4). The second described retinyl-ester synthase is acyl-CoA:retinol acyl-transferase (ARAT) (Saari and Bredberg, 1994). ARAT has not been purified or cloned. Similar to the 11-cis-retinyl-ester synthase in chicken and ground-squirrel retinas, ARAT uses palm-CoA as an acyl donor. However, ARAT is strongly inhibited by 1 mM dithiothreitol (DTT) (Saari and Bredberg, 1994), while assays for 11-cis-retinyl-ester synthase in the current study were done in the presence of 1 mM DTT. Deleting DTT reduced the long-term stability of 11-cis-retinyl-ester synthase but had no effect on its catalytic activity. Also, ARAT readily acylates free all-trans-retinol to form all-trans-retinyl esters (Helgerud et al., 1983), whereas the new 11-cis-retinyl-ester synthase formed only 11-cis-retinyl esters from all-trans-retinol. Together, these observations establish the new 11-cis-retinyl-ester synthase as a novel enzyme, distinct from LRAT and ARAT.
An 11-cis-Retinol Dehydrogenase in Retinas that Utilizes NADP+
11-cis-retinol dehydrogenase activity has never been previously described in retina. We detected an abundant 11-cis-retinol dehydrogenase activity in microsomal membranes from chicken and ground-squirrel retinas (Figure 6). Unlike the major 11-cis-retinol dehydrogenase in RPE (11-cis-retinol dehydrogenase type-5), which uses NAD+ as a cofactor, the 11-cis-retinol dehydrogenase described here used NADP+. A minor 11-cis-retinol dehydrogenase activity that uses NADP+ and exhibits similar pro-S stereospecificity for both 11-cis-retinol and dinucleotide substrates has been observed in RPE from rdh5−/− knockout mice (Jang et al., 2001). This may represent the same enzyme in a different tissue, or contamination of rdh5−/− RPE with outer segments. We observed a tight correlation between the catalytic efficiency of 11-cis-retinol dehydrogenase and the percentage of cone photoreceptors in each retinal tissue (Figure 6F). These observations suggest that this NADP+-specific 11-cis-retinol dehydrogenase is present in cones of both rod- and cone-dominant species.
We also assayed for all-trans-retinol dehydrogenase in retinal microsomes from the same four species. Although all-trans-retinol dehydrogenase is present in both rods and cones (Rattner et al., 2000), we observed significantly higher Vmax values in the cone-dominant compared to the rod-dominant species (Table 1), suggesting that cones can reduce all-trans-retinaldehyde at a higher maximal rate than rods. These data agree with the observed faster onset of all-trans-retinol fluorescence in isolated salamander cones compared to rods following a laser photobleach (Cornwall et al., 2000). Cones recover sensitivity following a photobleach much faster than do rods. The etiology of this difference in response kinetics is undoubtedly complex. However, one factor may be faster clearance of all-trans-retinaldehyde in cones, as suggested by the higher Vmax values for all-trans-retinol dehydrogenase in cone-dominant retinas.
An Alternate Visual Cycle that Mediates Pigment Regeneration in Cones
The data presented in the current study can be integrated by the hypothesized visual cycle presented in Figure 7. According to this model, the all-trans-retinol isomerase and 11-cis-retinyl-ester synthase are in Müller cells. This explains the previous observation that cultured Müller cells take up all-trans-retinol and release 11-cis-retinol into the medium (Das et al., 1992). We observed similar all-trans-retinol isomerase and 11-cis-retinyl-ester synthase activities in immortalized human Müller cells (Limb et al., 2002), consistent with the hypothesized cellular localization of these enzymes (N.L.M. and G.H.T., unpublished observations). The model predicts further that the new 11-cis-retinol dehydrogenase is present in cones, explaining the previous observation that isolated cones, but not rods, recover light sensitivity following a photobleach with addition of 11-cis-retinol (Jones et al., 1989). The likely source of substrate for the isomerase in Müller cells is all-trans-retinol released by rods and cones during light exposure. Müller cells contain the retinoid binding proteins, CRALBP (Bunt-Milam and Saari, 1983) and CRBP (Bok et al., 1984), observations that did not make sense until now.
Figure 7. Proposed Visual Cycle for Regeneration of Cone Opsin.
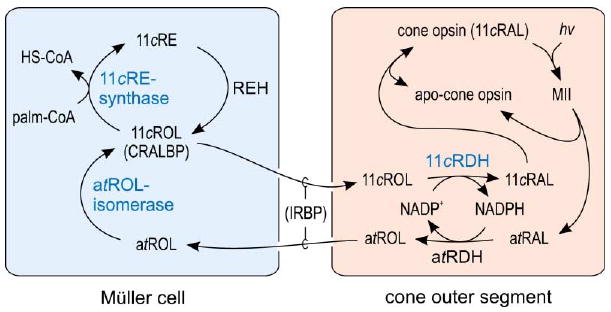
Absorption of a photon (hv) induces 11-cis to all-trans isomerization of the retinaldehyde chromophore, resulting in activated opsin (MII) inside a cone outer segment. Decay of MII releases all-trans-retinaldehyde (atRAL), which is reduced to all-trans-retinol (atROL) by NADPH-dependent all-trans-retinol dehydrogenase (atRDH). all-trans-retinol released into the extracellular space by rods and cones is taken up by Müller cells, which contain the novel all-trans-retinol isomerase activity. This enzyme catalyzes passive isomerization between all-trans-retinol and 11-cis-retinol. Müller cells also contain CRALBP, which binds 11-cis-retinol but not all-trans-retinol (Bunt-Milam and Saari, 1983). Isomerization of all-trans-retinol to 11-cis-retinol (11cROL) is driven by mass-action through the activity of a novel 11-cis-retinyl ester (11cRE) synthase, which catalyzes esterification of 11-cis-retinol using palm-CoA (or other fatty-acyl-CoA's) as an acyl-donor. Retinyl ester hydrolase (REH) is activated by apo-CRALBP to yield 11-cis-retinol, which is released by the Müller cell and taken up by cones. The all-trans-retinol and 11-cis-retinol within the extracellular space in transit between cones and Müller cells are bound to IRBP. Within the cone outer segment is a new NADP+-dependent 11-cis-retinol dehydrogenase (11cRDH), which oxidizes 11-cis-retinol to 11-cis-retinaldehyde. 11-cis-retinaldehyde combines with apo-opsin to regenerate cone opsin pigment. The novel enzymatic activities presented in this paper are displayed in blue.
Kinetics of Retinoid Isomerization for Rods and Cones
Vertebrates are capable of sustained vision under daylight conditions. This capacity requires that the rate of all-trans to 11-cis re-isomerization matches the rate of 11-cis-retinaldehyde photoisomerization under steady-state conditions. Is there a requirement for a supplementary visual cycle, or can the previously described visual cycle in RPE cells (Figure 1B) keep up with photoisomerization under daylight conditions? Cones have been shown to retain light sensitivity at background photoisomerization rates of 1,000,000 per second (Schnapf et al., 1990). This level of illumination roughly corresponds to bright sunshine on a field of snow (Rodieck, 1998). The average density of photoreceptors in an adult chicken retina is 0.15 per μm2 (Meyer and May, 1973). If we assume an average retinal area of 1.0 cm2, we can estimate the total number of photoreceptors in an adult chicken retina as 1.5 × 107. The rate of photoisomerization in the entire retina at this level of illumination is 1.5 × 1013 per second or 9.0 × 1014 per minute. Dividing by Avogadro's number, we can express this rate as 1,500 pmol-photoisomerizations per minute per eye. Can the visual cycle in RPE cells keep up with this rate?
We determined the Vmax for isomerohydrolase in total chicken RPE membranes as 18.5 pmol/min/mg protein. Total membranes from the RPE of one chicken eye contain 1.5 mg protein. Thus, the maximal rate of all-trans to 11-cis isomerization catalyzed by an entire chicken RPE is 28 pmols per minute per eye. This rate is 50-fold slower than the maximal rate required for full pigment regeneration in bright daylight. However, cones operate in bright light with a significant fraction of bleached photopigments (Burkhardt, 1994). Thus, the actual rate of chromophore delivery required by cones is probably lower. Still, the visual cycle in RPE is likely too slow to sustain light sensitivity at high levels of illumination.
A similar calculation can be performed for the hypothesized visual cycle in retinas. Since only cones can use 11-cis-retinol to regenerate photopigment, we must first correct the target rate of photoisomerization to reflect only photoisomerizations in cones. Chicken retinas contain 60% cones, hence 0.6 × 1,500 = 900 pmol-photoisomerizations per minute per eye. The maximal rate of all-trans to 11-cis isomerization by chicken retinal membranes in the presence of palm-CoA and apo-CRALBP was 430 pmol/min/mg protein (Figure 5). The protein content of these membranes was 1.4 mg per chicken retina. Thus, the visual cycle subserving cone-pigment regeneration can sustain isomerization rates of 600 pmols per minute per eye. Although 20-fold faster than the maximal rate of isomerization in RPE from the same species, even the alternate pathway can only provide chromophore at two-thirds the theoretical maximum rate of photoisomerization in brightest daylight. This final difference in isomerization rates may be made up by RGR opsin, which has been shown to effect all-trans-retinaldehyde to 11-cis-retinaldehyde “reverse” photoisomerization at high light intensities (Chen et al., 2001; Hao and Fong, 1999).
Competition for Chromophore between Rods and Cones
Although the all-trans-retinol isomerase and 11-cis-retinyl-ester synthase activities were only observed in the two cone-dominant retinas, we suggest that the alternate visual cycle is also present at lower abundance in the retinas of rod-dominant species. In fact, this pathway may play a more critical role in retinas that contain only a small fraction of cones. The photon capture efficiencies of rods and cones are similar (Gupta and Williams, 1990; Makino et al., 1991). Thus, in a rod-dominant retina under daylight conditions, the vast majority of photoisomerization events contribute nothing to useful vision. However, under these circumstances, cones must compete with an excess of rods for the limited supply 11-cis-retinaldehyde. This competition becomes more critical when we consider that recombination of 11-cis-retinaldehyde with apo-rhodopsin is hugely favored thermodynamically over recombination with apo-cone opsin, as discussed above. Rods thus have a strong tendency to “steal” chromophore from cones. The visual cycle presented here provides a possible mechanism for cones to escape this fierce competition. By expressing 11-cis-retinol dehydrogenase, cones gain access to 11-cis-retinol released by Müller cells. This represents an exclusive source of chromophore precursor for cones, since rods cannot utilize 11-cis-retinol. The local formation of 11-cis-retinaldehyde in cone outer segments would result in high regional concentrations and a short mean diffusion path to apo-cone opsin. These effects would protect cones from “chromophore theft” by rods.
Reciprocal Retinol Oxio-Reduction in Cones
The hypothesized visual cycle offers an additional advantage to cones. Since reduction of all-trans-retinaldehyde and oxidation of 11-cis-retinol must occur with 1:1 stoichiometry, the presence of reciprocal NADP+/NADPH-specific dehydrogenases in cones (Figure 7) affords a self-renewing supply of dinucleotide substrate at all rates of photoisomerization. The similar Vmax values for the 11-cis- and all-trans-retinol dehydrogenases in cone-dominant retinas (Table 1) suggests that these enzymes operate with similar turnover rates in vivo. Eliminating the need for energy-dependent synthesis of NADPH may remove the metabolic “bottleneck” of all-trans-retinaldehyde reduction observed in rods (Saari et al., 1998).
A Novel Role for Interphotoreceptor Retinoid Binding Protein
Interphotoreceptor retinoid binding protein (IRBP) is the major extracellular retinoid binding protein in retinas (Chen and Noy, 1994; Lai et al., 1982). It has been suggested that the primary function of IRBP is to bind all-trans-retinol and 11-cis-retinaldehyde during translocation of these retinoids between rod outer segments and the RPE. If true, we would predict delayed recovery of rhodopsin regeneration following a photobleach in knockout mice that lack IRBP. Rod function, however, is virtually normal in irbp−/− mice (Palczewski et al., 1999). Might IRBP be playing another role? The endogenous ligands of IRBP have been studied. In light-adapted frog and bovine retinas, IRBP contains, in addition to all-trans-retinol, higher levels of 11-cis-retinol than 11-cis-retinaldehyde (Adler and Spencer, 1991; Lin et al., 1989). The mean distance between apical processes of Müller cells and cone outer segments is much greater than the mean distance between apical processes of RPE cells and rod outer segments (Young, 1971). These observations suggest that the critical function of IRBP may not be exchange of all-trans-retinol and 11-cis-retinaldehyde between rods and RPE cells, as previously assumed, but rather exchange of all-trans-retinol and 11-cis-retinol between cones and Müller cells. A prediction of this alternate role for IRBP is reduced cone sensitivity in light-adapted irbp−/− mice.
The number of Müller cells is similar in rod-dominant rat and cone-dominant ground-squirrel retinas (Rasmussen, 1975). Thus, the large observed differences between rod- and cone-dominant species in all-trans-retinol isomerase and 11-cis-retinyl-ester synthase activities are not correlated with the number of Müller cells in each retina. Instead, we suggest that these activities are induced by the presence of cones. Utilization of 11-cis-retinol may stimulate expression of the pathway.
Summary
We present evidence in this paper for an alternate visual cycle that augments pigment regeneration in cones. Analysis of retinoid turnover reveals that the previously described visual cycle in RPE cells my be too slow to keep up with chromophore demand under daylight conditions. In contrast, the retinoid pathway presented here is capable of keeping up with cone photoisomerization in all but the brightest of light conditions. The new enzymatic activities that define this pathway were observed in two phylogenetically divergent cone-dominant species. This suggests that these activities are correlated with a high percentage of cones and are not a peculiarity of a particular animal species. We suggest that this pathway permits sustained vision in vertebrates under daylight conditions.
Experimental Procedures
Tissue Dissection and Preparation
Retinas and RPE were dissected from fresh bovine, mouse, chicken, and ground-squirrel eyes in PBS on ice. For combined RPE + retina preparations, the retina and RPE were removed together from the sclera of sectioned eyeballs. Dissected tissues were centrifuged at 10,000 × g, 10 min and the pellets disrupted in a glass-to-glass homogenizer in 25 mM Tris-HCl (pH 7.2), 1 mM EDTA, 1 mM DTT, 10 μM leupeptin, and 250 mM sucrose. Total membrane fractions were prepared from the tissue homogenates by centrifugation at 125,000 × g, 60 min. Microsomal-enriched fractions were prepared by differential centrifugation, as described (Mata et al., 1992). Membrane proteins were suspended in 10 mM Tris-HCl (pH 7.5), 2 mM CaCl2, 2 mM MgCl2, 1 μM leupeptin, 1 mM DTT (protein buffer), and stored at −80°C.
UV Treatment
Membrane fractions (2–3 mg protein per ml) in 500 μl aliquots were irradiated for 2–5 min in quartz cuvettes on ice with 365 nm light from a Spectroline Model EN-140L UV light source to destroy endogenous retinoids.
Preparation of Apo-CRALBP
Recombinant apo-CRALBP was expressed in E. coli and purified to homogeneity by anion exchange (DEAE) or Ni2+ affinity chromatography, as described (Crabb et al., 1998).
Analysis of Endogenous Retinoids
Retinoids were extracted from 1–3 mg of microsomal protein, as described (Saari and Bredberg, 1988; Weng et al., 1999). Samples were analyzed on an Agilent 1100 series liquid chromatograph equipped with a photo-diode array detector. Spectral data (450–210 nm) were acquired for all eluted peaks. Retinyl-ester fractions were chromatographed on a Supelcosil LC-Si column (4.6 × 250 mm, 5 μm) using 0.3% ethyl acetate in hexane at a flow rate of 1 ml/min. Retinols were chromatographed on an identical column using 4% dioxane in hexane at 1 ml/min. Identity of the eluted peaks was established spectrally and by co-elution with authentic retinoid standards. The identity of the retinyl-ester isomers was further confirmed by saponification of the retinyl-ester peaks in 2% KOH in methanol and re-analysis in the retinol mobile phase. Quantitation was performed by comparison of sample peak areas to calibration curves established with authentic retinoid standards.
Analysis of In Vitro Synthesized Retinoids
Analysis of [3H]- and [14C]-labeled retinoids was performed on the same HPLC system with an online flow scintillation analyzer (Packard 505TR) to monitor radioactivity in the eluted peaks. The instrument was configured to monitor [3H]- and [14C]-labeled compounds simultaneously using the energy ranges 0–15 keV and 18–256 keV, respectively. In these analyses, cis and trans isomers of retinyl esters, retinaldehydes, and retinols were separated on a Microsorb Si column (4.6 × 250 mm, 5 μm) during gradient elution (0.2%–10% dioxane in hexane at 2 ml/min). Quantitation was performed as described above using either sample peak area units or peak height dpm and the appropriate calibration curve.
Endogenous Fatty-acyl CoA's
Fatty-acyl esters of CoA were extracted and purified from ground-squirrel and chicken retinal membranes according to described procedures (Deutsch et al., 1994; Tardi et al., 1992). Analysis of the eluted acyl-CoA esters was performed by HPLC on a 4.6 × 150 mm Source 5RPC ST column (Amersham Pharmacia Biotech) using a gradient elution system consisting of 25 mM KH2PO4 (pH 4.9), and acetonitrile, flow = 1 ml/min. The column was equilibrated with 80% 25 mM KH2PO4/20% acetonitrile. The proportion of acetonitrile was then increased to 45% over 8 min, then to 60% over the next 22 min followed by re-equilibration to the initial starting conditions over 2 min. Three to four sample extracts were independently analyzed and data were quantified by relating sample peak area to calibration curves established with authentic acyl CoA esters.
Enzyme Assays
LRAT
In vitro synthesis of retinyl esters by RPE membranes was determined following published procedures (Saari and Bredberg, 1988, 1989). For the LRAT inhibitor studies, tRBA was synthesized (Mata et al., 1992) and added at 10 μM to retinyl-ester synthesis reaction mixtures in 1 μl of DMF 1 min after addition of [3H]-all-trans-retinol. To determine the effects of NEM on retinyl-ester synthesis, membrane proteins (100 μg/ml) were treated with either NEM (5 μM) in dimethylsulfoxide (DMSO) or DMSO alone for 60 min at 4°C on a nutator. Membrane proteins were separated from the unreacted NEM and DMSO by several centrifugations (250,000 × g, 30 min) and rehomogenization of the pellets in protein buffer. The washed membrane proteins at 350 μg/ml were used in retinyl ester synthesis assays.
All-trans-Retinol Isomerase
Production of 11-cis-retinyl esters from all-trans-retinol by chicken or ground-squirrel membranes was monitored in the presence or absence of palm-CoA. Reaction mixtures consisted of 100 mM Tris-HCL (pH 8.0), 1 mM DTT, 2 mM CaCl2, 2 mM MgCl2, 1% BSA, and protein to give a final concentration of 0.25–0.50 mg/ml (final volume = 988 μl). Samples were pre-incubated at 37°C for 2 min and [14C]-palm-CoA (100 μM, 40,000 dpm/nmol) was added in 10 μl of 25 mM NaOAc (pH 6.5) followed by [3H]-all-trans-retinol (10 μM; 1–2 × 105 dpm/nmol) in 2 μl DMF. Reactions were quenched and the products were extracted as described above. In some experiments, 30 μM apo-CRALBP was included in the assay buffer in the absence and presence of palm-CoA.
Isomerohydrolase
The assay for production of 11-cis-retinol was performed as previously described (Winston and Rando, 1998) with modifications to determine the apparent kinetic constants at different concentrations of in situ synthesized all-trans-retinyl-ester substrate. Retinoids were extracted and analyzed by HPLC as described above. Values for Vmax and Km were obtained by secondary transformation (Eadie-Hofstee) of the calculated specific activities.
Retinol Dehydrogenase
Stereospecifically labeled [3H]-dinucleotide and [3H]-retinol substrates were prepared as described (Cideciyan et al., 2000; Jang et al., 2000). Retinol dehydrogenase assays were performed on microsomal membranes from the indicated retinal tissues, as described (Jang et al., 2000). Dinucleotide substrate specificities were determined by monitoring formation of [3H]-11-cis-retinol during incubations of 11-cis-retinaldehyde (0–8 μM) with either [3H]-NADH or [3H]-NADPH (40 μM). Dinucleotide stereospecificities were determined by monitoring formation of [3H]-11-cis-retinol during incubations of 11-cis-retinaldehyde (60 μM) with either pro-S- or pro-R-[3H]-NADPH (0–40 μM). Retinoid stereospecificities were determined by monitoring formation of [3H]-NADPH during incubation of NADP+ (40 μM) with either pro-S- or pro-R-[3H]-11-cis-retinol (0–8 μM). Specificities of the 11-cis-retinol dehydrogenase reactions were determined as described above with addition of all-trans-retinaldehyde (40 μM) as a competing substrate.
Acknowledgments
We thank Jung Lee and Tam Bui for their outstanding technical support. We thank Sanjoy Bhattacharya and John Crabb for their gift of a plasmid that expresses CRALBP. We thank Rosalie Crouch for her gift of 11-cis-retinaldehyde. We thank Dean Bok, Catherine Kaschula, and Garen Vartanian for their valuable comments on the manuscript. This work is supported by grants from the National Eye Institute, the Foundation Fighting Blindness, the Ruth & Milton Steinbach Fund, and the Macula Vision Research Foundation. G.H.T. is the Charles Kenneth Feldman and Jules & Doris Stein Research to Prevent Blindness Professor.
References
- Adler AJ, Spencer SA. Effect of light on endogenous ligands carried by interphotoreceptor retinoid-binding protein. Exp Eye Res. 1991;53:337–346. doi: 10.1016/0014-4835(91)90239-b. [DOI] [PubMed] [Google Scholar]
- Baylor DA, Lamb TD, Yau KW. The membrane current of single rod outer segments. J Physiol. 1979;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Nunn BJ, Schnapf JL. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok D, Ong DE, Chytil F. Immunocytochemical localization of cellular retinol binding protein in the rat retina. Invest Ophthalmol Vis Sci. 1984;25:877–883. [PubMed] [Google Scholar]
- Bunt-Milam AH, Saari JC. Immunocytochemical localization of two retinoid-binding proteins in vertebrate retina. J Cell Biol. 1983;97:703–712. doi: 10.1083/jcb.97.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt DA. Light adaptation and photopigment bleaching in cone photoreceptors in situ in the retina of the turtle. J Neurosci. 1994;14:1091–1105. doi: 10.1523/JNEUROSCI.14-03-01091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J Comp Neurol. 1979;188:245–262. doi: 10.1002/cne.901880204. [DOI] [PubMed] [Google Scholar]
- Chen Y, Noy N. Retinoid specificity of interphotoreceptor retinoid-binding protein. Biochemistry. 1994;33:10658–10665. doi: 10.1021/bi00201a013. [DOI] [PubMed] [Google Scholar]
- Chen P, Hao WS, Rife L, Wang XP, Shen DW, Chen J, Ogden T, Van Boemel GB, Wu LY, Yang M, Fong HKW. A photic visual cycle of rhodopsin regeneration is dependent on Rgr. Nat Genet. 2001;28:256–260. doi: 10.1038/90089. [DOI] [PubMed] [Google Scholar]
- Cideciyan AV, Haeseleer F, Fariss RN, Aleman TS, Jang GF, Verlinde CL, Marmor MF, Jacobson SG, Palczewski K. Rod and cone visual cycle consequences of a null mutation in the 11-cis-retinol dehydrogenase gene in man. Vis Neurosci. 2000;17:667–678. doi: 10.1017/s0952523800175029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall MC, Poitry S, Tsacopoulos M, Wiggert B, Crouch RK. Spatial and temporal resolution of retinol fluorescence in salamander rods and cones. Invest Ophthalmol Vis Sci. 2000;41:S598. [Google Scholar]
- Crabb JW, Carlson A, Chen Y, Goldflam S, Intres R, West KA, Hulmes JD, Kapron JT, Luck LA, Horwitz J, Bok D. Structural and functional characterization of recombinant human cellular retinaldehyde-binding protein. Protein Sci. 1998;7:746–757. doi: 10.1002/pro.5560070324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SR, Bhardwaj N, Kjeldbye H, Gouras P. Muller cells of chicken retina synthesize 11-cis-retinol. Biochem J. 1992;285:907–913. doi: 10.1042/bj2850907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defoe DM, Bok D. Rhodopsin chromophore exchanges among opsin molecules in the dark. Invest Ophthalmol Vis Sci. 1983;24:1211–1226. [PubMed] [Google Scholar]
- Deigner PS, Law WC, Canada FJ, Rando RR. Membranes as the energy source in the endergonic transformation of vitamin A to 11-cis-retinol. Science. 1989;244:968–971. doi: 10.1126/science.2727688. [DOI] [PubMed] [Google Scholar]
- Deutsch J, Grange E, Rapoport SI, Purdon AD. Isolation and quantitation of long-chain acyl-coenzyme A esters in brain tissue by solid-phase extraction. Anal Biochem. 1994;220:321–323. doi: 10.1006/abio.1994.1344. [DOI] [PubMed] [Google Scholar]
- Fukada Y, Okano T, Shichida Y, Yoshizawa T, Trehan A, Mead D, Denny M, Asato AE, Liu RS. Comparative study on the chromophore binding sites of rod and red-sensitive cone visual pigments by use of synthetic retinal isomers and analogues. Biochemistry. 1990;29:3133–3140. doi: 10.1021/bi00464a033. [DOI] [PubMed] [Google Scholar]
- Fulton BS, Rando RR. Biosynthesis of 11-cis-retinoids and retinyl esters by bovine pigment epithelium membranes. Biochemistry. 1987;26:7938–7945. doi: 10.1021/bi00398a059. [DOI] [PubMed] [Google Scholar]
- Goldstein EB, Wolf BM. Regeneration of the green-rod pigment in the isolated frog retina. Vision Res. 1973;13:527–534. doi: 10.1016/0042-6989(73)90022-9. [DOI] [PubMed] [Google Scholar]
- Gulcan HG, Alvarez RA, Maude MB, Anderson RE. Lipids of human retina, retinal pigment epithelium, and Bruch's membrane/choroid: comparison of macular and peripheral regions. Invest Ophthalmol Vis Sci. 1993;34:3187–3193. [PubMed] [Google Scholar]
- Gupta BD, Williams TP. Lateral diffusion of visual pigments in toad (Bufo-marinus) rods and in catfish (Ictalurus-punctatus) cones. J Physiol. 1990;430:483–496. doi: 10.1113/jphysiol.1990.sp018303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao WS, Fong HKW. The endogenous chromophore of retinal G protein-coupled receptor opsin from the pigment epithelium. J Biol Chem. 1999;274:6085–6090. doi: 10.1074/jbc.274.10.6085. [DOI] [PubMed] [Google Scholar]
- Helgerud P, Petersen LB, Norum KR. Retinol esterification by microsomes from the mucosa of human small intestine. Evidence for acyl-Coenzyme A retinol acyltransferase activity. J Clin Invest. 1983;71:747–753. doi: 10.1172/JCI110822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood DC, Hock PA. Recovery of cone receptor activity in the frog's isolated retina. Vision Res. 1973;13:1943–1951. doi: 10.1016/0042-6989(73)90065-5. [DOI] [PubMed] [Google Scholar]
- Jang GF, McBee JK, Alekseev AM, Haeseleer F, Palczewski K. Stereoisomeric specificity of the retinoid cycle in the vertebrate retina. J Biol Chem. 2000;275:28128–28138. doi: 10.1074/jbc.M004488200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang GF, Van Hooser JP, Kuksa V, McBee JK, He YG, Janssen JJM, Driessen C, Palczewski K. Characterization of a dehydrogenase activity responsible for oxidation of 11-cis-retinol in the retinal pigment epithelium of mice with a disrupted RDH5 gene - A model for the human hereditary disease fundus albipunctatus. J Biol Chem. 2001;276:32456–32465. doi: 10.1074/jbc.M104949200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GJ, Crouch RK, Wiggert B, Cornwall MC, Chader GJ. Retinoid requirements for recovery of sensitivity after visual-pigment bleaching in isolated photoreceptors. Proc Natl Acad Sci USA. 1989;86:9606–9610. doi: 10.1073/pnas.86.23.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalov VK, Crouch RK, Cornwall MC, Yau KW. Dark exchange of chromophore in amphibian red cones. Invest Ophthalmol Vis Sci. 2001;42:S119. [Google Scholar]
- Krebs W, Krebs IP. Quantitative morphology of the bovine eye. In: Hollyfield JG, editor. The Structure of the Eye. New York: Elsevier Biomedical; 1982. pp. 175–182. [Google Scholar]
- Lai YL, Wiggert B, Liu YP, Chader GJ. Interphotoreceptor retinol-binding proteins: possible transport vehicles between compartments of the retina. Nature. 1982;298:848–849. doi: 10.1038/298848a0. [DOI] [PubMed] [Google Scholar]
- Limb GA, Salt TE, Munro PMG, Moss SE, Khaw PT. In vitro characterization of a spontaneously immortalized human Muller cell line (MIO-M1) Invest Ophthalmol Vis Sci. 2002;43:864–869. [PubMed] [Google Scholar]
- Lin ZS, Fong SL, Bridges CD. Retinoids bound to interstitial retinol-binding protein during light and dark-adaptation. Vision Res. 1989;29:1699–1709. doi: 10.1016/0042-6989(89)90152-1. [DOI] [PubMed] [Google Scholar]
- Lion F, Rotmans JP, Daemen FJ, Bonting SL. Biochemical aspects of the visual process. XXVII. Stereospecificity of ocular retinol dehydrogenases and the visual cycle. Biochim Biophys Acta. 1975;384:283–292. doi: 10.1016/0005-2744(75)90030-3. [DOI] [PubMed] [Google Scholar]
- MacDonald PN, Ong DE. Evidence for a lecithin-retinol acyltransferase activity in the rat small intestine. J Biol Chem. 1988;263:12478–12482. [PubMed] [Google Scholar]
- Makino CL, Taylor WR, Baylor DA. Rapid charge movements and photosensitivity of visual pigments in salamander rods and cones. J Physiol. 1991;442:761–780. doi: 10.1113/jphysiol.1991.sp018818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata NL, Tsin AT, Chambers JP. Hydrolysis of 11-cis- and all-trans-retinyl palmitate by retinal pigment epithelium microsomes. J Biol Chem. 1992;267:9794–9799. [PubMed] [Google Scholar]
- Meyer DB, May HC., Jr The topographical distribution of rods and cones in the adult chicken retina. Exp Eye Res. 1973;17:347–355. doi: 10.1016/0014-4835(73)90244-3. [DOI] [PubMed] [Google Scholar]
- Mondal MS, Ruiz A, Bok D, Rando RR. Lecithin retinol acyltransferase contains cysteine residues essential for catalysis. Biochemistry. 2000;39:5215–5220. doi: 10.1021/bi9929554. [DOI] [PubMed] [Google Scholar]
- Nicotra CMA, Gueli MC, De Luca G, Bono A, Pintaudi AM, Paganini A. Retinoid dynamics in chicken eye during pre- and postnatal development. Mol Cell Biochem. 1994;132:45–55. doi: 10.1007/BF00925674. [DOI] [PubMed] [Google Scholar]
- Palczewski K, Jager S, Buczylko J, Crouch RK, Bredberg DL, Hofmann KP, Asson-Batres MA, Saari JC. Rod outer segment retinol dehydrogenase: substrate specificity and role in phototransduction. Biochemistry. 1994;33:13741–13750. doi: 10.1021/bi00250a027. [DOI] [PubMed] [Google Scholar]
- Palczewski K, Van Hooser JP, Garwin GG, Chen J, Liou GI, Saari JC. Kinetics of visual pigment regeneration in excised mouse eyes and in mice with a targeted disruption of the gene encoding interphotoreceptor retinoid-binding protein or arrestin. Biochemistry. 1999;38:12012–12019. doi: 10.1021/bi990504d. [DOI] [PubMed] [Google Scholar]
- Perry RJ, McNaughton PA. Response properties of cones from the retina of the tiger salamander. J Physiol. 1991;433:561–587. doi: 10.1113/jphysiol.1991.sp018444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh EN, Lamb TD. Phototransduction in vertebrate rods and cones: molecular mechanisms of amplification, recovery and light adaptation. In: Stavenga DG, DeGrip WJ, Pugh EN Jr, editors. Handbook of Biological Physics. Elsevier Science B.V.; 2000. pp. 184–255. [Google Scholar]
- Rando RR, Chang A. Studies on the catalyzed interconversion of vitamin A derivatives. J Am Chem Soc. 1983;105:2879–2882. [Google Scholar]
- Rasmussen KE. A morphometric study of the Muller cell in rods and cone retinas with and without retinal vessels. Exp Eye Res. 1975;20:151–166. doi: 10.1016/0014-4835(75)90153-0. [DOI] [PubMed] [Google Scholar]
- Rattner A, Smallwood PM, Nathans J. Identification and characterization of all-trans-retinol dehydrogenase from photoreceptor outer segments, the visual cycle enzyme that reduces all-trans-retinal to all-trans-retinol. J Biol Chem. 2000;275:11034–11043. doi: 10.1074/jbc.275.15.11034. [DOI] [PubMed] [Google Scholar]
- Rodieck RW. The First Steps in Seeing. Sunderland, MA: Sinauer Associates, Inc.; 1998. [Google Scholar]
- Rodriguez KA, Tsin AT. Retinyl esters in the vertebrate neuroretina. Am J Physiol. 1989;256:R255–R258. doi: 10.1152/ajpregu.1989.256.1.R255. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Winston A, Lim YH, Gilbert BA, Rando RR, Bok D. Molecular and biochemical characterization of lecithin retinol acyltransferase. J Biol Chem. 1999;274:3834–3841. doi: 10.1074/jbc.274.6.3834. [DOI] [PubMed] [Google Scholar]
- Saari JC, Bredberg DL. Photochemistry and stereoselectivity of cellular retinaldehyde-binding protein from bovine retina. J Biol Chem. 1987;262:7618–7622. [PubMed] [Google Scholar]
- Saari JC, Bredberg DL. CoA- and non-CoA-dependent retinol esterification in retinal pigment epithelium. J Biol Chem. 1988;263:8084–8090. [PubMed] [Google Scholar]
- Saari JC, Bredberg DL. Lecithin:retinol acyltransferase in retinal pigment epithelial microsomes. J Biol Chem. 1989;264:8636–8640. [PubMed] [Google Scholar]
- Saari JC, Bredberg DL. Retinoids: From Basic Science to Clinical Applications. Basel, Switzerland: Birkhauser Verlag; 1994. Characterization of acyl-coenzyme A-dependent retinol esterification in bovine retinal pigment epithelium; pp. 43–52. [Google Scholar]
- Saari JC, Bredberg DL, Farrell DF. Retinol esterification in bovine retinal pigment epithelium: reversibility of lecithin:retinol acyltransferase. Biochem J. 1993;291:697–700. doi: 10.1042/bj2910697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saari JC, Garwin GG, Van Hooser JP, Palczewski K. Reduction of all-trans-retinal limits regeneration of visual pigment in mice. Vision Res. 1998;38:1325–1333. doi: 10.1016/s0042-6989(97)00198-3. [DOI] [PubMed] [Google Scholar]
- Schnapf JL, Nunn BJ, Meister M, Baylor DA. Visual transduction in cones of the monkey Macaca fascicularis. J Physiol. 1990;427:681–713. doi: 10.1113/jphysiol.1990.sp018193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YQ, Hubacek I, Rando RR. Kinetic mechanism of lecithin retinol acyl transferase. Biochemistry. 1993;32:1257–1263. doi: 10.1021/bi00056a009. [DOI] [PubMed] [Google Scholar]
- Simon A, Hellman U, Wernstedt C, Eriksson U. The retinal pigment epithelial-specific 11-cis retinol dehydrogenase belongs to the family of short chain alcohol dehydrogenases. J Biol Chem. 1995;270:1107–1112. [PubMed] [Google Scholar]
- Stecher H, Gelb MH, Saari JC, Palczewski K. Preferential release of 11-cis-retinol from retinal pigment epithelial cells in the presence of cellular retinaldehyde-binding protein. J Biol Chem. 1999;274:8577–8585. doi: 10.1074/jbc.274.13.8577. [DOI] [PubMed] [Google Scholar]
- Tardi PG, Mukherjee JJ, Choy PC. The quantitation of long-chain acyl-CoA in mammalian tissue. Lipids. 1992;27:65–67. doi: 10.1007/BF02537062. [DOI] [PubMed] [Google Scholar]
- Trehan A, Canada FJ, Rando RR. Inhibitors of retinyl ester formation also prevent the biosynthesis of 11-cis-retinol. Biochemistry. 1990;29:309–312. doi: 10.1021/bi00454a001. [DOI] [PubMed] [Google Scholar]
- Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- West RW, Dowling JE. Anatomical evidence for cone and rod-like receptors in the gray squirrel, ground squirrel, and prairie dog retinas. J Comp Neurol. 1975;159:439–460. doi: 10.1002/cne.901590402. [DOI] [PubMed] [Google Scholar]
- Winston A, Rando RR. Regulation of isomerohydrolase activity in the visual cycle. Biochemistry. 1998;37:2044–2050. doi: 10.1021/bi971908d. [DOI] [PubMed] [Google Scholar]
- Young RW. Shedding of discs from rod outer segments in the rhesus monkey. J Ultrastruct Res. 1971;34:190–203. doi: 10.1016/s0022-5320(71)90014-1. [DOI] [PubMed] [Google Scholar]


