Abstract
Inhibitory NK cell receptors are recognized as important determinants of NK cell activity in hematopoietic cell transplantation (HCT). The role of activating receptors and their acquisition after HCT are less certain. Therefore, we comprehensively evaluated both inhibitory and activating receptors in 59 patients receiving unrelated donor HCT. NK cell numbers normalized quickly relative to B- and T-cells, however the expression of both inhibitory and activating isoforms of killer immunoglobulin-like receptors (KIRs) was delayed. Most NK cells expressed an immature phenotype during the first six months post HCT; however, we found high expression of activating NKp46 and NKp44 natural cytotoxicity receptors (NCR), and cytotoxicity was preserved. Early reconstituting NK cells from unmanipulated grafts showed lower cytotoxicity than those from T-cell depleted grafts. Differences in NK cell reconstitution had significant effects on clinical outcomes. Patients whose NK cells reconstituted earlier had better survival and lower relapse rates. The best survival group was recipients who possessed HLA-C2 but their donor lacked the cognate activating KIR2DS1. Collectively, our data underscore the clinical relevance of reconstituting NK cells and their activating KIRs and NCRs. In addition to NK cell quantification and genotyping, comprehensive assessment of NK cell functions and phenotypes including activating receptors is essential.
Keywords: Hematopoietic Cell Transplant, Leukemia, NK cell receptors, NK cell reconstitution post SCT.
INTRODUCTION
Allogeneic hematopoietic cell transplantation (HCT) is an established treatment for many hematologic and malignant disorders. Because only about one-third of patients have an HLA-identical sibling donor (MSD), an HLA-matched unrelated donor (URD) is often sought.(1) Due in large part to advancement in HLA typing to a high resolution allelic level and improvement in supportive care, recent studies have demonstrated that the outcomes of URD HCT are as favorable as MSD HCT.(2,3) Broadened indications for transplant and improvement in outcomes have led to increased use of URD HCT. In 2006 over 3200 URD HCTs were performed through the National Marrow Donor Program (NMDP), representing a 10 fold increase over 1990 numbers.(4)
Success of HCT is typically defined as the absence of the primary disease and complete lymphohematopoietic reconstitution. A normal, reconstituted immune system is important for the control of infection, establishment of tolerance, and mediation of graft-versus-leukemia (GVL) effects. Previous studies have provided extensive, valuable information concerning T- and B-cells reconstitution and their effects on HCT outcomes.(5) More recently, studies have shown that reconstituting NK cells may also be important in infection, graft vs. host disease (GVHD), and GVL effects.(6)
NK cell function is regulated by integration of signals provided through numerous activating and inhibitory receptors expressed on its surface.(7) Inhibitory receptors, such as long-tailed killer immunoglobulin-like receptor (KIR) and CD94/NKG2A, have been recognized as important determinant of NK cell activity in HCT.(8) The role of activating receptors, such as short-tailed KIR and natural cytotoxicity receptors (NCR), is less certain. Therefore, we evaluated comprehensively both inhibitory and activating receptors in 59 patients receiving unrelated donor HCT, including the donor NK cell repertoire, and the recipient NK cell reconstitution, natural cytotoxicity, receptor acquisition kinetics, and clinical outcomes.
PATIENTS AND METHODS
Patients
Between 10/1993 and 4/2005, 184 patients with hematologic malignancy received an URD HCT at the St. Jude Children's Research Hospital. Among these 184 patients, 59 were included in this study because donor blood samples were archived and contained viable mononuclear cells. The St. Jude Institutional Review Board (IRB) and the NMDP IRB have approved the use of these archived samples for this study. Their clinical characteristics are listed in Table 1.
Table 1.
Patient characteristics (n=59).
| Median Age (range) | 11 (1.6-24) |
| Male / Female | 36 / 23 |
| Disease | |
| ALL | 23 |
| AML | 19 |
| CML/JMML | 10 |
| MDS | 5 |
| Other | 2 |
| Disease Status | |
| CR1 | 18 |
| CR2/CR3 | 20 |
| Stable/First chronic phase | 12 |
| Relapse/Refractory | 9 |
| Donor | |
| Median age (range) | 35 (19-55) |
| Male / Female | 31 / 28 |
| Graft | |
| T-cell deplete | 40 |
| Unmanipulated | 19 |
| Median cell dose (range) | |
| TNC (108/kg) | 2.03 (0.02-8.5) |
| CD34+ cell (106/kg) | 5.12 (0.18-23.9) |
| CD3+ cell (106/kg) | |
| T-cell deplete | 1.52 (0.37-7.7) |
| Unmanipulated | 49.4 (19.1-100) |
All patients in this study received a myeloablative conditioning before HCT, containing either 12Gy of TBI, 10mg/kg of Thiotepa, and 120mg/kg of Cyclophosphamide, or 14.4 Gy of TBI, 18gm/m2 of Ara-C, and 90mg/kg of Cyclophosphamide. Patients also received either 10mg/kg of rabbit antithymocyte globulin (ATG) or 90mg/kg of horse ATG.
All patient and donor pairs were matched at 6 of 6 HLA-A, -B, and -DRB1 loci at the antigen level. At allelic level, and including HLA-C, 32 patients (54%) were HLA matched at 8 of 8 loci; 14 (24%) in 7 loci; 11 (19%) in 6 loci; 1 (1.5%) in 5 loci; and 1 (1.5%) in 4 loci. Mismatches were identified in 6 patient at HLA-A, 12 at HLA-B, 24 at HLA-C, and 1 at DRB1. GVHD prophylaxis included ATG as described above, T-cell depletion by using either T10B9 complement mediated lysis (n=27) or immunomagnetic CD34+ cell selection (n=13), cyclosporine A beginning on day −2 (n=59), and Methotrexate on a day 1, 3, 6, 11 schedule (n=46). Fourteen patients had grade I acute GvHD, 8 had grade II, and 2 had grade III-IV. Five patients experienced chronic GvHD; only one was extensive.
NK cell assays
Methods for KIR genotyping and phenotyping for quantitative transcript measurement and surface protein expression have been described previously.(9) Briefly, KIR genotyping was performed by PCR using SSP. Phenotyping for surface expression of KIRs was determined by flow cytometric analysis using mAbs. KIR3DL1 was detected by DX9 (BD Immunocytometry Systems), KIR2DL1 by EB6B (Immunotech) and HP-3E4 (BD Immunocytometry Systems), and KIR2DL2/KIR2DL3 by CH-L (BD Pharmingen), GL 183 (Immunotech), and Dx27 (BD Pharmingen). Expression of CD94/NKG2A was measured by HP-3D9 (BD Pharmingen) and Z199 (Beckman Coulter), NKp44 by Z231 (Beckman Coulter), and NKp46 by BAB281 (Beckman Coulter).
Quantification of KIR transcripts was performed by real-time quantitative PCR (RQ-PCR) using the ABI Prism 7700 Sequence Detector Systems (Applied Biosystems) and the SYBR Green I Dye assay chemistry, as suggested by the manufacturer. cDNA was obtained by performing reverse transcription on RNA using OligoDTmers (Invitrogen). PCR reactions were performed with 2.5μl (75ng) of cDNA, 12.5 μl of SYBR GREEN PCR master mix (Applied Biosystems) and 0.5 μM forward and reverse primers in a final reaction volume of 25μl. Primer sets used were as per Uhrberg et al,(10) except for 3DL1. The primer sequence for KIR3DL1 was: 5′ AGGACAAGCCCTTCCTGTCT, and 3′ GGCTCATGTTGAAGCTCTCC. The amplified product is 174bp. Cycling parameters were as previously described.(11) Each of the 4 inhibitory KIR genes, as well as KIR2DS1 and KIR2DS2, were cloned to allow generation of standard curves for each RQ-PCR run.
Cloning of the KIR genes were performed using the TOPO TA Cloning kit from Invitrogen (Carlsbad, CA) as suggested by the manufacturer. Briefly, the purified PCR product was ligated and transformed into One Shot competent cells and plated. PCR from various colonies identified the presence of the desired gene determined by gel electrophoresis. Miniprep from Qiagen (Valencia, CA) was performed using the overnight culture for the desired colony. The resulting product was confirmed with DNA sequencing analysis using Applied Biosystems 3700 DNA Analyzers (Foster City, CA).
The number of lymphocyte subsets was calculated by multiplying the lymphocyte counts to the percentage of CD3+CD56− T cells, CD19+ B cells, or CD3−CD56+ NK cells. Natural cytotoxicity of NK cells was monitored by a standard in vitro europium release method as described previously using K562 cells as targets.(12)
Statistics
NK cell, B cell, and T cell were considered to be normalized at the time when their number exceeded the lower limit of normal for the first time after HSCT. The normal range for each lymphocyte subset was determined by testing 57 healthy children at our institution.
The time-to-recover to the donor-specific level of expression for each NK receptor was assessed using flow cytometry and RQ-PCR analyses. NK-cell receptor expression after HCT was considered to reconstitute to the donor-specific level when the difference in quantity of the receptor was within 50% of the average of the sample obtained from the donor pre-HCT and from the recipient post-HCT, {i.e., if (donor − recipient) ÷ ½(donor + recipient) is <0.5}. This cutoff was estimated from data of identical twins and was used in our previous study.(9) Similar equations were used to assess the time-to-reconstitution for total KIRs, inhibitory KIRs or activating KIRs as a group by summing the total number of NK cells expressing any KIR, the total number of inhibitory KIR transcripts, or the total number of activating KIR transcripts, respectively.
Overall survival was estimated using Kaplan-Meier method and the cumulative incidence of relapse was calculated by considering relapse and death due to other causes as competing events.(13,14) The time during which a patient is at risk of relapse was defined as the period from the date of HCT to the date of relapse, the date of death, or the date of last follow-up, which ever occurs first. Cox regression was be used to assess the hazard function of primary variables including the time-to-normalization of NK cells and donor-recipient KIR-HLA relationship, adjusting for the patient's leukemia risk group. The standard risk category consisted of patients with acute lymphoblastic leukemia (ALL) or acute myelogenous leukemia (AML) in first or second remission and patients with chronic myelogenous leukemia (CML) in first chronic phase.(15) The high-risk category consisted of patients with ALL or AML in third or subsequent remission or in relapse; patients with secondary leukemia; patients with CML other than those with a standard risk; and those with juvenile myelomonocytic leukemia or myelodysplastic syndrome.
RESULTS
Reconstitution of lymphocyte subsets after HCT
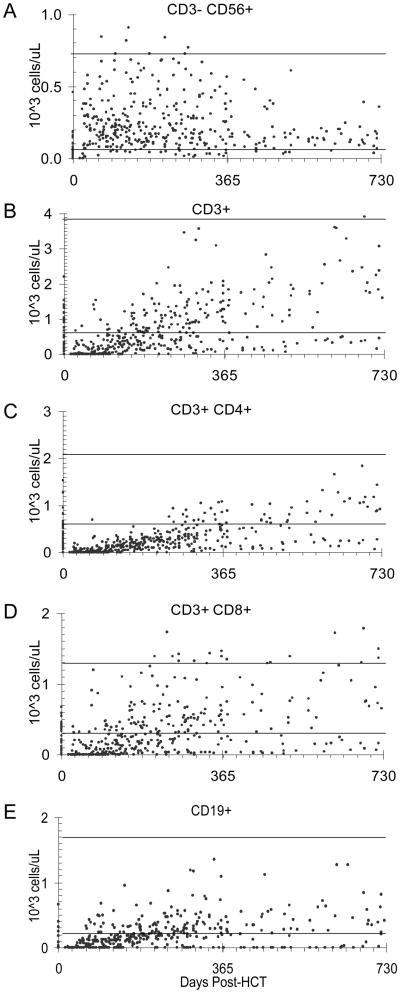
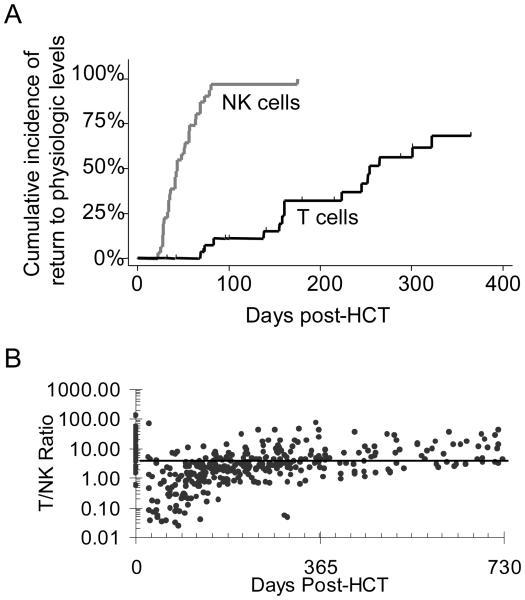
NK cells reconstituted to a normal level much faster than that of T cells and B cells (Figure 1). While 97% of the patients had a normal number of NK cells by day 100 after HCT, only 68% of the patients had a normal number of T cells by 1 year (Figure 2). The T cell to NK cell ratio was generally depressed throughout the first year after HCT. The reconstitution of CD4+ T cells was remarkably slow (Figure 1C).
Figure 1. Reconstitution of lymphocyte subsets.
Multicolor flow cytometry analyses were used for enumeration of (A) CD3−CD56+ NK cells, (B) CD3+ T cells, (C) CD3+CD4+ T cells, (D) CD3+CD8+ T cells, and (E) CD19+ B cells per microliter of blood. The solid lines in each panel represent the upper and lower limits of normal for each lymphocyte subset. The normal range was determined by testing 57 healthy children older than 12 months at our institution.
Figure 2. Comparison of NK-cell and T-cell reconstitution.
(A) Time-to-reconstitution to normal range for NK cells and T cells. (B) T:NK cell ratio. The solid line represent the median T:NK cell ration (5.4) in normal children. The normal range and normal ratio were determined using blood samples from 57 healthy children as described in the legend of Figure 1.
KIR expression after HCT
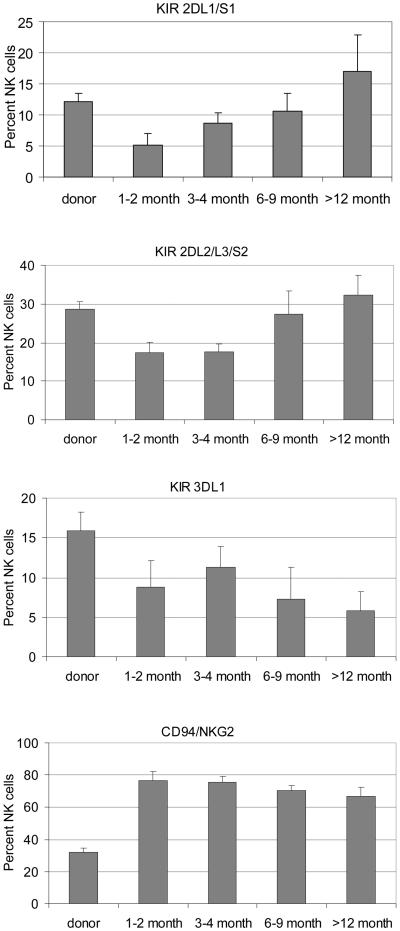
Although NK cell number returned to a normal level quite early, KIR expression on NK cells did not normalize for most patients until one year after HCT, especially that of KIR3DL1 which was the slowest to normalize (Figure 3). During the first 6 months, most of the NK cells were KIR− and CD94/NKG2A+, signifying an immature phenotype.(7) As the percentage of NK cells expressing KIR increase over time in the following 6 months, the percentage of NK cells expressing CD94/NKG2A decreased. However, for most patients, the CD94/NKG2A level remained elevated beyond one year after HCT, even when their KIR level has already normalized. Thus, the time-to-normalization was significantly longer for CD94/NKG2A than for KIRs (54% of the patients required longer than one year to normalize their CD94/NKG2A expression on NK cells, compared with only 12% for KIRs, p<0.006).
Figure 3. Surface expression of KIR and CD94/NKG2A.
Mean percentages of NK cells expressing different KIR genes and CD94/NKG2A as determined by multicolor flow cytometry.
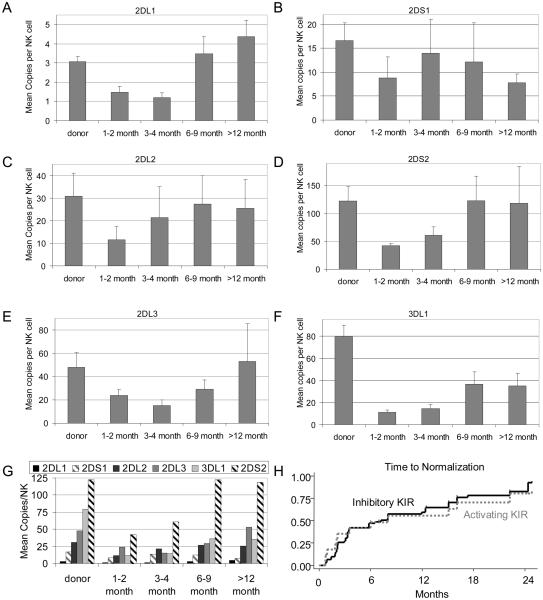
Because the role of activating KIR genes and their expression after HCT have not been elucidated, and the antibodies currently available cannot differentiate inhibitory KIR2DL1 from activating KIR2DS1, and inhibitory KIR2DL2/2DL3 from activating KIR2DS2, we used RQ-PCR to independently evaluate the transcription of inhibitory and activating KIR genes in the reconstituting NK cells (Figure 4). We found that the patterns at the four time-points after HCT were similar between RQ-PCR and flow cytometry analyses. The sum of the RQ-PCR results for KIR2DL1 and KIR2DS1 mimic that of KIR2DL1/2DS1 by flow cytometry. Similarly, those summing the RQ-PCR results for KIR2DL2, KIR2DL3, and KIR2DS2 mimicked that of KIR2DL2/2DL3/2DS1 by flow analyses. All of which showed a pattern of reduced expression during the first 6 months after HCT and then recovery towards normal levels by 1 year, with two notable exceptions: KIR2DS1, which recovered the fastest and was normal in most patients by 4 months with a subsequent decline, and KIR3DL1, which recovered the slowest and was still subnormal by one year. Indeed, the transcript levels for the KIR3DL1 gene were most severely diminished, in agreement with its level of surface expression as measured by flow cytometry. Thus, while the hierarchy-of-abundance for the transcripts of each KIR gene was generally preserved throughout the post-HCT period, KIR3DL1 was the only exception (Figure 4G).
Figure 4. Amount of KIR gene transcripts.
(A-F) The mean number of copies of each KIR gene transcript per NK cell in the donor and in comparison to those in the patient at various time-points after HCT. (G) Relative abundance of KIR gene transcripts over time. (H) Time-to-normalization of inhibitory versus activating KIR genes.
Because it is not known whether the activating and inhibitory families of KIRs recover in parallel after HCT, we compared their tempo of reconstitution to examine their natural balance. Overall, we found that the speed of reconstitution of inhibitory KIR receptors as a group was similar to that of the activating receptors (Figure 4H). The rate of acquisition of each receptor was not influenced by either the donor or the recipient HLA KIR-ligand groups [Data not shown].
Natural cytotoxicity and activating NCR expression after HCT
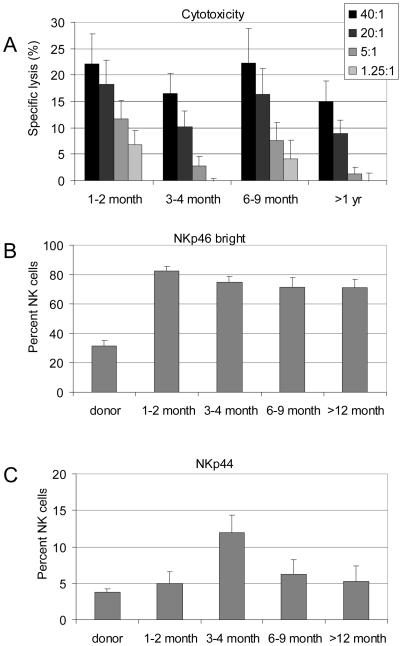
Besides KIR genes, stimulatory NCRs are critical determinant of NK cell activity. As the KIR and CD94/NKG2A phenotypes suggested an immature phenotype in the first year after HCT, concerns were raised on the natural cytotoxicity of these NK cells and their NCR expression. Therefore, we evaluated the natural cytotoxicity against K562 cells and measured the NKp46 levels as it is known that the cytotolytic activity of NK cells against NK-susceptible targets correlated strictly with the NKp46-bright phenotypes.(16) We also measured the NKp44 levels to asses the level of cytokine-activated NK cells, which also contribute to the general cytotoxicity of the NK cell population.(17) Propitiously, we found that although the NK cells possessed an immature phenotype, their cytotoxicity was preserved, as indicated by a normal level of lytic activity, which may be in part related to the high level of NKp46 bright and NKp44 positive NK cells (Figure 5).
Figure 5. Natural cytotoxicity and NCR expression.
(A) Mean cytotoxicity against K562 cells. (B) Mean percentages of NK cells that were NKp46bright, or (C) that were NKp44+, as determined by multicolor flow cytometry.
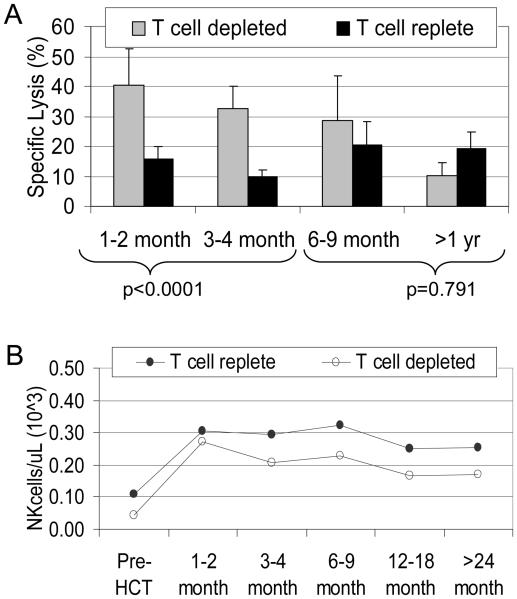
As we have previously demonstrated that T-cell alloreactivity dominated that of NK cells,(18) we sought to investigate the difference in NK cytotoxic function among patients who had received a T-cell depleted versus a T-cell replete graft. We found that although the number of NK cells and their receptor expression were similar in the T-cell replete group at all time-points (all p>0.24), the natural cytotoxicity of NK cells obtained from this group of patients in the first 4 months after HCT was lower than that of patients who had received a T-cell depleted graft (Figure 6).
Figure 6. Comparison of natural cytotoxicity and NK cell numbers between patients who had received a T cell depleted graft versus those with a T cell replete graft.
(A) In the first 4 months post-HCT, patients who received a T cell depleted graft showed a greater degree of natural cytotoxicity than patients who received T cell replete grafts. This difference was statistically significant (p<0.0001). There was no difference between the two graft types after 6 months. There was no difference in the quantitative reconstitution of NK cells in T-cell depleted grafts (-○-) versus unmanipulated grafts (-●-) (p>0.24 on all time points).
Clinical Outcomes
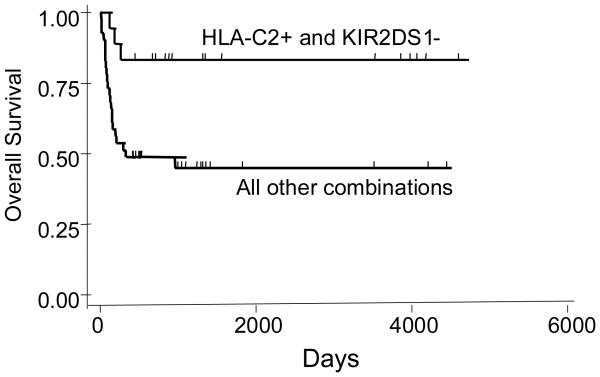
Of the 59 patients, 17 experienced disease relapse, 13 died of transplant complications, and 29 (49%) were surviving disease-free. As expected, outcomes were related to the disease-risk category (Table 2). When analysis was performed on the clinical effect of NK cell reconstitution, patients whose NK cell reconstituted to normal level earlier had a better survival and a lower relapse rate (Table 2). In addition, when each donor KIR gene was analyzed accounting for the presence or absence of its ligand in the recipient, HLA-C interactions were particularly important. Survival was found to be better for those recipients who were positive for HLA-C2 but their donor did not have the corresponding cognate receptor KIR2DS1 (Table 2, Figure 7). Improvement in survival in this group when compared with other patients was related to both lower relapse rate (16.7% vs. 26.8%) and lower transplant-related mortality (0% vs 29.3%). Survival outcomes were not affected by T-cell depletion, degree of donor-recipient HLA match, or a history of GVHD (p values = 0.83, 0.59, 0.16, respectively).
Table 2.
Multivariate analysis of risk factors
| Overall survival |
Leukemia relapse |
|
|---|---|---|
| Factor | Hazard ratio (95% CI), P value | |
| Disease Risk (high vs. low) | 13.2 (1.55 – 112.2), p=0.02 | 5.92 (1.12 – 31.06), p=0.04 |
| Time to normalization of NK cell number (1 mo vs 2+ mo) | 7.75 (1.22 – 49.2 ), p=0.03 | 4.63 (1.01 – 21.6), p=0.05 |
| KIR2DS1−/HLAC2+ vs. others | 0.23 (0.07 – 0.78), p=0.02 | 0.67 (0.32 – 1.39), p=0.28 |
Figure 7. Overall survival.
Patients who were positive for HLA-C group 2 and received a donor graft which was negative for KIR2DS1 had a significantly better survival (p=0.02).
DISCUSSION
With HLA-matched URD HCT for hematologic malignancy, approximately half of the patients are expected to be long-term survivors.(1) Further improvement in patient survival with this frequent HCT procedure will depend on the control of common causes of transplant failure, including infection, graft rejection, GVHD, and leukemia relapse. A vital component to control all these adverse events is an immune system that is effective in eliminating infectious agents and malignant cells on one hand, and in the development of bidirectional tolerance on the other. T cells were the first component of the immune system to be described as an important participant in these effects.(19) For instance, T-cell mediated GvL effect has been known for over 15 years.(20) Although donor T-cell activity is beneficial for the control of infection, leukemia relapse, and graft rejection, overaggressive T-cell alloreactivity may result in GVHD.
Similar to T cells, NK cells are known for their potent activity against virally infected cells and malignant cells.(7) Recently, animal models have also demonstrated that donor NK cells can have graft-facilitating and GVHD-prevention effects, based in part by TGF-β and their alloreactivity against recipient's T cells and dendritic cells.(21,22) Previous investigations in HCT have largely focused on the role of inhibitory receptors (extensively reviewed by Velardi recently).(8) “Missing self” effects have been demonstrated in KIR ligand mismatched allogeneic HCT and inhibitory KIR–HLA receptor–ligand mismatch effects have been observed in both allogeneic and autologous transplants.(9,23-26) High level of inhibitory CD94/NKG2A expression in donor or recipient NK cells was associated with poor transplant outcomes.(27-30) In contrast to inhibitory receptors, relatively little is known about the role of activating receptors such as short-tailed KIRs and NCRs. Therefore, we comprehensively evaluated both inhibitory and activating receptors in our cohort of patients. Using RQ-PCR to independently evaluate the transcription kinetics of inhibitory and activating KIR genes in the reconstituting NK cells, we found that both long-tailed KIRs and short-tailed KIRs typically showed a pattern of reduced expression during the first 6 months after HCT and then recovery towards normal levels by 1 year. We also demonstrated for the first time that the hierarchy-of-abundance of each KIR gene transcript was generally preserved throughout the post-HCT period, and the speed of reconstitution of inhibitory KIR receptors as a group was similar to that of the activating receptors. Thus, our data strongly support the hypothesis that human inhibitory and stimulatory KIR gene usage is tightly coordinated in general, even in the immediate post-transplant period, to maintain an appropriate level of biological responsiveness to normal and abnormal tissues. Future studies are essential to elucidate the molecular mechanisms that control such a tight balance. At the individual family member level, however, KIR2DS1 and KIR3DL1 were notable outliers. KIR2DS1 recovered the fastest and was normal in most patients by 4 months, whereas KIR3DL1 recovered the slowest and was still subnormal out to 12 months. This may be in part related to the differential methylation, direction, and strength of promoters associated with distinct KIR genes.(11,31-34) Recent studies have revealed the presence of several promoter polymorphisms in KIR3DL1, KIR3DS1, KIR2DL5, and KIR2DS4, but not in KIR2DL1 and KIR2DL3.(35) Functionally significant differences in forward and reverse promoter activities were especially observed among the polymorphic promoters of different KIR3DL1 alleles, which affect the binding sites of transcription factors such as YY1, E2F, and Sp1. These biological data are in line with our novel clinical observation that KIR3DL1 reconstitution was distinct from all other KIRs (Figure 4F and G). Our results should be generalizable, as the distribution of KIR3DL1 alleles in our study was representative of the general population, with approximately 20% of the individuals having the KIR3DL1 null phenotype as detected by DX9 antibody, 30% with intermediate staining, and 50% with high level of expression.(36)
Using KIR genotyping alone without expression analyses, previous studies have established an association between transplant outcomes and increasing number of donor activating KIR genes, which in turn is related to the presence of B haplotype that contains more activating KIR genes than the A haplotype that carriers a single activating KIR gene (KIR2DS4).(37) For instance, Cook et el found that donors with more than one activating KIR gene were associated with a reduction in CMV reactivation in MSD transplants.(38) De Santis showed that a greater number of activating KIRs in MUD protected against GVHD and improved survival.(39) Chen et al also demonstrated that additional activating KIR genes in MSD compared with the recipient genotype was associated with lower CMV reactivation, lower transplant-related mortality, and better survival.(40) Schellekens et al, Kroger et al, Bishara et al, and Clausen et al, however, found an increased risk of relapse, GVHD, or non-relapse mortality in patients with allogeneic donor having more activating KIRs.(41-44)
In terms of specific role for each individual activating KIR gene in HCT, Giebel et al and Kim et al have demonstrated in URD and MSD transplants that the presence of donor KIR2DS2 was associated with a high risk of GVHD and non-relapse mortality.(45-46) Yabe et al then went on to show that the association of KIR2DS2 with GVHD in URD transplant was particularly prominent in patients with the cognate C1 ligand and donor KIR ligand mismatch.(47) The detrimental effects of donor KIR2DS2 interaction with recipient C1 ligand has also been observed in an earlier MSD transplant study by Cook et al, in which better survival was seen if the recipients were homozygous for C2 (thus lacking C1 ligand) and the donor was KIR2DS2 negative.(48) Similarly, donor KIR2DS1 interaction with recipient C2 ligand has been shown by Schellekens et al to be associated with lower survival rate in MSD transplants.(41) The presence of donor KIR2DS1, however, might be advantageous if the donor was also positive for KIR2DS2, as demonstrated by Verheyden et al that the presence of these 2 activating KIRs in the donor was associated with a decrease leukemic relapse rate.(49) In vitro, the binding of C2 to KIR2DS1 has been clearly demonstrated, albeit at a lower affinity than C2-KIR2DL1 interaction; in contrast, C1 did not appear to bind to KIR2DS2.(50) Furthermore, NK clone analyses demonstrated evidently the ability of C2 ligand to activate KIR2DS1 dependent INF-gamma production.(51) Collectively, these data suggest a dominant role of C2–KIR2DS1 induction of alloresponse and are in agreement with our clinical findings that C2 positive patients had a better survival when the donor was negative for KIR2DS1. For example, the transplant-related mortality was much lower in these patients when compared with other patients (0% vs. 29%). Mechanistically, our clinical observations of the importance of C2-KIR2DS1 interaction was in corroboration with our laboratory finding that KIR2DS1 was the earliest KIR to reconstitute to normal level. By three to four months after transplantation, our patients had returned to nearly normal levels of donor KIR2DS1 expression. Because the recovery of the other KIR was slower, NK cells in which KIR2DS1 was the singular KIR expressed were prevalent. These data underscore the importance of not only HLA ligand typing or KIR genotyping in the investigation of the clinical effect of KIR mismatch, but also the requirement for direct KIR expression analyses to assess the availability of each KIR gene products for potential HLA interactions.
In addition to activating KIRs, other stimulatory NK cell receptors such as NKG2D, NTBA, and NCRs have been identified.(52) NCR expression, for example, has been shown to be significantly diminished at the time of diagnosis of leukemia and the level of expression correlated with patient outcomes.(53,54) Their role in HCT, however, has not been elucidated. In this study, we found that although reconstituting NK cells generally possessed an immature phenotype, as defined by other investigators as being KIR− CD94/NKG2A+,(7,27-30) their cytotoxicity was preserved, in part related to the high level of NKp46 bright and NKp44 positive NK cells. These data highlight the complexity of NK cell biology in HCT and encourage future comprehensive evaluation of NK cell function, repertoire, and phenotype after HCT, including other poorly characterized activating receptors such as DNAM-1, CD96 and 2B4.(55) In this regard, PVR and Nectin-2, the ligands for DNAM-1 and CD96, have been found to be highly expressed in myeloid leukemia cells.(56)
Besides NK cell determinants, other cellular and soluble factors should be considered. In this regards, we have previously demonstrated that in allogeneic HCT, T-cell alloreactivity dominates that of NK cells alloreactivity in pediatric patients.(18) Although natural cytotoxicity was generally preserved in our cohort as a whole, and the number of NK cells was similar among patients who received a TCD graft versus those who received a T-cell replete graft, the cytotoxicity in the first four months after transplantation was significantly lower in the group that received a T-cell replete graft. This is in agreement with previous studies that showed that NK cells expand and function the best in a severely T-cell lymphopenic environment,(57,58) and that when T-cells had already reconstituted, alloreactive NK cell clones could no longer be detectable.(59) These data provide a mechanistic explanation for the more prominent NK cell effects observed in T-cell depleted transplants as compared to transplants using T-cell replete grafts.(8,18) Besides T cells,(60) other factors including ATG, Campath, or steroid usage,(61-64) donor gender,(65) cytokine and growth factors such as IL12, IL15, IL18, GCSF, or Flt-3 ligand,(9,61,66,67) have been implicated as important predictors of NK activity.
Recently, Savani et al demonstrated that patients with quantitatively higher NK cells at day 30 after MSD transplants had lower myeloid leukemia relapse rates and improved survival.(68) In this study we demonstrated that patients who normalized their NK cell numbers in one month versus those who required two or more months had a reduction in the risk of leukemia relapse by more than fourfold and a reduction in overall mortality by more than 7 fold (Table 2). These data suggest that the tempo of NK cell reconstitution is another important determinant in the outcomes of patients and strongly encourage further investigation of new approaches to hasten NK cell recovery after transplantation. Taken together with our extensive data on both inhibitory and activating NK receptor expression, our study indicated that NK cell reconstitution after MUD HCT was a multifaceted process and has significant clinical implications in future attempts to further improve patient outcomes and prognostications. Novel data were obtained to provide evidence that NK cell quantity, function, phenotype, and tempo of KIR-NCR-NKG2A acquisition were all important measures of NK cell activity. Thus, this study does not only provide fundamental insights into the developmental biology and NK receptor reconstitution after URD transplantation, it also underscores the clinical relevance of comprehensive evaluation of NK cells including activating receptors in multiple transplant settings to optimize patient care and understanding of the role of NK cells in transplant immunology.
ACKNOWLEGEMENT
We would like to thank the NMDP for providing pre-transplant banked patient and donor samples. We would also like to thank Thasia Leming, Xioahua Chen, and James Knowles for their technical assistance.
This work was funded in part by Cancer Center Support (CORE) grant CA21765, by the Assisi Foundation of Memphis, and by the American Lebanese Syrian Associated Charities (ALSAC).
The funding sources had no role in data collection, data analysis, data interpretation, or writing of the report.
Footnotes
BMT and EMH are co-first authors. All work was done at St Jude Children's Research Hospital.
The authors have no financial conflict of interest.
REFERENCE LIST
- 1.Copelan E. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Hongeng S, Krance RA, Bowman LC, Srivastava DK, Cunningham JM, Horwitz EM, et al. Outcomes of transplantation with matched-sibling and unrelated-donor bone marrow in children with leukaemia. Lancet. 1997;350:767–771. doi: 10.1016/S0140-6736(97)03098-5. [DOI] [PubMed] [Google Scholar]
- 3.Yakoub-Agha I, Mesnil F, Kuentz M, Boiron J, Ifrah N, Milpied N, et al. Allogeneic marrow stem-cell transplantation from human leukocyte antigen-identical siblings versus human leukocyte antigen-allelic-matched unrelated donors (10/10) in patients with standard-risk hematology malignancy: a prospective study from the French society of bone marrow transplantation and cell therapy. J Clin Oncol. 2006;24:5695–5702. doi: 10.1200/JCO.2006.08.0952. [DOI] [PubMed] [Google Scholar]
- 4.National marrow donor program Trends in allogeneic transplants. http://www.marrow.org/PHYSICIAN/URD_Search_and_Tx/Number_of_Allogeneic_Tx_Perfor/index.html. Accessed February 6, 2008.
- 5.Auletta J, Lazarus H. Immune restoration following hematopoietic stem cell transplantation: an evolving target. Bone Marrow Transplant. 2005;35:835–857. doi: 10.1038/sj.bmt.1704966. [DOI] [PubMed] [Google Scholar]
- 6.Parham P, McQueen KL. Alloreactive killer cells: hindrance and help for haematopoietic transplants. Nat Rev Immunol. 2003;3:108–122. doi: 10.1038/nri999. [DOI] [PubMed] [Google Scholar]
- 7.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velardi A. Role of KIRs and KIR ligands in hematopoietic transplantation. Curr Opin Immunol. 2008;20:581–587. doi: 10.1016/j.coi.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Leung W, Iyengar R, Turner V, Lang P, Bader P, Conn P, et al. Determinants of antileukemia effects of allogeneic NK cells. J Immunol. 2004;172:644–650. doi: 10.4049/jimmunol.172.1.644. [DOI] [PubMed] [Google Scholar]
- 10.Uhrberg M, Valiante N, Shum B, Shilling H, Lienert-Weidenbach K, Corliss B, et al. Human diversity in killer cell inhibitory receptor genes. Immunity. 1997;7:753–763. doi: 10.1016/s1074-7613(00)80394-5. [DOI] [PubMed] [Google Scholar]
- 11.Leung W, Iyengar R, Triplett B, Turner V, Behm F, Holladay M, et al. Comparison of killer Ig-like receptor genotyping and phenotyping for selection of allogeneic blood stem cell donors. J Immunol. 2005;174:6540–6545. doi: 10.4049/jimmunol.174.10.6540. [DOI] [PubMed] [Google Scholar]
- 12.Blomberg K, Hautala R, Lovgren J, Mukkala V, Lindqvist C, Akerman K. Time-resolved fluorometric assay for natural killer activity using target cells labeled with a fluorescence enhancing ligand. J Immunol Methods. 1996;193:199–206. doi: 10.1016/0022-1759(96)00063-4. [DOI] [PubMed] [Google Scholar]
- 13.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Statist. 1988;16:1141–1154. [Google Scholar]
- 14.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. Wiley, John, and Sons, Inc; New York, New York: 1980. [Google Scholar]
- 15.Leung W, Turner V, Richardson S, Benaim E, Hale G, Horwitz E, et al. Effect of HLA class I or class II incompatibility in pediatric marrow transplantation from unrelated and related donors. Human Immunol. 2001;62:399–407. doi: 10.1016/s0198-8859(01)00220-8. [DOI] [PubMed] [Google Scholar]
- 16.Sivori S, Pende D, Bottino C, Marcenaro E, Pessino A, Biassoni R, et al. NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh of cultured human NK cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. Eur J Immunol. 1999;29:1656–1666. doi: 10.1002/(SICI)1521-4141(199905)29:05<1656::AID-IMMU1656>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 17.Vitale M, Bottino C, Sivori S, Sanseverino L, Castriconi R, Marcenaro E, et al. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J Exp Med. 1998;187:2065–2072. doi: 10.1084/jem.187.12.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowe E, Turner V, Handretinger R, Horwitz E, Benaim E, Hale G, et al. T-cell alloreactivity dominates natural killer cell alloreactivity in minimally T-cell-depleted HLA-non-identical paediatric bone marrow transplantation. Brit J Haem. 2003;123:323–326. doi: 10.1046/j.1365-2141.2003.04604.x. [DOI] [PubMed] [Google Scholar]
- 19.Appelbaum FR. Hematopoietic cell transplantation as immunotherapy. Nature. 2001;411:385–389. doi: 10.1038/35077251. [DOI] [PubMed] [Google Scholar]
- 20.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 21.Murphy WJ, Longo DL. The potential role of NK cells in the separation of graft-versus-tumor effects from graft-versus-host disease after allogeneic bone marrow transplantation. Immunol Rev. 1997;157:167–176. doi: 10.1111/j.1600-065x.1997.tb00981.x. [DOI] [PubMed] [Google Scholar]
- 22.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik W, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 23.Hsu KC, Keever-Taylor CA, Wilton A, Pinto C, Heller G, Arkun K, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105:4878–4884. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu KC, Gooley T, Malkki M, Pinto-Agnello C, Dupont B, Bignon JD, et al. International Histocompatibility Working Group. KIR ligands and prediction of relapse after unrelated donor hematopoietic cell transplantation for hematologic malignancy. Biol Blood Marrow Transplant. 2006;12:828–836. doi: 10.1016/j.bbmt.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Miller JS, Cooley S, Parham P, Farag SS, Verneris MR, McQueen KL, et al. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;109:5058–5061. doi: 10.1182/blood-2007-01-065383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leung W, Handgretinger R, Iyengar R, Turner V, Holladay MS, Hale GA. Inhibitory KIR-HLA receptor-ligand mismatch in autologous haematopoietic stem cell transplantation for solid tumour and lymphoma. Br J Cancer. 2007;97:539–542. doi: 10.1038/sj.bjc.6603913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shilling HG, McQueen KL, Cheng NW, Shizuru JA, Negrin RS, Parham P. Reconstitution of NK cell receptor repertoire following HLA-matched hematopoietic cell transplantation. Blood. 2003;101:3730–3740. doi: 10.1182/blood-2002-08-2568. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen S, Dhedin N, Vernant JP, Kuentz M, Jikakli AA, Rouas-Freiss N, et al. NK-cell reconstitution after haploidentical hematopoietic stem-cell transplantations: immaturity of NK cells and inhibitory effect of NKG2A override GvL effect. Blood. 2005;105:4135–4142. doi: 10.1182/blood-2004-10-4113. [DOI] [PubMed] [Google Scholar]
- 29.Cooley S, McCullar V, Wangen R, Bergemann T, Spellman S, Weisdorf D, et al. KIR reconstitution is altered by T cells in the graft and correlates with clinical outcomes after unrelated donor transplantation. Blood. 2005;106:4370–4376. doi: 10.1182/blood-2005-04-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao XY, Huang XJ, Liu KY, Xu LP, Liu DH. Reconstitution of natural killer cell receptor repertoires after unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation: analyses of CD94:NKG2A and killer immunoglobulin-like receptor expression and their associations with clinical outcome. Biol Blood Marrow Transplant. 2007;13:734–744. doi: 10.1016/j.bbmt.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Santourlidis S, Trompeter HI, Weinhold S, Eisermann B, Meyer KL, Wernet P, et al. Crucial role of DNA methylation in determination of clonally distributed killer cell Ig-like receptor expression patterns in NK cells. J Immunol. 2002;169:4253–4261. doi: 10.4049/jimmunol.169.8.4253. [DOI] [PubMed] [Google Scholar]
- 32.Chan HW, Kurago ZB, Stewart CA, Wilson MJ, Martin MP, Mace BE, et al. DNA methylation maintains allele-specific KIR gene expression in human natural killer cells. J Exp Med. 2003;197:245–255. doi: 10.1084/jem.20021127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trompeter HI, Gómez-Lozano N, Santourlidis S, Eisermann B, Wernet P, Vilches C, et al. Three structurally and functionally divergent kinds of promoters regulate expression of clonally distributed killer cell Ig-like receptors (KIR), of KIR2DL4, and of KIR3DL3. J Immunol. 2005;174:4135–4143. doi: 10.4049/jimmunol.174.7.4135. [DOI] [PubMed] [Google Scholar]
- 34.Davies GE, Locke SM, Wright PW, Li H, Hanson RJ, Miller JS, et al. Identification of bidirectional promoters in the human KIR genes. Genes Immun. 2007;8:245–253. doi: 10.1038/sj.gene.6364381. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Pascal V, Martin MP, Carrington M, Anderson SK. Genetic control of variegated KIR gene expression: polymorphisms of the bi-directional KIR3DL1 promoter are associated with distinct frequencies of gene expression. PLoS Genet. 2008;4:e1000254. doi: 10.1371/journal.pgen.1000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gardiner CM, Guethlein LA, Shilling HG, Pando M, Carr WH, Rajalingam R, et al. Different NK cell surface phenotypes defined by the DX9 antibody are due to KIR3DL1 gene polymorphism. J Immunol. 2001;166:2992–3001. doi: 10.4049/jimmunol.166.5.2992. [DOI] [PubMed] [Google Scholar]
- 37.McQueen KL, Dorighi KM, Guethlein LA, Wong R, Sanjanwala B, Parham P. Donor-recipient combinations of group A and B KIR haplotypes and HLA class I ligand affect the outcome of HLA-matched, sibling donor hematopoietic cell transplantation. Hum Immunol. 2007;68:309–323. doi: 10.1016/j.humimm.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cook M, Briggs D, Craddock C, Mahendra P, Milligan D, Fegan C, et al. Donor KIR genotype has a major influence on the rate of cytomegalovirus reactivation following T-cell replete stem cell transplantation. Blood. 2006;107:1230–1232. doi: 10.1182/blood-2005-03-1039. [DOI] [PubMed] [Google Scholar]
- 39.De Santis D, Bishara A, Witt CS, Nagler A, Brautbar C, Slavin S, et al. Natural killer cell HLA-C epitopes and killer cell immunoglobulin-like receptors both influence outcome of mismatched unrelated donor bone marrow transplants. Tissue Antigens. 2005;65:519–528. doi: 10.1111/j.1399-0039.2005.00396.x. [DOI] [PubMed] [Google Scholar]
- 40.Chen C, Busson M, Rocha V, Appert ML, Lepage V, Dulphy N, et al. Activating KIR genes are associated with CMV reactivation and survival after non-T-cell depleted HLA-identical sibling bone marrow transplantation for malignant disorders. Bone Marrow Transplant. 2006;38:437–444. doi: 10.1038/sj.bmt.1705468. [DOI] [PubMed] [Google Scholar]
- 41.Schellekens J, Rozemuller EH, Petersen EJ, van den Tweel JG, Verdonck LF, Tilanus MG. Activating KIRs exert a crucial role on relapse and overall survival after HLA-identical sibling transplantation. Mol Immunol. 2008;45:2255–2261. doi: 10.1016/j.molimm.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 42.Kröger N, Binder T, Zabelina T, Wolschke C, Schieder H, Renges H, et al. Low number of donor activating killer immunoglobulin-like receptors (KIR) genes but not KIR-ligand mismatch prevents relapse and improves disease-free survival in leukemia patients after in vivo T-cell depleted unrelated stem cell transplantation. Transplantation. 2006;82:1024–1030. doi: 10.1097/01.tp.0000235859.24513.43. [DOI] [PubMed] [Google Scholar]
- 43.Bishara A, De Santis D, Witt CC, Brautbar C, Christiansen FT, Or R, et al. The beneficial role of inhibitory KIR genes of HLA class I NK epitopes in haploidentically mismatched stem cell allografts may be masked by residual donor-alloreactive T cells causing GVHD. Tissue Antigens. 2004;63:204–211. doi: 10.1111/j.0001-2815.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- 44.Clausen J, Wolf D, Petzer AL, Gunsilius E, Schumacher P, Kircher B, et al. Impact of natural killer cell dose and donor killer-cell immunoglobulin-like receptor (KIR) genotype on outcome following human leucocyte antigen-identical haematopoietic stem cell transplantation. Clin Exp Immunol. 2007;148:520–528. doi: 10.1111/j.1365-2249.2007.03360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giebel S, Nowak I, Wojnar J, Markiewicz M, Dziaczkowska J, Wylezol I, et al. Impact of activating killer immunoglobulin-like receptor genotype on outcome of unrelated donor-hematopoietic cell transplantation. Transplant Proc. 2006;38:287–291. doi: 10.1016/j.transproceed.2005.11.091. [DOI] [PubMed] [Google Scholar]
- 46.Kim HJ, Choi Y, Min WS, Kim TG, Cho BS, Kim SY, et al. The activating killer cell immunoglobulin-like receptors as important determinants of acute graft-versus host disease in hematopoietic stem cell transplantation for acute myelogenous leukemia. Transplantation. 2007;84:1082–1091. doi: 10.1097/01.tp.0000285918.72930.35. [DOI] [PubMed] [Google Scholar]
- 47.Yabe T, Matsuo K, Hirayasu K, Kashiwase K, Kawamura-Ishii S, Tanaka H, et al. Japan Marrow Donor Program. Donor killer immunoglobulin-like receptor (KIR) genotype-patient cognate KIR ligand combination and antithymocyte globulin preadministration are critical factors in outcome of HLA-C-KIR ligand-mismatched T cell-replete unrelated bone marrow transplantation. Biol Blood Marrow Transplant. 2008;14:75–87. doi: 10.1016/j.bbmt.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 48.Cook MA, Milligan DW, Fegan CD, Darbyshire PJ, Mahendra P, Craddock CF, et al. The impact of donor KIR and patient HLA-C genotypes on outcome following HLA-identical sibling hematopoietic stem cell transplantation for myeloid leukemia. Blood. 2004;103:1521–1526. doi: 10.1182/blood-2003-02-0438. [DOI] [PubMed] [Google Scholar]
- 49.Verheyden S, Schots R, Duquet W, Demanet C. A defined donor activating natural killer cell receptor genotype protects against leukemic relapse after related HLA-identical stem cell transplantation. Leukemia. 2005;19:1446–1451. doi: 10.1038/sj.leu.2403839. [DOI] [PubMed] [Google Scholar]
- 50.Stewart CA, Laugier-Anfossi F, Vély F, Saulquin X, Riedmuller J, Tisserant A, et al. Recognition of peptide-MHC class I complexes by activating killer immunoglobulin-like receptors. Proc Natl Acad Sci U S A. 2005;102:13224–13229. doi: 10.1073/pnas.0503594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chewning JH, Gudme CN, Hsu KC, Selvakumar A, Dupont B. KIR2DS1-positive NK cells mediate alloresponse against the C2 HLA-KIR ligand group in vitro. J Immunol. 2007;179:854–868. doi: 10.4049/jimmunol.179.2.854. [DOI] [PubMed] [Google Scholar]
- 52.Moretta L, Bottino C, Pende D, Castriconi R, Mingari MC, Moretta A. Surface NK receptors and their ligands on tumor cells. Semin Immunol. 2006;18:151–158. doi: 10.1016/j.smim.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 53.Costello RT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci MJ, Reviron D, et al. Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood. 2002;99:3661–3667. doi: 10.1182/blood.v99.10.3661. [DOI] [PubMed] [Google Scholar]
- 54.Fauriat C, Just-Landi S, Mallet F, Arnoulet C, Sainty D, Olive D, et al. Deficient expression of NCR in NK cells from acute myeloid leukemia: Evolution during leukemia treatment and impact of leukemia cells in NCRdull phenotype induction. Blood. 2007;109:323–330. doi: 10.1182/blood-2005-08-027979. [DOI] [PubMed] [Google Scholar]
- 55.Vitale C, Chiossone L, Morreale G, Lanino E, Cottalasso F, Moretti S, et al. Human natural killer cells undergoing in vivo differentiation after allogeneic bone marrow transplantation: analysis of the surface expression and function of activating NK receptors. Mol Immunol. 2005;42:405–411. doi: 10.1016/j.molimm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 56.Pende D, Spaggiari GM, Marcenaro S, Martini S, Rivera P, Capobianco A, et al. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the Poliovirus receptor (CD155) and Nectin-2 (CD112) Blood. 2005;105:2066–2073. doi: 10.1182/blood-2004-09-3548. [DOI] [PubMed] [Google Scholar]
- 57.Prlic M, Blazar BR, Farrar MA, Jameson SC. In vivo survival and homeostatic proliferation of natural killer cells. J Exp Med. 2003;197:967–976. doi: 10.1084/jem.20021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jamieson AM, Isnard P, Dorfman JR, Coles MC, Raulet DH. Turnover and proliferation of NK cells in steady state and lymphopenic conditions. J Immunol. 2004;172:864–870. doi: 10.4049/jimmunol.172.2.864. [DOI] [PubMed] [Google Scholar]
- 59.Ruggeri L, Capanni M, Casucci M, Volpi I, Tosti A, Perruccio K, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94:333–339. [PubMed] [Google Scholar]
- 60.Nguyen S, Kuentz M, Vernant J-P, Dhedin N, Debre P, et al. Involvement of mature donor T cells in the NK cell reconstitution after haploidentical hematopoietic stem-cell transplantation. Leukemia. 2008;22:344–352. doi: 10.1038/sj.leu.2405041. [DOI] [PubMed] [Google Scholar]
- 61.Leung W, Iyengar R, Leimig T, Holladay MS, Houston J, Handgretinger R. Phenotype and function of human natural killer cells purified by using a clinical-scale immunomagnetic method. Cancer Immunol Immunother. 2005;54:389–394. doi: 10.1007/s00262-004-0609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Juliusson G, Theorin N, Karlsson K, Frödin U, Malm C. Subcutaneous alemtuzumab vs ATG in adjusted conditioning for allogeneic transplantation: influence of Campath dose on lymphoid recovery, mixed chimerism and survival. Bone Marrow Transplant. 2006;37:503–510. doi: 10.1038/sj.bmt.1705263. [DOI] [PubMed] [Google Scholar]
- 63.Penack O, Fischer L, Stroux A, Gentilini C, Nogai A, Muessig A, et al. Serotherapy with thymoglobulin and alemtuzumab differentially influences frequency and function of natural killer cells after allogeneic stem cell transplantation. Bone Marrow Transplant. 2008;41:377–383. doi: 10.1038/sj.bmt.1705911. [DOI] [PubMed] [Google Scholar]
- 64.Chiossone L, Vitale C, Cottalasso F, Moretti S, Azzarone B, Moretta L, et al. Molecular analysis of the methylprednisolone-mediated inhibition of NK-cell function: evidence for different susceptibility of IL-2- versus IL-15-activated NK cells. Blood. 2007;109:3767–3775. doi: 10.1182/blood-2006-07-037846. [DOI] [PubMed] [Google Scholar]
- 65.Stern M, Ruggeri L, Mancusi A, Bernardo ME, de Angelis C, Bucher C, et al. Survival after T cell-depleted haploidentical stem cell transplantation is improved using the mother as donor. Blood. 2008;112:2990–2995. doi: 10.1182/blood-2008-01-135285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boyiadzis M, Memon S, Carson J, Allen K, Szczepanski MJ, Vance BA, et al. Up-regulation of NK cell activating receptors following allogeneic hematopoietic stem cell transplantation under a lymphodepleting reduced intensity regimen is associated with elevated IL-15 levels. Biol Blood Marrow Transplant. 2008;14:290–300. doi: 10.1016/j.bbmt.2007.12.490. [DOI] [PubMed] [Google Scholar]
- 67.Chklovskaia E, Nowbakht P, Nissen C, Gratwohl A, Bargetzi M, Wodnar-Filipowicz A. Reconstitution of dendritic and natural killer-cell subsets after allogeneic stem cell transplantation: effects of endogenous flt3 ligand. Blood. 2004;103:3860–3868. doi: 10.1182/blood-2003-04-1200. [DOI] [PubMed] [Google Scholar]
- 68.Savani BN, Mielke S, Adams S, Uribe M, Rezvani K, Yong ASM, et al. Rapid natural killer cell recovery determines outcome after T-cell-depleted HLA-identical stem cell transplantation in patients with myeloid leukemias but not with acute lymphoblastic leukemia. Leukemia. 2007;21:2145–2152. doi: 10.1038/sj.leu.2404892. [DOI] [PubMed] [Google Scholar]