Abstract
Vertebrate retinas contain two types of light-detecting cells. Rods subserve vision in dim light, while cones provide color vision in bright light. Both contain light-sensitive proteins called opsins. The light-absorbing chromophore in most opsins is 11-cis-retinaldehyde, which is isomerized to all-trans-retinaldehyde by absorption of a photon. Restoration of light sensitivity requires chemical re-isomerization of retinaldehyde by an enzymatic pathway called the visual cycle in the retinal pigment epithelium. The isomerase in this pathway uses all-trans-retinyl esters synthesized by lecithin retinol acyl transferase (LRAT) as the substrate. Several lines of evidence suggest that cone opsins regenerate by a different mechanism. Here we demonstrate the existence of two catalytic activities in chicken retinas. The first is an isomerase activity that effects interconversion of all-trans-retinol and 11-cis-retinol. The second is an ester synthase that effects palmitoyl coenzyme A-dependent synthesis of all-trans- and 11-cis-retinyl esters. Kinetic analysis of these two activities suggests that they act in concert to drive the formation of 11-cis-retinoids in chicken retinas. These activities may be part of a new visual cycle for the regeneration of chromophores in cones.
Vision in vertebrates is mediated by two types of light-sensitive cells, rods and cones. Rods are specialized for vision in dim light, while cones provide high-resolution color vision in bright light. Despite the preponderance of rods in the human retina (∼95%), cones are more important for vision in civilized mankind. With the advent of artificial lighting, people spend most of their waking time under conditions where the rod response is saturated and vision is mediated entirely by cones.
The first event in light perception is absorption of a photon by an opsin pigment molecule in the outer segment of a rod or cone. This induces 11-cis to all-trans isomerization of the retinaldehyde chromophore, which activates the opsin pigment and stimulates the visual transduction cascade (1). After a brief period, the photopigment decays to yield apoopsin and free all-trans-retinaldehyde (atRAL).1 Before light sensitivity can be restored, the atRAL must be re-isomerized to 11-cis-retinaldehyde (11cRAL), which recombines with apoopsin to form a new rhodopsin or cone-opsin pigment molecule. The process of 11cRAL regeneration is called the visual cycle (Figure 1). This multistep pathway occurs within the retinal pigment epithelium (RPE), a single layer of cells adjacent to the photoreceptors. The enzyme that catalyzes the critical all-trans to 11-cis isomerization step uses fatty-acyl esters of all-trans-retinol as the substrate (2–4). These all-trans-retinyl esters (atREs) are generated within the RPE by lecithin retinol acyl transferase (LRAT), which transfers a fatty acid from the sn-1 position of phosphatidylcholine to retinol (5, 6). Direct enzymatic conversion of atROL to 11cROL without an atRE intermediate has never been observed in the RPE.
Figure 1.

Visual cycle for regeneration of rhodopsin. The light-sensitive protein in rods is rhodopsin, located in the membranes of outer-segment disks. 11-cis-Retinaldehyde (11cRAL) is coupled to a lysine residue in rhodopsin through a protonated Schiff base linkage. Absorption of a photon (hv) induces 11-cis to all-trans isomerization of retinaldehyde to yield metarhodopsin, which activates the visual transduction cascade. The all-trans-retinaldehyde (atRAL) subsequently dissociates from apoopsin and is reduced to all-trans-retinol (atROL) by all-trans-retinol dehydrogenase (atRDH), which uses NADPH as a cofactor. The atROL diffuses from the outer segment and is taken up by an RPE cell, where it is esterified with a fatty acid from phosphatidylcholine (PC) in a reaction catalyzed by lecithin retinol acyl transferase (LRAT), yielding an all-trans-retinyl ester (atRE) and lysophosphatidylcholine (lyso-PC). The atRE is converted to 11-cis-retinol (11cROL) and a free fatty acid (FFA) by the isomerase (Rpe65). The 11cROL is oxidized by an 11cROL dehydrogenase (11cRDH), which uses NAD+ as a cofactor, to yield 11cRAL. The 11cRAL diffuses back to the outer segment where it combines irreversibly with aporhodopsin to form a new rhodopsin pigment molecule.
Several observations suggest that visual pigments in cones may regenerate by an alternate visual cycle distinct from the pathway depicted in Figure 1. For example, when frog retinas were separated from RPE, cones but not rods recovered light sensitivity spontaneously (7, 8). Isolated salamander cones, but not rods, recovered sensitivity following a photobleach with addition of 11cROL (9). Finally, Müller glial cells contain cellular retinaldehyde binding protein (CRALBP), which specifically binds 11-cis-retinoids (10, 11). Cultured Müller glial cells were shown to isomerize atROL to 11cROL, which they secreted into the medium (12). These observations suggest that Müller cells may function to regenerate visual chromophore. We recently reported the discovery of a novel isomerase in cone-dominant chicken and ground squirrel retinas that was independent of LRAT and possessed kinetic properties distinct from those of the isomerase in RPE cells (13). It has been suggested, however, that the isomerase in chicken retinas is identical to the atRE-dependent isomerase in RPE (14).
We undertook this study to characterize biochemically the retinoid isomerase activity in chicken retinas and to resolve this disagreement. Here we show that LRAT, while present in RPE, is not expressed in retinas. Further, we demonstrate the presence in chicken retinas of an enzyme activity that isomerizes atROL to 11cROL, without an atRE intermediate. These data suggest that chicken retinas contain an alternate visual cycle, distinct from the pathway in RPE.
Materials and Methods
Tissue Dissection and Preparation
All procedures were performed at 4 °C. Eyes were enucleated from the heads of freshly slaughtered chickens and were dissected in ice-cold Dulbecco's phosphate-buffered saline (DPBS, pH 7.2). Retinas were detached from the underlying RPE by injecting DPBS into the interphotoreceptor matrix with a pasteur pipet. Microsomal membrane fractions were prepared from retinas and bovine RPE as previously described (13). Microsomes were stored at −80 °C for up to 7 days before being used. In some studies, fresh retina explants were used to determine vitamin A processing capacity, as described below.
Retinoid Extraction and Analysis by HPLC
Tissue samples were homogenized in a Duall glass homogenizer containing 1 mL of 0.1 M MOPS (pH 7.2) and 0.1 M hydroxylamine with 1 mL of ethanol. Following a 15 min incubation at room temperature, the homogenate was transferred to a 16 mm × 100 mm borosilicate tube and extracted twice with 5 mL of hexane. The pooled organic phases were dried under a stream of argon, and the sample was redissolved in 200 μL of hexane. Samples were analyzed with an Agilent 1100 series high-performance liquid chromatograph (HPLC) equipped with a photodiode array detector on an Agilent Zorbax Rx-Sil 4.6 mm × 250 mm, 5 μm column using a gradient of dioxane in hexane at a flow rate of 2 mL/min. Spectral data (450–210 nm) were acquired for all eluted peaks. The identity of each eluted peak was established spectrally and by coelution with authentic retinoid standards. Quantitation was performed by comparison of sample peak areas to calibration curves established with authentic retinoid standards. Analysis of radiolabeled retinoids generated during radiometric assays was carried out on a similar HPLC system equipped with an on-line flow scintillation analyzer (Packard 525TR). The instrument was configured to monitor 3H- and 14C-labeled compounds simultaneously using energy ranges of 0–18.6 and 18.6–256 keV, respectively.
Preparation of Apo-Cellular Retinaldehyde Binding Proteins (CRALBPs)
CRALBP was expressed in Escherichia coli as previously described (15). Proteins were purified to homogeneity using either anion exchange (DEAE) or Ni2+ affinity chromatography. After we had confirmed that the apo-CRALBP bound 11cROL (15), the purified protein was used immediately or stored in 20 mM MES (pH 6.5), 0.1 mM DTT, and 50 mM NaOAc at 4 °C for up to 2 days.
Preparation of Apo-Cellular Retinol Binding Protein Type I (CRBP1)
CRBP1 was expressed in E. coli (16) and purified from the bacterial culture using a previously published method (17). The purity of the CRBP1 was >95% as confirmed by SDS–PAGE (not shown). Samples were aliquoted and stored at −80 °C.
Northern Blot Analysis
One microgram of poly(A)+ RNA from bovine RPE, bovine retinas, chicken RPE, and chicken retinas was separated electrophoretically in a 1.2% agarose–formaldehyde gel and blotted onto Hybond-N nylon membranes (Amersham). The membranes were hybridized with 32P-labeled cDNA from bovine LRAT, as previously described (18).
Immunoblot Analysis
Ten micrograms of microsomal protein from bovine RPE, bovine retinas, chicken RPE, and chicken retinas were homogenized in buffer containing 1% β-mercaptoethanol. Samples were heated to 100 °C for 2 min and separated by SDS–PAGE as previously described (18). After the samples had been transferred to a nitrocellulose membrane (Amersham), the blot was reacted with an affinity-purified antibody against human LRAT, as described previously (19). Protein bands were detected by using the ECL system (Amersham).
Vitamin A Processing in Chicken Retina Explants
Freshly dissected, light-adapted chicken retinas were placed in 1 mL of MEM culture medium. The samples were transferred to darkness and preincubated for 5 min at 37 °C. [3H]atROL was added in 2 μL of DMSO (0.1 μM, 52 Ci/mmol), and incubation was resumed for up to 40 min with gentle agitation. Individual retinas were removed from the culture medium at the indicated incubation times and transferred to a new 24-well culture plate containing 1 mL of ice-cold PBS. The retinas were washed with three exchanges of 1 mL of PBS, placed in 1.5 mL cryo tubes, and snap-frozen in liquid nitrogen. Retinoids were extracted from the frozen retinas as described above.
Isomerase Assay
The isomerase assay was performed as previously described (13) with minor modifications. Reaction mixtures consisted of 10 mM Tris-HCl (pH 8.0), 1 mM DTT, 2 mM CaCl2, 2 mM MgCl2, 1% BSA, and protein, giving a final concentration of 0.5–1.0 mg/mL (150 μL final volume). Samples were preincubated at 37 °C for 2 min, and [14C]-palmitoyl-coenzyme A ([14C]palm-CoA) (20 000 dpm/nmol) was added in 5 μL of 50 mM NaOAc (pH 6.5) (100 μM) followed by [3H]atROL (40 000 dpm/nmol in 1 μL of DMSO, 10–20 μM). At the indicated times, reactions were quenched with 150 μL of ice-cold ethanol and the products extracted into 750 μL of hexane. Apo-CRALBP (20–30 μM) and apo-CRBP1 (55 μM), where indicated, were added to the assay buffers. Extracts were analyzed by HPLC as described above.
Kinetic Analyses
Apparent kinetic constants (KM and Vmax) for utilization of atROL, 11cROL, and palm-CoA were determined using the isomerase assay described above with the following modifications. Reaction mixtures contained increasing concentrations of either atROL (0–50 μM), 11cROL (0–50 μM), or palm-CoA (0–100 μM). Retinoids were delivered in dimethylformamide [final concentration of 0.5% (v/v)], and palm-CoA was delivered in 5 μL of 50 mM NaOAc (pH 6.5). Palm-CoA was present at 100 μM in reaction mixtures containing atROL and 11cROL. atROL was present at 50 μM in assays examining activity dependence on palm-CoA. Apo-CRALBP was present in all reaction mixtures at 30 μM. Samples were preincubated at 37 °C for 2 min, and chicken retinal membranes (200 μg) were added to give a final reaction volume of 150 μL. KM and Vmax values were obtained from the rate data following Eadie–Hofstee transformation.
Effect of CRBP1 on Vitamin A Processing
The effect of CRBP1 on vitamin A metabolism in chicken retinas was determined using the isomerase assay described above without palm-CoA. Briefly, homogenates of chicken retinas were preincubated with or without 55 μM apo-CRBP1 for 30 min at 4 °C. Following the preincubation, aliquots of the protein samples (250 μg) were added to reaction mixtures containing assay buffer and 20 μM holo-CRBP1 (150 μL final volume). The samples were incubated at 37 °C for 0–24 min. At the indicated time, reactions were quenched and retinoids extracted as described above.
Results
Synthesis of Retinyl Esters by Chicken Retina and Bovine RPE Membranes
RPE from cone-dominant chickens contains retinoid processing activities similar to that of RPE from rod-dominant cattle and mice (13). For this reason, we separated chicken retinas from RPE before attempting to detect new retinoid processing activities. Figure 2 shows a representative chicken retina after dissection from the RPE-containing eyecup. The absence of visible pigmentation in this tissue indicates negligible RPE contamination.
Figure 2.
Chicken eyecup and retina following dissection. The left panel shows the RPE-containing eyecup following dissection. Patches of the pigmented RPE were rinsed away during removal of the retina. The right panel shows a chicken retina after dissection from the posterior eyecup. Note the absence of melanin pigment, indicating clean separation from the RPE.
We prepared microsomal membranes from isolated bovine RPE and chicken retinas and incubated them with atROL (10 μM) and palmitoyl-CoA (palm-CoA) (100 μM). At different incubation times, we extracted the membranes and analyzed the retinoids by normal-phase HPLC. We confirmed identification of each retinoid species by UV spectral analysis. Bovine RPE membranes synthesized predominantly atREs from atROL substrate (Figure 3A). The small amount of 11cREs synthesized by bovine RPE membranes appeared after synthesis of significant atREs. In contrast, chicken retina membranes synthesized significantly more 11cREs than atREs from the atROL substrate (Figure 3B). In this tissue, synthesis of 11cREs preceded the appearance of atREs.
Figure 3.
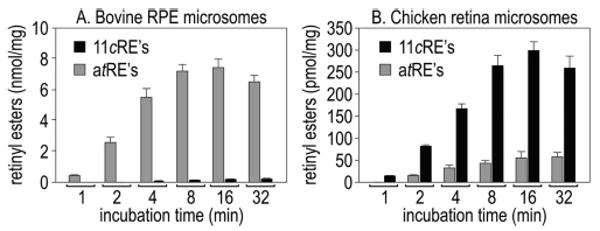
Synthesis of retinyl esters from atROL and palm-CoA by chicken retina and bovine RPE. (A) atREs and 11cREs produced by bovine RPE microsomes at the indicated times following incubation with 10 μM atROL and 100 μM palm-CoA. (B) atREs and 11cREs produced by chicken retina microsomes under similar incubation conditions. Error bars show standard deviations (n = 4). Note the predominant synthesis of atREs by bovine RPE. Also note the synthesis of 11cREs by chicken retina after a 1 min incubation, before the appearance of atREs.
Synthesis of Retinoids by Chicken Retina Explants
To analyze retinoid synthesis in a more native system, we incubated freshly dissected chicken retinas in medium containing [3H]atROL but no exogenous acyl donor. Following incubation, we homogenized the retina explants in hydroxylamine to protect the retinaldehydes, and extracted total retinoids for HPLC analysis. Figure 4A shows the levels of 3H-labeled 11cREs, 11cROL, 11cRAL, and atREs at the indicated incubation times. Representative chromatograms from the 5 and 40 min incubations are shown in Figure 4B. UV absorption spectra for each identified retinoid are shown in the insets. 11cROL and 11cRE levels peaked at 5 min and subsequently declined as the level of 11cRAL increased throughout the incubation period (Figure 4A). Here again, synthesis of 11-cis-retinoids preceded formation of atREs.
Figure 4.
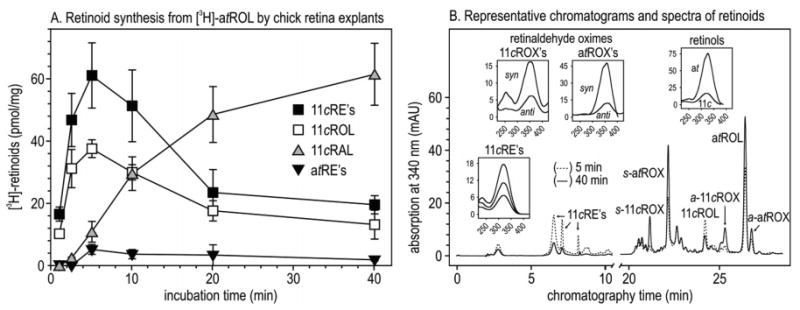
Synthesis of retinoids from [3H]atROL by chicken retina explants. (A) Time course of [3H]retinoids synthesized at the indicated incubation times from 0.1 μM [3H]atROL added to the culture medium. Error bars show standard deviations (n = 4). (B) Representative UV chromatograms at 340 nm of retinoids after 5 min (⋯) and 40 min (–) incubations. Extracts were incubated with hydroxylamine to protect the aldehydes during analysis. 11cRAL and atRAL were measured as the corresponding syn- and anti-oximes (s- or a-11cROX and s- or a-atROX). Insets show UV spectra of representative peaks from the 5 and 40 min chromatograms.
LRAT Is Expressed in RPE but Not in Retina
We attempted to identify the retinyl ester synthase responsible for the observed formation of 11cREs and atREs from atROL in chicken retinas. To test for LRAT, we analyzed RNA from bovine and chicken retinas and RPE by Northern blotting with an LRAT probe. As expected, we observed hybridization signals in the RPE samples from both species (Figure 5A). A second hybridizing band was present in the chicken RPE lane, possibly resulting from alternative polyadenylation of the lrat mRNA. However, we observed no LRAT hybridization in the lanes containing bovine or chicken retinal RNA. We also did immunoblot analysis of protein homogenates from the same four tissues with antisera against human LRAT. Immunoreactive bands with the predicted mass [25 kDa (18)] were detected in the lanes containing bovine and chicken RPE but not retina (Figure 5B). These data establish that LRAT either is not expressed in retina or is expressed at a much lower level than in RPE.
Figure 5.
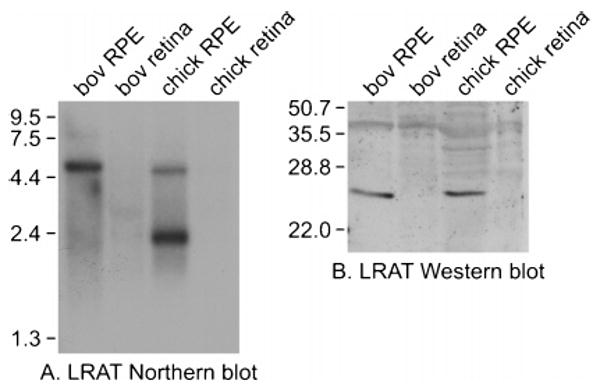
LRAT expression in RPE and retina. (A) Northern blot of RNA from chicken and bovine RPE and retinas probed with a cDNA for bovine LRAT. Each lane contained 1 μg of poly(A)+ RNA from the indicated tissue source. The mobilities of RNA size markers are shown on the left in kilobases. (B) Immunoblot of protein extracts from chicken and bovine RPE and retinas, reacted with an antibody to human LRAT. Each lane contained 10 μg of total protein from the indicated tissue source. The mobilities of protein size markers are shown at the left in kilodaltons.
Kinetics of Retinol Isomerization and Retinyl Ester Synthesis by Chicken Retina Membranes
A second retinyl ester synthase activity, called acyl CoA:retinol acyltransferase (ARAT), has been observed in several tissues (20), but never purified or cloned. Unlike LRAT, ARAT uses palm-CoA as an acyl donor (21). To analyze the substrate dependence of retinol isomerization and ester synthesis by chicken retina membranes, we measured the initial reaction velocity (V0) at different concentrations of atROL, 11cROL, and palm-CoA (Figure 6A–C). Eadie–Hofstee analysis of these data (Figure 6D–F) yielded the kinetic parameters Vmax and KM for each activity (Table 1). The synthesis of 11cREs and atREs was critically dependent upon the presence of palm-CoA (Figure 6C). This result, combined with the observation that LRAT is undetectable in chicken retinas (Figure 5), suggests that the dominant retinyl ester synthase in chicken retinas is ARAT. Unexpectedly, the rate of 11cRE synthesis from atROL was much higher than the rate of atROL-dependent synthesis of atREs (Figure 6A and Table 1) or 11cROL-dependent synthesis of 11cREs (Figure 6B and Table 1). The KM values of the four ester synthase reactions for retinols were similar (13.5, 12.1, 10.9, and 13.0 μM), suggesting that they are catalyzed by a single enzyme. These values are also similar to the reported KM (15 μM) for atROL-dependent synthesis of atREs by ARAT in mammary gland (20) and lipocytes (22).
Figure 6.
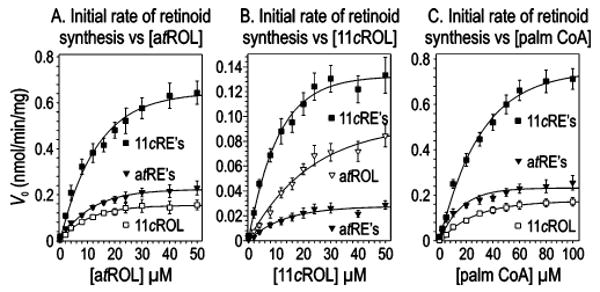
Substrate kinetics of retinoid synthesis by chicken retina microsomes. (A) Retinoids synthesized following 2 min incubations at the indicated concentrations of atROL. Palm-CoA (100 μM) was added to each reaction mixture. (B) Retinoids synthesized following 2 min incubations at the indicated concentrations of 11cROL. Palm-CoA (100 μM) was added to each reaction mixture. (C) Retinoids synthesized following 2 min incubations at the indicated concentrations of palm-CoA. atROL (50 μM) was added to each reaction mixture. Data are expressed as initial reaction velocities (V0). The Vmax and KM constants derived from Eadie–Hofstee transformations of these data are presented in Table 1.
Table 1.
Kinetics of Retinoid Synthesis for atROL, 11cROL, and Palm-CoA Substratesa
| Vmax (nmol min−1 mg−1) | KM (μM) | |||||||
|---|---|---|---|---|---|---|---|---|
| substrate | 11cREs | 11cROL | atREs | atROL | 11cREs | 11cROL | atREs | atROL |
| atROL | 0.82 | 0.19 | 0.28 | – | 13.5 | 10.1 | 10.9 | – |
| 11cROL | 0.17 | – | 0.04 | 0.14 | 12.1 | – | 13.0 | 35.0 |
| palm-CoA | 0.99 | 0.23 | 0.27 | – | 34.4 | 28.5 | 13.2 | – |
Determinations for atROL and 11cROL were carried out at 100 μM palm-CoA. Determinations for palm-CoA were carried out at 50 μM atROL.
Chicken Retina Homogenates Synthesize 11cROL from atROL without Formation of Retinyl Esters following Preincubation with Cellular Retinol Binding Protein Type I (CRBP1)
CRBP1 specifically binds atROL (23) and is present in RPE cells (24). Apo-CRBP1 is a potent competitive inhibitor of LRAT (17). To investigate the effects of apo-CRBP1 on the isomerase and ester synthase activities in chicken retina, we preincubated chicken retina homogenates with 55 μM apo-CRBP1 and assessed the time-dependent formation of 11cREs, atREs, and 11cROL from atROL. Interestingly, we observed significant conversion of atROL to 11cROL without formation of new 11cREs or atREs (Figure 7A). As a control, we assayed chicken retina homogenates under similar conditions without apo-CRBP1. Here we observed early synthesis of 11cREs with delayed formation of 11cROL (Figure 7B), as previously observed with the retina explants (Figure 4A). No atREs were synthesized during these reactions. These data suggest that the palm-CoA-dependent retinyl ester synthase in chicken retinas is inhibited by apo-CRBP1 while the isomerase is not. Further, synthesis of 11cROL from atROL without formation of atREs or 11cREs shows that the isomerase and ester synthase are independent catalytic activities.
Figure 7.

Effect of apo-CRBP1 on retinoids synthesized from atROL by chicken retinal homogenates. (A) Chicken retina homogenates were preincubated with 55 μM CRBP1 before addition of 20 μM holo-CRBP1 (atROL substrate with CRBP1). Retinoids were extracted and analyzed by HPLC at the indicated incubation times. (B) Chicken retina homogenates were incubated under conditions similar to those used for panel A without apo-CRBP1 preincubation. Results are shown for each retinoid detected as nanomoles per milligram of protein. Error bars show standard deviations (n = 4). Note the synthesis of 11cROL from atROL with no net synthesis of atREs or 11cREs in panel A.
Discussion
Regeneration of visual pigments following a photobleach requires re-isomerization of the chromophore. In rod-dominant retinas, this process occurs in the RPE and has been well-described. Previous studies suggest that cone visual pigments regenerate by an alternate pathway (7–9, 13). In the study presented here, we investigate the enzymology of retinoid processing in cone-dominant chicken retinas. Our results can be summarized by four observations. (i) Microsomes from isolated chicken retinas synthesized predominantly 11cREs from atROL, while bovine RPE microsomes synthesized predominantly atREs from the same substrate (Figure 3A,B). (ii) LRAT, although abundantly present in RPE from cattle and chickens, was undetectable in retinas from these species (Figure 5A,B). Further, the synthesis of retinyl esters by chicken retina microsomes was strongly dependent on palm-CoA (Figure 6C). The latter two observations implicate an ARAT-like activity as the major ester synthase in chicken retinas. (iii) Synthesis of 11-cis-retinoids from atROL by chicken retina explants preceded formation of atREs (Figure 4A), ruling out atREs being the substrate for this isomerase. Besides atROL-dependent synthesis of 11cREs, we also observed atROL-dependent synthesis of atREs and 11cROL-dependent synthesis of 11cREs by chicken retina microsomes (Figure 6A,B). The KM values for the three ester synthesis reactions were similar (Table 1), suggesting a single catalytic activity. The Vmax for atROL-dependent synthesis of 11cREs was severalfold higher than the Vmax for the other ester synthase reactions (Table 1). This was unexpected since atROL-dependent synthesis of 11cREs involved both all-trans to 11-cis isomerization and fatty acyl esterification of the retinoid, while the latter two reactions involved simple esterification. (iv) Finally, chicken retina homogenates preincubated with apo-CRBP1 converted atROL to 11cROL with no net synthesis of 11cREs or atREs (Figure 7A).
One conclusion of these results is that the mechanism of retinoid isomerization is different in chicken retinas and bovine RPE. An important difference is the substrate for isomerization. In RPE, the substrates are atREs (2–4). However, since 11-cis-retinoids are synthesized in the absence of atREs, these cannot be the substrates for the isomerase in chicken retinas. Instead, chicken retinas appear to catalyze the direct conversion of atROL to 11cROL. This poses a thermodynamics problem. Isomerization of retinol, from the planar all-trans to the sterically constrained 11-cis form, is endothermic with a free energy change of +4.1 kcal/mol (25). In RPE, the energy of isomerization comes from coupled hydrolysis of the carboxylate ester in an atRE (ΔG = −5 kcal/mol) (26). This energy source is unavailable if atROL is the substrate. What then is the driving force for retinoid isomerization?
We suggest that in chicken retinas, the isomerase reaction is driven by palm-CoA-dependent esterification of the 11cROL product. According to this model, the isomerization energy comes from hydrolysis of the thio ester in palm-CoA (ΔG = −7.5 kcal/mol). In support of this model, the major 11-cis-retinoids formed from atROL by chicken retinas were 11cREs, not 11cROL (Figures 4A and 6A). Oxidation of 11cROL to 11cRAL may also drive the synthesis of 11-cis-retinoids in chicken retinas. We observed formation of significant 11cRAL during incubation of chicken retina explants with atROL (Figure 4A), suggesting that oxidation also plays a role.
A model for integrating the results presented here is shown in Figure 8. We suggest that the isomerase machinery consists of two catalytic activities. One is an isomerase that catalyzes the passive interconversion of atROL and 11cROL. The second is a palm-CoA-dependent retinyl ester synthase. These enzymes operate in two catalytic modes. In the first mode, which probably involves formation of a physical complex, the enzymes are catalytically coupled. 11cROL produced by the isomerase is esterified by the synthase to yield an 11cRE. This is the dominant catalytic mode, since addition of atROL to chicken retina explants or microsomes results mainly in the formation of 11cREs (Figures 4A and 6A). For convenience, we have named this catalytic activity “isomerosynthase”. In the second mode, the enzymes work independently. For the isomerase, this mode is exemplified by the formation of 11cROL from atROL in total retina homogenates where retinyl ester synthesis is inhibited with apo-CRBP1 (Figure 7A). It is also exemplified by the reverse isomerization of added 11cROL to form atROL (Figure 6B). For the ester synthase, this second mode is represented by atROL-dependent synthesis of atREs (Figure 6A) and 11cROL-dependent synthesis of 11cREs (Figure 6B). The higher Vmax for atROL-dependent synthesis of 11cREs versus the other ester synthase reactions (Table 1) indicates enhanced catalytic efficiency for the enzymes operating in the first mode.
Figure 8.
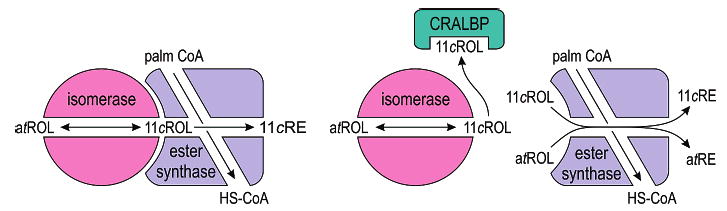
Hypothesized isomerosynthase and alternate visual cycle in chicken retinas. Schematic drawings of the retinol isomerase and palm-CoA-dependent retinyl ester synthase in chicken retinas. The isomerase catalyzes passive interconversion of atROL and 11cROL. The ester synthase uses palm-CoA as an acyl donor to catalyze formation of an atRE or 11cRE from 11cROL or atROL, respectively. In the isomerosynthase catalytic mode (depicted here as a complex between these enzymes), the 11cROL product of isomerization is directly esterified to drive the formation of 11-cis-retinoids. This results in higher catalytic efficiency than for uncoupled atROL-dependent synthesis of atREs or 11cROL-dependent synthesis of 11cREs.
To prevent reverse isomerization, the 11cROL product of 11cRE hydrolysis must be released into a cellular compartment discrete from the isomerase. This compartment may comprise apo-CRALBP, which specifically binds 11-cis-retinoids (10, 11). 11-cis-Retinyl ester hydrolase (11cREH) is located in the plasma membrane of RPE cells (27). A similar distribution of 11cREH in isomerase-containing chicken retina cells would facilitate delivery of 11cROL to interphotoreceptor retinoid-binding protein (IRBP) (28–30) in the extracellular space. This would deny access of the isomerase to 11cROL and prevent back-isomerization. 11cREH is activated by apo-CRALBP in RPE (31). In chicken retinas, this regulatory mechanism may prevent reverse isomerization by inhibiting hydrolysis of 11cREs when CRALBP is saturated with its 11cROL ligand.
In a recent publication, Gollapalli and Rando asserted that the isomerase in chicken retinas uses atREs, not atROL, as the substrate (14). However, they used chicken eyecups, containing both retina and RPE, as an enzyme source for their isomerase assays. They also used BSA as an acceptor protein for the 11cROL. BSA is a nonspecific binding protein with equal affinity for atROL and 11cROL (N. L. Mata, unpublished observation). Thus, BSA has no effect on the isomerization equilibrium. BSA works as an 11cROL acceptor for the isomerase in RPE (32) where the energy of isomerization comes from hydrolysis of atREs. BSA is ineffective, however, as an 11cROL acceptor for the chicken retina isomerase, which uses mass action to drive production of 11-cis-retinoids. The isomerase in chicken retinas requires either a specific 11cROL acceptor, such as apo-CRALBP, or an activated fatty acid as a substrate for the coupled ester synthase. Most experiments conducted by Gollapalli and Rando were carried out on mixed eyecup preparations without apo-CRALBP or palm-CoA. Since their assay conditions only supported the atRE-dependent isomerase activity in RPE, it is hardly surprising that this activity was detected. Interestingly, in one experiment where palm-CoA was added to the assay mixture without BSA, they observed dramatic production of 11cRP (14). This important observation was not discussed. In brief, Gollapalli and Rando prepared membranes from chicken retinas with RPE, assayed these membranes under conditions that supported the isomerase activity in RPE but not in chicken retinas, observed this RPE isomerase activity, and concluded that the isomerase in chicken retinas is identical to the RPE isomerase.
Cultured Müller cells from chicken retinas were previously shown to take up atROL and release 11cROL into the medium (12). These cells were also shown to contain CRALBP (10, 12). It is likely therefore that the retinol isomerase and palm-CoA-dependent retinyl ester synthase described here are located in Müller cells. Previously, we described an NADP+/NADPH-dependent 11cROL dehydrogenase (11cRDH) activity in cones (13). Together, these observations suggest the existence of an alternate visual cycle in chicken retinas. This cycle may function, in conjunction with the visual cycle in RPE cells, to provide cones with visual chromophore at the very high rates required in daylight.
Acknowledgments
We gratefully acknowledge Aarti Bagla and Hung Nguyen for their excellent technical assistance. We thank Dean Bok, Nick Desmond-Smith, Catherine Kaschula, and Steven Nusinowitz for their insightful comments and suggestions. We thank Rosalie Crouch for her gift of 11cRAL, John Crabb for his clone of CRALBP, and David Ong for his clone of CRBP1.
Footnotes
Abbreviations: 11cRAL, 11-cis-retinaldehyde; 11cRDH, 11cROL dehydrogenase; 11cREs, 11-cis-retinyl esters; 11cREH, 11cRE-hydrolase; 11cROL, 11-cis-retinol; 11cRP, 11-cis-retinyl palmitate; ARAT, acyl CoA:retinol acyltransferase; atRAL, all-trans-retinaldehyde; atRDH, all-trans-retinol dehydrogenase; atREs, all-trans-retinyl esters; atROL, all-trans-retinol; atRP, all-trans-retinyl palmitate; CoA, coenzyme A; CRALBP, cellular retinaldehyde binding protein; CRBP1, cellular retinol binding protein type I; HPLC, high-performance liquid chromatography; IRBP, interphotoreceptor retinoid binding protein; LRAT, lecithin retinol acyl transferase; lyso-PC, lysophosphatidylcholine; palm-CoA, palmitoyl-coenzyme A; PC, phosphatidylcholine; RPE, retinal pigment epithelium.
This work is supported by grants from the National Eye Institute (EY-11713) and the Foundation Fighting Blindness. G.H.T. is the Charles Kenneth Feldman and Jules & Doris Stein Research to Prevent Blindness Professor.
References
- 1.Arshavsky VY, Lamb TD, Pugh EN. G proteins and phototransduction. Annu Rev Physiol. 2002;64:153–87. doi: 10.1146/annurev.physiol.64.082701.102229. [DOI] [PubMed] [Google Scholar]
- 2.Moiseyev G, Crouch RK, Goletz P, Oatis J, Jr, Redmond TM, Ma JX. Retinyl esters are the substrate for isomerohydrolase. Biochemistry. 2003;42:2229–38. doi: 10.1021/bi026911y. [DOI] [PubMed] [Google Scholar]
- 3.Gollapalli DR, Rando RR. All-trans-retinyl esters are the substrates for isomerization in the vertebrate visual cycle. Biochemistry. 2003;42:5809–18. doi: 10.1021/bi0341004. [DOI] [PubMed] [Google Scholar]
- 4.Mata NL, Moghrabi WN, Lee JS, Bui TV, Radu RA, Horwitz J, Travis GH. Rpe65 is a retinyl ester binding protein that presents insoluble substrate to the isomerase in retinal pigment epithelial cells. J Biol Chem. 2004;279:635–43. doi: 10.1074/jbc.M310042200. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald PN, Ong DE. Evidence for a lecithin-retinol acyltransferase activity in the rat small intestine. J Biol Chem. 1988;263:12478–82. [PubMed] [Google Scholar]
- 6.Saari JC, Bredberg DL. Lecithin:retinol acyltransferase in retinal pigment epithelial microsomes. J Biol Chem. 1989;264:8636–40. [PubMed] [Google Scholar]
- 7.Goldstein EB, Wolf BM. Regeneration of the Green-rod Pigment in the Isolated Frog Retina. Vision Res. 1973;13:527–34. doi: 10.1016/0042-6989(73)90022-9. [DOI] [PubMed] [Google Scholar]
- 8.Hood DC, Hock PA. Recovery of cone receptor activity in the frog's isolated retina. Vision Res. 1973;13:1943–51. doi: 10.1016/0042-6989(73)90065-5. [DOI] [PubMed] [Google Scholar]
- 9.Jones GJ, Crouch RK, Wiggert B, Cornwall MC, Chader GJ. Retinoid requirements for recovery of sensitivity after visual-pigment bleaching in isolated photoreceptors. Proc Natl Acad Sci USA. 1989;86:9606–10. doi: 10.1073/pnas.86.23.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bunt-Milam AH, Saari JC. Immunocytochemical localization of two retinoid-binding proteins in vertebrate retina. J Cell Biol. 1983;97:703–12. doi: 10.1083/jcb.97.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saari JC, Bredberg DL. Photochemistry and stereoselectivity of cellular retinaldehyde-binding protein from bovine retina. J Biol Chem. 1987;262:7618–22. [PubMed] [Google Scholar]
- 12.Das SR, Bhardwaj N, Kjeldbye H, Gouras P. Muller cells of chicken retina synthesize 11-cis-retinol. Biochem J. 1992;285:907–13. doi: 10.1042/bj2850907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mata NL, Radu RA, Clemmons R, Travis GH. Isomerization and oxidation of vitamin a in cone-dominant retinas. A novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36:69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gollapalli DR, Rando RR. Molecular logic of 11-cis-retinoid biosynthesis in a cone-dominated species. Biochemistry. 2003;42:14921–9. doi: 10.1021/bi0356505. [DOI] [PubMed] [Google Scholar]
- 15.Crabb JW, Andrabi K, Shaw N, Wu Z, Bhattacharya S, West K, Burstedt M, Sandgren O, Noy N, Golovleva I. The R233W Mutation in Bothnia Dystrophy Does not Abolish CRALBP Retinoid Binding. Invest Ophthalmol Visual Sci. 2001;42:3524. [Google Scholar]
- 16.Stump DG, Lloyd RS, Chytil F. Site-directed mutagenesis of rat cellular retinol-binding protein. Alteration in binding specificity resulting from mutation of glutamine 108 to arginine. J Biol Chem. 1991;266:4622–30. [PubMed] [Google Scholar]
- 17.Herr FM, Ong DE. Differential Interaction of Lecithin-Retinol Acyltransferase with Cellular Retinol Binding Proteins. Biochemistry. 1992;31:6748–55. doi: 10.1021/bi00144a014. [DOI] [PubMed] [Google Scholar]
- 18.Ruiz A, Winston A, Lim YH, Gilbert BA, Rando RR, Bok D. Molecular and biochemical characterization of lecithin retinol acyltransferase. J Biol Chem. 1999;274:3834–41. doi: 10.1074/jbc.274.6.3834. [DOI] [PubMed] [Google Scholar]
- 19.Bok D, Ruiz A, Yaron O, Jahng WJ, Ray A, Xue L, Rando RR. Purification and characterization of a transmembrane domain-deleted form of lecithin retinol acyltransferase. Biochemistry. 2003;42:6090–8. doi: 10.1021/bi0342416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Randolph RK, Winkler KE, Ross AC. Fatty acyl CoA-dependent and -independent retinol esterification by rat liver and lactating mammary gland microsomes. Arch Biochem Biophys. 1991;288:500–8. doi: 10.1016/0003-9861(91)90227-a. [DOI] [PubMed] [Google Scholar]
- 21.Ross AC. Retinol esterification by rat liver microsomes. Evidence for a fatty acyl coenzyme A:retinol acyltransferase. J Biol Chem. 1982;257:2453–9. [PubMed] [Google Scholar]
- 22.Fortuna VA, Trugo LC, Borojevic R. Acyl-CoA: retinol acyltransferase (ARAT) and lecithin:retinol acyltransferase (LRAT) activation during the lipocyte phenotype induction in hepatic stellate cells. J Nutr Biochem. 2001;12:610–21. doi: 10.1016/s0955-2863(01)00179-6. [DOI] [PubMed] [Google Scholar]
- 23.MacDonald PN, Ong DE. Binding specificities of cellular retinol-binding protein and cellular retinol-binding protein, type II. J Biol Chem. 1987;262:10550–6. [PubMed] [Google Scholar]
- 24.Bok D, Ong DE, Chytil F. Immunocytochemical localization of cellular retinol binding protein in the rat retina. Invest Ophthalmol Visual Sci. 1984;25:877–83. [PubMed] [Google Scholar]
- 25.Rando RR, Chang A. Studies on the catalyzed interconversion of vitamin A derivatives. J Am Chem Soc. 1983;105:2879–82. [Google Scholar]
- 26.Deigner PS, Law WC, Canada FJ, Rando RR. Membranes as the energy source in the endergonic transformation of vitamin A to 11-cis-retinol. Science. 1989;244:968–71. doi: 10.1126/science.2727688. [DOI] [PubMed] [Google Scholar]
- 27.Mata NL, Tsin AT. Distribution of 11-cis LRAT, 11-cis RD and 11-cis REH in bovine retinal pigment epithelium membranes. Biochim Biophys Acta. 1998;1394:16–22. doi: 10.1016/s0005-2760(98)00078-2. [DOI] [PubMed] [Google Scholar]
- 28.Lai YL, Wiggert B, Liu YP, Chader GJ. Interphotoreceptor retinol-binding proteins: Possible transport vehicles between compartments of the retina. Nature. 1982;298:848–9. doi: 10.1038/298848a0. [DOI] [PubMed] [Google Scholar]
- 29.Redmond TM, Wiggert B, Robey FA, Nguyen NY, Lewis MS, Lee L, Chader GJ. Isolation and characterization of monkey interphotoreceptor retinoid-binding protein, a unique extracellular matrix component of the retina. Biochemistry. 1985;24:787–93. doi: 10.1021/bi00324a038. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Noy N. Retinoid specificity of interphotoreceptor retinoid-binding protein. Biochemistry. 1994;33:10658–65. doi: 10.1021/bi00201a013. [DOI] [PubMed] [Google Scholar]
- 31.Stecher H, Gelb MH, Saari JC, Palczewski K. Preferential release of 11-cis-retinol from retinal pigment epithelial cells in the presence of cellular retinaldehyde-binding protein. J Biol Chem. 1999;274:8577–85. doi: 10.1074/jbc.274.13.8577. [DOI] [PubMed] [Google Scholar]
- 32.Winston A, Rando RR. Regulation of isomerohydrolase activity in the visual cycle. Biochemistry. 1998;37:2044–50. doi: 10.1021/bi971908d. [DOI] [PubMed] [Google Scholar]


