Summary
Unc104 (KIF1A) kinesin transports membrane vesicles along microtubules in lower and higher eukaryotes. Using an in vitro motility assay, we show that Unc104 uses a lipid binding pleckstrin homology (PH) domain to dock onto membrane cargo. Through its PH domain, Unc104 can transport phosphatidylinositol(4,5)bisphosphate (PtdIns(4,5)P2)-containing liposomes with similar properties to native vesicles. Interestingly, liposome movement by monomeric Unc104 motors shows a very steep dependence on PtdIns(4,5)P2 concentration (Hill coefficient of ~20), even though liposome binding is noncooperative. This switch-like transition for movement can be shifted to lower PtdIns(4,5)P2 concentrations by the addition of cholesterol/sphingomyelin or GM1 ganglioside/cholera toxin, conditions that produce raft-like behavior of Unc104 bound to lipid bilayers. These studies suggest that clustering of Unc104 in PtdIns(4,5)P2-containing rafts provides a trigger for membrane transport.
Introduction
Long-range transport of membranes in higher eukaryotic cells occur along a microtubule network that is usually organized as a radial array emanating from the centrosome. A large multisubunit motor, cytoplasmic dynein, is primarily responsible for transport toward the microtubule minus end anchored at the centrosome, whereas plus-end-directed transport is driven by many motors belonging to the kinesin superfamily (Hirokawa, 1998). Mammals contain ~45 kinesin genes, 38 of which are expressed in brain tissue, and many of these motors are likely to be involved in transporting distinct cargoes in axons and dendrites (Miki et al., 2001). Understanding how specific kinesins become linked to particular cargoes and deciphering the regulatory mechanisms for membrane transport remain as major unsolved research questions.
Kinesin motor proteins contain two major functional domains: a “motor domain,” which powers motion along the microtubule and is conserved throughout the kinesin superfamily, and a “tail domain,” which recognizes a specific cargo and has a sequence that is unique to a particular kinesin class. Recently, several studies have identified vesicle-associated “receptor” proteins that bind to kinesin tail domains or tail-associated subunits (Goldstein, 2001). However, the role of membrane lipids in motor-driven transport has received less attention. A potential role for lipids in minus-end-directed membrane transport was proposed based upon findings showing that cytoplasmic dynein binds membranes via nonerythroid spectrin, which in turn binds phosphatidylinositol(4,5)bisphosphate (PtdIns(4,5)P2) lipids through a pleckstrin homology (PH) domain (Muresan et al., 2001). Candidate lipid binding PH domains also have been identified by sequence in the tail regions of the myosin-X (Berg et al., 2000) and the kinesin Unc104/KIF1A (Okada et al., 1995), although the functions of these domains have not been explored. Moreover, PtdIns(4,5)P2, the lipid target of many PH domains, has been implicated in membrane-trafficking events (Caroni, 2001; Martin, 2001; Simons and Ikonen, 1997). These findings raise the possibility that cargo packaging into vesicles/membrane tubules and the subsequent recruitment of motor proteins could potentially involve phosphoinositides.
To explore the roles of lipids in motor-driven transport, we investigated the mechanism of membrane movement driven by Unc104/KIF1A kinesin. Unc104/KIF1A kinesin was originally discovered as the gene underlying a paralyzed mutant (unc104) in worms (Hall and Hedgecock, 1991; Otsuka et al., 1991) and later cloned from mouse (KIF1A; Okada et al., 1995). In worms and mice, this kinesin plays a similar role in transporting synaptic vesicle precursors from the cell body to the nerve terminal (Hall and Hedgecock, 1991; Yonekawa et al., 1998). The direct visualization of Unc104-GFP in living worms reveals fast transport of fluorescent punctae (presumably vesicles) from the cell body into the axon (Zhou et al., 2001). Biochemical analysis further reveals that KIF1A binds to a synaptic vesicle precursor compartment and not to other axonally transported vesicles (Okada et al., 1995). Unc104/KIF1A relatives also are found in fungi (Sakowicz et al., 1999) and Dictyostelium (Pollock et al., 1999), and a disruption of the Dictyostelium gene causes defects in vesicle transport (Pollock et al., 1999). These results indicate that the involvement of Unc104/KIF1A motors in vesicle trafficking is ancient and precedes metazoan evolution.
Unc104/KIF1A kinesins (herein termed Unc104) have structural and motility properties that set them apart from other kinesins (Bloom, 2001). Adjacent to the N-terminal motor domain lies a 100 amino acid (aa) fork head homology-associated (FHA) domain that has been shown in other proteins to bind phosphothreonine residues. Following the FHA domain lies a long region of low sequence conservation and then a canonical PH domain (Lemmon, 1999). Despite containing regions of predicted coiled-coil sequence near the motor domain, mouse KIF1A (Okada et al., 1995) and Caenorhabditis elegans Unc104 (Pierce et al., 1999) purify as monomeric species, in contrast to most other kinesin motors which are dimers. However, Dictyostelium Unc104 (DdUnc104) has a ~600 aa coiled-coil extension beyond the PH domain that causes this motor to dimerize (Pollock et al., 1999). Unc104 is one of the fastest known kinesin motors, eliciting velocities of 1.2–2.5 μm/s, depending upon the organism source. While the biophysical and structural properties of mouse KIF1A (Kikkawa et al., 2001; Okada and Hirokawa, 1999, 2000) and C. elegans Unc104 (Pierce et al., 1999) motor domains have been studied extensively, the mechanism of vesicle transport by these kinesins has not been investigated.
In this study, we have used an in vitro motility assay to investigate how Unc104 transports membrane cargo. We report that the PH domain of this motor is necessary and sufficient for binding to and transporting native vesicles and PtdIns(4,5)P2-containing liposomes. We also demonstrate that liposome motility is highly cooperative with respect to the PtdIns(4,5)P2 content and is enhanced by agents that promote organization of lipids into raft-like domains (Simons and Ikonen, 1997). We propose that lipid raft formation may act as a regulatory mechanism for activating vesicle transport by monomeric Unc104 motors.
Results
DdUnc104 Transports PtdIns(4,5)P2-Containing Liposomes along Microtubules In Vitro
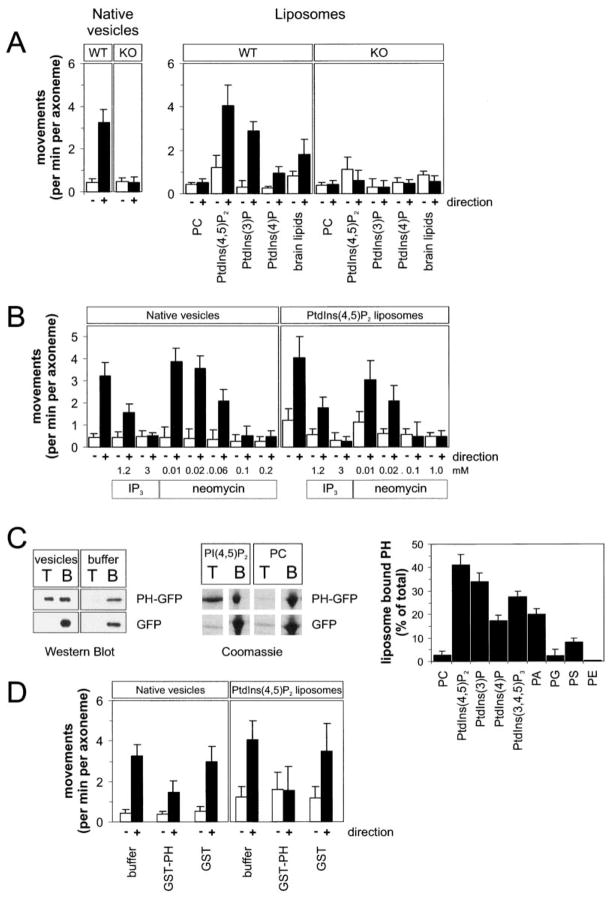
We have shown previously that the Dictyostelium ortholog of Unc104 (DdUnc104) transports Dictyostelium vesicles in vivo and in vitro (Pollock et al., 1999). A candidate membrane binding domain of DdUnc104 is the pleckstrin homology (PH) domain located in the C-terminal half of the protein. PH domains bind to acidic phospholipids, especially to phosphatidylinositolphosphates (PtdInsPn) (Kavran et al., 1998; Lemmon, 1999). We therefore tested whether DdUnc104 is capable of transporting artificial liposomes containing PtdInsPn. When combined with a microtubule-purified motor fraction from Dictyostelium, liposomes containing 10 mol% PtdIns(4,5)P2 moved predominantly to the microtubule plus end at a similar frequency to that observed for isolated Dictyostelium vesicles (Figure 1A). This plus-end-directed motion is largely generated by the DdUnc104 motor, since the frequencies of vesicle and liposome movement were reduced by >80% when the motor fraction was prepared from a DdUnc104 null cell line (KO cells). The properties of DdUnc104-mediated PtdIns(4,5)P2 liposome transport were similar to those of native Dictyostelium vesicles. The two cargoes exhibited similar processivity (travel distance of 5.4 ± 1.7 μm and 6.0 ± 1.8 μm for native vesicles and liposomes, respectively; mean and error of fit for n = 68 [Pierce et al., 1999]) as well as movement velocity (2.1 ± 0.4 μm/s and 2.0 ± 0.6 μm/s for native and liposomes, respectively; n = 40).
Figure 1. The PHDdUnc104 Domain Binds to Membranes and Enables DdUnc104 to Transport Native Vesicles and Artificial Liposomes In Vitro.
(A) Motility of liposomes and vesicles in an in vitro assay produced by a DdUnc104-containing motor fraction from wild-type or DdUnc104-knockout (KO) cells. PtdIns(4,5)P2, PtdIns(3)P, and PtdIns(4)P were added at 10 mol% fraction with the remainder being PC. The brain lipids were a mixture of 10% phosphoinositides, 50% phosphatidylserine, and several other brain lipids. The number of membranes moving per min per axoneme were scored. Values show the means and standard deviations (SD) of 3–8 independent experiments. Open bars show minus-end-directed movements; closed bars show plus-end-directed movements.
(B) Inhibition of plus-end-directed liposome and vesicle transport (produced by a DdUnc104-containing motor fraction from wild-type cells) by neomycin or soluble inositol(1,4,5)P3 (IP3). Values show the means and SD of four independent experiments.
(C) Binding of bacterially-expressed PHDdunc104-GFP, or GFP alone, to native vesicles or liposomes using a membrane floatation assay (Experimental Procedures). After a centrifugation step, membranes were recovered from the top (T) of sucrose step gradient while unbound protein remained at the bottom (B). The left gel (Western blot with an anti-GFP antibody) reveals recovery of PHDdUnc104-GFP in the top fraction only in the presence of vesicles and not in the buffer control. GFP was not recovered in the top fraction in the presence or absence of vesicles. The next image, a Coomassie-stained gel, reveals that PHDdUnc104-GFP binds to 10 mol% PtdIns(4,5)P2 liposomes but not liposomes composed only of PC. Right graph shows quantification of PHDdUnc104-GFP binding to liposomes of various compositions (all at 10 mol% fraction). Values show the mean and SD of three independent experiments.
(D) Inhibition of plus-end- but not minus-end-directed transport by addition of bacterially-expressed GST-PHDdunc104 domain (0.24 mg/ml). GST alone added at 0.3 mg/ml had little effect. The in vitro motility assays used a DdUnc104-containing motor fraction from wild-type cells and either 10 mol% PtdIns(4,5)P2 liposomes or native vesicles. Values are the mean and SD of three measurements.
We also tested the lipid specificity of DdUnc104 using the in vitro liposome transport assay. PtdIns(4,5)P2-containing liposomes were transported most efficiently (11-fold more frequently than liposomes composed exclusively of phosphatidylcholine [PC; Figure 1A]). PtdIns(4)P-containing liposomes moved less frequently with shorter run lengths (1.1 ± 0.1 μm) compared to PtdIns(4,5)P2 and PtdIns(3)P liposomes. These results also indicate that the position of a single phosphate moiety affects motor-driven transport. A crude brain lipid extract, a mixture of 10% PtdInsPn acidic phospholipids and 40%–50% phosphatidylserine, also showed a robust activity similar to PtdIns(4,5)P2 liposomes (Figure 1A) but with somewhat reduced processivity (3.7 ± 0.8 μm). With liposome compositions that supported motility, virtually all obvious microtubule binding events resulted in transport and static binding was rarely observed.
In contrast to DdUnc104, dynein-based movement of PtdIns(4,5)P2 liposomes toward the microtubule minus end was only slightly enhanced over PC liposomes and was much less pronounced compared to DdUnc104-generated motion. (Figure 1A, open bars). Dictyostelium also has a second plus-end-directed motor that moves vesicles in extracts from DdUnc104-KO cells (Pollock et al., 1999). This other plus-end-directed vesicle transport motor exhibited low levels of liposome transport and displayed only a slight preference for PtdIns(4,5)P2 over PC liposomes. Thus, among the microtubule-based vesicle motors in Dictyostelium extracts, DdUnc104 is the most active in transporting liposomes and displays the strongest preference for PtdIns(4,5)P2.
We also examined whether DdUnc104 utilizes phosphoinositides in its mechanism for transporting native vesicles. Figure 1B shows that transport of native vesicles and PtdIns(4,5)P2 liposomes were both reduced by ~85% by the addition of soluble inositol trisphosphate (IP3) (3 mM). Neomycin, an agent that binds to negatively charged lipid head groups, also inhibited the movement of native vesicles and PtdIns(4,5)P2 liposomes. These effects are specific for the DdUnc104 motor, since dynein-mediated transport of native vesicles was not inhibited by either IP3 or neomycin, which contrasts finding with squid dynein (Muresan et al., 2001). These agents, however, inhibited dynein-mediated liposome transport. In summary, these results suggest that DdUnc104 mediates transport of native vesicles, at least in part, by binding to phosphoinositide-containing lipids.
Purified PHDdUnc104 Binds Native Vesicles and PtdIns(4,5)P2-Containing Liposomes
Given the lipid specificity for liposome transport, DdUnc104’s PH domain was a likely candidate region for mediating membrane attachment. To test this directly, we bacterially expressed and purified a PHDdUnc104-GFP-His6 fusion protein (herein called PHDdUnc104) and assayed binding by sucrose gradient floatation. PHDdUnc104 bound to native Dictyostelium vesicles in the top membrane fraction, but GFP, as a control protein, did not. In the absence of vesicles, both PHDdUnc104 and GFP were recovered from the bottom fraction only (Figure 1C). These results indicate that PHDdUnc104 binds in a specific manner to native vesicles.
PHDdUnc104 also bound to 10 mol% PtdIns(4,5)P2, protein-free liposomes but not to liposomes composed entirely of PC (the same residual binding as the GFP control; Figure 1C). In a panel of different lipids tested (Figure 1C), PHDdUnc104 bound with greatest affinity to PtdIns(4,5)P2, followed by PtdIns(3)P, PtdIns(3,4,5)P3, phosphatidic acid, and PtdIns(4). PHDdUnc104 exhibited little binding to other phosphatidyl derivatives, such as phosphatidyl serine, phosphatidyl ethanolamine, and phosphatidyl glycerol. Therefore, PHDdUnc104 recognizes distinct inositol phosphate conformations, and this binding specificity mirrors that observed for the native DdUnc104 motor in the liposome transport assay (Figure 1A).
We also tested whether a bacterially expressed GST-PHDdUnc104 could compete with DdUnc104 for binding and transport of native vesicles and liposomes (Figure 1D). GST-PHDdUnc104 (0.24 mg/ml) reduced DdUnc104-mediated transport of both native vesicle and PtdIns(4,5)P2 liposomes by 60% (p < 0.02). GST alone at the same concentration had little inhibitory effect on plus-end-directed movement, and neither GST nor GST-PHDdUnc104 affected dynein-based minus-end-directed movements. The finding that GST-PHDdUnc104 specifically competes with the native motor suggests that DdUnc104 utilizes its PH domain to transport membrane cargo.
The PH Domain Is Necessary and Sufficient for Vesicle and Liposome Movement
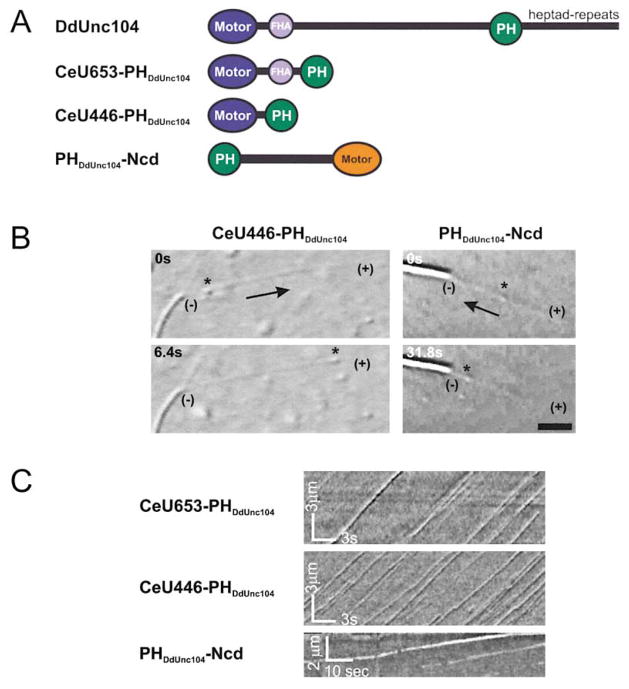
In order to further test the role of the PH domain through mutational analysis, we sought to reconstitute membrane transport using a recombinant DdUnc104. However, expression of the full-length DdUnc104 in Dictyostelium resulted in very poor yields. Instead, we attempted to reconstitute transport using a bacterially expressable “minimotor” that contains a motor domain and the minimal region necessary for cargo attachment. However, the Dictyostelium Unc104 motor domain could not be expressed in Escherichia coli due to insolubility. We therefore created a minimotor consisting of the motor and FHA domains from C. elegans Unc104, which expresses well in bacteria, and the PH domain from DdUnc104 (Figure 2A). This construct, termed CeU653-PHDdUnc104, lacks the long stalk region (~950 aa) that exhibits low amino acid sequence similarity among the Unc104 motors from Dictyostelium to mouse. This monomeric CeU653-PHDdUnc104 minimotor stimulated robust movement of both native Dictyostelium vesicles and PtdIns(4,5)P2 liposomes at similar frequencies to those produced by the native DdUnc104 motor from the microtubule-purified fraction (Table 1). When membrane movements generated by CeU653-PHDdUnc104 were analyzed using kymographs, long run lengths with only occasional pauses were observed (Figure 2C). The velocities of transport for CeU653-PHDdUnc104-mediated vesicles and liposomes were 1.6 μm/s and 1.5 μm/s, respectively, which is similar to the rates of microtubule gliding activity (Pierce et al., 1999) and in vivo vesicle transport (Zhou et al., 2001) produced by the C. elegans Unc104 motor. Importantly, membrane transport by the minimotor required the PH domain, since CeU653 protein lacking the PH domain only rarely moved membranous cargo (Table 1). CeU653-PHDdUnc104 exhibited the same selectivity for PtdIns(4,5)P2 liposomes as described for the native motor (data not shown).
Figure 2. The PHDdUnc104, Joined onto a Kinesin Motor Domain, Elicits Vesicle and Liposome Transport In Vitro.
(A) Schematic diagram of native DdUnc104 or minimotor constructs. The PH domain from DdUnc104 was fused C-terminal to the FHA domains (CeU653-PHDdUnc104) or to the motor/neck domain (CeU446-PHDdUnc104) of C. elegans Unc104. For the minus-end-directed, C-terminal motor Ncd, the DdUnc104 PH domain was fused at the N terminus of its elongate coiled-coil domain. See the Experimental Procedure for the precise fusion sites.
(B) DIC images show membrane movement (native vesicles indicated by an asterisk) being transported by CeU446-PHDdUnc104 toward the microtubule plus end (+) or by PHDdUnc104-Ncd toward the minus end (−). The axoneme (visible as the higher-contrast elongate object) has longer microtubules growing off of its plus end and shorter microtubules growing off of its minus end (not shown) as a marker of polarity. The scale bar equals 3 μm.
(C) Kymographs (see Experimental Procedures) demonstrate the processivity, velocity, and frequency of transport of PtdIns(4,5)P2 liposomes by minimotors. Movement of individual liposome is seen in this plot of the video data as continuous diagonal lines. Note the frequent liposome movement and the steady velocities indicated by parallel straight lines.
Table 1.
Properties of Native Vesicle and Liposome Transport by Native DdUnc104 and Bacterially Expressed Minimotors
| Native Vesicles |
Liposomes (10 mol% PtdIns(4,5)P2) |
|||
|---|---|---|---|---|
| Movements (per minute per axoneme) | Velocities (μm/s) | Movements (per minute per axoneme) | Velocities (μm/s) | |
| DdUnc104 | 3.3 ± 0.6 | 2.1 ± 0.4 | 4.1 ± 1.0 | 2.0 ± 0.6 |
| CeU653-GFP | 0.1 ± 0.1 | 0.5 ± 0.4 | 0.1 ± 0.1 | 0.4 ± 0.3 |
| CeU653-PHDdUnc104 | 7.8 ± 1.5 | 1.5 ± 0.2 | 54.8 ± 6.5 | 1.6 ± 0.1 |
| CeU446-PHDdUnc104 | 5.6 ± 1.2 | 1.4 ± 0.2 | 45.4 ± 4.3 | 1.4 ± 0.2 |
| PHDdUnc104-Ncd | 1.5 ± 0.6 | 0.15 ± 0.04 | 1.2 ± 0.5 | 0.17 ± 0.06 |
The minimotors and the assay conditions are described in Figure 2 and the Experimental Procedures. The means and standard deviations from 3–5 independent experiments are shown.
While the above experiments suggest that PH is important for cargo interaction, the CeU653-PHDdUnc104 protein also contained a fork head homology (FHA) domain next to the motor domain. To ascertain if the FHA domain played a role in membrane attachment, we created a minimotor construct that lacked the FHA domain (CeU446-PHDdUnc104) but contained the minimal motor domain necessary for eliciting maximal movement velocity (Y. Cui and R.D.V., unpublished observations) followed by the PHDdUnc104 domain. CeU446-PHDdUnc104 transported Dictyostelium vesicles and liposomes at the same rate and frequency as CeU653-PHDdUnc104 (Figure 2C and Table 1); movements were extremely processive and very rarely did the membranes release before reaching the microtubule plus end.
The above experiments, while strongly arguing that the PH domain is involved in membrane transport, do not exclude the possibility that a region in the Unc104 motor domain also is needed for cargo attachment. Therefore, we fused the PH domain to the motor-stalk domains of the minus-end-directed kinesin motor Ncd, which is involved in mitotic spindle function but not in vesicle transport (Figure 2A; Hirokawa, 1998). The PHDdUnc104-Ncd protein moved native vesicles and liposomes along microtubules toward their minus end at speeds of 0.15 μm/s, which is consistent with the previously observed direction and rates of Ncd motor activity (Figure 2B and Table 1). In the absence of a PH domain, Ncd did not move membranes (data not shown). These results indicate that the PH domain is sufficient for mediating membrane transport in an in vitro assay.
A Role for Membrane Proteins in DdUnc104-Mediated Vesicle Transport
While PtdIns(4,5)P2 lipids suffice in supporting binding of the PHDdUnc104 domain and membrane transport by the intact motor, membrane proteins in native vesicles also may be involved in the transport mechanism, and several PH domains have been shown to bind to specific protein partners. In order to dissect the roles of lipids and membrane proteins, we created several PHDdUnc104 mutants and identified one (KKK1531-3EEE) that is defective in PtdIns(4,5)P2 binding (90% reduction in liposome binding) (see Supplemental Data at http://www.cell.com/cgi/content/full/109/3/347/DC1). When this mutant PH domain was fused onto CeU653, liposome transport was decreased by ~90% (Table 2). When tested on native vesicles, on the other hand, the KKK1531-3EEE mutant was still capable of eliciting transport, albeit at ~50% of the level of the wild-type PH domain. This residual activity, which also argues that the triple lysine mutant is not misfolded, suggested that the mutant PH domain might elicit vesicle motility by binding to either membrane proteins or lipids other than PtdIns(4,5)P2. To examine this question, we proteolyzed surface-exposed membrane proteins with trypsin. Consistent with previous results for native DdUnc104 (Pollock et al., 1999), trypsinized vesicles showed a ~40% reduction in motility with wild-type PHDdUnc104, although transport velocity was not significantly changed. In contrast, the motility of trypsinized vesicles was reduced close to background levels with the KKK1531-3EEE mutant, presumably because of the lack of protein docking sites and its inability to bind lipids (Table 2). Taken together, these results suggest DdUnc104-mediated transport of native vesicles involves not only lipid binding but also proteins on the vesicle membrane.
Table 2.
Properties of Native Vesicle, Trypsinized Native Vesicles, and Liposome Transport by Bacterially Expressed Minimotors Containing Various PH Domains
| Native Vesicles |
Trypsinized Vesicles |
Liposomes |
||||
|---|---|---|---|---|---|---|
| Movements (per minute per axoneme) | Velocities (μm/s) | Movements (per minute per axoneme) | Velocities (μm/s) | Movements (per minute per axoneme) | Velocities (μm/s) | |
| 10 mol% PtdIns(4,5)P2 |
||||||
| CeU653-PHDdUnc104 | 7.8 ± 1.5 | 1.5 ± 0.2 | 3.0 ± 0.8 | 1.4 ± 0.2 | 5.8 ± 0.6 | 1.6 ± 0.1 |
| CeU653-PHDdUnc104 KKK1531-3EEE | 3.7 ± 0.8 | 1.4 ± 0.2 | 0.5 ± 0.2 | 1.0 ± 0.4 | 0.6 ± 0.3 | 1.1 ± 0.3 |
| CeU653-PHPLCδ1 | 3.9 ± 0.4 | 0.9 ± 0.2 | 4.0 ± 0.5 | 0.8 ± 0.2 | 10.5 ± 2.1 | 1.2 ± 0.1 |
| CeU653-PHDAPPI | 4.1 ± 0.3 | 0.8 ± 0.2 | 4.1 ± 0.6 | 1.0 ± 0.2 | 1.1 ± 0.5 | 0.3 ± 0.1 |
| 10 mol% PtdIns(3,4,5)P3 |
||||||
| CeU653-PHDAPPI | 5.2 ± 1.7 | 1.7 ± 0.1 | ||||
Minimotor CeU653 with a wild-type PHDdUnc104, a PtdIns(4,5)P2 binding-deficient PHDdUnc104 KKK1531-3EEE (see Supplemental Data), the domain from PLCδ1 (PHPLCδ1) which binds selectively to PtdIns(4,5)P2, or the PH domain from dual adaptor for phosphotyrosine and 3-phosphoinositides (PHDAPPI) which binds selectively to PtdIns(3,4,5)P3 was used in conjunction with the indicated membrane for in vitro transport assays (see Experimental Procedures). The means and standard deviations from four independent experiments are shown.
As a second means of investigating the roles of lipids and proteins in the microtubule-based transport of native vesicles, we created Unc104 minimotors bearing PH domains from other proteins. We chose the PH domain of phospholipase C-δ1 (PHPLCδ1), which, like PHDdUnc104, binds PtdIns(4,5)P2 but presumably has distinct protein binding partners, and the PH domain of the dual adaptor for phosphotyrosine and 3-phosphoinositides-1 (PHDAPPI), which has a distinct lipid specificity for PtdIns(3,4,5)P3 (Kavran et al., 1998). As expected from their lipid specificities, CeU653-PHPLCδ1 transported PtdIns(4,5)P2 liposomes efficiently, whereas CeU653-PHDAPPI elicited very few movements that were of low velocity (Table 2). In contrast, PtdIns(3,4,5)P3 liposomes were efficiently transported at maximal velocity by CeU653-PHDAPPI (Table 2). Neither of these minimotors moved PC liposomes efficiently (not shown). Interestingly, native vesicles were transported at similar frequencies (Table 2) and processivity (not shown) by CeU653-PHPLCδ1 and CeU653-PHDAPPI compared with CeU653-PHDdUnc104. However, the velocities of movement were significantly lower for these foreign PH domains, suggesting that protein interactions were somehow important for reconstituting normal cargo transport (Table 2). Importantly, trypsinization did not affect transport by either CeU653-PHPLCδ1 or PHDAPPI. This result indicates that these foreign PH domains mediate transport of native vesicle solely through lipid interactions and that trypsin diminishes PHDdUnc104-mediated transport by eliminating proteins that bind specifically to this PH domain.
Cooperativity of PtdIns(4,5)P2 Lipids in Transport
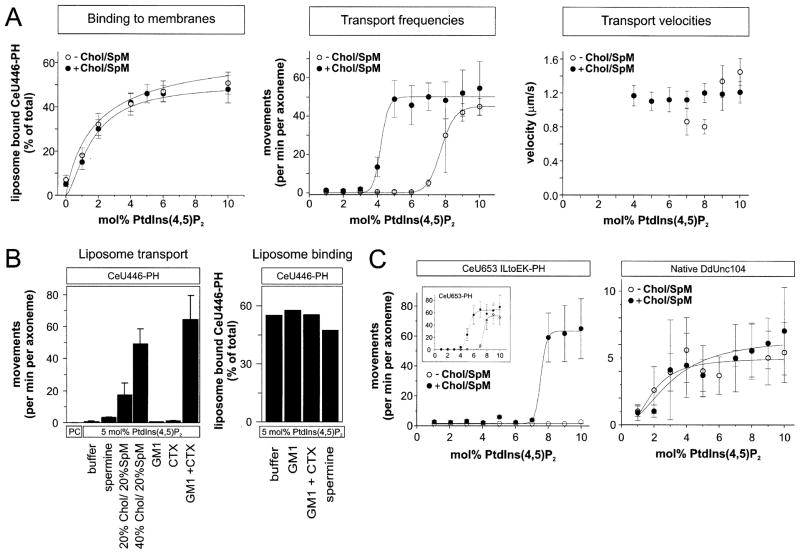
In order to better understand the requirements of PtdIns(4,5)P2 for Unc104-mediated membrane transport, we measured how the concentration of PtdIns(4,5)P2 influences the liposome binding and transport by CeU446-PHDdUnc104. With the motor concentration held constant and the PtdIns(4,5)P2 concentration varied between 0–10 mol%, the amount of liposome bound CeU446-PHDdUnc104 increased in a hyperbolic manner that is typical for a simple binding interaction with no apparent cooperativity (Figure 3A, Hill coefficient of 1.5 ± 0.5). In contrast, liposome transport was infrequently or not observed between 0 and 6 mol% PtdIns(4,5)P2, and then increased dramatically between 7 and 9 mol% (Figure 3A). This sharp transition with increasing PtdIns(4,5)P2 concentration indicates that liposome transport, but not motor binding, is a highly cooperative phenomenon (Hill coefficient of 20.1 ± 1.0). Once transport was observed, the velocity was not substantially affected by increasing PtdIns(4,5)P2 concentration.
Figure 3. Effects of PtdIns(4,5)P2 Concentration and Lipid Clustering Agents on Unc104-Mediated Liposome Transport.
(A) The effects of PtdIns(4,5)P2 concentration on motor (CeU446-PHDdUnc104) binding to liposomes (left), frequency of liposome movement (middle), and liposome transport velocity (right). Liposomes were prepared with the indicated concentration of PtdIns(4,5)P2 without (open circle) or with 40 mol% cholesterol/20 mol% sphingomyelin (closed circles), with the remainder being PC. The motor concentration was held fixed at 0.2 μM. Motor binding to liposomes with increasing PtdIns(4,5)P2 concentration showed little or no cooperativity (curves fitted with Hill coefficients of 1.5 ± 0.5 and 0.9 ± 0.7 in the absence and presence of cholesterol/sphingomyelin), while a high degree of cooperativity was observed for liposome transport (curves fitted with Hill coefficients of 20.1 ± 1.0 and 19.8 ± 8.7 in the absence and presence of cholesterol/sphingomyelin). Velocity was scored only when a minimum of ten events could be measured. The mean and SD from four independent experiments are shown.
(B) The effects of two lipid clustering agents, cholera toxin (CTX) or spermine, on liposome motility and binding. CTX (2 μg/ml) was used to cluster 10 mol% GM1 incorporated into 5 mol% PtdIns(4,5)P2/85 mol% PC liposomes; 2 mM spermine was added to assays with 5 mol% PtdIns(4,5)P2/95 mol% PC liposomes transported by the minimotor CeU446-PHDdUnc104 (0.2 μM). Chol/SpM liposomes were the same as described in (A). A representative binding experiment of CeU446-PHDdUnc104 to liposomes shows little or no increase in the presence of CTX or spermine.
(C) Left: The effect of a destabilized coiled-coil region on liposome transport. CeU653-PHDdUnc104 with the mutations I362E and L365K is indicated, and the behavior of wild-type CeU653-PHDdUnc104 is shown in the insert. Right: Transport of liposomes by a microtubule affinity-purified fraction containing native dimeric DdUnc104 transporting liposomes. The conditions were the same as those described in (A). The means and SD from three independent experiments are shown.
One potential explanation for the cooperativity is that a cluster of PtdIns(4,5)P2 with bound Unc104 motors is needed to elicit liposome transport. To investigate this idea, we tested whether agents that might facilitate the clustering of PtdIns(4,5)P2 affect liposome transport. Previous studies have shown that cholesterol and sphingomyelin enhance the formation of membrane subdomains called “rafts” (Simons and Ikonen, 1997). Cellular cholesterol levels can be relatively high (25–50 mol%) and vary among membranes (Sandhoff et al., 1999). When cholesterol (40 mol%) and sphingomyelin (20 mol%) were incorporated into liposomes with varying PtdIns(4,5)P2 concentrations, the binding of CeU446-PHDdUnc104 to membranes was not altered (Figure 3A); however, a dramatic change in the PtdIns(4,5)P2 dependency of liposome movement was observed. In the presence of cholesterol/sphingomyelin, transport frequencies increased sharply with increasing PtdIns(4,5)P2 concentrations (Hill coefficient of 19.8 ± 8.7), but this transition occurred at a 3 mol% lower PtdIns(4,5)P2 concentrations compared to cholesterol/sphingomyelin-free liposomes (Figure 3A). Reducing the cholesterol concentration to 20 mol% also enhanced motility substantially at a fixed PtdIns(4,5)P2 concentration of 5 mol%, although the effect was reduced by 65% (Figure 3B). Unc104 motor (CeU653) fused to the PH domain of PLCδ1 also displayed a sharp transition for movement that was shifted to lower PtdIns(4,5)P2 concentration by cholesterol/sphingomyelin (not shown).
If clustering of PtdIns(4,5)P2 by cholesterol/sphingomyelin-induced raft formation was responsible for facilitating transport, then lipid clustering achieved by other means might also produce similar effects. To test this notion, we prepared liposomes containing GM1, a lipid ganglioside that is recognized and clustered by binding of the pentameric B subunit of cholera toxin (CTX) (van Heyningen and King, 1976). Although GM1 is usually found only in the outer leaflet of biological membranes, we exploited the accessibility of GM1 in reconstituted liposomes in the in vitro motility assay. When CeU446-PHDdUnc104 was combined with liposomes consisting of 5 mol% PtdIns(4,5)P2 and 10 mol% GM1, the frequency of movement was unaltered compared with liposomes lacking GM1 (Figure 3B). However, addition of 2 μg/ml CTX increased movement frequencies 40-fold when GM1 was present but had no measurable effect in its absence (Figure 3B, left graph). We also tested the effects of spermine, a small polyamine compound that clusters acidic phospholipids in model liposome membranes (Denisov et al., 1998). Again, 2 mM spermine increased transport frequencies by 3-fold (Figure 3B) but had no effect when added to liposomes composed exclusively of PC alone (not shown). Neither spermine nor GM1/CTX affected the binding of CeU446-PHDdUnc104 to 5 mol% PtdIns(4,5)P2-liposomes (Figure 3B, right graph). Therefore, several diverse agents that have been reported to cluster lipids (cholesterol/sphingomyelin, GM1/CTB, and spermine) all increase transport frequencies without increasing the amount of motor bound to the membrane.
Raft-like Behavior of Unc104 Motors Bound to Liposomes and Supported Lipid Bilayers
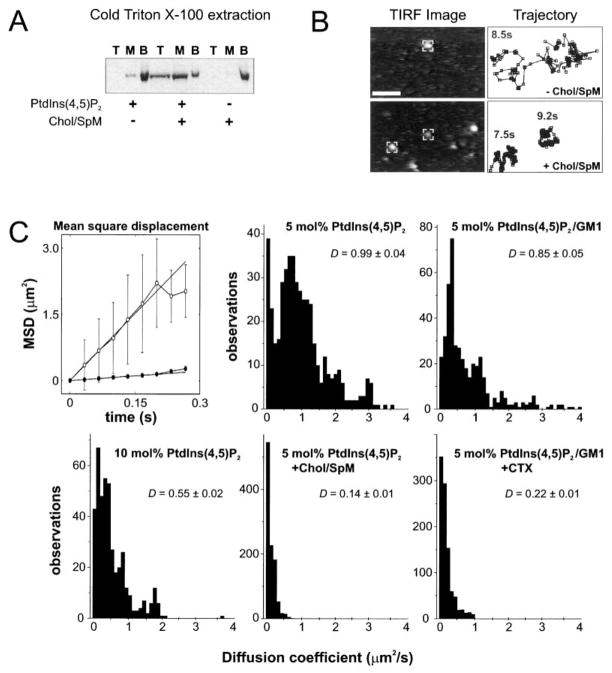
Previous studies have demonstrated that cholesterol and sphingomyelin promote the formation of lipid sub-domains (“rafts”) enriched in PtdIns(4,5)P2 (Caroni, 2001; Pike and Casey, 1996). Therefore, the enhanced Unc104-mediated liposome motility by cholesterol/sphingomyelin could be due to the partitioning of PtdIns(4,5)P2 bound motors into a lipid raft domain. Raft formation is most commonly tested by the partitioning of lipids/proteins into a cold 1% Triton X100-insoluble membrane fraction. In the presence of 5 mol% PtdIns(4,5)P2 without cholesterol/sphingomyelin, little CeU446-PHDdUnc104 was recovered from the 1% cold-Triton X100-resistant membrane fraction (Figure 4A). Similarly, little motor was recovered from this fraction with 40 mol% cholesterol/20 mol% sphingomyelin without 5 mol% PtdIns(4,5)P2, as the motor fails to bind to such liposomes. However, for liposomes composed of 5 mol% PtdIns(4,5)P2 and 40 mol% cholesterol/20 mol% sphingomyelin, the minimotor was recovered in the detergent-resistant fraction. The recovery in this fraction was ~50% of that observed with intact, nondetergent-treated liposomes (Figure 3A), indicating that a substantial fraction of Unc104 is bound to PtdIns(4,5)P2-associated lipid rafts under these conditions.
Figure 4. Raft-like Behavior of Unc104 Motor Bound to Liposomes or Supported Lipid Bilayers.
(A) 1% Triton X-100 (4°C) extraction of liposomes with bound CeU446-PHDdUnc104 as a biochemical assay of raft formation. The detergent extract was loaded at the bottom of a sucrose gradient, and after centrifugation, top, middle, and bottom (load) fractions were collected and analyzed by SDS-PAGE/Coomassie staining. The minimotor was recovered from detergent-resistant membrane top fraction only when both 5 mol% PtdIns(4,5)P2 and 40 mol% cholesterol/20 mol% sphingomyelin were present in liposomes.
(B) Visualization and tracking of single Cy3-labeled CeU446-PHDdUnc104 molecules bound to supported lipid bilayers containing 5 mol% PtdIns(4,5)P2 with or without 40 mol% cholesterol/20 mol% sphingomyelin (remainder PC). The left images show membrane bound motors as visualized by TIRF microscopy. The centroid positions of the “marked” fluorescent molecules were tracked using an automated procedure (Experimental Procedures); the centroid positions measured at 33 ms intervals are shown in the trajectory plots on the right (total time of measurement indicated). These typical plots reveal the restricted motion in the bilayers composed of 5 mol%Pt-dIns(4,5)P2/40 mol% cholesterol/20 mol% sphingomyelin. Very few fluorescent spots were observed on bilayers composed of 100% PC, indicating that binding was specific (not shown).
(C) Diffusion coefficients measured on supported membrane bilayers of different lipid coefficients. Examples of mean-square displacement plots calculated from single-molecule trajectories are shown in the absence (open) or presence (closed) of cholesterol/sphingomyelin. Diffusion coefficients (D) were measured as described in the Experimental Procedures; the histograms and the mean ± SD of the mean in μm2/s are shown. The lipid compositions and cholera toxin (CTX) concentration were the same as in Figure 3.
Although lipid raft subdomains have been reported to be small in size and highly dynamic (Pralle et al., 2000; Varma and Mayor, 1998), studies have demonstrated that proteins and lipids that partition into lipid rafts have lower two-dimensional diffusion coefficients compared to nonraft components (Dietrich et al., 2001; Pralle et al., 2000). We therefore examined whether the lateral diffusion of Unc104 motors bound to PtdIns(4,5)P2 is affected by cholesterol/sphingomyelin or GM1/CTX. To investigate this question, we labeled CeUnc446-PHDdUnc104 with Cy3 dye, visualized single fluorescently labeled motors bound to planar lipid bilayers supported on the surface of a quartz slide using total internal reflection microscopy, and tracked their movements using an automated centroid-tracking program (Figure 4B). Diffusion coefficients were determined from the initial slope of mean square displacement plots (Figure 4C, top left). CeUnc446-PHDdUnc104 molecules bound to 5 mol% PtdIns(4,5)P2 bilayers exhibited an average diffusion coefficient (D) of 0.99 ± 0.04 μm2/s (SEM) (Figure 4C). However, the histogram revealed several populations—a minor, less mobile population centered around 0.1 μm2/s, a major population centered around 0.9 μm2/s, and a minor, highly mobile population with diffusion coefficients of 1.5–3.5 μm2/s. In the presence of cholesterol/sphingomyelin, the average diffusion coefficient decreased ~7-fold to 0.14 ± 0.01 μm2/s, and the values were contained within a narrow range that corresponded to the minor, poorly mobile fraction observed with 5 mol% PtdIns(4,5)P2 alone (Figure 4C). Representative trajectories demonstrate the difference in the area roamed by a single motor in the absence or presence of cholesterol/sphingomyelin (Figure 4B). The addition of CTX to bilayers consisting of 5 mol% PtdIns(4,5)P2 and 10 mol% GM1 also resulted in a substantial reduction in D from 0.85 ± 0.05 μm2/s to 0.22 ± 0.01 μm2/s (Figure 4C). Multiple populations of diffusion coefficients were apparent again in 5 mol% PtdIns(4,5)P2/10 mol% GM1 (although different from 5 mol% PtdIns(4,5)P2 alone), but values were confined to a narrow range after CTX addition. A shift in D to lower values (0.55 ± 0.02 μm2/s) was also observed when the PtdIns(4,5)P2 content was increased from 5 to 10 mol%. Therefore, several conditions that enhanced the frequencies of Unc104-mediated liposome transport also restricted the lateral mobility of Unc104 bound to membrane surfaces.
The Molecular Basis of the Cooperative Switch for Transport
How could organized PtdIns(4,5)P2 lipid subdomains facilitate motor-driven transport? If Unc104 minimotors are nonprocessive, then a single molecule could not transport a liposome (Figure 5). However, a cluster of several Unc104 monomers could enable vesicle transport, as at least one motor would be contacting and producing movement along the microtubule during times when other motors are detached. An alternative possibility is that high local concentrations of Unc104 in a lipid raft facilitates motor dimerization (Figure 5), producing a processive Unc104 dimer that moves in a hand-over-hand manner as described for conventional kinesin (Vale and Milligan, 2000). The latter idea is suggested by the finding of a predicted coiled-coil adjacent to the motor domain in the same location as the neck coiled-coil of conventional kinesin (Vale and Fletterick, 1997). We therefore created two hydrophobic to charge mutations in the “a” (I362E) and “d” (L365K) positions of the first complete heptad of the predicted coiled-coil following the motor domain to inhibit or partially inhibit with motor dimerization and/or the mechanism of the motor dimer. Unlike wild-type CeU653-PHDdUnc104 (Figure 3C, insert), the mutant was unable to transport liposomes even at very high PtdIns(4,5)P2 concentrations (Figure 3C), although it bound to the same extent as the wild-type motor (not shown). Addition of cholesterol/sphingolipid allowed movement with a steep dependence on PtdIns(4,5)P2 but at much higher concentrations than observed with the nonmutated motor (Figure 3C). The transport velocity for the mutant was somewhat lower (1.1 ± 0.3 μm/s) than wild-type (1.6 ± 0.1 μm/s). We also investigated the behavior of native DdUnc104, which, unlike mouse KIF1A and C. elegans Unc104, is a constitutive dimer due to a coiled-coil extension at its C terminus. Liposome movement by dimeric native DdUnc104 did not display a cooperative transition, and cholesterol/sphingomyelin addition had little effect on movement (Figure 3C). Collectively, these results suggest that motor dimerization via coiled-coil formation may be facilitated when motor proteins become clustered in lipid raft domains.
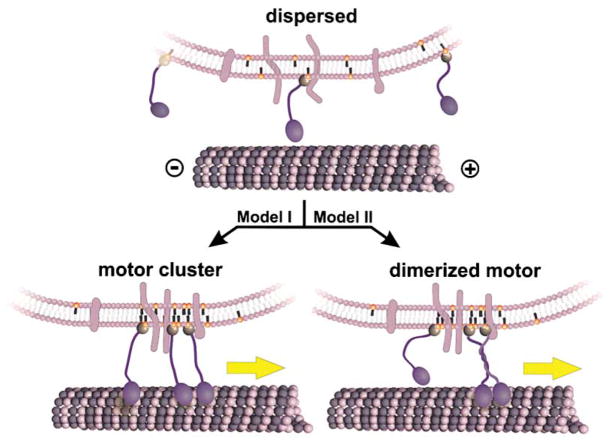
Figure 5. Models for the Regulation of Unc104-Mediated Membrane Transport by PtdIns(4,5)P2 Lipid Clustering.
A membrane cargo containing PtdIns(4,5)P2 lipids (orange head groups), transmembrane proteins (light blue), and bound Unc104 motors (dark blue) is shown. In the absence of sufficient PtdIns(4,5)P2 density or clustering, Unc104 motors are dispersed. Since the Unc104 motor is not processive, individual motors transiently interact with the microtubule, but this is insufficient to move the vesicle continuously along the microtubule. PtdIns(4,5)P2 organization into membrane rafts could activate transport by two potential mechanisms. (Model I) Several Unc104 monomeric motors are brought together in a cluster and acting together can maintain attachment to the microtubule and initiate transport. (Model II) Unc104 is a monomer in solution, but the concentration of several motors in a cluster may cause dimerization via predicted coiled-coil regions adjacent to the motor domain. A single, dimerized motor then could move along the microtubule by a hand-over-hand mechanism and processively transport the vesicle.
Discussion
In this study, we have dissected the mechanism of membrane transport by Unc104 kinesin, an important vesicle transport motor that is conserved from unicellular organisms to man. In contrast to other reported in vitro vesicle transport systems (Muresan et al., 2001; Schroer and Sheetz, 1991), Unc104-mediated transport can be reconstituted with purified vesicles and motor protein and no additional soluble components (Pollock et al., 1999). In this study, we have shown that the native motor can be substituted by bacterially-expressed minimotors containing a functional motor domain and a minimal membrane cargo binding region, which has enabled a structure-function dissection of membrane attachment through mutational analysis. We have also demonstrated that highly efficient transport can be achieved by liposomes in place of native vesicles, which has allowed a mechanistic dissection of how lipids influence transport. The combination of these methodologies have allowed us to identify the PH domain as the cargo binding region of Unc104, to analyze the roles for lipids and vesicle proteins in transport, and to uncover a regulatory mechanism for activating transport based upon lipid organization.
The PH Domain Is a Cargo Binding Region of Unc104
Unc104 motors are unusually large motors within the kinesin superfamily (>190 kDa). The motor domain is contained within the first ~40 kDa, but the functions of the remainder of the polypeptide chain have not been elucidated. Here, we demonstrate that the fusion of PHDdUnc104 to the motor domain of either C. elegans Unc104 or Ncd (a meiotic/mitotic motor with no known function in vesicle transport) is sufficient to reconstitute transport of native vesicles. These results indicate that neither the FHA domain nor the long region between the FHA and PH domains is necessary for membrane transport in this reconstituted assay, although these regions could play important regulatory roles in vivo. Our attempts to address these questions by rescuing DdUnc104 null cells with plasmids expressing this motor, however, have been unsuccessful to date.
Through binding and motility assays, we also show that the PHDdUnc104 domain is capable of binding directly to phoshoinositide lipids with selectivity for PtdIns(4,5)P2. The importance of lipid binding in the transport mechanism is suggested by the findings that neomycin (which binds PtdIns(4,5)2) and soluble IP3 both inhibit native vesicle movement by DdUnc104. In further support of this idea, a mutant PHDdUnc104 domain with diminished PtdIns(4,5)P2 binding capability cannot fully reconstitute native vesicle transport, and conversely, minimotors containing a PtdIns(4,5)P2 binding PH domain from an-other protein (PLCδ1) can reconstitute vesicle transport (albeit with lower velocity).
While lipid binding by the PH domain is important, vesicle-associated proteins also play a role in Unc104-mediated transport, since trypsinization of native vesicles decreases transport efficiency by half. Furthermore, a minimotor with a PH domain defective in PtdIns(4,5)P2 binding can still move native vesicles, albeit at 50% efficiency. The identities and functions of these proteins are unknown and will require further investigation.
Lipid Clustering Enhances Transport Efficiency
Using our defined liposome transport assay with monomeric motors, we unexpectedly discovered that liposome movement shows an “on-off” switch-like transition for transport as the concentration of PtdIns(4,5)P2 in the membrane is increased. Once transport occurs above the critical PtdIns(4,5)P2 threshold, movement takes place at near maximal velocity and processivity.
The switch-like behavior in transport is not due to cooperative binding of motors, but rather appears to involve the creation of clustered microdomains of PtdIns(4,5)P2. This hypothesis is supported by the finding that three distinct methods of clustering lipids (cholesterol/sphingomyelin, GM1 lipid in the presence of cholera toxin, and spermine) all enhance motility at low concentrations of PtdIns(4,5)P2. The steep transition point observed for motility could potentially reflect a “critical concentration” of PtdIns(4,5)P2 that allows the formation of raft microdomains.
The notion that lipid organization underlies the switch-like behavior in transport is supported by our biochemical and microscopic studies of Unc104 motors bound to membranes. Raft formation, as defined by detergent resistance, was observed for Unc104 motors bound to membranes containing PtdIns(4,5)P2 and cholesterol/sphingolipid. Furthermore, single Unc104 motors bound to supported lipid bilayers show lower diffusion coefficients in the presence of cholesterol/sphingolipid, GM1/CTX, or high concentrations of PtdIns(4,5)P2, conditions that all enhance motility at lower PtdIns(4,5)P2 concentrations. Although less pronounced than the results described here, other studies have measured a 2-fold decrease in the diffusion coefficients of lipids in raft domains in supported bilayers (Dietrich et al., 2001) and for raft-associated proteins in vivo (Pralle et al., 2000).
Vesicle Transport and the Biophysical Properties of Unc104 Motors
Monomeric mouse KIF1A motors have been reported to be processive using single-molecule fluorescence assays (Okada and Hirokawa, 1999, 2000). In such a case, a single motor should suffice for vesicle transport (Howard et al., 1989), and the type of cooperative transport that we observed would not be expected. We therefore believe that a single monomeric Unc104 from metazoan organisms is incapable of efficient, long-range transport. The processivity observed for mouse KIF1A resembles a biased diffusional motion, which results in a much lower velocity of motion (0.14 μm/s) than observed for native Unc104/KIF1A or longer motor constructs (Okada et al., 1995; Pierce et al., 1999; Pollock et al., 1999). Such biased diffusional motion has not been observed for vesicles either in our reconstituted assays or in axons of living worms (Zhou et al., 2001) and consequently may reflect an unusual behavior of the particular truncated KIF1A protein used in these studies.
Two possible models could allow Unc104 motors to transport a vesicle processively: Unc104 monomer clustering and facilitated dimerization (Figure 5). While the detachment of a single motor from the microtubule would interrupt vesicle transport, clustering of several monomeric motors could enable continuous motion. Such a mechanism has been suggested for a closely related monomeric kinesin motor called KIF1D (Rogers et al., 2001). The alternative possibility of motor dimerization is suggested by a predicted coiled-coil adjacent to the catalytic core in position corresponding to the neck coiled-coil of conventional kinesin (Vale and Fletterick, 1997). This putative Unc104 coiled-coil must be weak, since the motor behaves as a monomer under the concentrations in which hydrodynamic experiments are performed. However, when a high local concentration of motors is achieved in a lipid raft, the motor could dimerize and move by the hand-over-hand mechanism proposed for conventional kinesin (Vale and Milligan, 2000). In favor of this latter idea, introducing two point mutations destabilizing the first heptad of the predicted coiled-coil dramatically decreases the ability of motor to transport PtdIns(4,5)P2 liposomes. Moreover, the dimeric DdUnc104 motor does not show the switch-like behavior for transporting liposomes with increasing PtdIns(4,5)P2 concentration. However, additional experiments will be required to convincingly distinguish between these two proposed models.
Regulation of vesicle movement in cells has been generally thought to involve either turning the motor activity on or off (Reilein et al., 2001) or regulating motor interaction with a receptor (Sheetz, 1999). Our findings raise the possibility of yet another regulatory mechanism that involves the generation of a high local concentration of motors on a membrane surface through clustering into lipid subdomains or rafts. The steady-state cellular concentration of PtdIns(4,5)P2 is generally below 1 mol% (Cohn et al., 1988; Van der Kaay et al., 1990), and these levels would not be expected to allow transport by Unc104 motors based upon our in vitro transport results. However, high local concentrations of PtdIns(4,5)P2 could be achieved by recruiting PtdIns-kinases and -phosphatases to membranes (Toker, 1998) or proteins that can cluster PtdIns(4,5)P2 (Laux et al., 2000; Wang et al., 2001). Activating transport by generating high local motor concentrations represents a possible model for regulating the various kinesin motors belonging to the largely monomeric KIF1 class. However, the slime mold Dictyostelium has added an extended coiled-coil domain to the C terminus of its Unc104, which suggests that it has evolved an alternative regulatory mechanism.
Relevance to Other Systems
Many other proteins involved in membrane motility have the potential to bind PtdIns(4,5)P2 lipids directly, including the actin-based motor myosin X (Berg et al., 2000), the proposed dynein receptor axonal spectrin (Muresan et al., 2001), and N-WASP, which causes actin-based endosome rocketing (Taunton et al., 2000). In addition, a much larger group of proteins involved in signaling, trafficking, and motility have been shown to partition in lipid rafts (Ikonen, 2001; Noda et al., 2001; Simons and Ikonen, 1997). In many of these instances, aggregation of proteins into lipid subdomains has been proposed to facilitate biochemical reactions by increasing local protein concentrations. Our results illustrate how raft formation can control a process (in our case motility) if the process involves cooperative protein-protein interactions. Switch-like behavior, similar to the one described here, may occur for other proteins with cooperative activation mechanisms that tend to accumulate within lipid raft domains.
Experimental Procedures
Materials
The following lipids were purchased from Avanti Polar lipids (Birmingham, AL): egg PC(Cat. #840051), PtdIns(4)P (#840045), PtdIns(4,5)P2 (#840046), PS (#840032), PE (#840022), PG (#841138), and PA (#840101); PtdIns(3)P (#1773) and PtdIns(3,4,5)P3 (#1775) were from Matreya Inc. (State Collage, PA); and brain lipid extract (#B1627), sphingomyelin (#S0756), and cholesterol (#47127U) were obtained from Sigma (St. Louis, MO). Cholera toxin B subunit and spermine also were obtained from Sigma.
Cell Culture and Affinity Purification of DdUnc104
Dictyostelium strain Ax-2 and Ax-2 DdUnc104 null cells were grown, and a microtubule affinity-purified motor fraction was prepared as previously described (Pollock et al., 1998). The motor fraction was collected and either assayed for motility directly (see below) or frozen in liquid nitrogen. This fraction contains two predominant plus-end-directed vesicle transport kinesins, one of which is DdUnc104 and the second is a 160 kDa conventional kinesin (unpublished results). To determine the contribution of DdUnc104 in our assays, we prepared a microtubule affinity-purified motor fraction from DdUnc104 null cells by the same procedure.
Vesicle and Liposome Preparation
Dictyostelium vesicles were prepared as previously described (Pollock et al., 1998). Liposomes were prepared by dissolving 10 μmol lipids in chloroform followed by evaporation in 13 × 100 mm borosilicate screw cap vials under a constant Argon stream. The lipid film was dried under high vacuum overnight and lipids were rehydrated by addition of LBN (30 mM Tris [pH 8.0], 4 mM EGTA,150 mM NaCl, 5% sucrose). Ten freeze/thawing cycles were performed, and the liposomes were extruded through a 100 nm pore polycarbonate filter (Avestin; Ottawa, Canada) using a miniextruder from Avanti Polar Lipids. Liposome diameter size was verified by light scattering using a Zetasizer 1000 (Malvern Instruments, Inc.; Southborough, MA). Liposomes were stored in the dark at 4°C under an atmosphere of argon and used within a week.
Bacterial Protein Expression and Purification
The PH domain (aa 1523–1623) of DdUnc104 was cloned using standard PCR into a modified pET17b vector (Novagen; Madison, WI) with a 3′ flanking GFP followed by a His6 tag (PHDdUnc104-GFP). Lysines and arginines in the PHDdUnc104 domain were mutated using QuickChange mutagenesis (Stratagene; La Jolla, CA). Minimotors (CeU653-PHDdUnc104, CeU446-PHDdUnc104, CeU653-PHDAPPI, and CeU653-PHPLCδ1) were cloned from a C. elegans Unc104 construct (Pierce et al., 1999) with the indicated PH domain-His6 extension following the C-terminal Unc104 residue. PHDdUnc104-Ncd (in pET17b) was prepared by cloning the PHDdUnc104 domain into the KpnI site adjacent to the His6-GFP-start methionine of the Ncd construct (aa 235–700) (Case et al., 1997). All constructs were verified by DNA sequencing.
Proteins were expressed and purified by Ni-NTA chromatography (QIAGEN; Valencia, CA) followed by HiTrap-Q or HiTrap-S ion exchange chromatography (Amersham Pharmacia Biotech; Piscataway, NJ) as described (Case et al., 1997). Immediately after a microtubule bind and release affinity-purification step (Case et al., 1997), motors were either assayed or were frozen with 10% sucrose added and stored in liquid nitrogen.
For single-molecule microscopy, CeU446-PHDdUnc104 was labeled to a stochiometry of ~1.5 dye per protein using a Cy3 dye coupled to a monofunctional NHS-ester which reacts with primary amine groups (Amersham Pharmacia Biotech). Labeled protein was separated from free dye by Sephadex G25 gel filtration.
Membrane Binding and Lipid Raft Assays
Vesicles (100 μl of A280 ~2) or liposomes (100 μl of 10 mM total lipid concentration) were incubated on ice for 30 min with 1.5 μg minimotor or 1 μg PHDdUnc104-GFP. LB buffer (30 mM Tris, 4 mM EGTA, 2 M sucrose [pH 8.0]) was added to the incubation reaction to bring the final sucrose concentration to 1.6 M, and this mixture was overlaid with cushions containing 1.4 M, 0.4 M, and 0.25 M sucrose in the same buffer in a TLS-55 tube. After centrifugation at 201,000 × g (4°C) for 30 min in a TLS-55 rotor (Beckman), the 0.25/0.4 M interphase (top fraction), 0.4 M/1.4 M interphase (middle fraction), and the loading fractions were collected and analyzed by SDS-PAGE followed by either Coomassie-stained or immunoblot analysis (GFP constructs were detected with affinity-purified, polyclonal GFP antibodies using chemiluminescence reagents. Gels or films were digitized by flatbed scanning, and protein bands were quantified using ImageJ 1.26 software (NIH; Bethesda, MD). Binding efficiency was calculated by comparing the specific binding values to PtdIns(4,5)P2 versus PC liposomes, subtracting the corresponding nonspecific binding value from the PtdIns(4,5)P2 binding, and was expressed as the ratio between wild-type and mutant PH domain.
Association of CeUnc446 with lipid rafts were analyzed by sucrose gradients as described above except that the liposomes were lysed with 1% Triton X-100 for 40 min on ice and 1% Triton X-100 was included in the gradient that was centrifuged at 201,000 × g for 3 hr at 4°C.
In Vitro Membrane Transport Assay and DIC Microscopy
The details of this assay have been described elsewhere (Pollock et al., 1998). In brief, sea urchin sperm axoneme-nucleated microtubule structures were prepared in flow cells (10 μl) as described previously. KI-washed vesicles (0.3 M), a mixed population isolated from the crude extract, were isolated as described (Pollock et al., 1998). For the membrane transport assay, the flow cell was first washed with 10 μl of LB [pH 8.0]/15% sucrose, followed by the introduction of a 10 μl assay mix consisting of 5 μl purified motors from native source or recombinant expression, 3.0 μl LB/15% sucrose, 1 μl KI-washed vesicles or 1 μl of liposomes (0.5 mM final lipid concentration), 0.5 μl casein (20 mg/ml), and 0.5 μl ATP-regenerating mix. The movement of membranes was observed using a microscope (Axioplan; Carl Zeiss, Inc.) equipped with differential interference contrast (DIC) optics, a Newvicon camera (Hamamatsu Photonics), and an image processor (Argus10; Hamamatsu Photonics). Recordings were made with a S-VHS video tape recorder. Directionality of membrane movement was determined on axonemes with clearly identified long (plus end) and short (minus end) microtubules emerging from their ends. Transport frequency (as movements per min per axoneme) and velocity was analyzed using special projections of the image stack (referred to as “kymographs”): in an image stack, a line was drawn over a microtubule of interest and the image of that line as a function of time was obtained using ImageJ 1.26 software (NIH; Bethesda, MD). Velocities were calculated from these kymograph records by measuring the slope of the line for periods of persistent movement.
Single-Molecule Microscopy on Lipid Bilayers
Individual Unc104 minimotors were visualized using a custom-built total internal reflection fluorescence microscope as described previously (Pierce et al., 1999). A liposome suspension (5 μl of a 10 mM total lipid concentration, average liposome diameter 100 nm) was added to acid-washed quartz slides and incubated for 20 min. The spontaneous formation of supported bilayers was ended by removing excess liposomes with four buffer washes (200 μl of 10 mM Tris [pH 8.0], 4 mM EGTA, 5% sucrose, 2 mg/ml casein). Labeled Cy3-motors at 0.2 nM was added to the bilayer, incubated for 20 min at room temperature, and washed once with 200 μl of buffer. Cy3-molecules were illuminated with an argon laser (514 nm) at 5 mW. The fluorescent images were recorded onto S-VHS tape, and images were digitized (30 frames/s) and analyzed on a computer using the following custom macros. Fluorescent particles were first tracked in digitized video sequences using crosscorrelation with an experimentally obtained, diffraction-limited spot image and centroid analysis as described (Gelles et al., 1988). The algorithm was implemented in a set of Matlab (The Mathworks, Inc.; Natick, MA) scripts. In all cases, the accuracy of the tracking algorithm was checked visually, and, when needed, input parameters (search field for the crosscorrelation analysis, minimum correlation coefficient, and minimum average pixel intensity for endpoint determination) were adjusted to provide optimal tracking. Particle trajectories over 0.5 s long overlapping time windows were used to calculate diffusion coefficients by linear least square fitting of the mean square displacement of the particle using the first four data points (0.033–0.132 s) of the mean square displacement. Only those diffusion coefficients with a maximum error between the fitted and observed data less than 7.5% of the observed data were recorded. Between 30 and 50 individual particles were tracked for each bilayer composition, resulting in more than 400 diffusion coefficients for all conditions.
Supplementary Material
Acknowledgments
We would like to thank Adam Douglass for advice and expertise on lipid bilayers, Dr. Frank Szoka for advice on liposome preparation, Jeremy Epstein for data analysis, and Dr. Mark Lemmon for providing PH constructs. This work was supported by a European Molecular Biology Organization long-term fellowship (ALTF161-1999) to D.R.K. and a National Institutes of Health grant (38499) to R.D.V.
References
- Berg JS, Derfler BH, Pennisi CM, Corey DP, Cheney RE. Myosin-X, a novel myosin with pleckstrin homology domains, associates with regions of dynamic actin. J Cell Sci. 2000;113:3439–3451. doi: 10.1242/jcs.113.19.3439. [DOI] [PubMed] [Google Scholar]
- Bloom GS. The UNC-104/KIF1 family of kinesins. Curr Opin Cell Biol. 2001;13:36–40. doi: 10.1016/s0955-0674(00)00171-x. [DOI] [PubMed] [Google Scholar]
- Caroni P. Actin cytoskeleton regulation through modulation of PI(4,5)P(2) rafts. EMBO J. 2001;20:4332–4336. doi: 10.1093/emboj/20.16.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case RB, Pierce DW, Hom-Booher N, Hart CL, Vale RD. The directional preference of kinesin motors is specified by an element outside of the motor catalytic domain. Cell. 1997;90:959–966. doi: 10.1016/s0092-8674(00)80360-8. [DOI] [PubMed] [Google Scholar]
- Cohn RC, Poncz L, Waller RL, Dearborn DG. Phosphoinositide content of erythrocyte membranes in cystic fibrosis. J Lab Clin Med. 1988;111:336–340. [PubMed] [Google Scholar]
- Denisov G, Wanaski S, Luan P, Glaser M, McLaughlin S. Binding of basic peptides to membranes produces lateral domains enriched in the acidic lipids phosphatidylserine and phosphatidylinositol 4,5-bisphosphate: an electrostatic model and experimental results. Biophys J. 1998;74:731–744. doi: 10.1016/S0006-3495(98)73998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich C, Bagatolli LA, Volovyk ZN, Thompson NL, Levi M, Jacobson K, Gratton E. Lipid rafts reconstituted in model membranes. Biophys J. 2001;80:1417–1428. doi: 10.1016/S0006-3495(01)76114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelles J, Schnapp BJ, Sheetz MP. Tracking kinesin-driven movements with nanometre-scale precision. Nature. 1988;331:450–453. doi: 10.1038/331450a0. [DOI] [PubMed] [Google Scholar]
- Goldstein LS. Molecular motors: from one motor many tails to one motor many tales. Trends Cell Biol. 2001;11:477–482. doi: 10.1016/s0962-8924(01)02143-2. [DOI] [PubMed] [Google Scholar]
- Hall DH, Hedgecock EM. Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell. 1991;65:837–847. doi: 10.1016/0092-8674(91)90391-b. [DOI] [PubMed] [Google Scholar]
- Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- Howard J, Hudspeth AJ, Vale RD. Movement of microtubules by single kinesin molecules. Nature. 1989;342:154–158. doi: 10.1038/342154a0. [DOI] [PubMed] [Google Scholar]
- Ikonen E. Roles of lipid rafts in membrane transport. Curr Opin Cell Biol. 2001;13:470–477. doi: 10.1016/s0955-0674(00)00238-6. [DOI] [PubMed] [Google Scholar]
- Kavran JM, Klein DE, Lee A, Falasca M, Isakoff SJ, Skolnik EY, Lemmon MA. Specificity and promiscuity in phosphoinositide binding by pleckstrin homology domains. J Biol Chem. 1998;273:30497–30508. doi: 10.1074/jbc.273.46.30497. [DOI] [PubMed] [Google Scholar]
- Kikkawa M, Sablin EP, Okada Y, Yajima H, Fletterick RJ, Hirokawa N. Switch-based mechanism of kinesin motors. Nature. 2001;411:439–445. doi: 10.1038/35078000. [DOI] [PubMed] [Google Scholar]
- Laux T, Fukami K, Thelen M, Golub T, Frey D, Caroni P. GAP43, MARCKS, and CAP23 modulate PI(4,5)P(2) at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism. J Cell Biol. 2000;149:1455–1472. doi: 10.1083/jcb.149.7.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA. Structural basis for high-affinity phosphoinositide binding by pleckstrin homology domains. Biochem Soc Trans. 1999;27:617–624. doi: 10.1042/bst0270617. [DOI] [PubMed] [Google Scholar]
- Martin TF. PI(4,5)P(2) regulation of surface membrane traffic. Curr Opin Cell Biol. 2001;13:493–499. doi: 10.1016/s0955-0674(00)00241-6. [DOI] [PubMed] [Google Scholar]
- Miki H, Setou M, Kaneshiro K, Hirokawa N. All kinesin superfamily protein, KIF, genes in mouse and human. Proc Natl Acad Sci USA. 2001;98:7004–7011. doi: 10.1073/pnas.111145398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muresan V, Stankewich MC, Steffen W, Morrow JS, Holzbaur EL, Schnapp BJ. Dynactin-dependent, dynein-driven vesicle transport in the absence of membrane proteins: a role for spectrin and acidic phospholipids. Mol Cell. 2001;7:173–183. doi: 10.1016/s1097-2765(01)00165-4. [DOI] [PubMed] [Google Scholar]
- Noda Y, Okada Y, Saito N, Setou M, Xu Y, Zhang Z, Hirokawa N. KIFC3, a microtubule minus end-directed motor for the apical transport of annexin XIIIb-associated Triton-insoluble membranes. J Cell Biol. 2001;155:77–88. doi: 10.1083/jcb.200108042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Hirokawa N. A processive single-headed motor: kinesin superfamily protein KIF1A. Science. 1999;283:1152–1157. doi: 10.1126/science.283.5405.1152. [DOI] [PubMed] [Google Scholar]
- Okada Y, Hirokawa N. Mechanism of the single-headed processivity: diffusional anchoring between the K-loop of kinesin and the C terminus of tubulin. Proc Natl Acad Sci USA. 2000;97:640–645. doi: 10.1073/pnas.97.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Yamazaki H, Sekine-Aizawa Y, Hirokawa N. The neuron-specific kinesin superfamily protein KIF1A is a unique monomeric motor for anterograde axonal transport of synaptic vesicle precursors. Cell. 1995;81:769–780. doi: 10.1016/0092-8674(95)90538-3. [DOI] [PubMed] [Google Scholar]
- Otsuka AJ, Jeyaprakash A, Garcia-Anoveros J, Tang LZ, Fisk G, Hartshorne T, Franco R, Born T. The C. elegans unc-104 gene encodes a putative kinesin heavy chain-like protein. Neuron. 1991;6:113–122. doi: 10.1016/0896-6273(91)90126-k. [DOI] [PubMed] [Google Scholar]
- Pierce DW, Hom-Booher N, Otsuka AJ, Vale RD. Single-molecule behavior of monomeric and heteromeric kinesins. Biochemistry. 1999;38:5412–5421. doi: 10.1021/bi9830009. [DOI] [PubMed] [Google Scholar]
- Pike LJ, Casey L. Localization and turnover of phosphatidylinositol 4,5-bisphosphate in caveolin-enriched membrane domains. J Biol Chem. 1996;271:26453–26456. doi: 10.1074/jbc.271.43.26453. [DOI] [PubMed] [Google Scholar]
- Pollock N, Koonce MP, de Hostos EL, Vale RD. In vitro microtubule-based organelle transport in wild-type Dictyostelium and cells overexpressing a truncated dynein heavy chain. Cell Motil Cytoskeleton. 1998;40:304–314. doi: 10.1002/(SICI)1097-0169(1998)40:3<304::AID-CM8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Pollock N, de Hostos EL, Turck CW, Vale RD. Reconstitution of membrane transport powered by a novel dimeric kinesin motor of the Unc104/KIF1A family purified from Dictyostelium. J Cell Biol. 1999;147:493–506. doi: 10.1083/jcb.147.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pralle A, Keller P, Florin EL, Simons K, Horber JK. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J Cell Biol. 2000;148:997–1008. doi: 10.1083/jcb.148.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilein AR, Rogers SL, Tuma MC, Gelfand VI. Regulation of molecular motor proteins. Int Rev Cytol. 2001;204:179–238. doi: 10.1016/s0074-7696(01)04005-0. [DOI] [PubMed] [Google Scholar]
- Rogers KR, Weiss S, Crevel I, Brophy PJ, Geeves M, Cross R. KIF1D is a fast non-processive kinesin that demonstrates novel K-loop-dependent mechanochemistry. EMBO J. 2001;20:5101–5113. doi: 10.1093/emboj/20.18.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakowicz R, Farlow S, Goldstein LS. Cloning and expression of kinesins from the thermophilic fungus Thermomyces lanuginosus. Protein Sci. 1999;8:2705–2710. doi: 10.1110/ps.8.12.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhoff R, Brugger B, Jeckel D, Lehmann WD, Wieland FT. Determination of cholesterol at the low picomole level by nano-electrospray ionization tandem mass spectrometry. J Lipid Res. 1999;40:126–132. [PubMed] [Google Scholar]
- Schroer TA, Sheetz MP. Two activators of microtu-bule-based vesicle transport. J Cell Biol. 1991;115:1309–1318. doi: 10.1083/jcb.115.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz MP. Motor and cargo interactions. Eur J Biochem. 1999;262:19–25. doi: 10.1046/j.1432-1327.1999.00340.x. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Taunton J, Rowning BA, Coughlin ML, Wu M, Moon RT, Mitchison TJ, Larabell CA. Actin-dependent propulsion of endosomes and lysosomes by recruitment of N-WASP. J Cell Biol. 2000;148:519–530. doi: 10.1083/jcb.148.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker A. The synthesis and cellular roles of phosphatidylinositol 4,5-bisphosphate. Curr Opin Cell Biol. 1998;10:254–261. doi: 10.1016/s0955-0674(98)80148-8. [DOI] [PubMed] [Google Scholar]
- Vale RD, Fletterick RJ. The design plan of kinesin motors. Annu Rev Cell Dev Biol. 1997;13:745–777. doi: 10.1146/annurev.cellbio.13.1.745. [DOI] [PubMed] [Google Scholar]
- Vale RD, Milligan RA. The way things move: looking under the hood of molecular motor proteins. Science. 2000;288:88–95. doi: 10.1126/science.288.5463.88. [DOI] [PubMed] [Google Scholar]
- Van der Kaay J, Draijer R, Van Haastert PJ. Increased conversion of phosphatidylinositol to phosphatidylinositol phosphate in Dictyostelium cells expressing a mutated ras gene. Proc Natl Acad Sci USA. 1990;87:9197–9201. doi: 10.1073/pnas.87.23.9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heyningen WE, King CA. The role of gangliosides in the action of cholera toxin. Adv Exp Med Biol. 1976;71:205–214. doi: 10.1007/978-1-4614-4614-9_13. [DOI] [PubMed] [Google Scholar]
- Varma R, Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- Wang J, Arbuzova A, Hangyas-Mihalyne G, McLaughlin S. The effector domain of myristoylated alanine-rich C kinase substrate binds strongly to phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2001;276:5012–5019. doi: 10.1074/jbc.M008355200. [DOI] [PubMed] [Google Scholar]
- Yonekawa Y, Harada A, Okada Y, Funakoshi T, Kanai Y, Takei Y, Terada S, Noda T, Hirokawa N. Defect in synaptic vesicle precursor transport and neuronal cell death in KIF1A motor protein-deficient mice. J Cell Biol. 1998;141:431–441. doi: 10.1083/jcb.141.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HM, Brust-Mascher I, Scholey JM. Direct visualization of the movement of the monomeric axonal transport motor UNC-104 along neuronal processes in living Caenorhabditis elegans. J Neurosci. 2001;21:3749–3755. doi: 10.1523/JNEUROSCI.21-11-03749.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.