Abstract
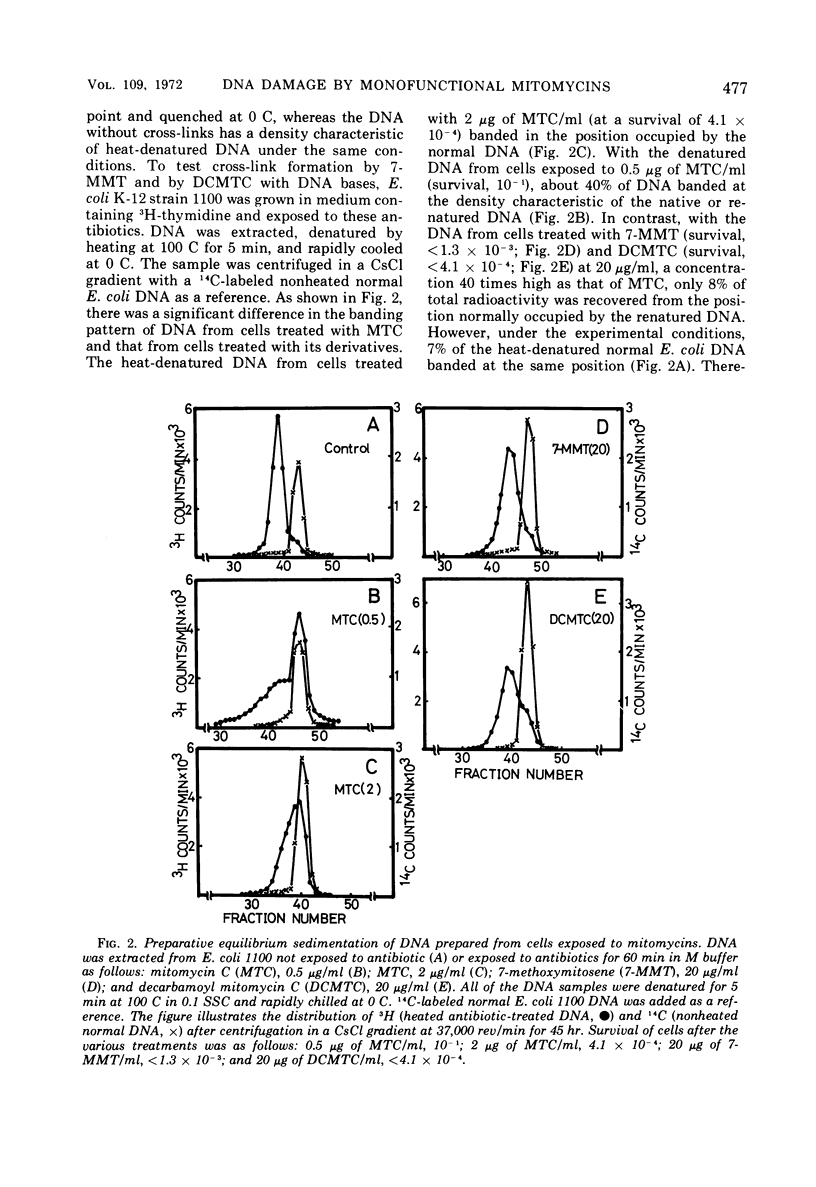
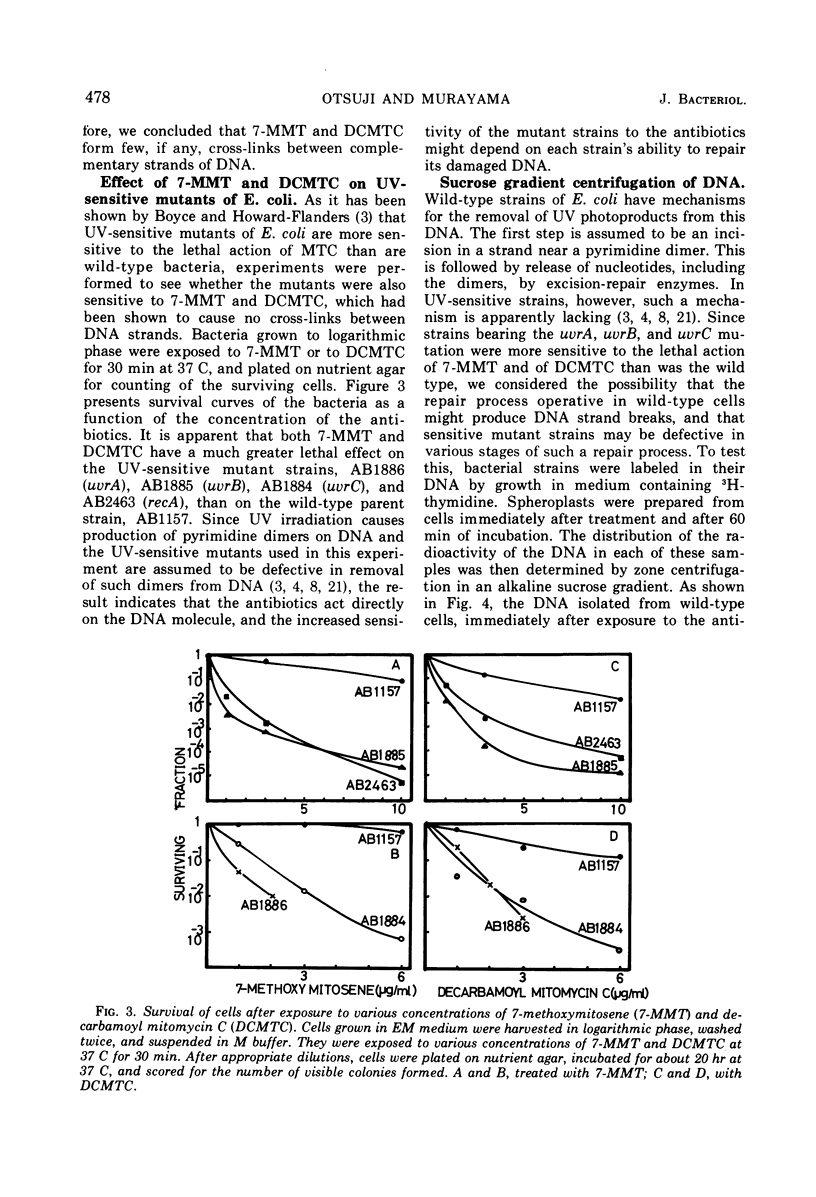
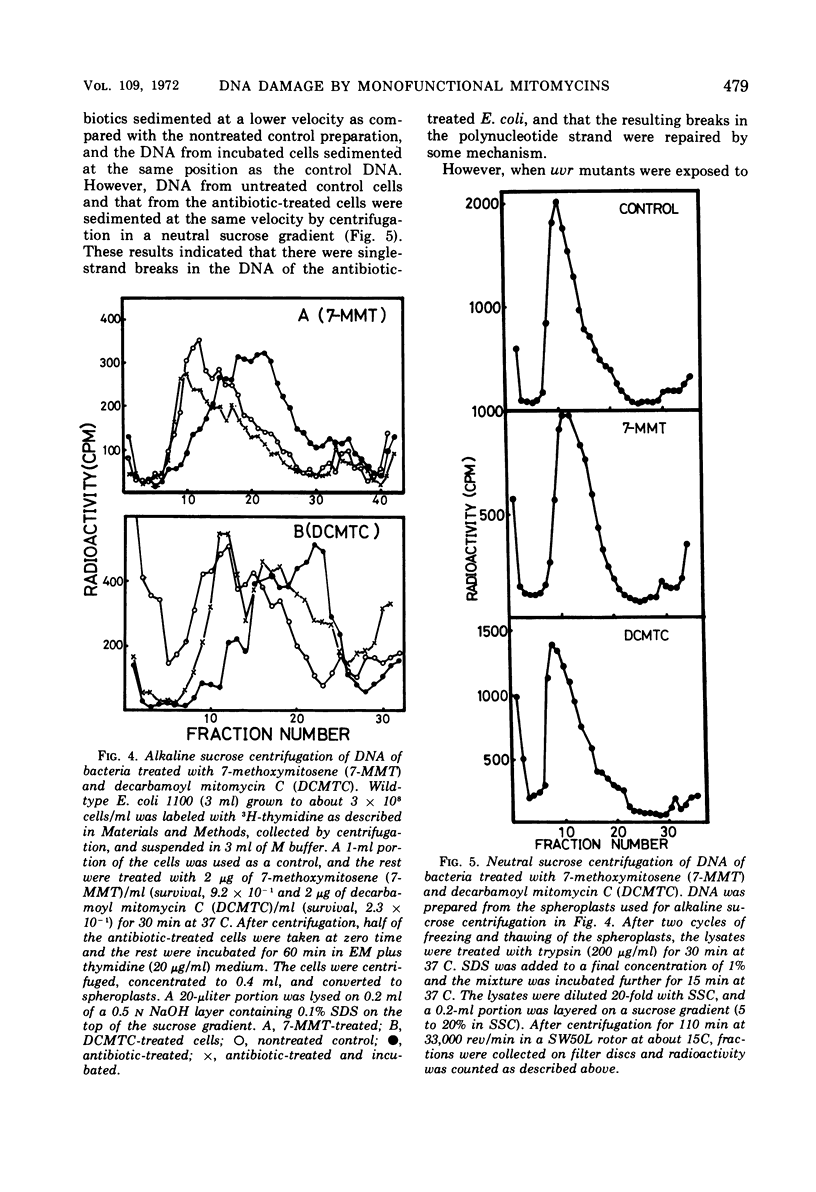
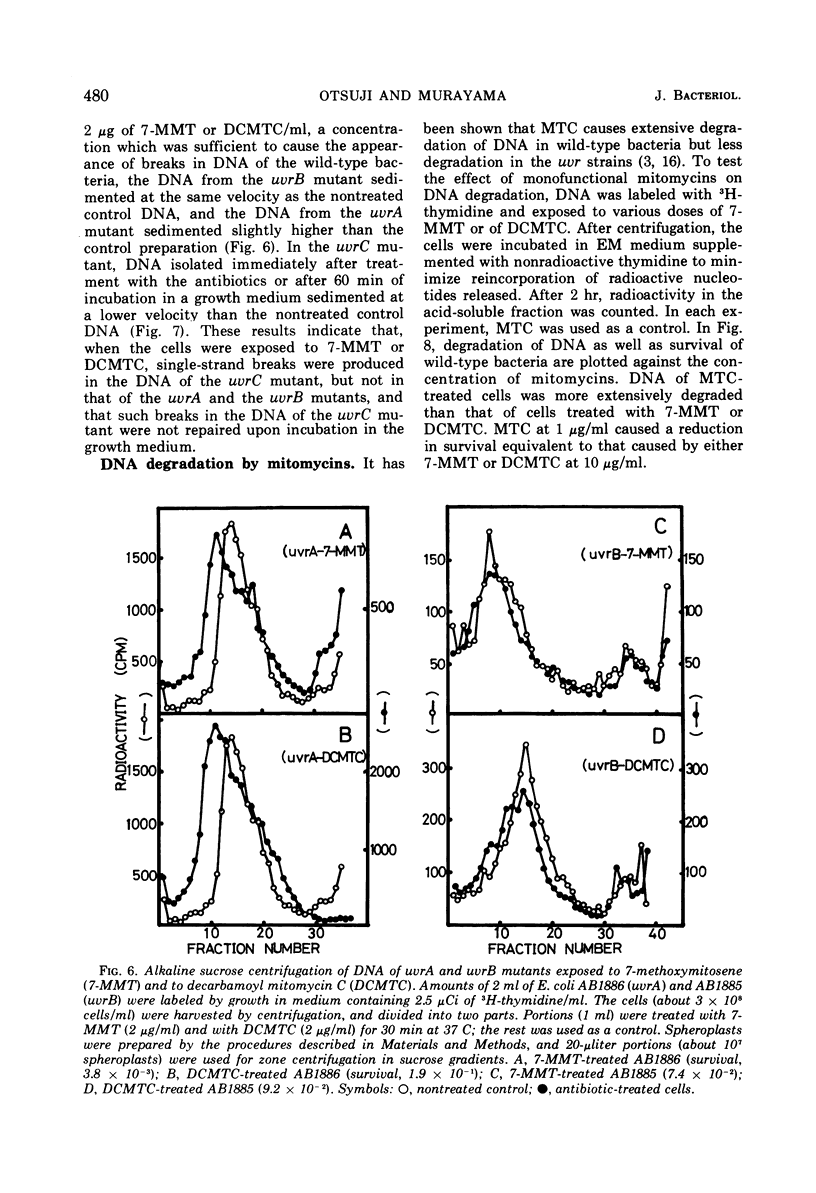
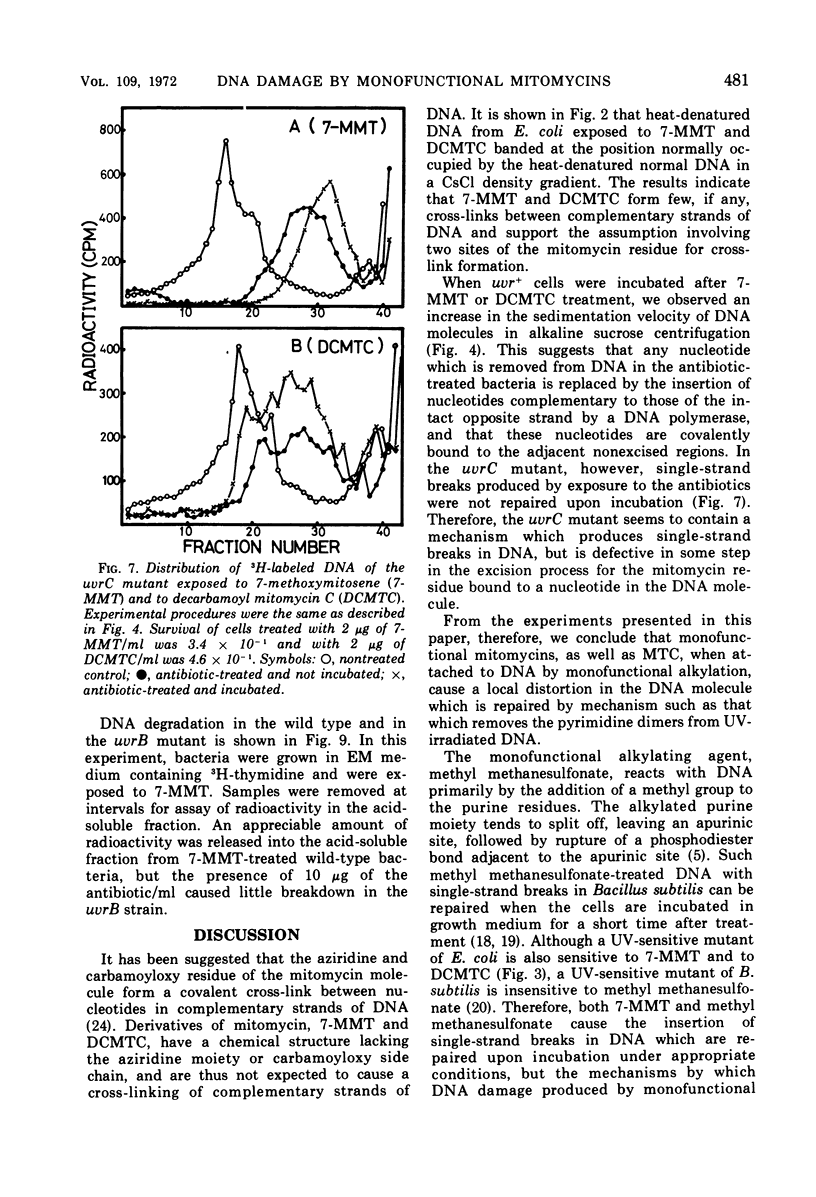
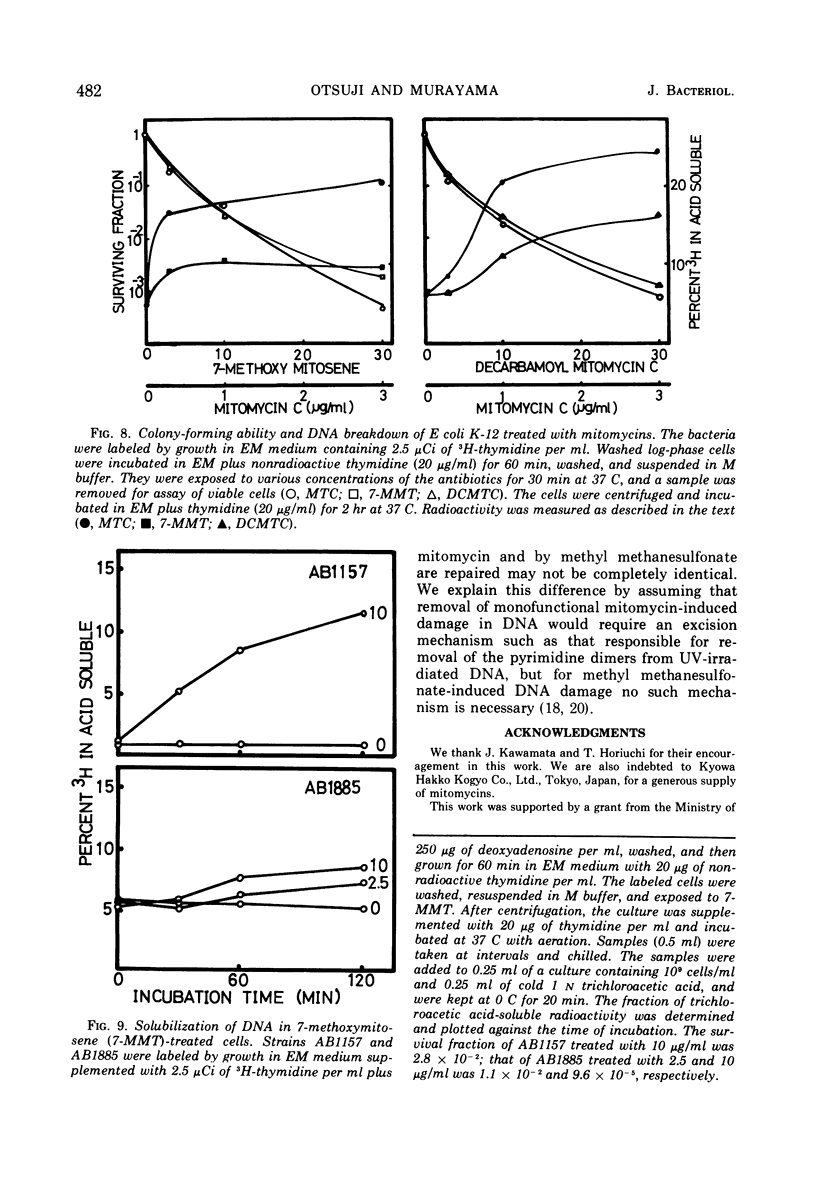
Exposure of Escherichia coli to the antibiotic mitomycin C (MTC) at a concentration of 0.5 μg/ml caused cross-linkage between complementary strands of deoxyribonucleic acid (DNA). Derivatives of mitomycin, 7-methoxymitosene (7-MMT) and decarbamoyl mitomycin C (DCMTC), at a level as high as 20 μg/ml formed no cross-links between DNA strands. Ultraviolet light-sensitive mutants of E. coli K-12 bearing uvrA, uvrB, uvrC, or recA mutations were more sensitive to the lethal action of 7-MMT and of DCMTC than was the wild-type strain. Treatment of wild-type cells with these antibiotics resulted in the production of single-strand breaks in DNA, which were repaired upon incubation in a growth medium. Such breaks in DNA were not produced in the uvrA and the uvrB mutants. In the uvrC mutant, single-strand breaks were produced by 7-MMT or by DCMTC, but these breaks were not repaired upon incubation. These results are discussed in connection with the mechanism for removal of pyrimidine dimers in ultraviolet-irradiated bacteria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. R., Jr, Poletto J. F., Weiss M. J. The mitomycin antibiotics. Synthetic studies. V. Preparation of 7-methoxymitosene. J Org Chem. 1965 Sep;30(9):2897–2904. doi: 10.1021/jo01020a006. [DOI] [PubMed] [Google Scholar]
- BOYCE R. P., HOWARD-FLANDERS P. GENETIC CONTROL OF DNA BREAKDOWN AND REPAIR IN E. COLI K-12 TREATED WITH MITOMYCIN C OR ULTRAVIOLET LIGHT. Z Vererbungsl. 1964 Dec 30;95:345–350. doi: 10.1007/BF01268667. [DOI] [PubMed] [Google Scholar]
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes P., Lawley P. D. The reaction of mono- and di-functional alkylating agents with nucleic acids. Biochem J. 1961 Sep;80(3):496–503. doi: 10.1042/bj0800496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBERG J., MANDELL J. D., WOODY P. L. Resistance and cross-resistance of Escherichia coli mutants to antitumour agent mitomycin C. J Gen Microbiol. 1961 Nov;26:509–520. doi: 10.1099/00221287-26-3-509. [DOI] [PubMed] [Google Scholar]
- HATA T., HOSHI T., KANAMORI K., MATSUMAE A., SANO Y., SHIMA T., SUGAWARA R. Mitomycin, a new antibiotic from Streptomyces. I. J Antibiot (Tokyo) 1956 Jul;9(4):141–146. [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- IYER V. N., SZYBALSKI W. A MOLECULAR MECHANISM OF MITOMYCIN ACTION: LINKING OF COMPLEMENTARY DNA STRANDS. Proc Natl Acad Sci U S A. 1963 Aug;50:355–362. doi: 10.1073/pnas.50.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IYER V. N., SZYBALSKI W. MITOMYCINS AND PORFIROMYCIN: CHEMICAL MECHANISM OF ACTIVATION AND CROSS-LINKING OF DNA. Science. 1964 Jul 3;145(3627):55–58. doi: 10.1126/science.145.3627.55. [DOI] [PubMed] [Google Scholar]
- Kinoshita S., Uzu K., Nakano K., Shimizu M., Takahashi T. Mitomycin derivatives. 1. Preparation of mitosane and mitosene compounds and their biological activities. J Med Chem. 1971 Feb;14(2):103–109. doi: 10.1021/jm00284a005. [DOI] [PubMed] [Google Scholar]
- Kinoshita S., Uzu K., Nakano K., Takahashi T. Mitomycin derivatives. 2. Derivatives of decarbamoylmitosane and decarbamoylmitosene. J Med Chem. 1971 Feb;14(2):109–112. doi: 10.1021/jm00284a006. [DOI] [PubMed] [Google Scholar]
- LEVINE M. Effect of mitomycin C on interactions between temperate phages and bacteria. Virology. 1961 Apr;13:493–499. doi: 10.1016/0042-6822(61)90280-x. [DOI] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- OTSUJI N., SEKIGUCHI M., IIJIMA T., TAKAGI Y. Induction of phage formation in the lysogenic Escherichia coli K-12 by mitomycin C. Nature. 1959 Oct 3;184(Suppl 14):1079–1080. doi: 10.1038/1841079b0. [DOI] [PubMed] [Google Scholar]
- OTSUJI N. The effect of glucose on the induction of lambda phage formation by mitomycin C. Biken J. 1961 Dec;4:235–241. [PubMed] [Google Scholar]
- Otsuji N. Properties of mitomycin C-sensitive mutants of Escherichia coli K-12. J Bacteriol. 1968 Feb;95(2):540–545. doi: 10.1128/jb.95.2.540-545.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash L., Strauss B. Repair of alkylation damage: stability of methyl groups in Bacillus subtilis treated with methyl methanesulfonate. J Bacteriol. 1970 Jun;102(3):760–766. doi: 10.1128/jb.102.3.760-766.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter H., Strauss B. Repair of damage induced by a monofunctional alkylating agent in a transformable, ultraviolet-sensitive strain of Bacillus subtilis. J Mol Biol. 1965 Nov;14(1):179–194. doi: 10.1016/s0022-2836(65)80239-x. [DOI] [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIBA S., TERAWAKI A., TAGUCHI T., KAWAMATA J. Selective inhibition of formation of deoxyribonucleic acid in Escherichia coli by mitomycin C. Nature. 1959 Apr 11;183(4667):1056–1057. doi: 10.1038/1831056a0. [DOI] [PubMed] [Google Scholar]
- SUGIURA K. Studies in a tumor spectrum. VIII. The effect of mitomycin C on the growth of a variety of mouse, rat, and hamster tumors. Cancer Res. 1959 May;19(4):438–445. [PubMed] [Google Scholar]
- Searashi T., Strauss B. Relation of the repair of damage induced by a monofunctional alkylating agent to the repair of damage induced by ultraviolet light in Bacillus subtilis. Biochem Biophys Res Commun. 1965 Sep 22;20(6):680–687. doi: 10.1016/0006-291x(65)90069-0. [DOI] [PubMed] [Google Scholar]
- Terawaki A., Greenberg J. Post-treatment breakage of mitomycin C induced cross-links in deoxyribonucleic acid of Escherichia coli. Biochim Biophys Acta. 1966 Jun 22;119(3):540–546. doi: 10.1016/0005-2787(66)90130-4. [DOI] [PubMed] [Google Scholar]



