Abstract
The objective of this study was to determine the effect of vaccinating susceptible animals on the transmission of Mycoplasma hyopneumoniae from experimentally infected pigs during the chronic phase of infection. Thirty-six seeder pigs were experimentally infected with M. hyopneumoniae. Eighty and 200 d post-infection (dpi) 18 seeder pigs were placed in direct contact with 15 vaccinated and 15 unvaccinated age-matched naïve animals. Direct animal contact occurred over 14 d. Pigs were euthanized at the end of the contact period and bronchial swabs were collected and lung tissue examined. At 94 dpi, 15 out of 15 unvaccinated sentinels and 14 out of 15 vaccinated sentinels tested positive for M. hyopneumoniae by nested polymerase chain reaction (N-PCR). At 214 dpi, M. hyopneumoniae DNA was detected by PCR in 8 out of 15 unvaccinated and 6 out of 15 vaccinated sentinels. Vaccination against M. hyopneumoniae did not prevent colonization of sentinels in contact with infected animals. Transmission of M. hyopneumoniae from asymptomatic carriers to unvaccinated and vaccinated sentinels was not different.
Résumé
L’objectif de la présente étude était de déterminer l’effet de la vaccination d’animaux susceptibles sur la transmission de Mycoplasma hyopneumoniae durant la phase chronique de l’infection à partir d’animaux infectés expérimentalement. Trente-six porcs excréteurs ont été infectés expérimentalement avec M. hyopneumoniae. Quatre-vingts et 200 j post-infection (dpi), 18 porcs excréteurs ont été mis en contact direct avec des animaux naïfs d’âge correspondant, soit 15 porcs vaccinés et 15 porcs non-vaccinés. Les contacts directs entre animaux se sont produits sur une période de 14 j. Les porcs ont été euthanasiés à la fin de la période de contact et des écouvillons bronchiaux ont été prélevés et les poumons examinés. Au jour 94 dpi, les 15 animaux sentinelles non-vaccinés et 14 des 15 animaux vaccinés se sont révélés positifs à M. hyopneumoniae par réaction d’amplification en chaîne par la polymérase (PCR). Au jour 214 dpi, l’ADN de M. hyopneumoniae a été détecté par PCR chez 8 des 15 porcs non-vaccinés et chez 6 des 15 porcs vaccinés. La vaccination contre M. hyopneumoniae n’a pas empêché la colonisation des animaux sentinelles en contact avec les animaux infectés. Il n’y avait pas de différence dans la transmission de M. hyopneumoniae des porteurs asymptomatiques à des animaux vaccinés ou non-vaccinés.
(Traduit par Docteur Serge Messier)
Mycoplasma hyopneumoniae is the causative agent of Enzootic pneumonia (1–2), a disease that is prevalent in every country where pigs are raised (3–5). The economic impact of M. hyopneumoniae infections on the swine industry is a composite of the direct effect of infection and a high incidence of secondary respiratory infections (6).
Mycoplasma hyopneumoniae causes chronic respiratory disease characterized by growth retardation and a dry non-productive cough when uncomplicated by other agents (7). The cough initiates approximately 10 to 16 d after experimental infection and ceases within 2 mo (8). Pig-to-pig transmission by direct contact is one of the most common routes of M. hyopneumoniae infection (9). Pigs infected with M. hyopneumoniae transmit the bacteria to susceptible animals during the acute (10) and chronic phases of infection (11). One of the most important features of M. hyopneumoniae infection is the persistence of bacteria within the respiratory tract of the pig for long periods of time and that persistently infected animals become asymptomatic carriers capable of infecting susceptible pigs (11–12).
Control of M. hyopneumoniae infections involves the application of 1 or several strategies (6). Intervention strategies are used in order to avoid direct contact between different groups, to decrease the amount of bacteria shed by infected animals, or to protect susceptible animals. Commercial M. hyopneumoniae bacterins are often used as means of protection against disease for uninfected animals, although vaccination has not been reported to prevent colonization (10,13). A comparison between the transmission of M. hyopneumoniae from infected to susceptible unvaccinated or vaccinated pigs during the acute phase of infection demonstrated that the vaccine did not change the transmission rate of the bacteria among the 2 groups (14). However, the effect of vaccination of susceptible pigs on transmission during the chronic phase of infection has not been investigated. Our hypothesis is that vaccination of replacement gilts does not prevent M. hyopneumoniae colonization of susceptible pigs after contact with asymptomatic carriers. Therefore, the objective of this study was to determine the effect of vaccination of susceptible animals on the transmission of M. hyopneumoniae from experimentally infected pigs during the chronic phase of infection.
To test our hypothesis, pigs were obtained from a source known to be negative to M. hyopneumoniae and porcine respiratory and reproductive syndrome virus. The experiments were performed at the University of Minnesota Swine Disease Eradication Center Research Farm (Appleton, Minnesota, USA) and animals were cared for according to the guidelines of the Institutional Animal Care and Committee, University of Minnesota. A total of 96 pigs were used in this study. Animals were distributed into 3 experimental groups. Group 1 were seeder pigs (n = 36), 15-week-old female pigs experimentally infected with M. hyopneumoniae. Group 2 were unvaccinated sentinels (n = 30), age and sex-matched with seeders, naïve to M. hyopneumoniae. Group 3 were vaccinated sentinels (n = 30), age and sex-matched with seeders.
The experimental design for this research is presented in Table I. The study consisted of the experimental infection of seeders with M. hyopneumoniae on day 0. Pigs were intra-tracheally inoculated with a lung homogenate suspension containing 1 × 105 color-changing units of M. hyopneumoniae strain 232 (obtained from Iowa State University, Ames, Iowa USA). Vaccinated sentinels were injected twice (15 and 30 d prior to contact with seeders) with 2 mL of a commercial bacterin for M. hyopneumoniae (RespiSure; Pfizer, New York, New York, USA).
Table I.
Experimental design for the evaluation Mycoplasma hyopneumoniae transmission from asymptomatic carriers to unvaccinated and vaccinated populations
| Days post-infection | Study activity | Experimental group |
|---|---|---|
| 0 | Experimental infection | Seedersa (n = 36) |
| 80 to 94 | Transmission assessment 1 | Seeders (n = 9) + Unvaccinated sentinelsb (n = 15) |
| Seeders (n = 9) + Vaccinated sentinelsc (n = 15) | ||
| 200 to 214 | Transmission assessment 2 | Seeders (n = 9) + Unvaccinated sentinels (n = 15) |
| Seeders (n = 9) + Vaccinated sentinels (n = 15) |
Gilts experimentally infected with M. hyopneumoniae strain 232.
M. hyopneumoniae negative and unvaccinated gilts.
M. hyopneumoniae negative gilts, vaccinated with a commercial bacterin 15 and 30 d prior to the first day of the transmission experiment.
Sentinels were age-matched with seeders.
At 80 and 200 d post-infection (dpi) a subset of 18 seeders was moved to a different barn, where they were placed in direct contact with 15 vaccinated and 15 unvaccinated sentinels. Direct contact between seeders and vaccinated and unvaccinated sentinels was only allowed during the 2 14-day transmission assessments. The first transmission assessment went from 80 to 94 dpi and a second one from 200 to 214 dpi. Each transmission assessment group was divided into 2 subgroups. Subgroup 1 was made up of 9 seeders and 15 unvaccinated sentinels and subgroup 2 was made up of 9 seeders and 15 vaccinated sentinels. Subgroups were physically separated. Group size was estimated based on a previously described algorithm (15).
A subgroup of seeders was monitored for confirmation of infection during the acute phase of infection. Nasal swabs were collected at 0, 4, 7, 11, 13, 18, 25, and 36 dpi for this purpose. Clinical signs in the groups were evaluated by daily observation.
Serum samples were collected from all seeders at 0, 34, and 60 dpi, and from seeders and sentinels at the beginning and at the end of the transmission assessments (80 to 94 and 200 to 214 dpi). Serum samples were analyzed for M. hyopneumoniae antibodies using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (DAKO; DakoCytomation California, Carpinteria, California, USA), following manufacturer’s instructions.
Bronchial swabs were collected from seeders and from all sentinels at the end of the transmission assessments (94 and 214 dpi). Swabs were evaluated for the presence of a region of the M. hyopneumoniae 16S gene using a nested-polymerase chain reaction (PCR) assay (16).
Gross and microscopic lung lesions were evaluated in seeders and sentinels at the end of the transmission assessments (94 and 214 dpi). Gross lesions suggestive of M. hyopneumoniae infection were scored based on the proportion of lung tissue involved in the pneumonic process (17). Lung samples were obtained from macroscopic lesions and from a consistent location in pigs without visible lesions. Tissues were fixed, stained with hematoxylin and eosin, and processed for microscopic examination.
All animals used for this study were negative to M. hyopneumoniae upon arrival at the facilities, as confirmed by nested-PCR from nasal swabs and the lack of clinical signs and antibodies to M. hyopneumoniae. The successful infection of seeders was confirmed by detection of M. hyopneumoniae DNA, antibodies, and clinical signs (such as coughing in the group). Thirty-nine percent of the experimentally infected animals seroconverted to M. hyopneumoniae by 34 dpi, while the entire group was positive by 60 dpi. Mycoplasma hyopneumoniae DNA from nasal swabs was detected at 13, 18, 25, and 34 dpi in a subset of seeders. A non-productive cough was detected in the seeder pigs’ barn from 11 until 68 dpi (data not shown).
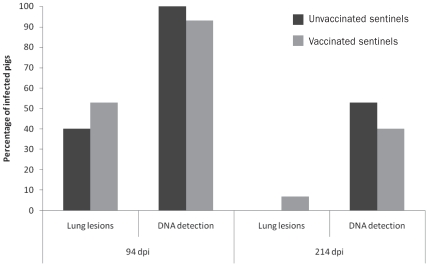
Detection of M. hyopneumoniae in all experimental groups during the transmission assessments is presented in Table II. During the first transmission assessment (80 to 94 dpi) 17 out of 18 seeders had detectable antibodies to M. hyopneumoniae, as evaluated at 80 and 94 dpi. Mycoplasma hyopneumoniae DNA was detected in bronchial swabs of 18 out of 18 animals at 94 dpi. No unvaccinated sentinels (0 out of 15) had antibodies to M. hyopneumoniae at 80 and 94 dpi, while M. hyopneumoniae DNA was detected in 15 out of 15 bronchial swabs and gross lesions were observed in 6 out of 15 of those animals. All 15 vaccinated sentinels had detectable antibodies to M. hyopneumoniae at 80 dpi, while 10 out of 15 had antibodies at 94 dpi. Mycoplasma hyopneumoniae DNA was detected in bronchial swabs and lung lesions in 14 out of 15 and 8 out of 15 vaccinated sentinels, respectively, as evaluated at 94 dpi. The proportion of unvaccinated and vaccinated sentinels infected with M. hyopneumoniae at 94 dpi by nested-PCR and the proportion of lung lesions is shown in Figure 1. Statistical comparison of the proportion of infected animals, obtained by using the Fisher’s exact test, did not show a significant difference (P > 0.05). The proportion of lung lesions in animals was similar (P > 0.05).
Table II.
Detection of Mycoplasma hyopneumoniae in experimental pigs during the transmission assessments
| Transmission assessment 1 |
Transmission assessment 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Experimental groups | Antibody detectiona 80 dpi | Antibody detection 94 dpi | DNA detectionb 94 dpi | Lung lesionsc 94 dpi | Antibody detection 200 dpi | Antibody detection 214 dpi | DNA detection 214 dpi | Lung lesions 214 dpi |
| Seeder pigs | 17/18d | 17/18 | 18/18 | 7/18 | 1/18 | 4/18 | 11/18 | 0/18 |
| Unvaccinated sentinels | 0/15 | 0/15 | 15/15 | 6/15 | 0/15 | 0/15 | 8/15 | 0/15 |
| Vaccinated sentinels | 15/15 | 15/15 | 14/15 | 8/15 | 15/15 | 15/15 | 6/15 | 1/15 |
DAKO ELISA.
Nested-PCR from bronchial swabs.
Gross and microscopic lesions.
Number of pigs positive/number of pigs tested.
Figure 1.
Proportion of Mycoplasma hyopneumoniae infected sentinels at 94 and 214 dpi (transmission assessments 1 and 2). Mycoplasma hyopneumoniae DNA was detected by nested-PCR from bronchial swabs. Gross lung lesions associated with M. hyopneumoniae infection were confirmed by histopathology. The proportion of unvaccinated and vaccinated sentinels in which M. hyopneumoniae DNA was detected was not statistically different (P > 0.05) at either 94 or 214 dpi. The proportion of unvaccinated and vaccinated sentinels in which lung lesions were detected at either 94 or 214 dpi was similar (P > 0.05).
At the second transmission assessment, 1 out of the 18 seeders had antibodies to M. hyopneumoniae at 200 dpi, while 4 out of 18 seeders had antibodies at 214 dpi. Mycoplasma hyopneumoniae DNA was detected in 11 out of 18 animals using bronchial swabs at 214 dpi. Unvaccinated sentinels did not have detectable antibodies to M. hyopneumoniae at 200 or 214 dpi. Mycoplasma hyopneumoniae DNA was detected in 8 out of 15 unvaccinated sentinels using bronchial swabs at 214 dpi. Lung lesions were not observed in the group. All vaccinated sentinels had detectable antibodies to M. hyopneumoniae at 200 dpi and 214 dpi. Mycoplasma hyopneumoniae DNA was detected in 6 out of 15 vaccinated pigs using bronchial swabs at 214 dpi. One animal presented gross lesions suggestive of M. hyopneumoniae infection at 214 dpi. A comparison of the proportion of infected sentinels (unvaccinated and vaccinated) and proportions of lung lesions demonstrated no significant differences, P > 0.05. The proportion of infected animals and lung lesions are shown in Figure 1.
Vaccination of replacement gilts with M. hyopneumoniae bacterins is a common practice in M. hyopneumoniae infected herds. The purpose of the vaccination is to decrease gilt mortality, lung lesions, and clinical signs in the incoming animals, and, ultimately, to decrease the transmission of the pathogen from infected to naïve animals. However, M. hyopneumoniae vaccination fails to protect naïve animals from colonization (13,14). It should be noted that experimental studies supporting these results have been performed during the acute phase of M. hyopneumoniae infection. Therefore, the objective of this study was to determine the effect of vaccination of susceptible animals on the transmission of M. hyopneumoniae from experimentally infected pigs during the chronic phase of infection.
In this study, M. hyopneumoniae transmission from asymptomatic carriers to sentinels was examined at either 94 or 214 dpi. This is the first time that a comparison of transmission between unvaccinated and vaccinated populations was performed using asymptomatic carriers as seeder pigs. Asymptomatic carriers have been demonstrated to be infected with M. hyopneumoniae for periods of up to 214 dpi (11).
Under the conditions of this study, the proportion of vaccinated replacement gilts infected from the seeder pigs differed numerically, but not statistically, from the proportion of unvaccinated gilts that became infected. These results were similar during the 2 transmission assessments. Another investigation, in which transmission of M. hyopneumoniae from experimentally infected pigs to vaccinated and unvaccinated populations was quantified, failed to demonstrate significant differences in the transmission rates (14). However, the study only looked at the transmission rate during the acute phase of infection.
The proportion of lungs with lesions suggestive of M. hyopneumoniae infection was numerically higher in the vaccinated sentinels than in the unvaccinated ones at the conclusion of the 2 transmission assessments. These results are contrary to reports that M. hyopneumoniae vaccination reduces lung lesions (6,13). Nevertheless, vaccine efficacy and lung lesion reduction is often evaluated after extended periods of time following vaccination (approximately 3.5 mo). Here, lung lesions were evaluated 14 d after the first contact with the infective animals. This short time period may be a factor in conflicting results. It could be hypothesized that vaccination exacerbates the inflammatory response in animals after a very recent infection, but lung lesions in vaccinated animals heal sooner than in the unvaccinated ones. It is important to note that this study did not evaluate production parameters, such as average daily gain and feed conversion ratio, which are usually improved in infected vaccinated animals when compared with unvaccinated ones (6,13,18,19).
The original design for this research included the quantification of M. hyopneumoniae transmission by calculating the basic reproductive ratio (R0) (20) and comparing it between 2 populations. However, R0 could not be estimated for the 2 populations in this study, since certain assumptions for the model did not apply to the study population. Namely, at least 1 animal (sentinel) would have to be negative at the end of the contact period, which did not occur in the 1st transmission assessment and all infected animals would have to remain infective during the contact period, which did not occur during the 2nd transmission assessment.
One limitation of this study was the fact that the length of the transmission assessments (14 d each) did not allow for either seroconversion of the infected animals (unvaccinated) or for onset of clinical signs in any newly infected pigs. Therefore, animals were classified as infected using the parallel interpretation, based on detection of M. hyopneumoniae DNA or lung lesions due to M. hyopneumoniae. In all cases, animals with mycoplasmal lesions contained M. hyopneumoniae DNA, as detected by nested-PCR.
In conclusion, vaccination of replacement gilts did not prevent colonization with M. hyopneumoniae of sentinels in contact with asymptomatically infected animals. Moreover, transmission of M. hyopneumoniae did not differ between the unvaccinated and vaccinated pigs, demonstrating that vaccination with bacterins does not protect incoming animals from becoming infected. Therefore, different control strategies or a combination of several interventions need to be evaluated in order to develop more effective approaches for protecting naïve animals that are incorporated into herds infected with M. hyopneumoniae. Ultimately, new vaccine technologies should be used to develop commercial products that provide better protection against M. hyopneumoniae.
Acknowledgments
This project was funded by the National Pork Board grant 05-013C. Genetiporc provided the animals used in this study.
Footnotes
Dr. Pijoan passed away in January 2007.
References
- 1.Mare CJ, Switzer WP. New species: Mycoplasma hyopneumoniae, a causative agent of virus pig pneumonia. Vet Med. 1965;60:841–846. [PubMed] [Google Scholar]
- 2.Goodwin RFW, Pomeroy AP, Whittlestone P. Production of enzootic pneumoniae in pigs with a mycoplasma. Vet Rec. 1965;77:1247–1249. [Google Scholar]
- 3.Ross RF. Mycoplasmal diseases. In: Straw B, D’Allaire S, Mengeline W, Taylor D, editors. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State Univer Pr; 1999. pp. 495–510. [Google Scholar]
- 4.Desrosiers R. A review of some aspects of the epidemiology, diagnosis, and control of Mycoplasma hyopneumoniae infections. J Swine Health Prod. 2001;9:233–237. [Google Scholar]
- 5.Otto L, Kristensen CS. A biological network describing infection with Mycoplasma hyopneumoniae in swine herds. Prev Vet Med. 2004;66:141–161. doi: 10.1016/j.prevetmed.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Maes D, Segalés J, Meyns T, Sibila M, Pieters M, Haesebrouck F. Control of Mycoplasma hyopneumoniae infections in pigs. Review Vet Microbiol. 2008;126:297–309. doi: 10.1016/j.vetmic.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sibila M, Pieters M, Molitor T, Maes D, Haesebrouck F, Segalés J. Current perspectives on the diagnosis and epidemiology of Mycoplasma hyopneumoniae infection. Vet J. 2009;181:221–231. doi: 10.1016/j.tvjl.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorensen V, Ahrens P, Barfod K, et al. Mycoplasma hyopneumoniae infection in pigs: Duration of the disease and evaluation of four diagnostic assays. Vet Microbiol. 1997;54:23–34. doi: 10.1016/s0378-1135(96)01266-7. [DOI] [PubMed] [Google Scholar]
- 9.Marois C, Cariolet R, Morvan H, Kobisch M. Transmission of pathogenic respiratory bacteria to specific pathogen free pigs at slaughter. Vet Microbiol. 2008;129:325–332. doi: 10.1016/j.vetmic.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 10.Meyns T, Maes D, Dewulf J, Vicca J, Haesebrouck F, de Kruif A. Quantification of the spread of Mycoplasma hyopneumoniae in nursery pigs using transmission experiments. Prev Vet Med. 2004;66:265–275. doi: 10.1016/j.prevetmed.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Pieters M, Pijoan C, Fano E, Dee S. An assessment of the duration of Mycoplasma hyopneumoniae infection in an experimentally infected population of pigs. Vet Microbiol. 2009;134:261–266. doi: 10.1016/j.vetmic.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Fano E, Pijoan C, Dee S. Dynamics and persistence of Mycoplasma hyopneumoniae infection in pigs. Can J Vet Res. 2005;69:223–228. [PMC free article] [PubMed] [Google Scholar]
- 13.Haesebrouck F, Pasmans F, Chiers K, Maes D, Ducatelle R, Decostere A. Efficacy of vaccines against bacterial diseases in swine: What can we expect? Vet Microbiol. 2004;100:255–268. doi: 10.1016/j.vetmic.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Meyns T, Dewulf J, de Kruif A, Calus D, Haesebrouck F, Maes D. Comparison of transmission of Mycoplasma hyopneumoniae in vaccinated and non-vaccinated populations. Vaccine. 2006;24:7081–7086. doi: 10.1016/j.vaccine.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 15.De Jong MC, Kimman TG. Experimental quantification of vaccine-induced reduction in virus transmission. Vaccine. 1994;12:761–766. doi: 10.1016/0264-410x(94)90229-1. [DOI] [PubMed] [Google Scholar]
- 16.Calsamiglia M, Pijoan C, Trigo A. Application of a nested polymerase chain reaction assay to detect Mycoplasma hyopneumoniae from nasal swabs. J Vet Diagn Invest. 1999;11:246–251. doi: 10.1177/104063879901100307. [DOI] [PubMed] [Google Scholar]
- 17.Pointon AM, Davies P, Bahnson PB. Disease surveillance at slaughter. In: Straw B, D’Allaire S, Mengeling W, Taylor D, editors. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State Univer Pr; 1999. pp. 1111–1132. [Google Scholar]
- 18.Maes D, Deluyker H, Verdonck M, et al. Effect of vaccination against Mycoplasma hyopneumoniae in pig herds with a continuous production system. J Vet Med B. 1998;45:495–505. doi: 10.1111/j.1439-0450.1998.tb00820.x. [DOI] [PubMed] [Google Scholar]
- 19.Maes D, Deluyker H, Verdonck M, et al. Effect of vaccination against Mycoplasma hyopneumoniae in pig herds with an all-in/ all-out production system. Vaccine. 1999;17:1024–1034. doi: 10.1016/s0264-410x(98)00254-0. [DOI] [PubMed] [Google Scholar]
- 20.Dobson A, Carper R. Infectious diseases and human population history. BioScience. 1996;46:115–126. [Google Scholar]