Abstract
The purpose of this study was to detect cell-mediated and local humoral immune responses to Lawsonia intracellularis in pigs inoculated with a pure culture of the pathogenic isolate or with an intestinal mucosa homogenate. Twenty-four 5-week-old pigs were inoculated with a pure culture of L. intracellularis (n = 10), an intestinal mucosa homogenate from proliferative enteropathy diseased pigs (n = 10), or a control solution (n = 4). All animals were bled 0, 7, 14, and 20 d post-inoculation (pi). Serum was tested for immunoglobulin (Ig) G against L. intracellularis and for the production of interferon (IFN)-γ by peripheral blood mononuclear cells (PBMC) after inoculation with L. intracellularis total proteins. Delayed-type hypersensitivity (DTH) reactions were evaluated 24 and 48 h after intra-dermal injection of different concentrations of L. intracellularis antigen 20 d pi. All animals were euthanized on day 22, intestinal lavages of ileum and IgA titrations were done. Weak IFN-γ production was detected in 1 pig from the pure culture group and 2 pigs from the mucosal homogenate group 14 d pi, and in 2 animals from both groups 20 d pi. All pigs, in both inoculated groups, were seropositive for IgG on day 20. Inoculated pigs from both groups showed very weak dose-dependent DTH reactions, which were more evident at 24 h than 48 h pi. Eight pigs from the pure culture group and 7 from the mucosa homogenate group had detectable IgA titers in the intestinal lavage 22 d pi. In conclusion, specific local intestinal humoral and weak cell-mediated immune responses can be detected in pigs experimentally infected with L. intracellularis.
Résumé
L‘objectif de la présente étude était de détecter les réponses immunitaires à médiation cellulaire et humorale locale à Lawsonia intracellularis chez des porcs inoculés avec une culture pure de l’isolat pathogène ou avec un homogénat de la muqueuse intestinale. Vingt-quatre porcs âgés de 5 semaines ont été inoculés avec une culture pure de L. intracellularis (n = 10), un homogénat de la muqueuse intestinale de porcs atteints d’entéropathie proliférative (n = 10), ou une solution témoin (n = 4). Un prélèvement sanguin a été obtenu de tous les animaux aux jours 0, 7, 14 et 20 post-inoculation (pi). On vérifia la présence dans le sérum d’immunoglobulines (Ig) G envers L. intracellularis et la production d’interféron (IFN)-γ par les mononucléaires du sang périphérique (PBMC) après l’inoculation de protéines totales de L. intracellularis. Les réactions d’hypersensibilité retardée ont été évaluées 24 et 48 h après injections intra-dermiques de différentes concentrations d’antigène de L. intracellularis 20 j pi. Tous les animaux ont été euthanasiés au jour 22, des lavages de l’iléon et la détermination des titres d’IgA ont été effectués. Une faible production d’IFN-γ a été détectée chez 1 porc inoculé avec une culture pure et 2 porcs inoculés avec l’homogénat de muqueuse14 j pi, et de 2 animaux des deux groupes 20 j pi. Tous les porcs, dans les deux groupes inoculés, étaient séropositifs pour la présence d’IgG au jour 20. Les porcs inoculés des deux groupes ont montré de très faibles réactions de DTH dose-dépendante qui étaient plus évidentes à 24h qu’à 48h. Huit porcs inoculés avec une culture pure et 7 inoculés avec un homogénat de muqueuse avaient des titres d’IgA détectables dans le lavage intestinal 22 j pi. En conclusion, des réponses locales spécifiques d’immunité humorale et de faibles réponses à médiation cellulaire peuvent être détectées chez des porcs infectés expérimentalement avec L. intracellularis.
(Traduit par Docteur Serge Messier)
Introduction
Proliferative enteropathy (PE) is an infectious enteric disease caused by Lawsonia intracellularis, an obligate intracellular gram-negative bacterium. Based on current knowledge, intracellular organisms usually stimulate a cell-mediated immune response (1). Although L. intracellularis is an intracellular organism, much is known about systemic humoral immune response (serum IgG) in experimentally and naturally infected pigs, which is likely not protective. However, little is known about the cell-mediated immune (CMI), delayed type hypersensitivity (DTH), or local mucosal IgA immune responses of pigs infected with the organism.
The enzyme-linked immunosorbent spot (ELISPOT) T-cell assay detects the secretion of interferon-gamma (IFN-γ) produced by memory or activated T-lymphocytes and is used to evaluate the T-helper 1 (Th 1) response on the single-cell level (2). The ELISPOT T-cell assay was recently used to assess the onset and duration of cell-mediated immune response to L. intracellularis in pigs challenged with a pathogenic isolate or exposed to a commercial vaccine (Enterisol Ileitis; Boehringer Ingelheim, St. Joseph, Missouri, USA) (3). Results of this study showed the induction of cell-mediated immune response started at 21 dpi; however, as this was the first and only study using this assay, more data are necessary to corroborate these results.
The DTH reaction can be induced by a variety of intracellular bacterial pathogens (such as Brucella abortus, Listeria monocytogenes, Mycobacterium bovis), but it has not yet been studied during L. intracellularis infection. This reaction happens as a consequence of the antigen activation of T-helper lymphocytes (1). A granulomatous inflammatory reaction, in the intestines of Iberian pigs, was associated with L. intracellularis infection (4). Thus, use of intradermal injections of different types and concentrations of L. intracellularis antigens in experimentally infected pigs may demonstrate the existence of a DTH response involved in PE. The presence of a cell-mediated immune response against L. intracellularis infection suggests that further studies relating the DTH response to protection from infection are warranted.
Secretory IgA binds to bacteria and viral surface antigens in the lumen of the intestine and prevents pathogens from attaching to mucosal cells (1). In addition, it has been suggested that IgA is involved in the neutralization of intracellular organisms in the lamina propria and when passing through infected enterocytes (5). Enterocytes, mainly from the aboral part of the small intestines, are the permissive cells for the entry and maintenance of L. intracellularis resulting in persistent infection (6). Understanding the role of mucosal IgA, specific against L. intracellularis, may help explain the mechanism of protection from infections in future studies of PE.
The immune response induced by L. intracellularis infection was studied in pigs challenged with pure culture of a pathogenic isolate or with an intestinal mucosa homogenate extracted from PE-diseased pigs. In addition to the ELISPOT T-cell assay, 3 other methods were used to evaluate immune response: a skin test measuring the DTH reaction, titration of secretory IgA specific to L. intracellularis in intestinal lavages measuring local humoral immune response, and titration of serum IgG specific to L. intracellularis measuring systemic humoral immune response.
Materials and methods
Study design
All procedures were conducted in accordance with the guidelines of the Animal Care and Use Manual from the University of Minnesota and approved by the Institutional Animal Care and Use Committee. Twenty-four 5-week-old pigs weighing between 9 and 13.6 kg (20 and 30 lb) were assigned to one of 3 groups. The study design implemented was described in detail by Guedes and Gebhart (7). Briefly, 1 d prior to inoculation, the pigs were divided into 3 groups randomized by weight: 4 pigs in the control group; 10 pigs in the pure culture group; and 10 pigs in the intestine homogenate group. Each group was housed in different rooms in the isolation barns at the University of Minnesota. All animals were intragastrically dosed with 40 mL of inoculums, according to their respective group, using a stomach tube. On day 0, pigs in the control group received sucrose-potassium glutamate solution (SPG; 0.218M sucrose, 0.0038M KH2PO4, 0.0072M K2HPO4 and 0.0049M of glutamate, pH 7.0) with 5% fetal bovine serum (FBS). Each pig in the pure culture group received 8.9 × 108 L. intracellularis organisms in a SPG solution with 5% FBS. The 3rd group received a solution of 2.9 × 1010 L. intracellularis organisms per pig, scraped from the affected mucosa of a PE-affected gilt, diluted 1:1 w/v SPG with 5% FBS.
Fecal and blood sampling
Two days before challenge, all pigs were bled and serum was tested for exposure to L. intracellularis by an immunoperoxidase monolayer assay (IPMA) (8,9). In addition, fecal samples were collected and tested for L. intracellularis DNA using polymerase chain reaction (PCR) (10) to assure animals were negative for PE.
Serum and heparinized whole blood samples were collected from all pigs on days 7, 14, and 20 post-inoculation (pi) and tested by IPMA for IgG specific to L. intracellularis (8,9) and by ELISPOT assay for interferon-gamma (IFN-γ) (3).
Delayed-type hypersensitivity reaction
On day 20, all animals were sedated and 200 μL of 10 different solutions were injected intradermally in 10 different areas between the nipples. Solutions were as follows: (A) sterile phosphate buffer saline (PBS, pH 7.2); (B) 165 μg/mL of sonicated noninfected McCoy cell suspension; (C) 250 μg/mL of Phytohemagglutinin (PHA; Sigma, St. Louis, Missouri, USA) (positive control); (D) 109 L. intracellularis formalin-fixed organisms per mL; (E) 108 L. intracellularis formalin-fixed organisms per mL; (F) 107 L. intracellularis formalin-fixed organisms per mL; (G) 250 μg/mL of sonicated L. intracellularis; (H) 25 μg/mL of sonicated L. intracellularis; (I) 75 μg/mL of outer membrane protein of L. intracellularis; and (J) 7.5 μg/mL of outer membrane protein of L. intracellularis. The purification of L. intracellularis from an infected McCoy cell line and the preparation antigens for the DTH and ELISPOT tests are described in detail by Guedes and Gebhart (3). Outer membrane preparations were obtained as previously described (11,12). Twenty-four and 48 h later, the delayed-type hypersensitivity immune response was measured using a manual caliper evaluating double skin fold thickness and the erythema diameter, when present.
Intestinal mucosal IgA
All pigs were euthanized 22 d pi. Intestinal lavage of the aboral 25 cm of the small intestine (ileum) of each pig was done using 20 mL of cold PBS, which was then centrifuged at 150 × g for 5 min to eliminate solid material and the supernatant was frozen for posterior secretory IgA titration (described below).
The technique used to quantitate intestinal mucosal IgA was the immunoperoxidase monolayer assay (IPMA) (8,9), with some modifications. Briefly, 96-well plates (Nunclon, 167008; Nunc, Rochester, New York, USA) containing an acetone fixed monolayer of McCoy cells highly infected with L. intracellularis were rehydrated in a solution of PBS with 5% skim milk for 10 min at 37°C to block nonspecific reactions. The intestinal lavage samples were diluted in the same block solution in serial 4-fold dilutions (1:4, 1:16, 1:64, and 1:256). Then, 50 μL of each diluted sample was added to the test well. The plate was incubated for 30 min at 37°C and then washed 5 times with PBS containing 0.05% Tween 20 (PBST, Tween 20; Sigma). Goat anti-porcine IgA-peroxidase conjugate (A100-102P-11; Bethyl Laboratories, Montgomery, Texas, USA), diluted 1:1,000 in PBST, was added at 30 μL/well, and incubation proceeded for 45 min at 37°C. The plate was washed 5 times with PBST and 100 μL of pre-diluted chromogen (3-amino-9-ethyl-carbazole; AEC; A-6926; Sigma) solution was added to each well and incubated at room temperature for 20 min. The plate was washed with PBS 3 times, allowed to dry, and examined using an inverted light microscope. The presence of red stained L. intracellularis indicated a positive result.
Results
Intragastric inoculation of 5-week-old pigs with either a pure culture of L. intracellularis (PHE-MN/01) or an intestinal homogenate resulted in clinical signs of PE, pathological lesions, and infection determined using immunohistochemistry, as described in detail in a previous study (7). In brief, diarrhea was most commonly observed in inoculated pigs in the 3rd wk pi and reduced average daily gain in pure culture and homogenate groups (440 and 490 g, respectively) compared to the control group (650 g, P < 0.05). Macroscopic lesions typical of PE, ranging from 4 to 125 cm and from 5 to 140 cm, were observed in the small intestine (jejunum and ileum) in all pigs inoculated with pure culture and intestinal homogenate, respectively. Characteristic PE histologic lesions and positive immunohistochemistry results were observed in all ileum sections of animals from both inoculated groups. No lesions or signs of infection were detected in any of the control animals. All animals were negative by PCR analysis of fecal samples and by IPMA serology 2 d prior to inoculation.
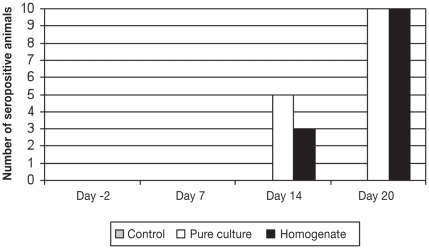
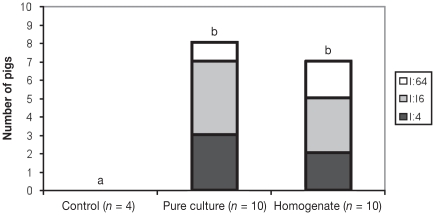
Serum conversion was first detected in some animals in both challenged groups at 14 d pi (Figure 1). All pigs in both challenged groups were seropositive on day 20 pi with titers ranging from 1:30 to 1:480 in the pure culture group and from 1:30 to 1:1920 in the intestinal homogenate group. Eight out of 10 pigs from the pure culture group and 7 out of 10 pigs from the intestine homogenate group had IgA titers specific for L. intracellularis in the intestinal lavage ranging from 1:4 to 1:64 (Figure 2). Using Mann-Whitney to compare 2 groups at a time, there was a statistically significant difference between the control and the 2 challenge groups (P < 0.05).
Figure 1.
Immunoperoxidase monolayer assay results for serum IgG against L. intracellularis in pigs from the control (n = 4), pure culture (n = 10), and intestinal homogenate (n = 10) groups on days −2, 7, 14, and 20 post-inoculation (pi).
Figure 2.
Number of pigs, from the control, pure culture, and intestine homogenate groups, showing various levels of specific IgA titers against L. intracellularis by ileum lavage, 22 d post-inoculation (pi). ab Significant difference between groups (P < 0.05)
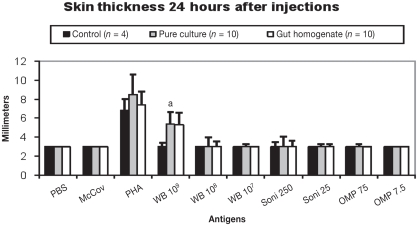
One pig in the pure culture group (6 spots) and 2 pigs in the intestine homogenate group (11 and 8 spots) had detectable production of IFN-γ in vitro, as measured by ELISPOT using 10 μg/mL of bacteria antigen on day 14 pi. Two animals in the pure culture group (6 and 5 spots), including 1 that had been detected on day 14, and the same 2 animals in the homogenate group (15 and 6 spots) had detectable production of IFN-γ on day 20 pi. No skin lesions were observed in control animals. The results of the DTH response are summarized in Figure 3. The skin reaction was more evident at 24 h than 48 h pi and only the higher concentration of whole bacteria (109 L. intracellularis organisms) induced detectably different (P < 0.01) results compared to PBS and sonicated McCoy cells injection sites in both L. intracellularis inoculated animal groups using ANOVA. No skin reaction was detected at either 24 or 48 h pi with 108 and 107 L. intracellularis organisms, 250 and 25 μg/mL of sonicated L. intracellularis, or 75 and 7.5 μg/mL of outer membrane protein of L. intracellularis. No difference in erythema diameter was observed among the pure culture, intestinal homogenate, or control groups.
Figure 3.
Delayed-type hypersensitivity (DTH) response against 10 different antigens in pigs from the control, pure culture, and intestinal homogenate groups on day 20 post-inoculation (pi). Skin thickness was measured 24 h pi.
a Significant difference (P < 0.01) between inoculated (pure culture and intestinal homogenate) and control groups, and between PBS and sonicated McCoy cells injection sites and 109 whole L. intracellularis sites in inoculated animal groups.
Antigens used in injection sites. PBS — Phosphate buffered saline (pH 7.2); McCoy — non-infected sonicated McCoy cells; PHA — phytohemagglutinin; WB 109–109 whole L. intracellularis organisms; WB 108–108 whole L. intracellularis organisms; WB 107–107 whole L. intracellularis organisms; Soni 250–250 μg of total L. intracellularis proteins after bacterial sonication; Soni 25–25 μg of total L. intracellularis proteins after bacterial sonication; OMP 75–75 μg of L. intracellularis outer-membrane proteins; OMP 7.5–7.5 μg of L. intracellularis outer-membrane proteins.
Discussion
Serum IgG titers detected in all challenged animals during the study were similar to those reported in other studies using intestine homogenate (8,9,13) or pure culture inocula (3,14), starting 2 wk pi and peaking at the 3rd wk. Specific L. intracellularis local intestinal humoral immune response, represented by IgA titers in intestine lavages, was demonstrated in the majority of the animals in both challenged groups using a modified IPMA technique, but it was absent in the control group. Large accumulations of IgA of unknown specificity in the apical cytoplasm of proliferating enterocytes in intestinal sections from pigs affected with the acute and chronic forms of PE was previously described (15,16). Immunoglobulin A accumulation was also described in the cytoplasm of plasma cells underlying proliferative lesions (17) and in peripheral areas of lymphoid Peyer’s patches (15). In addition to the well-known effect of specific IgA against infectious agents in the mucus of the intestinal lumen, IgA secreted by lymphocytes in the lamina propria can be effective against microorganisms present in the lamina propria and also in the cytoplasm of enterocytes, during IgA translocation through the epithelial cell layer toward the intestinal lumen (5). Lawsonia intracellularis is an obligate intracellular organism that enters intestinal epithelial cells and is found in the cytoplasm of enterocytes and often in the lamina propria (8). As a result, IgA probably plays an important role in protecting the intestine against L. intracellularis invasion and intracellular proliferation. This is the first report of detection of an intestinal IgA specific for L. intracellularis in infected animals. Future studies must be conducted to determine whether there is a correlation between intestinal lavage IgA titers and protection.
A weak production of IFN-γ by T helper cells using 10 μg/mL of L. intracellularis test antigen purified from pure cultures of the bacteria was initially detected 2 wk pi in just a few animals from both challenge groups in this study. These results are in agreement with a preliminary study (3) that showed that the onset and peak of cell-mediated immune response in the majority of the animals infected with L. intracellularis are delayed in relation to the systemic humoral immune response (serum IgG). In a mouse L. intracellularis challenge model, infection of IFN-γ receptor knockout animals was substantially higher than in the wild type mice (18). In this same study, infection level peaked on day 21 pi in both wild type and IFN-γ receptor knockout mice, but it was more prolonged in the latter, up to 35 d pi. The authors concluded that IFN-γ may play a significant role in limiting infection and cell proliferation of L. intracellularis. Further studies must be conducted to evaluate the peak response, duration of response, and correlation between cell-mediated immune response and protection in pigs.
The fact that a significant DTH reaction was detected with L. intracellularis whole bacteria, at a concentration of 109 organisms, suggests that this kind of immune response can be induced in PE-diseased pigs as early as 22 d pi. The non-responsiveness to lower concentrations of whole cell bacteria and bacterial antigens reflects the fact that the response is easily titrated out and so may not be easily detected. Granulomatous inflammatory reactions associated with L. intracellularis infection in pigs have been described (4), but there was no proof of a cause and effect association between them. Based on the results of the present study, further research should be conducted in this area using a higher concentration of bacteria for a longer period of time.
The ability to detect systemic cell-mediated immune response measured by the IFN-γ T-cell assay and local intestinal humoral immune response (secreted IgA) in intestinal lavages measured by the modified IPMA technique will certainly contribute to a better understanding of the immune response involved in PE. Future studies will address whether these specific immune responses reflect immune protection to L. intracellularis infection.
Acknowledgments
The authors thank Debra Swanson and Dr. Maria Isabel M. Coelho-Guedes for technical assistance. They also thank Dr. Gregers Jungersen for the critical evaluation of the manuscript.
References
- 1.Goldsby RA, Kindt TJ, Osborne BA. Kuby Immunology. 4th ed. New York, New York: WH. Freeman and Company; 2000. pp. 380–410. [Google Scholar]
- 2.Zuckermann FA, Martin S, Husmann RJ, Brandt J. Use of interleukin-12 to enhance the cellular immune response of swine to an inactivated herpesvirus vaccine. Adv Vet Med. 1999;41:447–461. doi: 10.1016/s0065-3519(99)80034-2. [DOI] [PubMed] [Google Scholar]
- 3.Guedes RMC, Gebhart CJ. Onset and duration of fecal shedding, cell-mediated and humoral immune responses in pigs after challenge with a pathogenic isolate or attenuated vaccine strain of Lawsonia intracellularis. Vet Microbiol. 2003;91:135–145. doi: 10.1016/s0378-1135(02)00301-2. [DOI] [PubMed] [Google Scholar]
- 4.Segalés J, Fernández-Salguero JM, Fructuoso G, et al. Granulomatous enteritis and lymphadenitis in Iberian pigs naturally infected with Lawsonia intracellularis. Vet Pathol. 2001;38:343–346. doi: 10.1354/vp.38-3-343. [DOI] [PubMed] [Google Scholar]
- 5.Lamm ME, Nedrud JG, Kaetzel CS, Mazane MB. IgA and mucosal defense. APMIS. 1995;103:241–246. doi: 10.1111/j.1699-0463.1995.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 6.Smith DGE, Lawson GHK. Lawsonia intracellularis: Getting inside of the pathogenesis of proliferative enteropathy. Vet Microbiol. 2001;82:331–345. doi: 10.1016/s0378-1135(01)00397-2. [DOI] [PubMed] [Google Scholar]
- 7.Guedes RMC, Gebhart CJ. Comparison of intestinal mucosa homogenate and pure culture of the homologous Lawsonia intracellularis isolate in reproducing proliferative enteropathy in swine. Vet Microbiol. 2003;93:159–166. doi: 10.1016/s0378-1135(03)00013-0. [DOI] [PubMed] [Google Scholar]
- 8.Guedes RMC, Gebhart CJ, Winkelman NA, Mackie-Nuss RA, Marsterlleres TA, Deen J. Comparison of different methods for diagnosis of porcine proliferative enteropathy. Can J Vet Res. 2002;66:99–107. [PMC free article] [PubMed] [Google Scholar]
- 9.Guedes RMC, Gebhart CJ, Winkelman NL, Mackie-Nuss RA. A comparative study of an indirect fluorescent antibody test and an immunoperoxidase monolayer assay for the diagnosis of porcine proliferative enteropathy. J Vet Diag Invest. 2002;14:420–423. doi: 10.1177/104063870201400512. [DOI] [PubMed] [Google Scholar]
- 10.Jones GF, Ward GE, Murtaugh MP, Lin G, Gebhart CJ. Enhanced detection of the intracellular organism of swine proliferative enteritis, Ileal symbiont intracellularis, in feces by polymerase chain reaction. J Clin Microbiol. 1993;31:2611–2615. doi: 10.1128/jcm.31.10.2611-2615.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filip C, Fletcher G, Wulff JL, Earhart CF. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973;115:717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barenkamp SJ, Munson RS, Granoff DM. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis. 1981;143:668–676. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- 13.Guedes RMC, Gebhart CJ, Deen J, Winkelman NL. Validation of an immunoperoxidase monolayer assay as a serologic test for porcine proliferative enteropathy. J Vet Diag Invest. 2002;14:528–530. doi: 10.1177/104063870201400618. [DOI] [PubMed] [Google Scholar]
- 14.Knittel JP, Jordan DM, Schwartz KJ, et al. Evaluation of ante-mortem polymerase chain reaction and serologic methods for detection of Lawsonia intracellularis exposed pigs. Am J Vet Res. 1998;59:722–726. [PubMed] [Google Scholar]
- 15.Lawson GHK, Rowland AC, Roberts L, Fraser G, McCartney E. Proliferative haemorrhagic enteropathy. Res Vet Sci. 1979;27:46–51. [PubMed] [Google Scholar]
- 16.McOrist S, MacIntyre N, Stokes CR, Lawson GHK. Immunocytological responses in porcine proliferative enteropathies. Infect Immun. 1992;60:4184–4191. doi: 10.1128/iai.60.10.4184-4191.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holyoake PK. Proliferative enteritis: Endemic in Australian piggeries? Aust Vet J. 1993;70:167–169. doi: 10.1111/j.1751-0813.1993.tb00854.x. [DOI] [PubMed] [Google Scholar]
- 18.Smith DGE, Mitchell SC, Nash T, Rhind S. Gamma interferon influences intestinal epithelial hyperplasia caused by Lawsonia intracellularis infection in mice. Infect Immun. 2000;68:6737–6743. doi: 10.1128/iai.68.12.6737-6743.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]