Abstract
Background
Individual differences in specific components of attention contribute to behavioral reactivity and regulation. Children with the temperament of behavioral inhibition (BI) provide a good context for considering the manner in which certain components of attention shape behavior. Infants and children characterized as behaviorally inhibited manifest signs of heightened orienting to novelty. The current study considers whether this attention profile moderates risk for clinical anxiety disorders among adolescents with a history of BI.
Methods
Participants were assessed at multiple time points for BI, beginning in early childhood. At adolescence, event-related potentials (ERPs) were recorded during a three-stimulus auditory novelty oddball task, which employed frequent-standard and infrequent-deviant tones as well as a set of complex, novel sounds. Clinical diagnosis was carried out using the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL). P3 and mismatch negativity (MMN) components were examined at midline frontal, central, and parietal electrode sites.
Results
Individuals who displayed high levels of BI during childhood and increased P3 amplitude to novelty in adolescence were more likely to have a history of anxiety disorders as adolescents compared to behaviorally inhibited adolescents with lower P3 amplitudes. Groups did not differ on measures of MMN.
Conclusions
Increased neural responses to novelty moderate risk for anxiety disorders amongst individuals with a history of BI.
Keywords: temperament, anxiety, adolescence, attention, risk factors
Introduction
Behavioral inhibition (BI), a well described temperamental disposition identified in early childhood, is marked by a tendency to withdraw in the face of novelty, particularly if social in nature (Fox, Henderson, Rubin, Calkins, & Schmidt, 2001b; Kagan, Reznick, Snidman, Gibbons, & Johnson, 1988). Behaviorally inhibited children typically avoid novelty and/or withdraw in unfamiliar situations and are often labeled as ‘shy’ by both adults and peers (Coplan, Girardi, Findlay, & Frohlick, 2007; Coplan, Rubin, Fox, & Calkins, 1994). Moreover, infants who remain high on measures of BI throughout childhood face particularly high risks for developing an anxiety disorder later in life (Chronis-Tuscano et al., in press; Hirshfeld et al., 1992; Schwartz, Snidman, & Kagan, 1999). Recently, it has been suggested that perturbations in specific components of attention moderate the association between early BI and later anxiety disorders such that BI children with an attention bias to threat or novelty are at increased risk for developing anxiety disorders (Fox, Hane, & Pine, 2007). This propensity to display increased vigilance to novelty among behaviorally inhibited children may prevent effective regulation of emotional responses to novel situations, and may sustain or exacerbate social and affective maladjustment (Fox, Russo, Bowles, & Dutton, 2001a; Fox, Russo, & Georgiou, 2005).
Mathews and MacLeod (1994) suggest that the ability to effectively override initial reactive tendencies distinguishes healthy high-trait anxious individual from counterparts who manifest anxiety disorders. An early-appearing temperamental disposition such as BI may involve perturbations in attention associated specifically with enhanced novelty detection (Derryberry & Reed, 1994). Indeed, Kagan (1994) suggested that hypervigilance to novelty in behaviorally inhibited children may lead to perturbations in attention mechanisms. Two sets of findings support this possibility. First, associations between anxiety and attention perturbations are found for indices of elaborative stimulus processing, as indexed by amplitude in the P3 component, a component associated with the orienting response (Cycowicz & Friedman, 1998; Friedman, Cycowicz, & Gaeta, 2001). Studies have shown that individuals with anxiety display increased P3 amplitude compared to controls (Bruder, 1992; Bruder et al., 2002; Daruna, Rau, & Strecker, 1991). As well, increasing state anxiety leads to increased P3 amplitude to novel stimuli (Grillon & Ameli, 1994). Second, focusing on earlier components of stimulus processing, Bar-Haim and colleagues (2003) linked BI in 10-year-olds to perturbed mismatch negativity (MMN), an electrophysiological marker of pre-perceptual detection of stimulus deviance (Naatanen & Alho, 1995; Naatanen, Paavilainen, Tiitinen, Jiang, & Alho, 1993). Marshall and colleagues (2009), using a somewhat different paradigm, demonstrated similar findings among 9-month-olds selected for characteristics related to BI. In addition, similar findings have been observed in anxious individuals (Cornwell et al., 2007; Hogan, Butterfield, Phillips, & Hadwin, 2007; Iwanami, Isono, Okajima, & Kamijima, 1997). Taken together, these results demonstrate associations between individual differences in anxious symptoms and novelty detection at various stages of cognitive processing.
Despite consistent demonstrations of associations between enhanced novelty detection and anxious behavior at various ages, virtually all research in this area examines cross-sectional associations between anxiety or BI and attention. Fox and colleagues (2007) hypothesize that the association between heightened attention to novelty and BI in relation to anxiety manifests across development. More specifically, they suggest that heightened attention to novelty will moderate the association between early BI and later anxiety disorders. Using P3 and MMN, two measures of novelty detection, the current study explores this hypothesis. We examined whether measures of BI during toddlerhood and childhood predict attention-based aspects of novelty processing and whether these measures of attention differentiate behaviorally inhibited adolescents with and without lifetime histories of anxiety disorders. We hypothesized that electrophysiological responses to novel and deviant stimuli would moderate the association between BI and clinical anxiety in adolescents.
Methods
Participants
One hundred and nine adolescents participated in the current study (M=15.1 years, SD=0.97 years, range 13.6 to 16.8 years). The adolescents were recruited from two cohorts participating in an ongoing longitudinal study of temperament and emotional reactivity (for details see Calkins, Fox, & Marshall, 1996; Fox et al., 2001b; Kagan et al., 1988). The original cohorts consisted of 166 children (81 male) who were selected at 4 months of age based on their reactions to novel auditory and visual stimuli.
At 14 and 24 months of age, BI was assessed via responses to novel objects and unfamiliar adults using standard laboratory procedures (Calkins et al., 1996; Fox et al., 2001b; Kagan, Reznick, Clarke, Snidman, & Garcia-Coll, 1984). Behaviors coded include proximity to the caregiver and latency to approach novel stimuli. At 48 and 84 months of age, social reticence was assessed during play with an unfamiliar peer via coding of unoccupied, on-looking behavior using the Play Observation Scale (POS; Coplan et al., 1994; Rubin, 1989). Inter-rater reliability was calculated from 30% of the sample and Cohen’s kappa scores ranged from .81 to .94.
Participants returned to the laboratory during mid-adolescence to complete a battery of social, emotional, and cognitive tasks. A total of 109 adolescents (54 male) participated in a novelty auditory oddball task. There were no significant differences between the participants who completed the ERP task and those who did not on measures of BI scores, psychopathology, age, gender, or ethnicity. This study was approved by the University of Maryland Institutional Review Board. All children and their parents provided written informed assent/consent prior to participating in the study.
Novelty Auditory Oddball Task
EEG data were collected while adolescents read a book or magazine while passively listening to auditory stimuli. Participants were instructed to ignore the stimuli. The task consisted of three types of stimuli: 576 standard tones, 72 deviant tones, and 72 unique complex novel sounds. Novel sounds included noises such as a door closing, dog barking, cork popping, and other environmental sounds. Stimuli were presented in two blocks, each containing 228 standard tones, 36 deviant tones, and 36 novel sounds. The standard tone was a 1000 Hz sine wave presented throughout the experiment. One block contained large deviant tones (800 Hz sine wave) while the other block contained small deviant tones (950 Hz sine wave). The order of the blocks was counter-balanced across participants. Each deviant or novel stimulus was preceded by a sequence of 3, 4, or 5 standard tones, with the deviant tones and novel sounds being presented in random order. All stimuli were 150 ms in duration and were presented binaurally through EAR-3A earphones at 75 dB peak SPL. The inter-stimulus interval was 450 ms onset-to-onset. There was a 20-s pause between blocks. The total duration of stimulus presentation was approximately 6.5 minutes.
ERP Collection and Scoring
During the novelty auditory oddball task, continuous EEG data were recorded from 14 scalp sites (F3, F4, F7, F8, Fz, C3, C4, P3, P4, Pz, O1, O2, T7, T8) and two mastoid sites (A1 and A2) using a Lycra stretch cap (Electro-Cap International Inc., Eaton, OH) with sewn-in tin electrodes according to the 10–20 system of electrode placement. After cap placement, each electrode site was gently abraded and electrolytic conducting gel was inserted into the space between the scalp and the electrode. EEG was referenced to Cz. Afz served as the ground electrode. Impedances were considered acceptable if they were at or below 10 kΩ.
Prior to the recording of EEG from each participant, a 50 μV 10-Hz signal was input into each of the channels and the amplified signal was recorded for calibration purposes. Sampling rate was 512 Hz with bioamplifier filter settings of 0.1 Hz (high-pass) and 100 Hz (low-pass). To monitor participant eye blinks, electrooculogram (EOG) was recorded from two mini-electrodes placed above and below the left eye. EEG and EOG signals were amplified by a custom bioamplifier (SA Instruments, San Diego, CA) by a factor of 5000 and 1000, respectively, and were digitized onto a PC using an Iotech (Cleveland, OH) Daqbook A/D converter (+/− 2.5 V input range) and Snap-Master acquisition software (HEM Data Corporation, Southfield, MI). All further processing was carried out using the EEG and ERP Analysis Systems from James Long Company (Caroga Lake, NY). EEG channels were re-referenced in software to an average-mastoids reference. Epochs in which the EEG signal exceeded +/− 150 μV were excluded from analyses while eye movement artifact was regressed. Next, a digital 15-Hz lowpass filter was applied for the primary analyses. All ERPs were calculated for each stimulus type using a 100 ms prestimulus baseline.
In order to quantify the novelty P3, a difference waveform was computed by subtracting the waveform elicited by standard tones from the waveform elicited by novel sounds. Maximal peak amplitude of the P3 was scored in the 180–280 ms time window based on the grand average ERP. To quantify the MMN component, a difference waveform was computed by subtracting the waveform elicited by standard tones from the waveform elicited by deviant tones. Peak amplitude of the MMN was scored as the negative most peak in the difference waveform in the 100–250 ms time window based on the grand average ERP. ERP responses at the midline sites over frontal, central, and parietal scalp regions (Fz, Cz, and Pz respectively) were used in the analyses.
Schedule for Affective Disorders and Schizophrenia for School-Age Children- Present and Lifetime Version (K-SADS-PL)
The K-SADS-PL (Kaufman et al., 1997) is a semi-structured diagnostic interview designed to assess current and past episodes of psychopathology in children and adolescents according the DSM-III-R and DSM-IV criteria. Probes and objective criteria are provided to rate individual symptoms. The current study focused on the lifetime prevalence of anxiety disorders including separation anxiety disorder, generalized anxiety disorder, social phobia, specific phobia, post-traumatic stress disorder, and obsessive-compulsive disorder. Interviews were conducted by advanced clinical psychology doctoral students under the close supervision of a board-certified child and adolescent psychiatrist and a licensed clinical psychologist, all of whom were blind to the subject’s BI classification. Final clinical diagnoses were discussed by the team and made by expert consensus. Reliability was computed from audiotapes of 39% of the interviews reviewed by the two experts (kappa = .92) for anxiety disorders.
Behavioral Inhibition Profiles
Preliminary analyses investigating the effect of early BI using either the 14- or the 24-month behavioral measures alone did not yield significant findings. Therefore, we constructed temperament profiles over the course of childhood by including measures of BI in toddlerhood and social reticence at 48 and 84 months. Although we have previously reported that stable maternal report of BI is predictive of later social anxiety disorders (Chronis-Tuscano et al., in press), the current analysis used observed behavior when creating our BI profiles. We chose behavioral observation as opposed to maternal report to enhance the independence of measurement methods and eliminate the impact of shared method variance due to the fact that the psychopathology assessment also included maternal report.
Longitudinal profiles of BI were obtained by performing latent class analysis (LCA) on measures of BI and social reticence at 14, 24, 48, and 84 months using Mplus version 4.1 (Muthen & Muthen, 2006). Models with 2 through 4 profiles were estimated. Best model fit was assessed using Bayesian information Criteria (BIC), where the smallest negative number indicates the best fit. The Lo-Mendell-Rubin Likelihood ratio test (LMRL) was also used to test the significance of the -2 Log likelihood difference between models with k and k-1 profiles (Lo, Mendell, & Rubin, 2001).
Due to the skewed distribution of the data, behavioral measures for 14, 24, 48, and 84 months were individually dichotomized at the median, where 0 denoted a child rated in the lower half of the sample at a particular time point and 1 denoted a child rated in the top half of the sample at that same time point. Model fit (Bayesian information criteria) for the current sample was −766.12 for one profile, −768.92 for two profiles, −792.71 for three profiles, and −817.55 for four profiles. The 2-profile model was selected as the best fitting model given the low BIC value and significant LMRL ratio test. The average posterior probabilities of membership ranged from .78 to .86 across the two profiles, reflecting a moderately high degree of confidence in profile assignment. Cases were assigned to the BI profile for which their posterior probability was highest. The “high” BI profile (n = 69) displayed high-average levels of BI at all four time points and 42% of the sample had a higher probability of membership in this profile than the other profile. The “low” BI profile (n = 97) displayed lower levels of BI at all four time points and 58% of the sample had a higher probability of membership in this profile than the other profile.
In the current study, 43 (20 male) adolescents were in the high BI group and 66 (34 male) adolescents were in the low BI group. Twenty-eight (13 male) and 45 (22 male) participants from the high BI and low BI groups, respectively, did not have anxiety diagnoses. Fifteen (7 male) adolescents in the high BI group (mean number of anxiety diagnoses: 1.47, SD=0.9) and 21 (12 male) adolescents in the low BI group (mean number of anxiety diagnoses: 1.33, SD=0.58) had a lifetime diagnosis of anxiety. Of these children, 8 (3 male) in the high BI group and 16 (10 male) in the low BI group had current anxiety diagnoses.
Data Analysis
Repeated measures ANOVA was used to examine the interaction between electrode site and BI profiles on P3 and MMN amplitude and latency. Hierarchical logistic regression analysis was used to examine the moderating role of attention (i.e., P3 and MMN) in the relation between BI and anxiety. Sex was entered as a covariate in both analyses. One participant was excluded as a statistical outlier from analysis of the P3 component.
Results
ERP Morphology
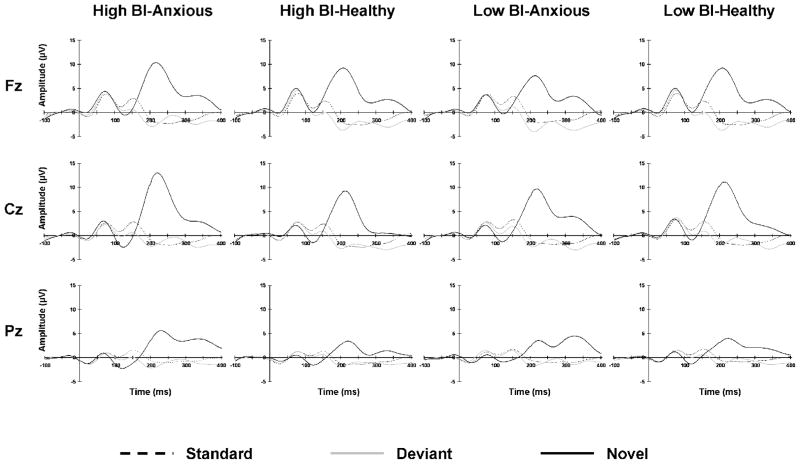
Figure 1 displays waveforms for standard, deviant, and novel stimuli for high and low BI adolescents with and without lifetime anxiety diagnosis. P1 and N1 components were elicited by all stimuli at approximately 75 ms and 125 ms after stimulus onset, respectively. P2 and N2 components were only observed following standard and deviant tones at approximately 150 and 200 ms after stimulus onset, respectively. The novel complex sound elicited a prominent positive peak between 200–250 ms after stimulus onset. This peak was not observed following presentations of standard or deviant stimuli. Preliminary analysis determined that the number of epochs used for data analysis for the different ERP components did not differ as a function of temperament or diagnosis.
Figure 1. Grand Mean Waveforms.
Grand mean ERP waveforms for the standard (black dashed line), deviant (gray solid line), and novel (black solid line) stimuli for high BI-anxious, high BI-healthy, low BI-anxious, and low BI-healthy.
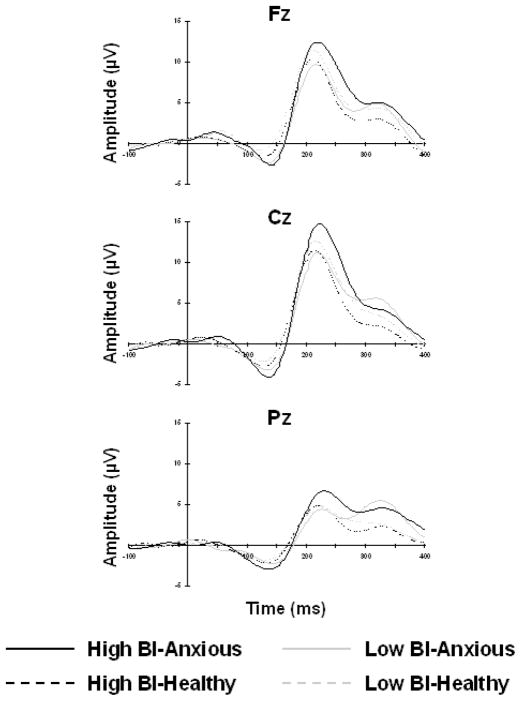
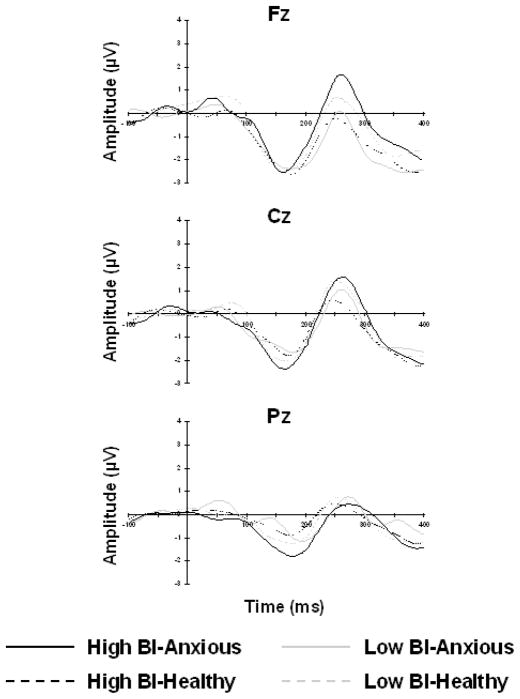
P3 and MMN Amplitude and Latency
Figure 2 displays the difference waveforms for novel sounds – standard tones and Figure 3 displays the difference waveforms of deviant – standard tones for Fz, Cz, and Pz separately. A repeated measures ANOVA with site (Fz, Cz, Pz) as the within-subjects measure, temperament (low BI, high BI) as the between-subjects measure, and sex as the covariate was computed for the P3 and MMN components separately. Analysis conducted on P3 peak amplitude and latency revealed a significant main effect of site for both P3 amplitude [F(2,210)=88.98, p<.001] and latency [F(2,210)=26.00, p<.001]. Post-hoc analysis revealed that P3 amplitude was significantly greater at Cz compared to both Fz [t(107)=4.18, p<.001] and Pz [t(107)=21.55, p<.001] and that P3 amplitude was significantly greater at Fz compared to Pz [t(107)=13.65, p<.001]. Significantly shorter peak latency for the P3 component was found for Cz compared to Fz [t(107)=3.60, p<.001] and Pz [t(107)=7.75, p<.001] as well as for Fz compared to Pz [t(107)=6.02, p<.001]. Analysis conducted on MMN amplitude and latency revealed a significant main effect of site for amplitude [F(2,212)=13.36, p<.001], but not for latency. Post-hoc analysis revealed that MMN amplitude was significantly more negative at Fz compared to Cz [t(108)=4.08, p<.001] and Pz [t(108)=6.12, p<.001] and at Cz compared to Pz [t(108)=4.84, p<.001]. No further significant interaction or main effects were found.
Figure 2. P3 Response.
Grand mean auditory response potential difference waveforms (novel – standard) at Fz (top), Cz (middle) and Pz (bottom) for high BI-anxious (black solid line), high BI-healthy (black dashed line), low BI-anxious (gray solid line), and low BI-healthy (gray dashed line).
Figure 3. MMN Response.
Grand mean tone-evoked response potential difference waveforms (deviant – standard) at Fz (top), Cz (middle) and Pz (bottom) for high BI-anxious (black solid line), high BI-healthy (black dashed line), low BI-anxious (gray solid line), and low BI-healthy (gray dashed line).
Moderation of anxiety by BI and attention to novelty
A dichotomous index of anxiety, indicating the lifetime presence or absence of an anxiety disorder, was regressed on the high and low BI indicator variable as well as on continuous values of the P3 response and the two-way interaction between the P3 response and the two temperament groups. Because a main effect of site was found, logistic regression analysis was conducted for Fz, Cz, and Pz separately. A significant two-way interaction between BI and P3 amplitude on lifetime anxiety was found only at Cz (OR=1.21; 95% CI=1.00–1.45; Wald X2 = 3.99; p<.05; Figure 4). Follow-up analysis revealed a trend effect for high behaviorally inhibited adolescents in which greater P3 amplitude was related to increased risk for anxiety (OR=1.16; 95% CI=.98–1.36; Wald X2=4.57; p=.08). There was no relation between P3 amplitude and anxiety diagnosis in the low BI group (p>.20). A similar analysis was conducted to determine if the same two-way interaction exists for current anxiety diagnosis and a trend effect was found at Cz (OR=1.19; 95% CI=.97–1.47; Wald X2 = 2.92; p=.09).
Figure 4. P3 amplitude moderates the relation between behavioral inhibition and anxiety.
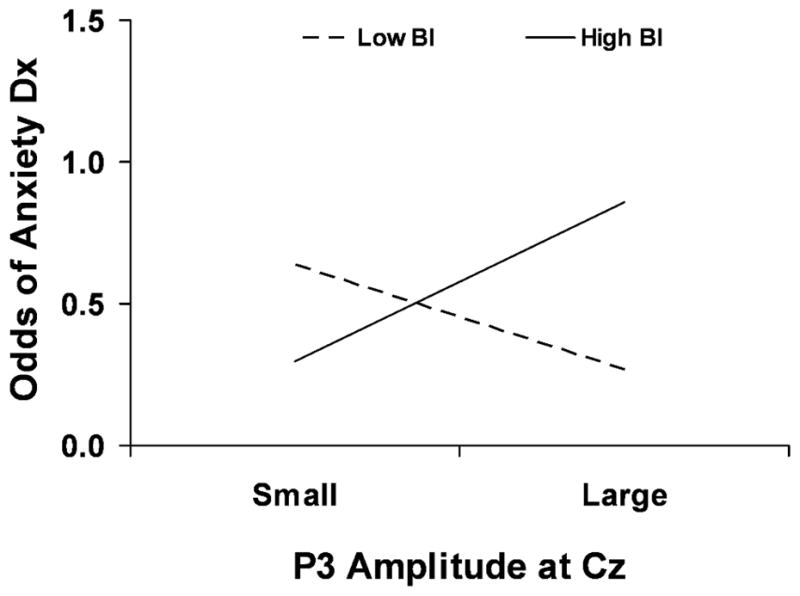
High BI adolescents with a large P3 response are at greater risk of an anxiety diagnosis compared to those with a small P3 response.
A similar regression analysis investigating the moderating role of MMN amplitude on the relation between BI and anxiety was not significant at any site (ps>.20). Main effects of BI and P3/MMN amplitude were not significant.
Discussion
In the current study, we found that the neural correlates associated with the processing of novel auditory stimuli moderate the association between BI and anxiety. Specifically, increased P3 amplitude differentiated adolescents with high levels of BI and a history of anxiety diagnosis from similar behaviorally inhibited adolescents with no such history. Surprisingly, the MMN response to deviant tones did not differ between the high and low BI groups, independent of their history of anxiety. This study is the first to examine the degree to which attention to novelty moderates the link between BI and anxiety. The current findings add to the growing body of research that suggests that specific components of attention moderate the relation between BI and risk for anxiety (McDermott, Perez-Edgar, Henderson, Pine, & Fox, 2009; Perez-Edgar, Fox, Bar-Haim, McDermott, & Pine, in press; Reeb-Sutherland et al., 2009).
In particular, the relations between early BI and anxiety may be moderated by perturbed attention to novelty. This hypothesis stems from a number of studies that have found heightened attention bias to threat among anxious adults (MacLeod, Rutherford, Campbell, Ebsworthy, & Holker, 2002; Mathews & MacLeod, 1994) and behaviorally inhibited children (Perez-Edgar & Fox, 2005; Perez-Edgar et al., in press; Schwartz, Snidman, & Kagan, 1996). As well, the current study suggests that behaviorally inhibited adolescents show enhanced attention to novel (but not necessarily threatening) stimuli. Thus, the attention bias to threat may be part of a more general tendency to identify novelty in the environment amongst behaviorally inhibited participants. This bias toward novelty in behaviorally inhibited children may contribute to their continued hypervigilance and social withdrawal when faced with novel social situations. Additionally, in the absence of appropriate or adaptive regulation it may lead to the manifestation of anxiety disorders. The current findings provide preliminary support for this theory in that high BI children with increased attention to novelty (i.e., increased P3 amplitude) were at greater risk for having an anxiety disorder than those high BI children who did not display increased attention to novelty.
Coping resources available to the child, through mechanisms of attention, may moderate the physiological and behavioral correlates of temperament (Nachmias, Gunnar, Mangelsdorf, Parritz, & Buss, 1996). Orienting to novel stimuli and, specifically, to perceived threatening stimuli in the environment may elicit behavioral avoidance and negative affect. Behaviorally inhibited children who can direct their attention away from novel stimuli may mitigate underlying reactive tendencies and avoid the deleterious effects of negative affect. In contrast, behaviorally inhibited children with poor attention control skills, whose attention is drawn frequently to novelty, may be more susceptible to maladaptive affective reactions. The current findings suggest that behaviorally inhibited children with an increased attention bias to novelty are at increased risk for developing an anxiety disorder. Although both the attention and clinical data were collected concurrently, three recent studies on perturbed attention suggest that the effects of attention as a moderator of BI are critical for understanding the developmental trajectories of children with this temperamental disposition. First, Marshall et al. (2009) reported that high negative-high motor 9-month-old infants, selected for their relation to BI, display heightened sensitivity to novel auditory stimuli. In two studies with adolescents characterized across childhood with BI, findings of heightened attention bias to threat (Perez-Edgar et al., in press) and increased error monitoring and error-related negativity (ERN) amplitude (McDermott et al., 2009) have been reported. Thus, multiple components of attention involved in orienting and monitoring appear perturbed in behaviorally inhibited samples.
Our lab has recently reported that children with high and stable maternal report of BI (but not observed BI) is a risk factor for developing social anxiety (Chronis-Tuscano et al., in press). In the current study, we did not find a significant main effect of observed BI on anxiety diagnosis. However, we did find that by using behavioral measures of BI in conjunction with psychophysiological measures of attention to novelty, we were able to predict adolescent risk for anxiety. The results of the current study parallel other recent findings from our lab in which observed BI is related to anxiety, but only when examined in combination with various psychophysiological measures (McDermott et al., 2009; Reeb-Sutherland et al., 2009). Taken together, these results suggest that one should consider using multiple levels of analysis (e.g., behavioral and physiological) when examining the relation between observed BI and anxiety (Kagan, Snidman, McManis, Woodward, & Hardway, 2002). In addition, future work should explore the specific mechanisms by which maternal report versus observational measures of BI directly and indirectly predict anxiety symptomatology and disorder across development.
Previous studies have shown that behaviorally inhibited children (Bar-Haim et al., 2003) and negatively reactive infants (Marshall et al., 2009) differ in their early cortical processing of change detection (i.e., MMN, mismatch response in infants) compared to non-inhibited children and positively reactive infants, respectively. In contrast to these studies, we did not observe differences in MMN amplitude between the low and high BI adolescents. The MMN is thought to reflect an automatic preattentive response to stimulus deviance while the P3 is considered an endogenously-driven orientation response to novelty (Friedman et al., 2001). It appears that, by adolescence, automatic preattentive processes may no longer distinguish between formerly high and low BI individuals.
This study should be considered in light of a number of limitations. First, while this is the largest longitudinal study to examine the moderating influence of P3 on risk for anxiety in BI, the overall sample size was relatively small. Accordingly, replication in larger samples is needed. Moreover, due to such a small sample size, we were forced to combine adolescents with any history of any anxiety disorder into a single group. Future studies with larger samples might consider associations with specific anxiety disorders. Because psychophysiological measures of novelty detection and anxiety were measured concurrently, this study was unable to predict whether increased response to novelty can be used as a predictor of anxiety or is the result of being both anxious and high on measures of BI. Future studies should examine attention to novelty in children at earlier ages in order to examine whether these differences exist prior to diagnosis.
Previous research has demonstrated that state anxiety during presentations of an auditory oddball task can significantly influence P3 amplitude (Grillon & Ameli, 1994). Therefore, an increase in state anxiety among behaviorally inhibited children with a history of anxiety, particularly those with a current anxiety diagnosis, may have influenced our findings. Because we did not assess state anxiety in the current study, we were not able to directly address this issue. However, a trend-level interaction effect of BI and P3 amplitude on current anxiety diagnosis was found, suggesting that having a current anxiety disorder may influence the current findings, but due to insufficient power we were unable to detect a significant effect.
In summary, this study provides evidence that the association between BI and anxiety diagnosis during adolescence may be moderated by attention bias toward novelty. While other studies have reported differences in attention processes between behaviorally inhibited and non-inhibited individuals and healthy and anxious patients, this is the first to employ a longitudinal design to demonstrate that high-risk children who develop anxiety many years later also display increased neural responses to novelty.
Key Points
Behavioral inhibition, a temperamental disposition characterized by an increased tendency to withdraw in the face of novelty, is a risk factor for the development of anxiety disorders.
Behaviorally inhibited children with an increased attention bias to threat or novelty may be at increased risk for developing anxiety disorders.
Electrophysiological correlates of attention to novelty were found to distinguish between behaviorally inhibited adolescents with and without a lifetime history of anxiety diagnosis.
Employing both neural and behavioral approaches to the measurement of temperament may help identify which behaviorally inhibited children are at heightened risk for developing anxiety disorders so that preventative treatment may begin prior to the manifestation of the disorder.
Acknowledgments
This project was supported by grants from the National Institutes of Health (MH074454 and HD 17899) to Nathan A. Fox. The authors would like to thank children and their families for their continued participation in our studies.
Abbreviations
- BI
behavioral inhibition
- MMN
mismatch negativity
- ERN
error-related negativity
- ERPs
event-related potential
- EEG
electroencephalogram
- EOG
electrooculogram
- K-SADS-PL
Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version
Footnotes
Disclosure statement: Dr. Chronis-Tuscano has received support from Ortho-McNeil-Janssen. The other authors report no conflicts of interest.
References
- Bar-Haim Y, Marshall PJ, Fox NA, Schorr EA, Gordon-Salant S. Mismatch negativity in socially withdrawn children. Biological Psychiatry. 2003;54:17–24. doi: 10.1016/s0006-3223(03)00175-6. [DOI] [PubMed] [Google Scholar]
- Bruder GE. P300 findings for depressive and anxiety disorders. Annals of the New York Academy of Sciences. 1992;658:205–222. doi: 10.1111/j.1749-6632.1992.tb22846.x. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Kayser J, Tenke CE, Leite P, Schneier FR, Stewart JW, et al. Cognitive ERPs in depressive and anxiety disorders during tonal and phonetic oddball tasks. Clinical Electroencaphalography. 2002;33:119–124. doi: 10.1177/155005940203300308. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Fox NA, Marshall TR. Behavioral and physiological antecedants of inhibited and uninhibited behavior. Child Development. 1996;67:523–540. [PubMed] [Google Scholar]
- Chronis-Tuscano A, Degnan K, Pine D, Perez-Edgar K, Henderson H, Diaz Y, et al. Stable, early maternal report of behavioral inhibition predicts lifetime social anixety disorder in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. doi: 10.1097/CHI.0b013e3181ae09df. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan RJ, Girardi A, Findlay LC, Frohlick SL. Understanding solitude: young children’s attitudes and responses toward hypothetical socially withdrawn peers. Social Development. 2007;16:390–409. [Google Scholar]
- Coplan RJ, Rubin KH, Fox NA, Calkins SD. Being alone, playing alone, and acting alone: distinguishing among reticence and passive, and active solitude in young children. Child Development. 1994;65:129–138. [PubMed] [Google Scholar]
- Cornwell BR, Baas JMP, Johnson L, Holroyd T, Carver FW, Lissek S, et al. Neural responses to auditory stimulus deviance under threat of electric shock revealed by spatially-filtered magnetoencephalography. Neuroimage. 2007;37:282–289. doi: 10.1016/j.neuroimage.2007.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cycowicz YM, Friedman D. Effect of sound familiarity on the event-related potentials elicited by novel environmental sounds. Brain and Cognition. 1998;36:30–51. doi: 10.1006/brcg.1997.0955. [DOI] [PubMed] [Google Scholar]
- Daruna JH, Rau AE, Strecker CD. P3 amplitude in young children: relation to anxiety. Biological Psychiatry. 1991;29:837–840. doi: 10.1016/0006-3223(91)90203-x. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Reed MA. Temperament and attention: orienting toward and away from positive and negative signals. Journal of Personality and Social Psychology. 1994;66:1128–1139. doi: 10.1037//0022-3514.66.6.1128. [DOI] [PubMed] [Google Scholar]
- Fox E, Russo R, Bowles R, Dutton K. Do threatening stimuli draw or hold visual attention in subclinical anxiety? Journal of Experimental Psychology: General. 2001a;130:681–700. [PMC free article] [PubMed] [Google Scholar]
- Fox E, Russo R, Georgiou GA. Anxiety modulates the degree of attentive resources required to process emotional faces. Cognitive, Affective & Behavioral Neuroscience. 2005;5:396–404. doi: 10.3758/cabn.5.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Hane AA, Pine DS. Plasticity for affective neurocircuitry. Current Directions in Psychological Science. 2007;16:1–5. [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: psychophysiological and behavioral influences across the first four years of life. Child Development. 2001b;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neuroscience and Biobehavioral Reviews. 2001;25:355–373. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R. P300 assessment of anxiety effects on processing novel stimuli. International Journal of Psycophysiology. 1994;17:205–217. doi: 10.1016/0167-8760(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Hirshfeld DR, Jerrold MA, Rosenbaum JF, Biederman J, Bolduc EA, Faraone SV, et al. Stable behavioral inhibition and its association with anxiety disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:103–111. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- Hogan AM, Butterfield EL, Phillips L, Hadwin JA. Brain response to unexpected novel noises in children with low and high trait anxiety. Journal of Cognitive Neuroscience. 2007;19:25–31. doi: 10.1162/jocn.2007.19.1.25. [DOI] [PubMed] [Google Scholar]
- Iwanami A, Isono H, Okajima Y, Kamijima K. Auditory event-related potentials in panic disorder. European Archives of Psychiatry and Clinical Neuroscience. 1997;247:107–111. doi: 10.1007/BF02900202. [DOI] [PubMed] [Google Scholar]
- Kagan J. Galen’s Prophecy. New York, NY: Basic Books; 1994. [Google Scholar]
- Kagan J, Reznick JS, Clarke C, Snidman N, Garcia-Coll C. The physiology and psychology of behavioral inhibition in children. Child Development. 1984;58:1459–1473. [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N, Gibbons J, Johnson MO. Childhood derivatives of inhibition and lack of inhibition to the unfamiliar. Child Development. 1988;59:1580–1589. doi: 10.1111/j.1467-8624.1988.tb03685.x. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N, McManis M, Woodward S, Hardway C. One measure, one meaning: multiple measures, clearer meaning. Development and Psychopathology. 2002;14:463–475. doi: 10.1017/s0954579402003048. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. [Google Scholar]
- MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L. Selective attention and emotional vulnerability: assessing the causal bases of their association through the experimental manipulation of attentional bias. Journal of Abnormal Psychology. 2002;111:107–123. [PubMed] [Google Scholar]
- Marshall PJ, Reeb BC, Fox NA. Electrophysiological responses to auditory novelty in temperamentally different 9-month-old infants. Developmental Science. 2009;12:568–582. doi: 10.1111/j.1467-7687.2008.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive approaches to emotion and emotional disorders. Annual Review of Psychology. 1994;45:25–50. doi: 10.1146/annurev.ps.45.020194.000325. [DOI] [PubMed] [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Pine DS, Fox NA. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry. 2009;65:445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen L, Muthen B. Mplus 4.1 User’s Guide. Los Angeles, CA: 2006. [Google Scholar]
- Naatanen R, Alho K. Mismatch negativity - a unique measure of sensory processing in audition. International Journal of Neuroscience. 1995;80:317–337. doi: 10.3109/00207459508986107. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Paavilainen P, Tiitinen H, Jiang D, Alho K. Attention and mismatch negativity. Psychophysiology. 1993;30:436–450. doi: 10.1111/j.1469-8986.1993.tb02067.x. [DOI] [PubMed] [Google Scholar]
- Nachmias M, Gunnar M, Mangelsdorf S, Parritz RH, Buss K. Behavioral inhibition and stress reactivity: the moderating role of attachment security. Child Development. 1996;67:508–522. [PubMed] [Google Scholar]
- Perez-Edgar K, Fox NA. A behavioral and electrophysiological study of children’s selective attention under neutral and affective conditions. Journal of Cognition and Development. 2005;6:89–118. [Google Scholar]
- Perez-Edgar K, Fox NA, Bar-Haim Y, McDermott J, Pine DS. Attention bias to threat link behavioral inhibition in early childhood to adolescent social withdrawal. Emotion. doi: 10.1037/a0018486. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeb-Sutherland B, Helfinstein S, Degnan K, Perez-Edgar K, Henderson H, Lissek S, et al. Startle response in behaviorally inhibited adolescents with a lifetime occurrence of anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:610–617. doi: 10.1097/CHI.0b013e31819f70fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin KH. The Play Observation Scale (POS) Ontario, Canada: University of Waterloo; 1989. [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. Early temperamental predictors of Stroop interference to threatening information at adolescence. Journal of Anxiety Disorders. 1996;10:89–96. [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1008–1015. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]