Abstract
The visual systems of most species contain photoreceptors with distinct spectral sensitivities that allow animals to distinguish lights by their spectral composition. In Drosophila, photoreceptors R1–R6 have the same spectral sensitivity throughout the eye and are responsible for motion detection. In contrast, photoreceptors R7 and R8 exhibit heterogeneity and are important for color vision. We investigated how photoreceptor types contribute to the attractiveness of light by blocking the function of certain subsets and by measuring differential phototaxis between spectrally different lights. In a “UV vs. blue” choice, flies with only R1–R6, as well as flies with only R7/R8 photoreceptors, preferred blue, suggesting a nonadditive interaction between the two major subsystems. Flies defective for UV-sensitive R7 function preferred blue, whereas flies defective for either type of R8 (blue- or green-sensitive) preferred UV. In a “blue vs. green” choice, flies defective for R8 (blue) preferred green, whereas those defective for R8 (green) preferred blue. Involvement of all photoreceptors [R1–R6, R7, R8 (blue), R8 (green)] distinguishes phototaxis from motion detection that is mediated exclusively by R1–R6.
Keywords: differential phototaxis, attractiveness function, R7/R8 sytem, color vision
Depending on its spectral composition and intensity, light can serve as an attractive or repulsive landmark for orientation. Accordingly, animals identify an object or a light source at a certain location in visual space and approach or retreat from it. Phototaxis in insects has been a useful paradigm to gain a better understanding of this behavior. Here we take advantage of Drosophila genetics to investigate the function of the different subtypes of photoreceptors in this behavior.
The Drosophila compound eye consists of about 750 ommatidia, each containing 8 photoreceptor cells (1). The six outer photoreceptors (R1–R6) contain a blue-sensitive rhodopsin (Rh1) and show a second sensitivity peak in the UV due to a sensitizing pigment (1–5). They are specialized for vision at low light levels and low pattern contrast (1, 6). They are necessary and sufficient for motion vision, and fly optomotor responses are abolished in their absence (7, 8). The two inner photoreceptors (R7 and R8) are heterogeneous. Along the dorsal margin of the eye, both R7 and R8 express rhodopsin Rh3 that is sensitive to shorter wavelength UV (9). This part of the retina is specialized for the detection of the e-vector of polarized light (10, 11). The remaining part of the retina contains two types of ommatidia called pale (p) and yellow (y). In p-type ommatidia, R7 cells contain Rh3 (R7p) and R8 express blue-sensitive Rh5 (R8p). In y-type ommatidia, R7 cells express Rh4 sensitive to longer wavelength UV (R7y) and R8 cells contain green-sensitive Rh6 (R8y) (Fig. 1A) (5, 12–15). R7y cells in the dorsal third of the eye coexpress Rh3 and Rh4 (16). Approximately 30% of the ommatidia are of the p- and 70% of the y-type, with the p- and y-subtypes distributed stochastically in the retina. R7/R8 cells in the main part of the retina appear to be involved in color vision (15), which requires the comparison of at least two photoreceptors with different spectral sensitivities. This comparison could be between R7 and R8 within one ommatidium or between different (p vs. y) ommatidia (17), or it could involve outer photoreceptors.
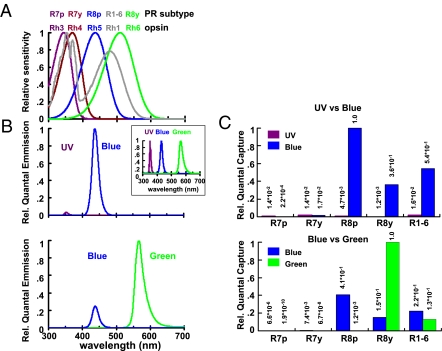
Fig. 1.
(A) Normalized spectral quantum sensitivity of the different rhodopsins in the photoreceptor subtypes R7p, R7y, R8p, R8y, and R1–R6 (R. Hardie, unpublished data). The height of the blue peak relative to the UV peak of R1–R6 (gray curve) might be lower than shown depending upon the β-carotene content of fly food (5, 38). (B) Spectral properties of the stimuli used in this study. The normalized spectral curves of LEDs are shown in the Upper Inset. (C) The relative number of quanta captured by each photoreceptor (PR) subtype was estimated for the light stimuli used in UV/B (Upper) and B/G experiments (Lower). Note that these normalized estimates refer to single photoreceptors. Neither their absolute sensitivities nor their relative numbers (e.g., R8p:R8y = 3:7) were taken into account.
Phototaxis can be tested in different ways (6, 18, 19). When flies in small tubes illuminated from one side (“light/dark”) are disturbed, they rush toward the light for an escape (“fast phototaxis”) (20). In a variant of this behavior, called “differential phototaxis,” flies choose between two light sources. Both paradigms have been used to determine the action spectra of phototaxis. Using a light/dark assay (21–23), two peaks of sensitivity were identified, one in the UV and one in the blue, representing mainly the sensitivity of the outer photoreceptors R1–R6 (24). In differential phototaxis (25–27), the UV peak is particularly pronounced.
Mutants with defects in phototactic behavior (20) affect phototransduction and/or cause photoreceptor degeneration [e.g., ninaE17 (28) rdgB (29, 30)]. sevenlessLY3 mutants (sev) (27) also display phototaxis defects. In these mutants, photoreceptor development is affected because R7 cells are missing, and photoreceptors R1–R6 and R8 are largely intact, although the latter are deprived of their proper optics, the rhabdomere of R7. The sev mutation has been useful for investigating the contribution of R7 to phototaxis behavior. The sensitivity of sev flies to UV measured by electroretinogram (27) or light/dark phototaxis (23) is comparable to that of wild-type flies. In differential phototaxis, however, sev flies prefer visible light at intensities where wild-type flies have a strong preference for UV (7, 27, 31). The “UV vs. visible” choice paradigm is a robust functional assay that was used for genetic screens that identified other genes essential for R7 development (6, 19).
Functional analyses of R8 cells have lagged behind. Due to their position behind R7 photoreceptors and their small size, their physiological properties are difficult to examine by electrophysiological recordings. The spectral properties of R8 opsins were measured by using the double-mutant rdgB sev, in which R8 were believed to be the only functional photoreceptors (27), and more recently by expressing R8 opsins in R1–R6 of ninaE17 mutant cells lacking Rh1 (5). It appears that R8 alone can mediate phototactic behavior at high intensities (32). However, the contribution of R8 in the presence of an intact R1–R6 system has not been investigated because R8 specific mutants were previously not available. Moreover, the distribution of R8 subtypes is altered in sev flies (33, 34), possibly confounding results that had assessed the contribution of R8 to phototaxis.
To study the contributions of photoreceptor types to phototaxis, we developed a variant of differential phototaxis involving choices between two different monochromatic lights: UV vs. blue or blue vs. green. We tested various mutants with altered or lost opsin expression, as well as flies in which specific types of photoreceptors were functionally switched off by specifically blocking synaptic transmission. We showed that R1–R6, R7, R8p, and R8y all affect phototaxis. The results are largely consistent with the prediction made by estimating the relative number of quanta captured by each photoreceptor subtype if each subtype is separately weighted. However, some photoreceptor combinations generated effects that could not be explained by simple summation of the responses of all functional photoreceptors.
These phototaxis experiments provide a first glimpse of a spectral-wavelength–dependent attractiveness function used in landmark discrimination. A quantitative description of this function would require measuring all the intensity dependencies of spectral preferences. It will be of interest to compare this function to color vision defined as intensity-invariant hue discrimination, which has been measured by learning and generalization tasks (35, 36).
Results
Differential Phototaxis.
To test the contribution of photoreceptor types to phototaxis, we used a T-maze apparatus (37), adding two different monochromatic light sources (LEDs) at the end of each of the test tubes. Approximately 100 flies were placed at the choice point, and the number of flies that had moved toward each light source was counted 20 s later. A preference index (PI) was calculated as PI = (NL − NS)/(NL + NS), where NL and NS were the number of flies on the side of the longer and shorter wavelength lights, respectively. To test the ability of the flies to distinguish lights of different spectral composition, we used two combinations of monochromatic lights: UV vs. blue (UV/B), to presumably assay R7 vs. R8 function, and blue vs. green (B/G) for R8p vs. R8y (see below). The wavelength of the lights was chosen such that they stimulate the respective photoreceptor types effectively (Fig. 1). R7p and R7y contain UV-sensitive rhodopsins that are tuned fairly closely, and we did not attempt to test the ability of the fly to distinguish in this range of wavelengths.
For the UV/B tests, UV intensity was set to ∼1,000 times the phototaxis threshold of wild-type flies to make sure that the lights could be detected by the R7 and R8 cells. This was more than five times the phototaxis threshold of ninaE17, a rh1 null mutant that completely blocks R1–R6 function (3), as tested in separate “light vs. dark” experiments. The intensity of the blue light was adjusted such that wild-type flies distributed equally between UV and blue (PI = 0). As UV is highly attractive for wild-type flies (7, 25–27), its intensity was ∼57 times lower than the blue (Fig. 1B, Upper). For B/G tests, green intensity was set to ∼500 times the phototaxis threshold of wild-type flies, i.e., 20 times the phototaxis threshold of ninaE17 mutants in the “light vs. dark” experiment. The blue intensity was 7.8 times lower than the green, reflecting the innate preference of flies for shorter wavelengths. In the UV/B test, blue was about 85 times more intense than in the B/G test (see SI Materials and Methods for further information).
Contributions of Photoreceptors in UV/B Choice Tests.
In UV/B choice tests, the UV (peak wavelength 350 nm) was chosen to maximize R7 responses. R7p contains Rh3 maximally absorbing around 345 nm, and R7y contains Rh4 with an absorption maximum around 375 nm (14). The UV light also optimally stimulated the R1–R6 photoreceptors in the spectral range of the sensitizing pigment (gray curve in Fig. 1A). The blue (peak wavelength 430 nm; Fig. 1B, Upper) effectively stimulated both subtypes of R8 cells as well as R1–R6 (Rh1 opsin peak at 478 nm). R8p expressed blue-absorbing Rh5 (437 nm), and R8y a green-absorbing Rh6 (508 nm) (5). Fig. 1A shows the normalized spectral properties of each photoreceptor subtype (R. Hardie, unpublished data; refs. 5 and 38). Fig. 1B indicates the spectral profile of each LED for UV/B and B/G choices.
The relative numbers of quanta captured by each subtype of the photoreceptors were estimated for the light intensities used (Fig. 1C; see also SI Materials and Methods) by taking into account the spectral properties of each photoreceptor subtype (Fig. 1A) and the relative irradiance of each LED (Fig. 1B).
Both R8p and R8y photoreceptors respond to blue much more strongly than to UV (Fig. 1C, Upper). Thus, in the UV/B choice test, mutants impaired in R8p and/or R8y are expected to prefer UV to blue, assuming a simple additive model of photoreceptor function. On the other hand, R7p receptors should respond more to UV than to blue, whereas R7y should respond similarly to UV and blue (Fig. 1C, Upper). Thus, switching off both R7p and R7y is expected to lead to a preference for blue. These assumptions imply, however, that the attractiveness function of differential phototaxis is not dominated by the R1–R6 photoreceptors which, due to their size, optics, and number are about 10–50 times more sensitive than R7/R8 cells.
Mutants Defective in R7 Function Prefer Blue in UV/B Choice.
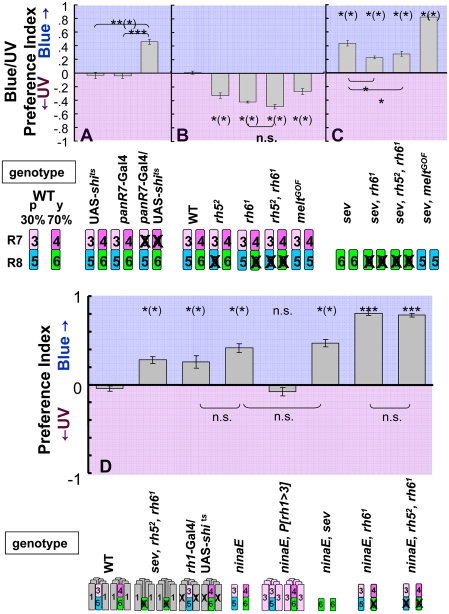
We first examined the contribution of R7 photoreceptors. sev mutant flies (27) preferred blue in the UV/B choice test (Fig. 2C), consistent with earlier experiments using a UV vs. green choice test (7). In addition to the loss of R7 cells, sev flies also have defects in R8: Because R7 cells are required during development for R8p subtype specification, ∼93% of R8 cells in sev mutant flies contain Rh6 (R8y subtype) (33, 34). To assess the contribution of R7 alone without affecting R8, we silenced R7 cells in the adult after they had correctly instructed R8p cells. We used the Gal4/UAS system (39) to switch off R7 cells using a late R7-specific driver (panR7-Gal4) (40) and, as effector, the temperature-sensitive semidominant allele of shibire (UAS-shits) (41, 42) that switches off synaptic transmission in photoreceptors (43). The result for panR7 > shits flies was not significantly different from that of sev flies: When R7 cells were blocked in this way, flies showed a strong preference for blue over UV (Fig. 2A), indicating that it is the impairment of R7 function alone that results in this phenotype.
Fig. 2.
UV/B choice tests. Pictograms below graphs represent the rhodopsin content of each photoreceptor subtype (1–6 refer to rh1-rh6) for each genotype. R1–R6 are omitted in Figs. 2 and 3. (A) Contribution of R7. panR7-Gal4/UAS-shits flies, in which R7 function is lost, preferred blue and control UAS-shits or panR7-Gal4 alone showed normal behavior. (B) Contribution of R8. All mutant flies defective for R8 (rh52, rh61, rh52 rh61, and meltGOF) preferred UV. The behavior of rh52 rh61 double-mutant flies was not significantly different from rh61 flies. (C) Mutant flies lacking R7 preferred blue. All mutant flies (sev, sev rh6, sev rh5 rh6, and sev meltGOF) showed preference for blue. Distinct phenotypes of meltGOF were observed in the presence or absence of R7 (compare meltGOFin B and sev meltGOF in C). In B and C, results from each mutant were compared to wild type in B. n.s, not significant; *P < 0.05, **P < 0.01, ***P < 0.001; n = 5–6. (D) Contribution of the R1–R6 system in differential phototaxis; both rh1-Gal4/UAS-shits and ninaE17 flies preferred blue to UV, whereas ninaE17 P[rh1 > 3] behaved similarly to wild type. Both ninaE17 and sev ninaE17 flies preferred blue, without detectable differences. Both ninaE17 rh61 and ninaE17 rh52 rh61 showed a strong blue preference. n = 5–10. Error bars are SEMs.
Mutants Defective in R8 Function Prefer UV in UV/B Choice.
Next, we investigated the contribution of R8 in the UV/B choice. As expected, all mutants defective for R8 function showed preference for UV over blue (Fig. 2B). Mutant rh52 flies completely lack Rh5 expression (8) and thus have nonfunctional R8p cells but have normal R8y cells. In the UV/B choice, rh52 flies preferred UV. Mutant rh61 flies lacking functional R8y cells also showed increased UV preference. The behavior of rh52 rh61 double-mutant flies (Fig. 2B) was not significantly different from that of rh61 flies.
The melted (melt) gene affects the specification of the R8p subset (40). In flies homozygous for a melt gain-of-function allele (meltGOF), R8p cells are dramatically expanded at the expense of R8y cells, without affecting R7 or R1–R6 (40). Rh6 is therefore nearly absent and almost all R8 express blue-sensitive Rh5, in contrast to rh61 mutants in which only R8p cells express Rh5 and R8v cells are nonfunctional. Unexpectedly, meltGOF flies also had enhanced preference for UV over blue (Fig. 2B). This result does not fit the assumption of a simple addition of the responses from each of the photoreceptor types. Because R8p photoreceptors are about 2.5 times as sensitive to blue light as R8y cells (Fig. 1C), transforming the 70% R8y into R8p cells in meltGOF flies should lead to an increased sensitivity to blue light compared to blue-light sensitivity in wild type. Yet, the preference was shifted toward the UV (see Discussion for possible interpretation).
Mutants Defective in Both R7 and R8 Prefer Blue in UV/B Choice.
Impairing R7 or R8 function resulted in opposite phenotypes. Therefore, impairing both R7 and R8 might cancel out their effects. To test this, we examined mutants defective for both R7 and R8 function. Because sev mutant flies lack R7 and have very few R8p cells, sev rh61 double mutants have only very few R8 cells expressing Rh5, and the sev rh52 rh61 triple mutant should have no functional opsins expressed in the inner photoreceptors; therefore, the contributions of R7 and R8 to the attractiveness function should be lost (8). These flies showed a preference for blue over UV. In both the double and triple mutants, the preference for blue was smaller than in sev flies (Fig. 2C), indicating a contribution of R8y cells in sev mutants. The preference for blue in mutants lacking both R7 and R8 functions indicates a low weight of the R1–R6 subsystem in the attractiveness function and a prevailing contribution of the R7/R8 subsystem for UV in wild type, as had been reported earlier (7, 23).
We wondered whether the replacement of R8y by R8p cells in the absence of R7 (sev meltGOF) would also enhance UV attractiveness in the UV/B choice. In this case, however, the preference for blue over UV was increased compared to sev or to panR7 > shits flies (P < 0.05) (Fig. 2C). The distinct phenotypes of meltGOF in the presence and absence of R7 support the notion of a nonadditive interaction between R7 and R8 (but see Discussion for meltGOF phenotype).
Taken together, these results show that flies impaired for R7 function prefer blue and that flies impaired for R8 function prefer UV. All flies lacking R7 function preferred blue regardless of R8 function (Fig. 2 A and C), confirming that the contribution of R7 cells is essential for UV attractiveness. Surprisingly, the effects of replacing R8y with R8p depended on R7 function. The UV/B choice behavior revealed an interaction between R7 and R8.
Mutants Defective in Photoreceptors R1–R6.
Flies lacking R1–R6 cells walk more slowly and exhibit severe defects in orientation behavior (44) not observed with any of the R7/R8 mutants, making the direct comparison of these mutants somewhat difficult. Yet, the outer photoreceptors R1–R6 are not essential for phototaxis (32). We confirmed this result by finding that ninaE17 and rh1 > shits flies preferred blue to UV in the UV/B choice (Fig. 2D). We could assess the attractiveness function of these mutants because their phototaxis scores were normal. Remarkably, both major subsystems, R1–R6 and R7/R8, showed a blue preference by themselves whereas their combination in wild type led to a balance between UV and blue preferences, indicating that these systems contribute nonadditively to the attractiveness function.
These results also show that the R1–R6 subsystem does contribute to the attractiveness function and is not switched off by the R7/R8 subsystem, as suggested by Jacob et al. (22). This conclusion was strengthened by replacing Rh1 in R1–R6 by the UV-sensitive opsin Rh3{ninaE17 P[rh1 > 3] (14)}. These flies behaved similarly to wild type in the UV/B choice (Fig. 2D), showing that the presence of Rh3 in R1–R6 does provide a preference shift toward short wavelengths as compared to flies lacking a functional R1–R6 subsystem (ninaE17or rh1 > shits flies). As these flies did not show a preference shift toward the UV compared to wild type, as would be expected from the change in photopigment, these results again are not compatible with a linear model.
Interestingly, comparing ninaE17 to flies with only R8y photoreceptors (sev ninaE17) did not reveal an effect of R7 cells. Both strains had the same blue preference in the UV/B choice (Fig. 2D), suggesting that without the R1–R6 subsystem the attractiveness function is dominated by the R8 photoreceptors: The contribution of R7 to the attractiveness function seems to require a functional R1–R6 system. If, however, in addition to R1–R6, R8y photoreceptors were also inactivated (ninaE17, rh61), flies showed a very strong blue preference, as did the flies of the triple mutant ninaE17, rh52, rh61. In other words, with only R7 photoreceptors left, flies preferred blue in the UV/B choice, and this preference was not affected by the presence or the absence of input from the blue receptor R8p.
Contribution of Photoreceptors in B/G Choice Tests.
In the B/G choice, the stimuli (blue peak wavelength 430 nm, green peak wavelength 565 nm; Fig. 1B, Lower) were chosen to characterize the attractiveness function in the spectral range of the two types of R8 photoreceptors, R8p (blue) and R8y (green). The relative numbers of quanta captured by each subtype were estimated for the B/G experiments (Fig. 1C, Lower) in the same way as for UV/B. Whereas R8p is expected to respond to blue more than to green, the response of R8y should be biased toward green (Fig. 1C, Lower). This predicts that mutants impaired in R8p should prefer green, whereas mutants impaired in R8y should prefer blue, again assuming that the R1–R6 subsystem does not dominate the attractiveness function.
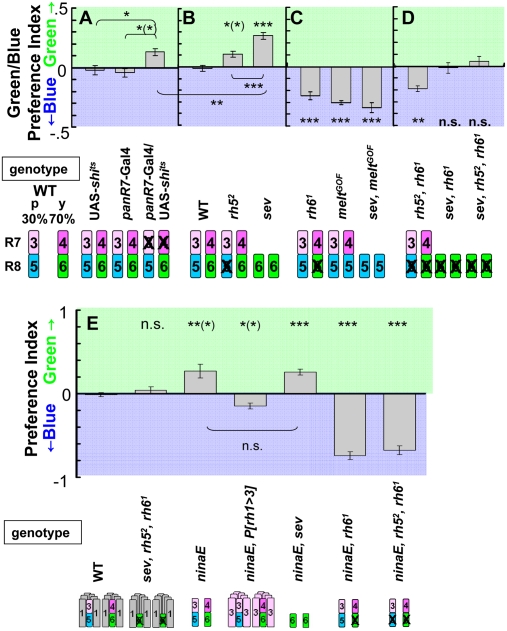
Because R7 cells are mostly sensitive to UV, mutants defective for R7 might show no preference shift in the B/G choice. To test this, we used panR7 > shits flies tested at the restrictive temperature, which exhibited a weak preference for green over blue (Fig. 3A). Attractiveness of blue is likely mediated in part by R7y whose spectrum of absorption overlaps with blue, but not with green (see also ninaE17 P[rh1 > 3] flies below).
Fig. 3.
B/G choice tests. (A) Contribution of R7; panR7-Gal4/UAS-shits flies showed preference for green. (B) Contribution of R8p. Flies in which R8p function is impaired (rh52 and sev) preferred green. sev flies showed a stronger preference for green over blue than panR7 > shits or rh52 alone. (C) Contribution of R8y. Flies in which R8y function is impaired (rh6, meltGOF, and sev meltGOF) preferred blue. (D) Flies lacking functional R8; rh5 rh6 mutant flies showed a preference for blue, whereas sev rh6 and sev rh5 rh6 flies showed no obvious preference. In B–D, the results from each mutant were compared to the wild type in B. (E) Contribution of the R1–R6 system in differential phototaxis. Both ninaE17 and sev ninaE17 flies preferred green, without detectable differences. ninaE17 P[rh1 > 3] flies showed a preference for blue. As in UV/B choice, both ninaE17 rh61 and ninaE17 rh52 rh61 showed a strong blue preference. n = 5–10. Error bars are SEMs.
As shown above, in panR7 > shits flies, R8p and R8y almost balanced each other in the B/G choice as they are sensitive to blue (Rh5) and to green (Rh6). We thus assayed their respective contribution. As predicted from Fig. 1C, rh52 mutant flies showed a preference for green (Fig. 3B). Consistent with the fact that sev flies lack all UV-sensitive R7 and most blue-sensitive R8p, they showed a stronger preference for green over blue than panR7 > shits (P < 0.01) or rh52 alone (P < 0.001; Fig. 3 A and B).
Both rh61 mutant and meltGOF flies lack functional R8y but have UV- and blue-sensitive photoreceptors, with meltGOF mutant flies exhibiting an expansion of blue-sensitive Rh5 to all R8 cells (40). Both mutants showed a strong preference for blue (Fig. 3C). Moreover, sev meltGOF flies, which lacked R7 as well, still showed a strong preference for blue, indicating that this preference is mostly due to R8p and that R7 cells contribute less in the B/G choice (Fig. 3C).
Because loss of blue-sensitive R8p or green-sensitive R8y results in opposite phenotypes in B/G choice, we examined flies in which both R8 subtypes were nonfunctional (rh52, rh61 double mutant). These flies still preferred blue over green, similar to the rh61 single mutant (Fig. 3D) and suggesting that R7 cells do contribute to the preference for short wavelengths in these flies. To confirm this, we assayed the contribution of R7 in the absence of functional R8. Both sev rh61 double-mutant or sev rh52 rh61 triple-mutant flies, in which the function of both R7 and R8 is largely or completely lost, did not show a preference for either blue or green (Fig. 3D).
Taken together, flies lacking R8p function preferred green, whereas flies in which R8y function was impaired preferred blue. It is also apparent that R8 cells play a major role in the B/G choice whereas R7 cells play only a minor role. In the B/G choice, a substantial shift to preference for blue light was observed for ninaE17, P[rh1 > 3] (14) in comparison with ninaE17 flies (Fig. 3E; P < 0.05), demonstrating that Rh3 rhodopsin is sufficiently stimulated by blue light in R1–R6 cells to contribute to the attractiveness function.
As in the UV/B choice, no differences were detected in the B/G choice between ninaE17 and sev ninaE17 flies. Both had a pronounced preference for green (Fig. 3E), suggesting that without R1–R6 the R7/R8 subsystem is dominated by R8 and in particular by R8y. No contribution of R7 to the attractiveness function was detectable under these conditions.
Finally, flies that had R1–R6 as well as R8y (ninaE17, rh61) or both types of R8 photoreceptors (ninaE17, rh52, rh61) inactivated showed a very strong blue preference as had been observed in the UV/B choice for these genotypes. As the latter flies had only R7 photoreceptors left in the compound eyes, we assume that, in the absence of functional R1–R6 and R8y photoreceptors, R7 cells mediated this preference in phototaxis (Discussion).
Discussion
Phototaxis consists of at least three behavioral components: (i) detection of an object or a light source in visual space; (ii) the attractiveness or aversiveness of the light stimulus at this location; and (iii) a motor output, in our case goal-directed walking and turning. Our genetic dissection of the visual input to phototaxis relies on the assumption that phototaxis stays intact even if some of these inputs are deleted, i.e., that the mutants can still detect the locations of the two stimuli and move toward or away from them. Although in mutants affecting the R1–R6 subsystem, walking speed, turning, and orientation toward stationary objects are affected (44, 45), these behaviors are sufficiently intact to support phototaxis. In our experiments, we chose light intensities such that all mutants had at least one photoreceptor type that could detect the light sources and guide walking and turning behavior. This allowed us to measure the second component, the differential attractiveness of the two stimuli, which determined the choice between the two monochromatic lights depending upon the photoreceptor types available in the respective fly strains. Flies could judge the attractiveness of a light source on the basis of its spectral composition and/or spectrally weighted brightness. In the following paragraphs we discuss the contributions of the four types of photoreceptors R1–R6, R7, R8p, and R8y to the attractiveness function.
All Four Photoreceptor Types Influence Attractiveness.
Blocking or removing any one of the photoreceptor types shifts the preference away from the point of neutrality in at least one of the choice tests. Silencing the R1–R6 cells leaves the flies with a modest blue preference in the UV/B choice, and replacing their broadly sensitive opsin Rh1 by the UV-sensitive opsin Rh3 shifts the preference in the B/G choice to blue. Removing or silencing R7 in the presence of R1–R6 shifts the preference in the UV/B choice to blue and in the B/G choice to green. Inactivating the green-sensitive Rh6 opsin in R8y cells shifts the preference in the B/G choice to blue and in the UV/B choice to UV. Inactivating blue-sensitive Rh5 in the R8p cells causes a green preference in the B/G choice and a UV preference in the UV/B choice.
Independence of Subsystems.
Each of the three photoreceptor subsystems (R1–R6, R7, R8) alone can drive phototaxis. Flies with only photoreceptors R1–R6 (sev rh52 rh61 triple mutants) show a blue preference in the UV/B choice, implying that this subsystem not only mediates optomotor responses and orientation but also can mediate the attractiveness function in phototaxis, i.e., that, with only R1–R6 photoreceptors, flies compare the quantum flux of two light sources 180° apart.
The R8 subsystem alone can also mediate the attractiveness function in phototaxis (sev ninaE17), as had been shown before for the sev rdgB double mutant (32). The sev ninaE17 flies have a pronounced green preference in the B/G choice test, consistent with the absorption spectrum of Rh6 expressed in all R8 photoreceptors.
Flies that have only R7 photoreceptors operating (ninaE17, rh52, rh61) show phototaxis. Surprisingly, they have a very strong preference for blue in both choice tests. This could be explained if R7p inhibit R7y photoreceptors. Otherwise, the flies would show a strong UV preference in the UV/B choice. As an alternative explanation, however, it is possible that phototaxis is mediated by the ocelli in flies with only R7 photoreceptors.
Nonlinear Interactions Between Subsystems.
As long as only one of the central photoreceptor subsystems R7 or R8 is inactivated, the results can be explained by a model in which the respective photoreceptors contribute roughly additively to the attractiveness function. In the honeybee, all three subtypes of photoreceptors (UV, blue, and green) also feed into phototaxis (46, 47). Spectral mixing experiments in phototaxis are consistent with simple summation of quantal fluxes (47), and the action spectra of phototactic behavior also suggest pooling of all three photoreceptor types. Color information, i.e., comparison between different photoreceptors, does not appear to be used in honeybee phototaxis (47).
A simple summation model thus can no longer explain the results of all of the genetic manipulations of photoreceptor types in Drosophila presented here (7, 23). When all four photoreceptor types are intact, UV light is more attractive than would be expected from the sum of the UV attractiveness values of the two isolated major subsystems, R1–R6 and R7/R8. In the UV/B choice test that is balanced for wild type, both flies with only the R1–R6 subsystem (sev rh52 rh61) and flies with only the R7/R8 subsystem (rh1 > shits or ninaE17) show a blue preference (Fig. 2D). We thus have to postulate that an interaction between the two major retinal subsystems R1–R6 and R7/R8 enhances UV attractiveness. The interaction cannot be explained by an attenuation of the attractiveness of blue because no increased blue preference of the two mutants is observed in the B/G choice (Fig. 3E). The interaction can unambiguously be traced to the R7 cells. In fact, the strength of the contribution of R7 to the attractiveness function in the UV/B choice depends upon which of the other photoreceptor types are functional. We cannot detect any effect of R7 on spectral preference without a functional R1–R6 subsystem (comparing ninaE17 and sev ninaE17 in both choice tests; Fig. 2D). We find a moderate effect in the presence of R1–R6 and R8 (comparing wild type and sev; Fig. 2 A and C) whereas the effect of R7 is large in the absence of the R8 subsystem (comparing rh52 rh61 and sev rh52 rh61 in UV/B choice; Fig. 2 B and C).
The most parsimonious explanation of our data are to assume that, in the presence of a functional R8 subsystem, neither the R1–R6 nor the R7 subsystems on their own have a direct input to the attractiveness function (Figs. 2D and 3E and Fig. S1). In the absence of R7 (mutant sev), the R1–R6 subsystem seems not to matter for attractiveness although the R8 subsystem is lacking spectral sensitivity in the UV. Likewise, in the absence of a functional R1–R6 subsystem, the R7 subsystem seems not to contribute. Interestingly, as soon as R8y photoreceptors (or R8p and R8y) are inactivated, the other two subsystems exert their influence on the attractiveness function independently of each other. A full model of these interactions would require a more complete investigation.
R1–R6 rhabdomeres degenerate in ninaE17 mutants (48), and this degeneration may have secondary effects on the R7/R8 cells. However, as shown in Fig. 2D, there is no significant difference between ninaE17 mutants and flies expressing rh1>shits (P > 0.05), in which R1–R6 function is disrupted without affecting rhabdomere structures. Moreover, it was recently reported that R8y are functional in ninaE17 mutants because circadian entrainment to red light, which is still observed in ninaE17, is abolished in ninaE17 rh61 double mutants (49).
Our data reveal a further deviation from a simple summation of photoreceptor inputs to the attractiveness function. In the meltGOF mutant, green-sensitive R8y cells are transformed into blue-sensitive R8p cells. This should shift their preference in the UV/B choice to blue. Yet, a UV shift is observed. In contrast, in the absence of R7 cells, the transformation of R8y to R8p photoreceptors (comparison between sev and sev meltGOF) increases the blue preference in the UV/B choice as would be expected from spectral sensitivities (Fig. 1C). This might indicate that R8p cells enhance the input of the UV channel (R1–R6 × R7) to the attractiveness function. These findings, however, should be treated with caution as unknown developmental defects in the sev and meltGOF mutants might account for the phenotypes (50).
Using a different phototaxis paradigm, Jacob et al. (22) proposed a model to explain the nonadditive effects observed in their phototaxis experiments. They postulated an inhibition of R1–R6 by R7/R8 and suggested that R7 cells function only when the R1–R6 system is intact. Our results ask for revision of this model. We found no general inhibition of the R1–R6 receptor subsystem by R7/R8. Our data suggest that neither the R1–R6 nor the R7 subsystems have access to the attractiveness function on their own, except in the absence of functional R8y photoreceptors. Moreover, R8p cells in our tests had an effect only in the presence of functional R8y cells.
Rh3-expressing R8 cells in the dorsal rim area account for about 10% of all Rh3 expressing cells. These R8 are still present in sev flies, but are switched off in panR7> shits flies. The comparison of these two lines does not reveal an effect of the Rh3-expressing R8 cells in the UV/B choice. Taking these cells into consideration therefore does not change any of the above conclusions.
Concluding Remarks.
We have shown that all four photoreceptor types [R1–R6, R7(p, y), R8p, and R8y] are involved in phototaxis, in contrast to motion detection, which relies exclusively on R1–R6 photoreceptors (7, 8). The wild-type attractiveness function cannot be described as the sum of the attractiveness functions of flies lacking one or more functional photoreceptor types. We observe a multiplicative interaction between photoreceptors R1–R6 and R7. In the absence of functional R1–R6 or R7, the attractiveness function is governed by R8. Only in the absence of R8 and one of the other two subsystems does the remaining subsystem govern the attractiveness function. A recent study on the neuronal substrate of spectral preference identified postsynaptic interneurons in the medulla (51) that are good candidates for mediating some of these interactions. Their involvement in differential phototaxis can now be tested.
The attractiveness function of differential phototaxis is easy to record, robust, and sensitive (for example, Rh5-expressing blue-sensitive cells account for only ∼4% of all photoreceptors, yet yield a significant phenotype when they are not functional). Differential phototaxis may lend itself to mutant screens affecting other chromaticity computations in the brain, including color vision, at the circuit level.
Materials and Methods
Fly Stocks.
Fly stocks were raised as described previously (8). See SI Materials and Methods for mutant lines and more details.
Behavioral Assays.
Flies that were 2–7 days old were used. Two fresh plastic tubes (Falcon 352017) were attached to the T-maze, which is similar to the one used for the olfactory paradigm (37) but is black to avoid light transmission. Flies (50–150) were put in the hole of the lift, the light stimuli were turned on, and then the lift was opened for 20 s. The number of flies found in each tube was counted. The PI was calculated as described in Results. See SI Materials and Methods for details about “light vs. dark” experiments and shibire experiments. Error bars are SEMs.
LEDs and Spectroradiometry.
The LEDs used in this study are the following: UV (RT350-05–15; Roithner Laser Technik), blue (LED435-12–30; Roithner Laser Technik), and green (E1503SGC, eLED). See SI Materials and Methods for more details.
Supplementary Material
Acknowledgments
We thank Konrad Öchsner and Daniel Vasiliauskas for building the experimental apparatus; Terry Blackman for help with the flies; Reinhard Wolf for helpful advice and constant support; Roger Hardie for providing data for the normalized spectral properties of each photoreceptor subtype; and people in the Desplan and Heisenberg laboratory, in particular Nina Vogt for establishing the rh52 rh61 ninaE17 mutant and Jens Rister for helpful discussion. We thank Tetsuya Tabata for his kind support and the people in his laboratory, in particular Yuko Maeyama-Kamoshida for her generous assistance and Satoshi Murakami for his help. We thank Shuji Hanai for providing us with the rh61 ninaE17 double mutant. Funding for this work was provided by the Uehara memorial foundation postdoctoral fellowship and a Human Frontier Science Program postdoctoral fellowship (to S.Y.), National Institutes of Health Grant EY017916 (to C.D.), and Sonder-Forschungs-Bereich Grant 554 (to M.H.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809398107/DCSupplemental.
References
- 1.Hardie RC. In: Progress in Sensory Physiology. Autrum H, editor. Berlin: Springer-Verlag; 1985. pp. 1–79. [Google Scholar]
- 2.Kirschfeld K, Franceschini N, Minke B. Evidence for a sensitising pigment in fly photoreceptors. Nature. 1977;269:386–390. doi: 10.1038/269386a0. [DOI] [PubMed] [Google Scholar]
- 3.O'Tousa JE, et al. The Drosophila ninaE gene encodes an opsin. Cell. 1985;40:839–850. doi: 10.1016/0092-8674(85)90343-5. [DOI] [PubMed] [Google Scholar]
- 4.Zuker CS, Cowman AF, Rubin GM. Isolation and structure of a rhodopsin gene from D. melanogaster. Cell. 1985;40:851–858. doi: 10.1016/0092-8674(85)90344-7. [DOI] [PubMed] [Google Scholar]
- 5.Salcedo E, et al. Blue- and green-absorbing visual pigments of Drosophila: Ectopic expression and physiological characterization of the R8 photoreceptor cell-specific Rh5 and Rh6 rhodopsins. J Neurosci. 1999;19:10716–10726. doi: 10.1523/JNEUROSCI.19-24-10716.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heisenberg M, Wolf R. Vision in Drosophila. Berlin: Springer-Verlag; 1984. [Google Scholar]
- 7.Heisenberg M, Buchner E. The role of retinula cell types in visual behavior of Drosophila melanogaster. J Comp Physiol. 1977;117:127–162. [Google Scholar]
- 8.Yamaguchi S, Wolf R, Desplan C, Heisenberg M. Motion vision is independent of color in Drosophila. Proc Natl Acad Sci USA. 2008;105:4910–4915. doi: 10.1073/pnas.0711484105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fortini ME, Rubin GM. Analysis of cis-acting requirements of the Rh3 and Rh4 genes reveals a bipartite organization to rhodopsin promoters in Drosophila melanogaster. Genes Dev. 1990;4:444–463. doi: 10.1101/gad.4.3.444. [DOI] [PubMed] [Google Scholar]
- 10.Labhart T, Meyer EP. Detectors for polarized skylight in insects: A survey of ommatidial specializations in the dorsal rim area of the compound eye. Microsc Res Tech. 1999;47:368–379. doi: 10.1002/(SICI)1097-0029(19991215)47:6<368::AID-JEMT2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 11.Wernet MF, et al. Homothorax switches function of Drosophila photoreceptors from color to polarized light sensors. Cell. 2003;115:267–279. doi: 10.1016/s0092-8674(03)00848-1. [DOI] [PubMed] [Google Scholar]
- 12.Montell C, Jones K, Zuker C, Rubin G. A second opsin gene expressed in the ultraviolet-sensitive R7 photoreceptor cells of Drosophila melanogaster. J Neurosci. 1987;7:1558–1566. doi: 10.1523/JNEUROSCI.07-05-01558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuker CS, Montell C, Jones K, Laverty T, Rubin GM. A rhodopsin gene expressed in photoreceptor cell R7 of the Drosophila eye: Homologies with other signal-transducing molecules. J Neurosci. 1987;7:1550–1557. doi: 10.1523/JNEUROSCI.07-05-01550.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feiler R, et al. Ectopic expression of ultraviolet-rhodopsins in the blue photoreceptor cells of Drosophila: Visual physiology and photochemistry of transgenic animals. J Neurosci. 1992;12:3862–3868. doi: 10.1523/JNEUROSCI.12-10-03862.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook T, Desplan C. Photoreceptor subtype specification: From flies to humans. Semin Cell Dev Biol. 2001;12:509–518. doi: 10.1006/scdb.2001.0275. [DOI] [PubMed] [Google Scholar]
- 16.Mazzoni EO, et al. Iroquois complex genes induce co-expression of rhodopsins in Drosophila. PLoS Biol. 2008;6:e97. doi: 10.1371/journal.pbio.0060097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morante J, Desplan C. The color-vision circuit in the medulla of Drosophila. Curr Biol. 2008;18:553–565. doi: 10.1016/j.cub.2008.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McEwen RS. The reactions to light and to gravity in Drosophila and its mutants. J Exp Zool. 1918;25:49–106. [Google Scholar]
- 19.Choe KM, Clandinin TR. Thinking about visual behavior; learning about photoreceptor function. Curr Top Dev Biol. 2005;69:187–213. doi: 10.1016/S0070-2153(05)69007-2. [DOI] [PubMed] [Google Scholar]
- 20.Benzer S. Behavioral mutants of Drosophila isolated by countercurrent distribution. Proc Natl Acad Sci USA. 1967;58:1112–1119. doi: 10.1073/pnas.58.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fingerman M, Brown FAJ. A “Purkinje shift” in insect vision. Science. 1952;116:171–172. doi: 10.1126/science.116.3007.171. [DOI] [PubMed] [Google Scholar]
- 22.Jacob KG, Willmund R, Folkers E, Fischbach KF, Spatz HC. T-maze phototaxis of Drosophila melanogaster and several mutants in the visual system. J Comp Physiol. 1977;116:209–225. [Google Scholar]
- 23.Fischbach KF. Simultaneous and successive colour contrast expressed in “slow” phototactic behaviour of walking Drosophila melanogaster. J Comp Physiol. 1979;130:161–171. [Google Scholar]
- 24.Stark WS. Spectral selectivity of visual response alterations mediated by interconversions of native and intermediate photopigments in Drosophila. J Comp Physiol. 1975;96:343–356. [Google Scholar]
- 25.Bertholf LM. The extent of the spectrum for Drosophila and the distribution of stimulative efficiency in it. Z Vgl Physiol. 1932;18:32–64. [Google Scholar]
- 26.Schuemperli RA. Evidence for colour vision in Drosophila melanogaster through spontaneous phototactic choice behaviour. J Comp Physiol. 1973;86:77–94. [Google Scholar]
- 27.Harris WA, Stark WS, Walker JA. Genetic dissection of the photoreceptor system in the compound eye of Drosophila melanogaster. J Physiol. 1976;256:415–439. doi: 10.1113/jphysiol.1976.sp011331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pak WL. In: Neurogenetics: Genetic Approaches to the Nervous System. Breakefield X, editor. New York: Elsevier; 1979. pp. 67–99. [Google Scholar]
- 29.Hotta Y, Benzer S. Genetic dissection of the Drosophila nervous system by means of mosaics. Proc Natl Acad Sci USA. 1970;67:1156–1163. doi: 10.1073/pnas.67.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pak WL. Drosophila in vision research. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1995;36:2340–2357. [PubMed] [Google Scholar]
- 31.Gerresheim F. PhD thesis. Munich, Germany: Munich University; 1981. Isolation and characterization of mutants with altered phototactic reaction to monochromatic light in Drosophila melanogaster. [Google Scholar]
- 32.Hu KG, Stark WS. Specific receptor input into spectral preference in Drosophila. J Comp Physiol. 1977;121:241–252. [Google Scholar]
- 33.Chou WH, et al. Identification of a novel Drosophila opsin reveals specific patterning of the R7 and R8 photoreceptor cells. Neuron. 1996;17:1101–1115. doi: 10.1016/s0896-6273(00)80243-3. [DOI] [PubMed] [Google Scholar]
- 34.Papatsenko D, Sheng G, Desplan C. A new rhodopsin in R8 photoreceptors of Drosophila: Evidence for coordinate expression with Rh3 in R7 cells. Development. 1997;124:1665–1673. doi: 10.1242/dev.124.9.1665. [DOI] [PubMed] [Google Scholar]
- 35.Menne D, Spatz HC. Colour vision in Drosophila melanogaster. J Comp Physiol. 1977;114:301–312. [Google Scholar]
- 36.Hernandez de Salomon C, Spatz HC. Colour vision in Drosophila melanogaster: Wavelength discrimination. J Comp Physiol. 1983;150:31–37. [Google Scholar]
- 37.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 38.Govardovskii VI, Fyhrquist N, Reuter T, Kuzmin DG, Donner K. In search of the visual pigment template. Vis Neurosci. 2000;17:509–528. doi: 10.1017/s0952523800174036. [DOI] [PubMed] [Google Scholar]
- 39.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 40.Mikeladze-Dvali T, et al. The growth regulators warts/lats and melted interact in a bistable loop to specify opposite fates in Drosophila R8 photoreceptors. Cell. 2005;122:775–787. doi: 10.1016/j.cell.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 41.Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- 42.Waddell S, Armstrong JD, Kitamoto T, Kaiser K, Quinn WG. The amnesiac gene product is expressed in two neurons in the Drosophila brain that are critical for memory. Cell. 2000;103:805–813. doi: 10.1016/s0092-8674(00)00183-5. [DOI] [PubMed] [Google Scholar]
- 43.Rister J, Heisenberg M. Distinct functions of neuronal synaptobrevin in developing and mature fly photoreceptors. J Neurobiol. 2006;66:1271–1284. doi: 10.1002/neu.20284. [DOI] [PubMed] [Google Scholar]
- 44.Strauss R, Renner M, Gotz K. Task-specific association of photoreceptor systems and steering parameters in Drosophila. J Comp Physiol A. 2001;187:617–632. doi: 10.1007/s003590100234. [DOI] [PubMed] [Google Scholar]
- 45.Rister J, et al. Dissection of the peripheral motion channel in the visual system of Drosophila melanogaster. Neuron. 2007;56:155–170. doi: 10.1016/j.neuron.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 46.Kaiser W, Seidl R, Vollmar J. The participation of all three colour receptors in the phototactic behaviour of fixed walking honeybees. J Comp Physiol. 1977;122:27–44. [Google Scholar]
- 47.Menzel R, Greggers U. Natural phototaxis and its relationship to color-vision in honeybees. J Comp Physiol A. 1985;157:311–321. [Google Scholar]
- 48.Kumar JP, Ready DF. Rhodopsin plays an essential structural role in Drosophila photoreceptor development. Development. 1995;121:4359–4370. doi: 10.1242/dev.121.12.4359. [DOI] [PubMed] [Google Scholar]
- 49.Hanai S, Hamasaka Y, Ishida N. Circadian entrainment to red light in Drosophila: Requirement of Rhodopsin 1 and Rhodopsin 6. Neuroreport. 2008;19:1441–1444. doi: 10.1097/WNR.0b013e32830e4961. [DOI] [PubMed] [Google Scholar]
- 50.Morey M, et al. Coordinate control of synaptic-layer specificity and rhodopsins in photoreceptor neurons. Nature. 2008;456:795–799. doi: 10.1038/nature07419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao S, et al. The neural substrate of spectral preference in Drosophila. Neuron. 2008;60:328–342. doi: 10.1016/j.neuron.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.