Abstract
The mitotic (or spindle assembly) checkpoint system ensures accurate segregation of chromosomes by delaying anaphase until all chromosomes are correctly attached to the mitotic spindle. This system acts by inhibiting the activity of the anaphase-promoting complex/cyclosome (APC/C) ubiquitin ligase to target securin for degradation. APC/C is inhibited by a mitotic checkpoint complex (MCC) composed of BubR1, Bub3, Mad2, and Cdc20. The molecular mechanisms of the inactivation of the mitotic checkpoint, including the release of APC/C from inhibition, remain obscure. It has been reported that polyubiquitylation by the APC/C is required for the inactivation of the mitotic checkpoint [Reddy SK, Rape M, Margansky WA, Kirschner MW (2007) Nature, 446:921–924]. We confirmed the involvement of polyubiquitylation, but found that another process, which requires ATP cleavage at the β–γ position (as opposed to α–β bond scission involved in ubiquitylation), is essential for the release of APC/C from checkpoint inhibition. ATP (β–γ) cleavage is required both for the dissociation of MCC components from APC/C and for the disassembly of free MCC, whereas polyubiquitylation is involved only in the former process. We find that the requirement for ATP (β–γ) cleavage is not due to the involvement of the 26S proteasome and that the phenomena observed are not due to sustained activity of protein kinase Cdk1/cyclin B, caused by inhibition of the degradation of cyclin B. Thus, some other energy-consuming process is needed for the inactivation of the mitotic checkpoint.
Keywords: cell cycle, mitosis, spindle checkpoint
The mitotic (or spindle assembly) checkpoint system is a surveillance mechanism that ensures accurate chromosome segregation by delaying anaphase until all chromosomes are correctly attached to the mitotic spindle via their kinetochores (reviewed in refs. 1–3). This system is activated by improperly attached kinetochores and acts by inhibiting the action of the anaphase-promoting complex/cyclosome APC/CCdc20 ubiquitin ligase to target cyclin B and securin for degradation. In spite of its biological importance, the molecular mechanisms by which the mitotic checkpoint system is activated, maintained, and finally inactivated remain obscure. It is generally assumed that a primary diffusible signal is formed at unattached kinetochores, but the nature of the signal and the mechanisms of its amplification are poorly understood. It is known that the binding of the checkpoint proteins Mad2 and BubR1 to the APC/C activator Cdc20 is required for the inhibition of APC/C (1, 2). However, these checkpoint proteins do not seem to act just by the sequestration of Cdc20 (4), but rather by the formation of a mitotic checkpoint complex (MCC) composed of BubR1, Bub3, Mad2, and Cdc20, which inhibits the activity of APC/C (5). Another mitotic checkpoint inhibitory complex, mitotic checkpoint factor 2 (MCF2), has been separated from MCC, but its composition is not known (6). We have shown that both MCC an MCF2 are tightly associated with APC/C and inhibit it in a manner competitive with Cdc20 (6). In fact, a recent study on the structure of the APC/C-MCC complex showed that MCC occupies a site that partially overlaps with the Cdc20-binding site of APC/C (7). The checkpoint-inhibited state of APC/C is also maintained by additional mechanisms, such as by the inhibitory phosphorylation of Cdc20 by several protein kinases (8–11). We showed that the maintenance of the checkpoint-arrested state requires protein kinase action, as indicated by the observations that the protein kinase inhibitor staurosporine shortens the time of exit from checkpoint and that the ATP analogue adenosine-5′-(γ- thio)triphosphate (ATPγS) stabilizes the checkpoint-arrested state, possibly by stable thiophosphorylation of some relevant proteins (4).
Another important problem is the identification of the molecular mechanisms by which the checkpoint is inactivated, i.e., the pathway through which APC/C is released from inhibition after the checkpoint has been satisfied. It appears reasonable to assume that inactivation of the mitotic checkpoint is also tightly regulated to ensure high fidelity of chromosome segregation. Several processes that target Mad2 and may stimulate exit from mitotic checkpoint have been described, such as inhibitory phosphorylations of Mad2 (12) and its binding to CMT2/p31comet (13, 14). However, it is not known whether these processes are regulated during release from the checkpoint. In addition, it has been reported that ubiquitylation by APC/C, possibly of Cdc20, is required to turn off the mitotic checkpoint (15). However, it was subsequently shown that exit from checkpoint was not affected when Cdc20 was replaced by a nonubiquitylatable derivative (16). We now show that cleavage of ATP at the β–γ bond (which is distinct from the α–β bond scission involved in ubiquitylation) is essential for exit from mitotic checkpoint arrest and for the disassembly of MCC.
Results
Dependence of Exit from Mitotic Checkpoint on ATP.
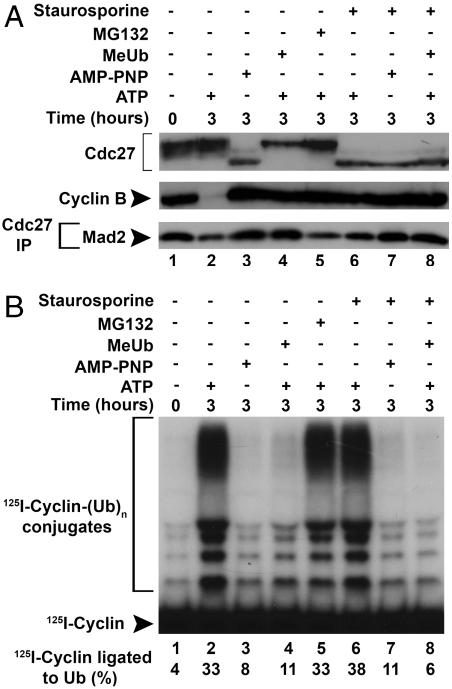
It has been shown previously that extracts from nocodazole-arrested HeLa cells faithfully reproduce downstream events of the mitotic checkpoint system (4). APC/C is inactive in such extracts, presumably due to the effects of checkpoint-specific inhibitory factors such as MCC and MCF2 (5, 6). When checkpoint extracts were incubated in the presence of ATP, APC/C was converted to an active form, following a lag period (4, 6). We found that this activation of APC/C was strongly dependent upon ATP hydrolysis. In the experiment shown in Fig. 1A, extracts were incubated in the presence or absence of ATP, or with certain analogues of ATP, and then APC/C was isolated by immunoprecipitation with an antibody directed against its Cdc27 subunit. The activity of APC/C was estimated in immunoprecipitated material by the ligation of 125I-cyclin to ubiquitin, in the presence of all other components necessary for ubiquitylation (see Methods). As observed previously (6), upon incubation with ATP at 23 °C there was a lag at 1 h, and by 3 h APC/C became strongly activated (lanes 2 and 3). By contrast, after incubation without ATP, little if any activation of APC/C took place (Fig. 1A, lanes 4 and 5).
Fig. 1.
Cleavage of ATP at the β–γ bond is required for the release of APC/C from the checkpoint-inhibited state and for the disassembly of MCC. (A) Effects of incubation of extracts with ATP or its analogues on the release of APC/C from inhibition by the mitotic checkpoint. Extracts from nocodazole-arrested cells were incubated with the indicated additions as described under Methods. APC/C was isolated by immunoprecipitation with anti-Cdc27 and its activity in the ligation of 125I-cyclin to ubiquitin was determined. (B) Effects of ATP or its analogues on the phosphorylation state of Cdc27 and on the degradation of endogenous cyclin B. Samples of extracts (30 μg of protein) from the incubations described in A were blotted with antibodies to the indicated proteins. (C) Influence of ATP and its analogues on the release of MCC components from APC/C. Extracts were incubated as described in A and were subjected to immunoprecipitation with anti-Cdc27. Samples were resolved by SDS-PAGE and were blotted with the antibodies indicated. Blots were also probed for Cdc27 as the loading control, following phosphatase treatment of samples as described under Methods. (D) Quantitation of data from C. (E) Effects of ATP and its analogues on the dissociation of free MCC. Extracts were incubated as in A and then were subjected to sequential immunoprecipitations as described in Methods. Samples from anti-Cdc20 (Upper) and anti-BubR1 (Lower) immunoprecipitations were immunoblotted as indicated.
We examined possible explanations for the ATP dependence of exit from mitotic checkpoint arrest. It has been reported that APC/C-dependent ubiquitylation is required for this process (15). Ubiquitylation involves scission of the α–β bond of ATP for the E1-catalyzed reaction (17). Therefore, ATP can be replaced for ubiquitylation by the analogue andenosine-5′-(β–γ-imido)triphosphate (AMP-PNP) that is nonhydrolyzable in the β–γ position, but can be cleaved in the α–β position. We found that when ATP was replaced by AMP-PNP, activation of APC/C did not take place (Fig. 1A, lanes 6 and 7), suggesting that an additional process, that involves scission at the β–γ bond of ATP, is also required for exit from mitotic checkpoint. Another ATP analogue that works well with E1, ATPγS, also could not replace ATP in the activation of APC/C (Fig. 1A, lanes 8 and 9). This could be due to the lack of compatibility of this ATP analogue with a reaction that requires β–γ bond cleavage, or to stable thiophosphorylation of some proteins that prevents exit from the checkpoint-arrested state, as suggested previously (4). The difference in the mode of the action of these two ATP analogues was demonstrated by their influence on the state of the phosphorylation of the Cdc27 subunit of APC/C. As shown in Fig. 1B, upper row, incubation with AMP-PNP for 3 h increased the dephosphorylation of Cdc27, as indicated by the increase in its electrophoretic migration (lane 7). This was presumably due to the action of this analogue to prevent phosphorylation by competition with residual endogenous ATP. By contrast, incubation with ATPγS caused Cdc27 to be retained in a slowly migrating form (Fig. 1B, lane 9), presumably reflecting hyperthiophosphorylation. As would be expected, both ATP analogues prevented the degradation of endogenous cyclin B (Fig. 1B, lower row).
It should be noted that these results appear to be different from our previous findings that only ATPγS, but not AMP-PNP, stabilizes the checkpoint-arrested state (4). However, this is due to differences in conditions of assay of APC/C activity. In the present study, we used immunopurified APC/C for activity assay (Fig. 1A), whereas previously we determined APC/C activity by the degradation of 35S-securin in crude extracts. This required the supplementation of ATP to extracts following preincubation with ATP analogues (4). Under those conditions, the effect of AMP-PNP was reversed by subsequently added ATP, whereas that of ATPγS was not reversible, possibly due to stable protein thiophosphorylation.
Because MCC is a checkpoint inhibitor of APC/C, and because MCC components are released from APC/C during exit from mitotic checkpoint arrest (4, 6), we next examined the question of whether ATP hydrolysis is required for the release of MCC components from APC/C. For this purpose, extracts from nocodazole-arrested cells were incubated for different time periods in the presence of ATP or its analogs as described above, APC/C was immunoprecipitated with anti-Cdc27 antibody, and MCC components associated with APC/C were detected by immunoblotting. The results are shown in Fig. 1C, and their quantitation in Fig. 1D. As observed previously (6), Mad2 is rapidly released from APC/C upon incubation with ATP. We found that the release of Mad2 from APC/C-associated material was completely prevented when extracts were incubated with AMP-PNP or ATPγS. BubR1 was dissociated from APC/C slower than Mad2, and this was also prevented by ATP analogues (Fig. 1C and D). The data obtained with AMP-PNP suggest that hydrolysis at β–γ position of ATP is required for the release of MCC components form APC/C.
We next examined whether ATP β–γ cleavage is also required for the dissociation of free MCC (that is not associated with APC/C) into its components during exit from mitotic checkpoint. For this purpose, checkpoint-arrested extracts were incubated with ATP or its analogues as described above and then APC/C and associated material were removed by immunoprecipitation with anti-Cdc27. Subsequently, the remaining supernatants were subjected to immunoprecipitation with anti-BubR1 or anti-Cdc20 polyclonal antibodies and these immunoprecipitates were probed with monoclonal antibodies directed against MCC subunits. The results are shown in Fig. 1E. Upon incubation of extracts with ATP, both BubR1 and Mad2 were rapidly released from anti-Cdc20 immunoprecipitates (Fig. 1E, Upper, lanes 2 and 3), indicating disassembly of free MCC. The dissociation of both BubR1 and Mad2 from Cdc20 was prevented by AMP-PNP (lanes 4 and 5) and ATPγS (lanes 6 and 7). Similarly, Mad2 was rapidly released from anti-BubR1 immunoprecipitates upon incubation with ATP (Fig. 1E, Lower, lanes 2 and 3), but not with ATP analogues (lanes 4–7). In this case, the release of Cdc20 from BubR1 could not be determined with sufficient confidence, due to some nonspecific interaction of Cdc20 with the anti-BubR1 antibodies used. The cumulative results indicated that scission at the β–γ bond of ATP was required both for the release of MCC components from APC/C and for the dissociation of MCC into its components.
Polyubiquitylation is Required for the Release of MCC from APC/C, but Not for the Disassembly of Free MCC.
As noted above, the influence of the nonhydrolyzable β–γ ATP analogue could not be ascribed to the reported requirement for polyubiquitylation of exit from mitotic checkpoint (15). We therefore compared the characteristics of the effects of prevention of β–γ cleavage of ATP with those of inhibition of polyubiquitylation. We first confirmed that polyubiquitylation is required for checkpoint exit. As shown in Fig. 2A, the addition of methylated ubiquitin (MeUb) [which prevents polyubiquitin chain formation, (18)] to extracts incubated with ATP prevented to a considerable extent the activation of APC/C, as assayed by cyclin-ubiquitin ligase activity of immunoprecipitated APC/C. As would be expected, MeUb also blocked the degradation of endogenous cyclin B (Fig. 2B, Lower). It prevented the partial dephosphorylation of Cdc27 that took place by 3 h of incubation (Fig. 2B, Upper, lane 7 vs. lane 4), presumably by preventing the inactivation of protein kinase Cdk1.
Fig. 2.
Polyubiquitylation is required for the release of MCC components from APC/C, but not for the dissociation of free MCC. (A) Influence of incubation of extracts with MeUb on the release of APC/C from inhibition by the mitotic checkpoint. Extracts were incubated with ATP in the absence (●) or presence (▪) of 100 μM MeUb. APC/C was immunopurified and its activity in the ligation of 125I-cyclin to ubiquitin was determined (see Methods). (B) Effects of MeUb on the degradation of endogenous cyclin B and on the phosphorylation state of Cdc27. Samples (30 μg of protein) of extracts from the incubation described in A were immunoblotted as indicated. (C) Effect of inhibition of polyubiquitylation on the release of MCC components from APC/C. Anti-Cdc27 immunoprecipitates from an incubation similar to A were subjected to phosphatase treatment (see Methods), followed by SDS-PAGE and immunoblotting for the proteins indicated. (D). Quantitation of data from C. (E) Dissociation of free MCC is not affected by inhibition of polyubiquitylation. Extracts were incubated as in A, and then were subjected to sequential immunoprecipitation with anti-Cdc27, followed by anti-Cdc20 (Upper) and anti-BubR1 (Lower) antibodies. Samples were immunoblotted as indicated. (F). Quantitation of data from E.
We next tested the effects of MeUb on the release of MCC components from APC/C and on the dissociation of free MCC in checkpoint extracts incubated in the presence of ATP. These were done by consecutive immunoprecipitations with anti-Cdc27, followed by anti-Cdc20 and anti-BubR1, as described above. As shown in Fig. 2C and D, MeUb prevented the release of Mad2 and of BubR1 from APC/C. By contrast, it had little effect on the dissociation of free MCC, as shown either by the release of BubR1 and Mad2 from anti-Cdc20 immunoprecipitates (Fig. 2E, Upper) or by the release of Mad2 from anti-BubR1 immunoprecipitates (Fig. 2E, Lower). MeUb caused slight retardation in the decay of BubR1 and Mad2 immunoprecipitated with anti-Cdc20 (Fig. 2E). However, as would be expected, MeUb also prevented the degradation of Cdc20 that took place in extracts incubated with ATP (Fig. 2E). Thus, when the ratio of BubR1 or Mad2 to Cdc20 was calculated following quantitation of immunoblots, little if any effect of MeUb on their dissociation from Cdc20 could be detected (Fig. 2F). Thus, in contrast to ATP (β–γ) bond cleavage, which is required both for the release of MCC components from APC/C and for the dissociation of free MCC, polyubiquitylation is involved mainly in the former process.
Sustained Activity of Protein Kinase Cdk1/cyclin B is Not Sufficient to Prevent Exit from Mitotic Checkpoint Inhibition of APC/C.
In the experiments described above, all treatments that prevented exit from mitotic checkpoint also blocked the degradation of endogenous cyclin B (Figs. 1B and 2B). It seemed possible, therefore, that exit from mitotic checkpoint is prevented under these conditions by sustained high activity of protein kinase Cdk1/cyclin B. In fact, it was shown that Cdk1/cyclin B phosphorylated and inactivated Cdc20 in vitro (8), and this process was suggested to have a possible role in mitotic checkpoint inhibition of APC/C (10). To examine this possibility, we tested the effects of staurosporine, an inhibitor of many protein kinases including Cdks (19), alone or in combination with AMP-PNP or MeUb. We have also tested the effect of the proteasome inhibitor MG-132, which blocks the degradation of cyclin B (Fig. 3A, Middle, lane 5) as do the other agents (lanes 3 and 4). The addition of staurosporine caused drastic dephosphorylation of Cdc27 in the presence of all agents (Fig. 3A, Upper), suggesting that Cdk1/cyclin B protein kinase activity was effectively blocked in spite of high levels of cyclin B. The addition of staurosporine slightly stimulated APC/C activity as compared to the control incubation (Fig. 3B, lane 6 vs. lane 2, 38% vs. 33% 125I-cyclin ligated to ubiquitin). This is in accordance with our previous observations that this protein kinase inhibitor shortens the lag period in exit of APC/C activity from the checkpoint-inhibited state (4). However, treatment with staurosporine in the presence of MeUb or AMP-PNP did not relieve the inhibition of APC/C activation (Fig. 3B, lanes 7 and 8 vs. lanes 3 and 4). Similarly, incubation with staurosporine did not stimulate the release of Mad2 from APC/C in the presence of MeUb or AMP-PNP (Fig. 3A, Lower, lanes 7 and 8).These results indicate that the influence of these agents to prevent exit from the checkpoint-arrested state of APC/C is not due to sustained Cdk1/cyclin B activity. This conclusion was corroborated by the observation that high concentrations of the proteasome inhibitor MG132, which effectively inhibited the degradation of endogenous cyclin B (Fig. 3A, lane 5), did not block the activation of APC/C (Fig. 3B, lane 5). The lack of influence of proteasome inhibition on exit from the checkpoint-arrested state is in accordance with previous observations of Reddy et al. (15).
Fig. 3.
Sustained activity of cyclin-dependent kinase is not sufficient to prevent release of APC/C from checkpoint inhibition. (A) Influence of incubation with staurosporine in combination with other inhibitors on the phosphorylation state of Cdc27, the degradation of cyclin B and the release of Mad2 from APC/C. Extracts were incubated with the additions indicated and samples of total extracts (Upper and Middle rows) or of anti-Cdc27 immunoprecipitates (Lower row) were subjected to immunoblotting. (B) Effects of staurosporine in combination with other inhibitors and of the proteasome inhibitor MG-132 on the release of APC/C from checkpoint inhibition. Samples were incubated as in A, and then APC/C was isolated by immunoprecipitation with anti-Cdc27 beads and its activity in cyclin-ubiquitin ligation was determined.
Polyubiquitylation Cannot Replace ATP (β–γ) Cleavage-Requiring Process to Overcome Arrest by the Mitotic Checkpoint.
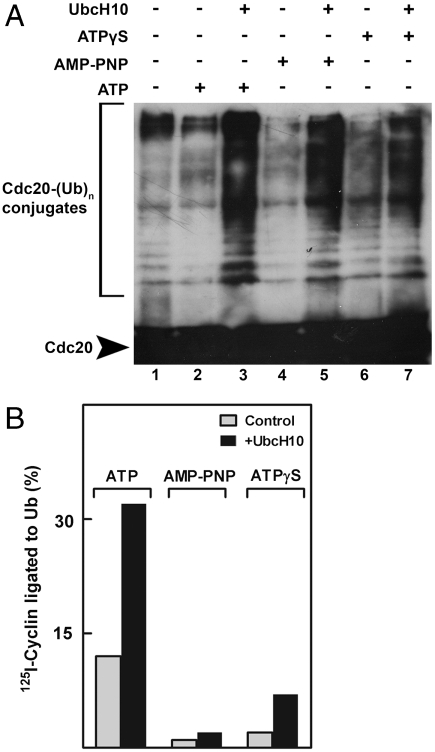
We next asked whether polyubiquitylation and a reaction that requires ATP (β–γ) bond cleavage stimulate, by different mechanisms, a similar process that is required for exit from checkpoint. In such case, it would be expected that strong stimulation of polyubiquitylation may overcome the inhibition of checkpoint exit produced by ATP analogues. High rates of APC/C-mediated polyubiquitylation can be obtained by the supplementation of high concentrations of UbcH10/E2C, the specific E2 partner of APC/C (20). It has been shown previously that supplementation of UbcH10 at 5 μM (which is 50-fold higher than physiological levels) strongly accelerated escape from mitotic checkpoint inhibition of APC/C (15). In the experiment shown in Fig. 4A, extracts from nocodazole-arrested cells were first incubated with ATP or its analogues, and then UbcH10 was added and the formation of polyubiquitylated Cdc20 was examined. It has been shown that high concentrations of UbcH10 stimulate the polyubiquitylation of Cdc20 (15), although the relevance of this process to exit from mitotic checkpoint has been questioned (16). As expected, UbcH10 stimulated the polyubiquitylation of Cdc20 in the presence of either ATP or its analogues, which allow the E1 reaction to take place. However, although UbcH10 at high concentration strongly stimulated the activation of APC/C following incubation with ATP, it had little influence on this process following treatment with ATP analogues (Fig. 4B). We concluded that stimulation of polyubiquitylation cannot, by itself, promote exit from the ATP-arrested state in the absence of β–γ bond cleavage of ATP.
Fig. 4.
Stimulation of ubiquitylation by high concentration of UbcH10 does not overcome checkpoint inhibition of APC/C in the presence of ATPγS or AMP-PNP. (A) High concentrations of UbcH10 stimulate polyubiquitylation of Cdc20 in the presence of ATP or its analogues. Extracts from nocodazole-arrested cells were incubated with ATP or its analogues for 3 h at 23 °C, and then 5 μM UbcH10 was added as indicated. Following further incubation of 5 min, samples were immunoprecipitated with rabbit polyclonal anti-Cdc20 antibody and immunoblotted with mouse monoclonal anti-Cdc20. (B) Incubation conditions were similar to A, except that extracts were incubated with ATP analogues for 30 min prior to the addition of UbcH10, and then were incubated for further 2 h. Subsequently, samples were immunoprecipitated with anti-Cdc27 and cyclin-ubiquitin ligation was determined as described in Methods, except that the reaction was carried out at 20 °C for 10 min.
Discussion
We report that the release of APC/C from inhibition by the mitotic checkpoint and various steps in the disassembly of mitotic checkpoint complexes require the energy of the cleavage of the β–γ phosphoanhydride bond of ATP. It is not surprising that the disassembly of the mitotic checkpoint complexes may require energy, in view of the highly stable structure of at least some among these complexes. The Mad2-Cdc20 complex contains a “safety belt” structure (21), in which a strand in a closed conformation of Mad2 (“C-Mad2”) is tightly wrapped around a specific segment of Cdc20. The structure of the BubR1-Cdc20 complex is not known, but the tight interaction of these proteins may involve a similar conformational transition to an energetically favorable stable complex. Thus, an energy-dependent dissociation mechanism may be required to overcome kinetic barriers in the breakup of mitotic checkpoint complexes in the rapid inactivation of the checkpoint. Because the inactivation of the mitotic checkpoint is a tightly regulated process, as is the activation of this system, the energy-dependent step may also have regulatory roles.
The specific mechanism that employs ATP hydrolysis to cause the disassembly of mitotic checkpoint complexes is not known, but we have ruled out several possibilities. It has been reported that polyubiquitylation by APC/C is required for exit from the checkpoint-arrested state and for the release of Mad2 and BubR1 from APC/C (15). We have confirmed these observations but found that an additional process, which involves β–γ bond cleavage of ATP, is essential for checkpoint inactivation and for the disassembly of mitotic checkpoint complexes. This conclusion is based on the findings that the nonhydrolyzable β–γ ATP analogue AMP-PNP, which effectively carries out ubiquitylation (because the scission of the α–β bond of ATP takes place in the E1 reaction), cannot replace ATP in checkpoint inactivation and in complex disassembly (Fig. 1). Because the action of the 26S proteasome requires β–γ bond scission of ATP (18), it was still possible that polyubiquitylation-dependent degradation of certain proteins is required for release from the checkpoint-arrested state. However, we found that polyubiquitylation is required only for the release of MCC components from APC/C (Fig. 2), whereas β–γ bond cleavage of ATP is involved in both the release from APC/C and for the dissociation of free MCC into its components. This indicates that, at least in the latter case, requirement for ATP is not due to ubiquitylation-dependent protein degradation. We also confirmed the findings of Reddy et al. (15) that the proteasome inhibitor MG-132 has no influence on APC/C activation (Fig. 3B), as opposed to the effects of the polyubiquitylation inhibitor MeUb. The target of polyubiquitylation remains to be unknown. It does not seem to be Cdc20 (16), but it could be another component of the mitotic checkpoint system, a subunit if APC/C or a protein that influences APC/C-MCC interaction. We also ruled out the possibility that the effects of ATP analogues and of MeUb are due to sustained Cdk1/cyclin B activity, caused by prevention of the degradation of cyclin B, because inhibition of protein kinase activity by staurosporine did not reverse the effects of any of these agents (Fig. 3).
The requirement for β–γ bond cleavage of ATP in the disassembly of mitotic checkpoint complexes may reflect the involvement of chaperone proteins that utilize the energy of ATP hydrolysis for the conformational changes necessary for complex dissociation. It is also possible that β–γ-cleavable ATP is required for some phosphorylation reaction necessary for the breakdown of checkpoint complexes. For example, phosphorylation of Mad2 at its C-terminal region has been shown to increase during exit from mitosis and to prevent its interaction with APC/C (12). The protein kinase that carries out the phosphorylation of Mad2 has not been identified. We noted previously (6), and observed again in the present work (Figs. 1 and 2) that Mad2 is released most rapidly from mitotic checkpoint complexes. It is possible that the release of Mad2 is the initial event that allows the dissociation and/or inactivation of mitotic checkpoint complexes. If the phosphorylation of Mad2, or of some other checkpoint protein is involved in complex disassembly, it will have to be assumed that the reaction is carried out by a staurosporine-insensitive protein kinase, because this agent does not inhibit escape from checkpoint arrest (Fig. 3), but actually shortens the lag in the activation of APC/C (4). Staurosporine is one of the most generally active protein kinase inhibitors: In a recent extensive survey of the interaction of many protein kinase inhibitors with 317 protein kinases (representing > 50% of the predicted human “kinome”), staurosporine was found to bind tightly to 87% of protein kinases (19). Thus, if a specific protein kinase action is involved in complex disassembly, its insensitivity to staurosporine may facilitate its identification. Obviously, much more work is necessary to elucidate the molecular mechanisms of exit from mitotic checkpoint.
Methods
Extracts from nocodazole-arrested HeLa cells were prepared as described previously, without addition of ATP (4). Extracts were stored at -70 °C in small samples. Prior to use, extracts were thawed on ice for ∼30 min, and then were centrifuged (14,000 rpm for 6 min at 4 °C) to remove particulate material. Extracts were incubated with 10 mM Tris·HCl (pH 7.6), 5 mM MgCl2 and 1 mM DTT. Where indicated, ATP (1 mM) was supplemented together with 10 mM phosphocreatine and 100 μg/mL creatine phophokinase (Sigma C3755). ATPγS (Roche) was added at 2 mM, and AMP-PNP at 5 mM. MeUb was prepared as described (18), and was added to incubations at 100 μM. Staurosporine and MG-132 were purchased from Sigma and were added at concentrations of 10 and 100 μM, respectively. These agents were dissolved in DMSO; the final concentration of DMSO solvent did not exceed 0.5%. Control experiments showed that this concentration of DMSO had no influence any of the processes examined in this study. Samples were incubated at 23 °C for the time periods indicated in figures.
Incubated extracts were subjected to sequential immunoprecipitations, to separate between pools of MCC associated with APC/C and free MCC. Samples of 130 μL of incubated extracts (∼2.5 mg of protein) were first rotated (4 °C, 2 h) with 20 μL of Affi-prep protein A beads (BioRad) covalently linked to rabbit polyclonal anti-Cdc27 antibodies, as described (4). The resulting supernatants, which usually contained not more than 15% of total APC/C, were divided to two ∼1-mg portions for parallel immunoprecipitations with affinity-purified rabbit polyclonal anti-BubR1 (5) and anti-Cdc20 (Santa Cruz, sc-8358) antibodies. In each case, 5 μg of antibody was bound noncovalently to 10 μL of Affi-prep protein A beads and samples were rotated for an additional 2 h at 4 °C. In all cases, beads were washed 3 times with 1-mL portions of a buffer consisting of 50 mM Tris·HCl (pH 7.2), 1 mg/mL BSA, 20% (vol/vol) glycerol, and 0.5 mM DTT and were resuspended in 2 volumes of the same buffer. Samples were subjected to SDS-PAGE, transferred to polyvinylidene fluoride membranes (Immobilon P, Millipore) and were blotted with mouse monoclonal antibodies. The following monoclonal antibodies were used for immunoblotting: antibodies against Cdc27, cyclin B, and BubR1 were from BD Transduction Laboratories; anti-Cdc20 was from Santa Cruz (E7, sc-13162); and anti-Mad2 was from Medicinal Biological Laboratories. Immunoblots were visualized with enhanced chemiluminescence (SuperSignal, Pierce) and were quantified with an ImageQuant RT ECL instrument (GE Healthcare). By this procedure, the intensity of the signals was not strictly linear with antigen concentration, and therefore results cannot be regarded as strictly quantitative. In the case of immunoblotting of anti-Cdc27 immunoprecipitates, blotting for Cdc27 was used for loading controls. In various samples, Cdc27 was phosphorylated to different extents and we observed that phoshorylated Cdc27 yielded higher ECL signals than the unphosphorylated protein. Therefore, samples of anti-Cdc27 immunoprecipitates were dephosphorylated prior to SDS-PAGE by phosphatase treatment as follows. One microliter of packed anti-Cdc27 beads were suspended in 10 μL of reaction mixture that contained 50 mM Tris·HCl (pH 7.6), 2 mg/mL BSA, 10% (vol/vol) glycerol, 0.5 mM DTT, 2 mM MnCl2, and 100 units lambda phosphatase (New England Biolabs). Samples were incubated at 30 °C for 1 h with shaking at 1,000 rpm.
APC/C activity was assayed in 1-μL samples (packed beads) of anti-Cdc27 immunoprecipitates, prepared as described above, by the ligation of 125I-cyclin to ubiquitin, as described previously (6). Unless otherwise stated, incubations were carried out at 23 °C for 30 min with shaking at 1,000 rpm.
Acknowledgments.
We thank Ilana Braunstein and Elena Dumin for help and advice. The skillful and devoted technical assistance of Jonathan Gromis is greatly appreciated. Parts of this work were carried out during the stay of A.H. at the Marine Biological Laboratory, Woods Hole, MA. This work was supported by the Israel Science Foundation and the Diane and Guilford Glazer Distinguished Chair of the Israel Cancer Research Fund.
Footnotes
The authors declare no conflict of interest.
References
- 1.Bharadwaj R, Yu H. The spindle checkpoint, aneuploidy and cancer. Oncogene. 2004;23:2016–2027. doi: 10.1038/sj.onc.1207374. [DOI] [PubMed] [Google Scholar]
- 2.Musacchio A, Salmon ED. The spindle checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 3.Ciliberto A, Shah JV. A quantitative systems view of the spindle assembly checkpoint. EMBO J. 2009;28:2162–2173. doi: 10.1038/emboj.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braunstein I, Miniowitz S, Moshe Y, Hershko A. Inhibitory factors associated with the anaphase-promoting complex-cyclosome in mitotic checkpoint. Proc Natl Acad Sci USA. 2007;104:4870–4825. doi: 10.1073/pnas.0700523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudakin V, Chan GKT, Yen TJ. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BubR1, Bub3, Cdc20 and Mad2. J Cell Biol. 2001;154:925–936. doi: 10.1083/jcb.200102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eytan E, et al. Two different mitotic checkpoint inhibitors of the anaphase-promoting complex/cyclosome antagonize the action of the activator Cdc20. Proc Natl Acad Sci USA. 2008;105:9181–9185. doi: 10.1073/pnas.0804069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herzog F, et al. Structure of the anaphase-promoting complex/cyclosome interacting with a mitotic checkpoint complex. Science. 2009;323:1477–1481. doi: 10.1126/science.1163300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yudkovsky Y, Shteinberg M, Listovsky T, Brandeis M, Hershko A. Phosphorylation of Cdc20/Fizzy negatively regulates the mammalian cyclosome/APC in the mitotic checkpoint. Biochem Biophys Res Commun. 2000;271:299–304. doi: 10.1006/bbrc.2000.2622. [DOI] [PubMed] [Google Scholar]
- 9.Chung E, Chen RH. Phosphorylation of Cdc20 is required for its inhibition by the spindle checkpoint. Nat Cell Biol. 2003;5:748–753. doi: 10.1038/ncb1022. [DOI] [PubMed] [Google Scholar]
- 10.D’Angiolella V, Mari C, Nocera D, Rametti L, Grieco D. The spindle checkpoint requires cyclin-dependent kinase activity. Genes Dev. 2003;17:2520–2525. doi: 10.1101/gad.267603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang Z, Shu H, Oncel D, Chen S, Yu H. Phosphorylation of Cdc20 by Bub1 provides a catalytic mechanism for APC/C inhibition by the spindle checkpoint. Mol Cell. 2004;16:387–397. doi: 10.1016/j.molcel.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 12.Wassman K, Liberal V, Benezra R. Mad2 phosphorylation regulates its association with Mad1 and APC/C. EMBO J. 2003;22:797–806. doi: 10.1093/emboj/cdg071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habu T, Kim SH, Weinstein J, Matsumoto T. Identification of a Mad2-binding protein, CMT2, and its role in mitosis. EMBO J. 2002;21:6419–6428. doi: 10.1093/emboj/cdf659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia G, et al. Conformation-specific binding of p31comet antagonizes the functions of Mad2 in spindle checkpoint. EMBO J. 2004;23:3133–3143. doi: 10.1038/sj.emboj.7600322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy SK, Rape M, Margansky WA, Kirschner MW. Ubiquitination by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature. 2007;446:921–925. doi: 10.1038/nature05734. [DOI] [PubMed] [Google Scholar]
- 16.Nilsson J, Yekezare M, Minshull J, Pines J. The APC/C maintains the spindle assembly checkpoint by targeting Cdc20 for destruction. Nat Cell Biol. 2008;10:1411–1420. doi: 10.1038/ncb1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 18.Hershko A, Heller H. Occurrence of a polyubiquitin structure in ubiquitin-protein conjugates. Biochem Biophys Res Commun. 1985;128:1079–1086. doi: 10.1016/0006-291x(85)91050-2. [DOI] [PubMed] [Google Scholar]
- 19.Karaman MW, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 20.Peters JM. The anaphase-promoting complex/cyclosome: A machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- 21.Sironi L, et al. Crystal structure of the Mad1-Mad2 core complex: Implications of a “safety belt” binding mechanism for the spindle checkpoint. EMBO J. 2002;21:2496–2506. doi: 10.1093/emboj/21.10.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]