Abstract
Environmental temperature impacts the physical activity and ecology of ectothermic animals through its effects on muscle contractile physiology. Sprinting, swimming, and jumping performance of ectotherms decreases by at least 33% over a 10 °C drop, accompanied by a similar decline in muscle power. We propose that ballistic movements that are powered by recoil of elastic tissues are less thermally dependent than movements that rely on direct muscular power. We found that an elastically powered movement, ballistic tongue projection in chameleons, maintains high performance over a 20 °C range. Peak velocity and power decline by only 10%–19% with a 10 °C drop, compared to >42% for nonelastic, muscle-powered tongue retraction. These results indicate that the elastic recoil mechanism circumvents the constraints that low temperature imposes on muscle rate properties and thereby reduces the thermal dependence of tongue projection. We propose that organisms that use elastic recoil mechanisms for ecologically important movements such as feeding and locomotion may benefit from an expanded thermal niche.
Keywords: biomechanics, muscle physiology, elastic storage, thermal ecology, Chamaeleonidae
Temperature influences diverse physiological processes, including metabolic rate, muscle dynamics, and nerve conduction velocity, which in turn can affect whole-organism performance. Ectothermic animals are particularly vulnerable to the effects of low ambient temperatures, because their body temperature (Tb) is dictated by environmental conditions. The effect of Tb on muscle physiology has a clear impact on an organism's ability to move, escape predators, and engage in foraging behavior (1–6); for example, a 10 °C drop in Tb reduces sprint speed in lizards, swimming speed in fish, and jumping distance in frogs by at least 33% (2, 5). We find that, unlike these other dynamic movements, ballistic tongue projection in chameleons maintains extremely high performance over a Tb range of 20 °C.
The mechanism of chameleon prey capture is unique among lizards, relying on ballistic projection of the tongue up to twice the length of the body in as little as 0.07 second (7, 8). This feeding mechanism is common to all chameleons and gives these slow, cryptic, sit-and-wait predators the element of surprise. Chameleons feed over a wider range of Tb than other lizards, using ballistic tongue projection in habitats ranging from deserts, where Tb exceeds 39 °C (9), to alpine zones above 3,500 m with temperatures below freezing (10). Some chameleon species feed at a Tb of 3.5 °C (9), exploiting an early morning peak in alpine insect activity (10) before sympatric lizard species become active (11). This ability to feed at low Tb has not been explained; we propose that the elastic-recoil mechanism of tongue projection confers this temperature insensitivity.
Ballistic tongue projection in chameleons achieves its extreme performance by rapid elastic recoil of collagen tissue within the tongue—tissue that is first stretched by slow contraction of the tongue accelerator muscle (7). This “bow and arrow” mechanism decouples muscle contraction temporally from tongue launch and thereby allows kinetic energy to be imparted to the tongue at a rate far exceeding that possible via direct muscle contraction (7). Once launched—at accelerations exceeding 400 ms−2 (41 g)—the tongue travels to the target on its momentum alone and then adheres to the prey. Tongue retraction relies on neither ballistic launch nor elastic recoil to bring prey to the mouth, but rather is driven by continuous contraction of the lengthy hyoglossus muscle (8).
The differing mechanisms of tongue projection and retraction in chameleons provide an opportunity to evaluate the hypothesis that the elastic-recoil mechanism confers low thermal dependence to tongue projection. We tested whether elastically powered tongue projection has a lower thermal dependence than nonelastic tongue retraction by examining the effects of temperature on performance parameters of these two movements. In addition, we propose that our findings can be generalized to explosive ballistic movements in other ectotherms, and that elastic-recoil mechanisms may serve to expand the thermal niche of ectotherms that use them for critical movements.
Results
Veiled chameleons (Chamaeleo calyptratus) were able to project the tongue and capture prey across the same range of distances regardless of temperature (15 °C–35 °C). Overall, projection distances ranged from 6.6 cm to 19.6 cm. Individual average projection distances ranged from 10.4 cm to 14.2 cm, with an overall average of 12.5 cm. No significant effect of temperature on prey distance, tongue projection distance, or tongue overshoot distance was found.
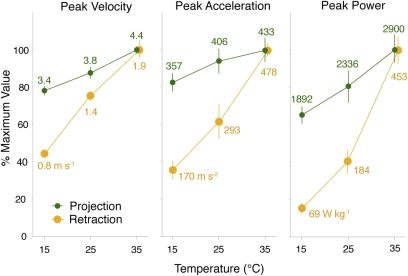
Inverse dynamic analysis of tongue movements revealed that as temperature increased, performance increased significantly (Table 1) for both tongue projection and retraction. Nonetheless, peak performance measures of ballistic tongue projection were maintained at a high level at all temperatures (Table 2). At the low end of our experimental Tb range (15 °C), peak projection velocity averaged 3.4 ms−1, peak acceleration averaged 357 ms−2, and peak power averaged 1,892 Wkg−1. At 35 °C, values were somewhat higher: peak velocity averaged 4.4 ms−1, peak acceleration averaged 433 ms−2, and peak power averaged 2,900 Wkg−1. In contrast, performance parameters of retraction increased markedly at higher temperature. At 15 °C, peak velocity averaged 0.8 ms−1, peak acceleration averaged 170.3 ms−2, and peak power averaged 34.4 Wkg−1, whereas at 35 °C, peak velocity averaged 1.9 ms−1, peak acceleration averaged 478 ms−2, and peak power averaged 453 Wkg−1 (Table 2). The average power of projection also was maintained at a high level, averaging 1,092 ± 78 Wkg−1 at 15 °C (mean ± SE) and 1,911 ± 156 Wkg−1 at 35 °C. The order of experimental temperatures experienced by an individual had no significant effect on projection or retraction performance.
Table 1.
Results from repeated-measures ANCOVA examining the performance parameters peak velocity, peak acceleration, and peak power for effects of temperature, individual, feeding phase (projection vs. retraction), and projection distance (covariate)
| Peak velocity | Peak acceleration | Peak power | |||||||
| df | F value | P value | df | F value | P value | df | F value | P value | |
| 15 °C vs. 25 °C | |||||||||
| Individual | 4 | 7.384 | <0.0001* | 4 | 5.585 | 0.0003* | 4 | 1.832 | 0.1253 |
| Temperature | 1 | 436.1 | <0.0001* | 1 | 77.56 | <0.0001* | 1 | 244.5 | <0.0001* |
| Projection distance | 1 | 44.86 | <0.0001* | 1 | 5.477 | 0.0205 | 1 | 0.589 | 0.4438 |
| Phase | 1 | 8118 | <0.0001* | 1 | 294.8 | <0.0001* | 1 | 8356 | <0.0001* |
| Individual × temperature | 4 | 2.58 | 0.0395 | 4 | 2.439 | 0.0493 | 4 | 4.283 | 0.0026* |
| Individual × projection distance | 4 | 1.665 | 0.1609 | 4 | 0.697 | 0.5952 | 4 | 1.002 | 0.4083 |
| Temperature × projection distance | 1 | 0.030 | 0.8633 | 1 | 2.781 | 0.0974 | 1 | 10.96 | 0.0012* |
| Individual × phase | 4 | 3.159 | 0.0157* | 4 | 5.433 | 0.0004* | 4 | 4.351 | 0.0023* |
| Temperature × phase | 1 | 250.5 | <0.0001* | 1 | 73.86 | <0.0001* | 1 | 195.5 | <0.0001* |
| Projection distance × phase | 1 | 6.371 | 0.0126* | 1 | 30.84 | <0.0001* | 1 | 9.627 | 0.0023* |
| 25 °C vs. 35 °C | |||||||||
| Individual | 4 | 10.10 | <0.0001* | 4 | 2.345 | 0.0571 | 4 | 6.580 | <0.0001* |
| Temperature | 1 | 222.2 | <0.0001* | 1 | 45.15 | <0.0001* | 1 | 132.4 | <0.0001* |
| Projection distance | 1 | 77.65 | <0.0001* | 1 | 0.207 | 0.6494 | 1 | 16.50 | <0.0001* |
| Phase | 1 | 6464 | <0.0001* | 1 | 26.25 | <0.0001* | 1 | 7719 | <0.0001* |
| Individual × temperature | 4 | 2.884 | 0.0244 | 4 | 1.759 | 0.1398 | 4 | 1.113 | 0.3523 |
| Individual × projection distance | 4 | 6.300 | <0.0001* | 4 | 1.636 | 0.1679 | 4 | 1.528 | 0.1966 |
| Temperature × projection distance | 1 | 4.605 | 0.0334 | 1 | 3.252 | 0.0732 | 1 | 0.025 | 0.8759 |
| Individual × phase | 4 | 5.597 | 0.0003* | 4 | 2.652 | 0.0353 | 4 | 2.093 | 0.0843 |
| Temperature × phase | 1 | 78.38 | <0.0001* | 1 | 21.21 | <0.0001* | 1 | 54.06 | <0.0001* |
| Projection distance × phase | 1 | 37.25 | <0.0001* | 1 | 14.22 | 0.0002* | 1 | 1.536 | 0.217 |
Note the significant temperature × phase interaction effects, which indicate that tongue projection and tongue retraction are affected differently by changes in temperature.
*Significant difference in ANCOVA at Benferroni-corrected α = 0.017, indicating significant effect.
Table 2.
Kinematic performance variables during projection and retraction at 15 °C, 25 °C, and 35 °C
| Peak velocity, mean ±SEM | Peak acceleration, mean ± SEM | Peak power, mean ± SEM | |
| Projection | |||
| 15 °C | 3.4 ± 0.1 | 357 ± 20 | 1,892 ± 123 |
| 25 °C | 3.8 ± 0.1 | 406 ± 27 | 2,336 ± 239 |
| 35 °C | 4.4 ± 0.1 | 433 ± 27 | 2,900 ± 235 |
| Retraction | |||
| 15 °C | 0.8 ± 0.03 | 170 ± 21 | 69 ± 12 |
| 25 °C | 1.4 ± 0.04 | 293 ± 43 | 184 ± 27 |
| 35 °C | 1.9 ± 0.1 | 478 ± 14 | 453 ± 32 |
Values were.calculated as the mean ± SE of each individual's predicted performance at a projection distance of 12.5 cm based on each individual's performance regressed against projection distance.
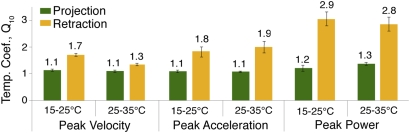
Although tongue projection and retraction both showed effects of temperature, retraction showed a significantly stronger effect. For each 10 °C increment in temperature between 15 °C and 35 °C, a significant interaction effect of temperature (Tb) and phase (i.e., projection vs. retraction) on performance was found (Table 1). Q10 values and percent decrease of average performance reveal that tongue projection maintained performance with decreasing temperature to a greater extent than did tongue retraction (Figs. 1 and 2). Performance parameters declined by only 10%–19% over the 15 °C–25 °C interval at a projection distance of 12.5 cm (Fig. 2). Temperature coefficients (Q10) for projection parameters never exceeded 1.3 (Fig. 2) and varied by no more than 0.04 across all distances. In contrast, tongue retraction was strongly affected by temperature; it slowed visibly at 15 °C, and its performance variables showed Q10 values of 1.7–2.9 and declined by 42%–63% over 10 °C (Figs. 1 and 2).
Fig. 1.
Mean temperature coefficients (Q10) with SE bars for tongue projection (green) and retraction (gold), indicating the factor by which each performance parameter changes over 10 °C. Note the consistently lower values for projection versus retraction. Q10 was calculated as the average of each individual's Q10 value for that parameter; individual Q10 values were calculated from interpolated performance values at an average projection distance of 12.5 cm (from performance values regressed against projection distance).
Fig. 2.
Performance parameters (mean ± SE) as a percent of maximum for tongue projection and retraction, showing low thermal dependence of projection (green) compared with retraction (gold). Absolute values of means are shown in native units. Values were calculated as the average of each individual's value for that parameter; individual values were interpolated at an average projection distance of 12.5 cm (from performance values regressed against projection distance).
Discussion
Remarkably, C. calyptratus achieved extremely high-performance tongue projection even when cold. At a Tb of 15 °C, time-averaged muscle-mass–specific power output averaged 1,092 Wkg−1, and peak instantaneous muscle-mass–specific power output during projection averaged 1,892 Wkg−1. This peak value is well in excess of peak power output of muscle tissue during active contraction as measured or estimated in other vertebrates operating at higher Tb, including flying quail during vertical takeoff (1,121 Wkg−1) (12), sprinting lizards (952 Wkg−1) (13), and jumping frogs (373 Wkg−1) (6). High power outputs for rapid movements using the elastic-recoil mechanism, including jumping in bushbabies (14) and insects (15, 16), predatory strikes of mantis shrimp (17), and tongue projection in salamanders (18) and chameleons (7), have been documented in numerous kinematic studies; little focus has been given to the maintenance of performance at low Tb, however.
The Q10 values for tongue projection (1.1–1.3; Fig. 1) are well below the Q10 values of contractile rate properties of isolated muscles and of other dynamic behaviors, which generally exceed 1.5 (1–6). This degree of temperature independence is similar to that of static contractile muscle properties, such as maximum isometric tetanic tension (3, 6), and of static behaviors, such as exertion of peak bite force (1); however, the extent of temperature dependence on tongue retraction (Q10 = 1.7–2.9; Figs. 1 and 2) resembles that of contractile rate properties of isolated muscles and of dynamic behaviors, such as sprinting (1–6). Jump distance in frogs, for example, exhibits a Q10 value of 1.6 over 14 °C–25 °C, and the power generated by the muscles activated during jumping has a Q10 value of 2.7 (5). Similarly, sprint speed in lizards has an average Q10 value of 1.5 at temperatures below the estimated optimal temperature (2).
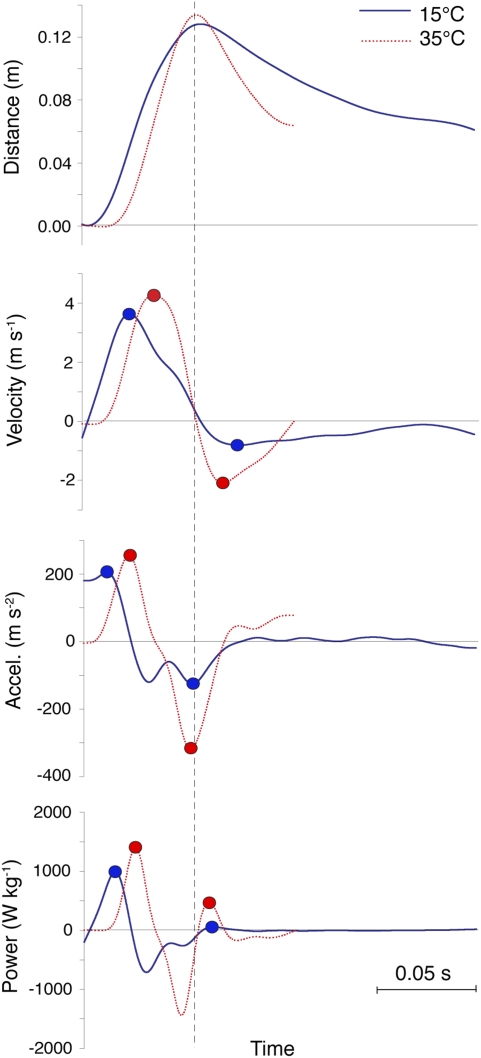
The contrasting thermal dependence of tongue projection and retraction (Fig. 3 and Movie S1) supports the hypothesis that the low thermal dependence of tongue projection in chameleons is due to the elastic-recoil mechanism, in which temperature-dependent muscle shortening occurs during the loading phase before tongue launch, and is temporally decoupled from the temperature-independent elastic recoil of connective tissue that powers ballistic tongue projection. This mechanism not only endows chameleons with spectacular performance, but also liberates projection from the constraints on muscle rate properties imposed by low temperature. Thus, the thermal dependence of the contractile rate properties of the tongue accelerator muscle need not be unusually low to maintain high performance at low temperature. In contrast, tongue retraction declines at low temperature, because it relies on direct muscle power output, which is thermally dependent. Projection performance depends instead on peak muscle tension and the elastic modulus of collagen, both of which show low thermal dependence or complete thermal independence (3, 5, 6, 19). Peak isometric muscle tension typically exhibits Q10 values of 1.0–1.2 (5), and the load–strain relationship of collagenous tendon exhibits a Q10 of 1 across the large physiological temperature range of 0 °C–37 °C (19).
Fig. 3.
Kinematic and dynamic profiles from two representative feedings of similar projection distance showing similar peak values for projection at 15 °C and 35 °C, compared with dissimilar values for retraction at the two temperatures. Retraction is analyzed only until the tongue reaches the entoglossal process. Profiles are overlaid at the time of maximum projection distance (dashed line). Power values are not corrected for muscle mass (2× for projection and 4× for retraction).
Studies of other animal systems that use elastic structures to power movements lend additional support to the conclusion that elastic-recoil mechanisms confer relative thermal independence compared with movements that rely on muscle rate properties. Among ballistic systems, jumping in frogs is powered partially by recoil of in-series elastic elements that supplements muscle power output (20). Frog jumping appears to show a reduced effect of temperature on performance (5), but it is not liberated to the same extent as tongue projection in chameleons, probably because elastic recoil and muscle contraction overlap temporally (20). Among cyclical systems, wingbeat frequency of beetles shows very low temperature sensitivity, apparently because frequency is determined by the resonant frequency of the flight system, which is dictated by its physical properties rather than by its muscle rate properties (21).
Because the mechanical properties of elastic tissues are known to have low thermal sensitivity (19, 22, 23), temperature manipulation may be a valuable methodological approach to test for the presence or prevalence of elastic recoil in powering movements. Elastic recoil is implicated if performance of a movement is maintained at a high level over a wide range of body temperatures. Our findings on chameleons thus serve as independent validation for the presence of an elastic-recoil mechanism in tongue projection.
Finally, chameleons have increased the thermal breadth of their feeding mechanism by decreasing the temperature effects on performance of ballistic tongue projection and thus are able to feed at very low Tb (9–11, 24, 25). This ability likely grants them an expanded thermal niche, allowing them to feed early in the morning when effective thermoregulation is not possible (10) and enabling them to be active over a wider temperature range than other sympatric lizard species (11). The ability of chameleons to forage at low temperatures also may reduce thermoregulatory behavior and its ecological costs (26). Other ectothermic organisms that use explosive, ballistic movements for prey capture or locomotion across a range of temperatures may similarly benefit from the relative thermal independence of elastic recoil mechanisms.
Materials and Methods
Five Chamaeleo calyptratus (12.5–14.0 cm snout–vent length) were imaged at 3 kHz at a Tb of 15 °C, 25 °C, and 35 °C while feeding on crickets at a range of distances, using a Photron Fastcam high-speed digital camera. Crickets were placed on a square of insect screen suspended vertically from above by thread. This “cricket trapeze” allowed the chameleon's tongue to complete its trajectory naturally without being stopped by an immovable target, and thus permitted examination of performance and physiological parameters at a range of actual tongue projection distances.
To control Tb, after an acclimation period of at least 1 h, imaging trials were conducted in an environmental chamber set to the experimental Tb. Supplemental lighting was switched on immediately before tongue projection and turned off immediately after tongue retraction to prevent elevation of body temperature through light source radiation. During the prey reduction phase, immediately after tongue retraction, Tb was verified orally using a calibrated Sixth Sense LT300 infrared thermometer (± 1 °C accuracy). Only feeding sequences with a postfeeding Tb of the target experimental temperature ± 1 °C were included in the analysis.
Ten feeding sequences were collected from each of four individuals at each experimental Tb, for a total of 120 feedings. Five feeding sequences from a fifth individual were collected at each experimental Tb before this animal was removed from the experiment due to illness. Between one and five feeding events were collected per individual at each feeding session. The sequence of experimental Tb for each individual was selected randomly, and no two animals were exposed to an identical Tb sequence. To account for natural variation in the distance between the prey and the chameleon's snout because of the distance that the chameleon leaned its body forward off the perch for any given feeding event, distance to the “cricket trapeze” was varied within a normal range of projection distances. Thus, feedings were collected over an 8- 20-cm range of tongue projection distances. Distance to the prey was adjusted to elicit maximal tongue projection length for each individual at each experimental Tb. Effects of temperature on preprojection distance to the target and overshoot distance of the tongue beyond the target were examined using repeated-measures ANOVA.
An inverse-dynamics approach was used to compute the instantaneous velocity, acceleration, and power of tongue projection and retraction. Using National Institutes of Health Image J software (http://reb.info.nih.gov/ij), the distance of tongue projection for each scale-calibrated feeding sequence was recorded. Image J software was used to record the x,y coordinates of the tip of the tongue on each frame throughout the tongue projection sequence. Using a custom script for the P-Spline package of R statistical software (R Project for Statistical Computing), a quintic spline was fitted to the position trace of the tongue and smoothed to remove secondary oscillation artifacts from the first and second derivatives of position. From these smoothed position data, instantaneous velocity (ms−1) and acceleration (ms−2) (i.e., first and second derivatives of the position) were calculated. For tongue retraction, coordinates of four positions along the length of the retractor muscle were recorded on each frame of the retraction sequence. These coordinate data were used to quantify the length of the retractor muscle in each frame, and this length was then used to compute the length change through the retraction sequence. These length data were then smoothed and subjected to the same inverse dynamics analysis as the tongue projection position data. Mass-specific power (in Wkg−1) was calculated as the product of velocity and acceleration (7) and corrected for the mass of the active muscle in each phase. As in other species (7), dissection and mass measurements of the tongue apparatus of seven C. calyptratus (12.0–15.5 cm snout–vent length) determined that the circular portion of the accelerator muscle accounts for ∼50% (mean, 48.2% ± 2.9%) of the mass of the accelerator muscle complex and tongue pad, whereas the retractor muscle accounts for ∼25% (mean, 25.8% ± 1.7%) of the mass of the accelerator muscle complex, tongue pad, and retractor muscle. Thus, mass-specific power is multiplied by a factor of 2 for projection and by a factor of 4 for retraction (7).
To examine the effects of temperature on performance, tongue projection distance, peak velocity (ms−1), peak acceleration (ms−2) and peak mass specific power (Wkg−1) for both tongue projection and retraction were computed for each feeding sequence. Performance was log-transformed and examined for effects of temperature (fixed effect), phase of feeding (fixed effect), and individual (random effect) using repeated-measures ANCOVA with projection distance as a covariate. The temperature × phase interaction term of the model allowed us to examine whether tongue projection and tongue retraction responded differently to temperature changes. In addition, the influence of experimental temperature sequence on performance was assessed using repeated-measures ANOVA to test for an effect of previous temperature on the projection distance residuals of each performance parameter.
Least squares regression of performance parameters during both projection and retraction, with projection distance as the independent variable, was performed for each individual at each temperature. The interpolated value of each performance parameter at the overall average projection distance (12.5 cm) was calculated for each individual and used to calculate temperature coefficient (Q10) values using the equation Q10 = (R2/R1)[10/(t2 − t1)], where R1 and R2 are the interpolated performance values at temperatures t1 and t2, respectively, and t2 is greater than t1. The Q10 values for each individual were then used to calculate an average Q10 value with SE.
Supplementary Material
Acknowledgments
We thank S. T. Hsieh, U. Müller, J. C. O'Reilly, W. Ryerson, and P. Sandusky for helpful suggestions on drafts of this manuscript. This work was supported by National Science Foundation Grant IOS 0842626 (to S.M.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910778107/DCSupplemental.
References
- 1.Herrel A, James RS, Van Damme R. Fight versus flight: Physiological basis for temperature-dependent behavioral shifts in lizards. J Exp Biol. 2007;210:1762–1767. doi: 10.1242/jeb.003426. [DOI] [PubMed] [Google Scholar]
- 2.Huey RB, Bennett AF. Phylogenetic studies of coadaptation: Preferred temper-atures versus optimal performance temperatures of lizards. Evolution. 1987;41:1098–1115. doi: 10.1111/j.1558-5646.1987.tb05879.x. [DOI] [PubMed] [Google Scholar]
- 3.Bennett AF. Temperature and muscle. J Exp Biol. 1985;115:333–344. doi: 10.1242/jeb.115.1.333. [DOI] [PubMed] [Google Scholar]
- 4.Huey RB, Stevenson RD. Integrating thermal physiology and ecology of ectotherms: A discussion of approaches. Am Zool. 1979;19:357–366. [Google Scholar]
- 5.Rome LC. Influence of temperature on muscle recruitment and muscle function in vivo. Am J Physiol. 1990;259:R210–R222. doi: 10.1152/ajpregu.1990.259.2.R210. [DOI] [PubMed] [Google Scholar]
- 6.Lutz GJ, Rome LC. Muscle function during jumping in frogs, II. Mechanical properties of muscle: implications for system design. Am J Physiol. 1996;271:C571–C578. doi: 10.1152/ajpcell.1996.271.2.C571. [DOI] [PubMed] [Google Scholar]
- 7.de Groot JH, van Leeuwen JL. Evidence for an elastic projection mechanism in the chameleon tongue. Proc R Soc Lond B Biol Sci. 2004;271:761–770. doi: 10.1098/rspb.2003.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrel A, Meyers JJ, Nishikawa KC, De Vree F. Morphology and histochemistry of the hyolingual apparatus in chameleons. J Morphol. 2001;249:154–170. doi: 10.1002/jmor.1047. [DOI] [PubMed] [Google Scholar]
- 9.Burrage BR. Comparative ecology and behaviour of Chamaeleo pumilus pumilus (Gmelin) and C. namaquensis A. Smith (Sauria: Chamaeleonidae) Ann S Afr Mus. 1973;61:33–71. [Google Scholar]
- 10.Reilly SM. Ecological notes on Chamaeleo schubotzi from Mount Kenya. J Herpetol Assoc Afr. 1982;28:1–3. [Google Scholar]
- 11.Hebrard JJ, Reilly SM, Guppy M. Thermal ecology of Chamaeleo hoehnelii and Mobuya varia in the Aberdare Mountains: Constraints of heterothermy in an alpine habitat. J East Afr Nat Hist Soc. 1982;176:1–6. [Google Scholar]
- 12.Askew GN, Marsh RL. The mechanical power output of the pectoralis muscle of blue-breated quail (Coturnix chinensis): The in vivo length cycle and its implications for muscle performance. J Exp Biol. 2001;204:3587–3600. doi: 10.1242/jeb.204.21.3587. [DOI] [PubMed] [Google Scholar]
- 13.Curtin NA, Woledge RC, Aerts P. Muscle directly meets the vast power demands in agile lizards. Proc R Soc Lond B Biol Sci. 2005;272:581–584. doi: 10.1098/rspb.2004.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aerts P. Vertical jumping in Galago senegalensis: The quest for an obligate mechanical power amplifier. Philos Trans R Soc Lond B Biol Sci. 1998;353:1607–1620. [Google Scholar]
- 15.Bennet-Clark HC. In: Perspectives in Evolutionary Biology, Vol 1: Zoology. Spencer Davies P, editor. Oxford, UK: Pergamon; 1975. pp. 467–479. [Google Scholar]
- 16.Burrows M. Froghopper insects leap to new heights. Nature. 2003;424:509. doi: 10.1038/424509a. [DOI] [PubMed] [Google Scholar]
- 17.Patek SN, Korff WL, Caldwell RL. Deadly strike mechamism of a mantis shrimp. Nature. 2004;428:819–820. doi: 10.1038/428819a. [DOI] [PubMed] [Google Scholar]
- 18.Deban SM, O'Reilly JC, Dicke U, van Leeuwen JL. Extremely high-power tongue projection in plethodontid salamanders. J Exp Biol. 2007;210:655–667. doi: 10.1242/jeb.02664. [DOI] [PubMed] [Google Scholar]
- 19.Rigby BJ, Hirai N, Spikes JD, Eyring H. The mechanical properties of rat tail tendon. J Gen Physiol. 1959;43:265–283. doi: 10.1085/jgp.43.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts TJ, Marsh RL. Probing the limits to muscle-powered accelerations: Lessons from jumping bullfrogs. J Exp Biol. 2003;206:2567–2580. doi: 10.1242/jeb.00452. [DOI] [PubMed] [Google Scholar]
- 21.Oertli JJ. Relationship of wing beat frequency and temperature during take-off flight in temperate-zone beetles. J Exp Biol. 1989;145:321–338. [Google Scholar]
- 22.Alexander RM. Rubber-like properties of the inner hinge-ligament of Pectinidae. J Exp Biol. 1966;44:119–130. doi: 10.1242/jeb.44.1.119. [DOI] [PubMed] [Google Scholar]
- 23.Denny M, Miller L. Jet propulsion in the cold: Mechanics of swimming in the Antarctic scallop Adamussium colbecki. J Exp Biol. 2006;209:4503–4514. doi: 10.1242/jeb.02538. [DOI] [PubMed] [Google Scholar]
- 24.Bennett AF. In: Animals and Environments: Proceedings of the Third International Conference of Comparative Physiology and Biochemistry, Interna-tional Congress Series, Vol 1275. Morris S, Vosloo A, editors. Amsterdam: Elsevier; 2004. pp. 234–241. [Google Scholar]
- 25.Andrews RM. Lizards in the slow lane: Thermal biology of chameleons. J Therm Biol. 2008;33:57–61. [Google Scholar]
- 26.Huey RB. Behavioral thermoregulation in lizards: Importance of associated costs. Science. 1974;184:1001–1003. doi: 10.1126/science.184.4140.1001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.