Abstract
The widely used nonsteroidal anti-inflammatory drugs block the cyclooxygenase enzymes (COXs) and are clinically used for the treatment of inflammation, pain, and cancers. A selective inhibition of the different isoforms, particularly COX-2, is desirable, and consequently a deeper understanding of the molecular basis of selective inhibition is of great demand. Using an advanced computational technique we have simulated the full dissociation process of a highly potent and selective inhibitor, SC-558, in both COX-1 and COX-2. We have found a previously unreported alternative binding mode in COX-2 explaining the time-dependent inhibition exhibited by this class of inhibitors and consequently their long residence time inside this isoform. Our metadynamics-based approach allows us to illuminate the highly dynamical character of the ligand/protein recognition process, thus explaining a wealth of experimental data and paving the way to an innovative strategy for designing new COX inhibitors with tuned selectivity.
Keywords: nonsteroidal anti-inflammatory drugs, COX selectivity, coxibs, path collective variables, metadynamics
Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used as therapeutic agents for the treatment of pain and inflammation, and in addition several evidences have been very recently reported on their chemopreventive effect in colorectal cancer (1). Their mechanism of action is based on the blockage of the cyclooxygenase enzymes (COXs) (2) by sterically hindering the entrance of the physiological binder arachidonic acid. The classical NSAIDs such as aspirin, ibuprofen, or flurbiprofen are nonselective and inhibit indifferently all the COXs isoforms. In the last few decades, the interest of scientists has been mostly focused on a selective inhibition between COX-1/COX-2. In fact, the inhibition of COX-1, particularly in the gastrointestinal system, may lead to dangerous side effects such as ulcers. As a consequence, a selective inhibition of COX-2 has been sought for decades, and recently a new generation of NSAIDs, namely coxibs, was found. However, some of them, such as rofecoxib (Vioxx®), have been withdrawn from the market due to their cardiotoxicity (3–5). This was not the case of other COX-2 selective drugs such as celecoxib and nimesulide, with the latter one largely used as an anti-inflammatory agent in many diseases. As a consequence, today, in the rational design of new COXs binders, medicinal chemists have to pay attention to the selectivity properties of the designed drugs, and a fine-tuning of the COX selectivity profile might be necessary to generate novel effective drugs with reduced side effects. In the rational modulation of the binders selectivity precious help comes from the x-ray crystallography. In fact, the crystallographic structures show that selective and nonselective inhibitors generally bind in two different patterns. In COX-1, the space of the selectivity pocket is reduced due to the presence of Ile523, while in COX-2 the presence of Val523 augments the available space providing a more stable binding possibility for selective inhibitors (6). The kinetics of selective and nonselective inhibitors are also different in general, and in recent years, much effort has been paid in elucidating the dynamic binding mechanism of COX inhibitors (7–9). Lanzo et al. (8) have found, by means of the fluorescence quenching technique, that while the association of the COX-2 selective inhibitor SC-299 with COX-1 and COX-2 occurs at similar rates, the dissociation of SC-299 from COX-2 takes hours, which is thousandsfold slower than in COX-1 (≈30 s). This finding clearly shows that there is a correlation between the relative rates of dissociation and the selectivity of the isoenzyme inhibition, and this correlation is confirmed also in additional experiments on other COX-2 selective inhibitors (9). These phenomena have been so far ascribed to a more stable binding mode of selective inhibitors into the COX-2 isoform due to the presence of diverse residues such as Val434 in COX-2 instead of Isoleucine in COX-1. However, this represents a very simplistic way to address the different rates of dissociation of an inhibitor from an enzyme, and very likely other molecular mechanisms might be involved (8–11).
On the other hand, it has to be pointed out that processes that take from microseconds to hundreds of seconds, such as binding and unbinding of ligands into protein, are impossible to simulate with standard computational techniques such as molecular dynamics (MD), whose typical timescale is hundreds of nanoseconds. Thus, computer simulation of the binding and especially of the unbinding, which for COX-2 selective inhibitors is responsible of the differences in the rates of dissociation, is an ongoing challenge we decided to deal with.
Thus, to reveal at an atomic level what might happen during the complex formation and dissociation and with the aim to overcome the large free-energy barriers toward the inhibitor-enzyme undocking process in an affordable computational time, we used the recently developed well-tempered metadynamics (12), which is an evolution of standard metadynamics (13). For this purpose, one of the most potent and selective COX-2 inhibitors, SC-558 (IC50 = 9.3 nM) (6), was selected.
We have found a previously unreported alternative binding mode of SC-558 into COX-2, which is very similar to that experimentally found for some nonselective COX inhibitors (14, 15). This has important consequences on the classical way of performing rational drug design, which is mainly based on understanding only the main binding mode. Here we show that in so doing one might miss crucial bits of information and one cannot compare the results of modeling with the experiments. In fact, our model of ligand binding allows one to rationalize many previously unexplained experimental results, for instance, the key role played by some residues in the binding path of the ligand as well as the kinetic models for the binding mechanism of closely related analogues of SC-558 (8, 9, 16). Additionally, our study highlights the importance of the three alpha-helices’ flexibility at the entrance of the enzyme, in line with what has been discussed in literature (15). Finally, we contrast the behavior of COX-2 with that of COX-1, in which only one binding mode for SC-558 has been found.
Results
Biased MD Simulations.
The well-tempered metadynamics is an evolution of standard metadynamics (13), able of enhancing the sampling and reconstructing the free-energy profile of the process of interest by adding an adaptive bias on a selected number of collective variables (CVs) (12). The user defined CVs must be able to discriminate the initial and final states of the system and take into account all the slow modes of the process (17–19). One of the very first problems encountered in our undocking study, which has been also one of the major topics in COX inhibition, was the identification of the part of the protein that allows COX binders to exit from the inner cyclooxygenase site. On the basis of apo and ligated x-ray structures of COX-2, it was stated that the enzyme can assume a relaxed and a tightened state (SI Text) where Arg120 is free to move or makes a salt bridge with the Glu524, respectively (16). In the latter case the substrate is locked into a catalytically competent conformation. Moreover, the evidence that, when complexed with certain inhibitors, the COX-2 x-ray structures show some changes in the Cα position at the base of one of the helices A–D (20) led experimentalists to make the hypothesis that the group of helices A–D forms the door through which the arachidonic acid and other ligands pass (14).
This is a slow mode that needs to be sampled in a biased MD by the introduction of an appropriate CV. In order to address this effect we use a path collective variable (21) constructed with the contact map (22) between the residues that determine the gate flexibility (see Methods and SI Text). In addition we use a distance and a dihedral angle CV to identify the position and orientation of the ligand relative to the enzyme (SI Text). This last set of CVs has been already successfully used in several metadynamics simulations of undocking processes of small ligands from their binding sites (23, 24). The final choice of CV is the result of a lengthy investigation reported in SI Text.
SC-558 Dissociation Process in COX-2.
Under the action of metadynamics, the ligand leaves the starting position, which corresponds to the x-ray structure of the SC-558/COX-2 complex (PDB ID code 1cx2) (6), and explores the whole binding site and finally takes its way out from the enzyme through the helices gate. We describe in detail the relevant minima found along the exiting path in the following paragraphs.
The crystallographic pose.
The first energy minimum, basin A in the free-energy surface (FES) depicted in Fig. 1, is the deepest and corresponds to the x-ray conformation. This means that basin A represents the most energetically stable pose where the ligand has the highest probability to be found once docked in agreement with the crystallographic structure. The bromophenyl ring is placed in a hydrophobic cavity surrounded by Phe381, Leu384, Tyr385, Trp387, Phe518, and Ser530. Also the Gly526 and Ala527 backbone takes part in these hydrophobic contacts. The trifluoromethyl moiety resides in a close pocket surrounded by Met113, Val116, Tyr355, Leu359, and Leu531 (Fig. 1). This is referred to as the common pocket in Fig. 2 since it is the same that hosts the aromatic ring bearing the carboxylate function of many COX nonselective compounds such as ibuprofen. Finally, the phenylsulphonamide moiety inside the selectivity pocket assumes a conformation in which one of the oxygen atoms H-bonds with Arg513 and is close enough to interact with His90 while the other oxygen forms a H-bond with a water molecule. The amide hydrogens of the sulphonamide group interact with the backbone of Phe518 via two water bridges. Unfortunately, x-rays have not been able to resolve the conformation of the sulphonamide group in the selectivity pocket, and conformations dissimilar to the x-rays’ one have been already reported in previous theoretical studies (25, 26).
Fig. 1.
The FES of the dissociation process as a function of the distance and dihedral CVs is shown at the bottom using isosurfaces of 2 kcal/mol. The four main energy basins A–D found during the metadynamics simulations are highlighted in the FES graph. The four snapshots of the complex SC-558/COX-2 displayed in the surrounding boxes represent the following binding poses: basin A, crystallographic pose; basin B, alternative pose; basin C, poses at gate site; basin D, external pose. The ligand and the main interacting residues are displayed as licorice, while the protein is represented as a green cartoon with the α-helices forming the gate colored in orange. The interacting waters are shown as spheres, while hydrogens are not displayed for clarity. On the basis of the information derived from the FES, many aspects of the complex inhibition kinetics of COX-2 selective inhibitors can be elucidated. In fact, the presence of the second deep minimum (B) can explain the time-dependent behavior of many COX-2 selective inhibitors and, consequently, their long residence time inside the protein. In this respect we have been encouraged by the interpretation of flourescence quenching experiments of Lanzo et al., who have suggested for a SC-558 analogue an in and out movement from the selectivity pocket (8). They proposed that while in COX-1 the binding is a two-step process (see Fig. 4), in COX-2 a further not yet identified step takes place. Our calculations reveal the nature of this additional step, which considerably slows the off rate.
Fig. 2.
Schematic representation of the main binding sites found during our metadynamics simulations. The common and the selectivity pocket represent the SC-558 binding site in the crystallographic pose (basin A in Fig. 1), while the same common pocket with the side pocket represents the site for the alternative pose (basin B in Fig. 1). The gate site (basin C in Fig. 1) is when the ligand is in proximity of the protein gate assuming several similar conformations. Finally, the lobby-like site represents the binding site of SC-558 in its external pose (basin D in Fig. 1).
The alternative pose.
Under the action of metadynamics, the ligand leaves the crystallographic pose and while exploring the catalytic site it finds another minimum (SI Text). The presence of this second minimum (basin B in Fig. 1) was a surprise and reveals the presence of a pose of great interest for several reasons. Such a pose was also found in metadynamics where different CV settings have been used (SI Text). First of all, the depth of this second basin suggests a good thermodynamic stability of the ligand at the site. We confirmed the stability of this alternative pose by carrying out a 5-ns standard MD run. During this simulation, the complex was very stable with an average rmsd of the ligand heavy atoms of only 1.06 Å. We also measured an rmsd of 2.24 Å for the heavy atoms of the residues that interact with the ligand, with respect to their x-ray coordinates (SI Text). This large value reflects the fact that the ligand induces local conformational changes in the protein. It is important to note that standard docking programs such as AutoDock (27, 28), which assume the protein to be rigid, fail to predict this second binding pose, as we have explicitly tested. In order to check if basin B is an artifact of the potential applied we used a different force field (parmff99SB) to validate this second minimum through a standard MD run of over 10 ns. The results are very close to those obtained using the parm99 force field with all the principal ligand/protein interactions conserved and with comparable average rmsd values calculated for the ligand heavy atoms [1.07 (parmff99SB) vs. 1.06 (parm99)].
In view of these data, it is natural to suggest that this unique pose can provide an alternative way for SC-558 to bind to COX-2. Several interactions conspire to make this pose stable. The bromophenyl moiety is in the highly hydrophobic cage defined by Ile345, Val349, Leu359, Leu531, and Met535, while the trifluoromethylpyrazole occupies approximately the same cavity as the crystallized pose but is rotated by 180°. In such a way, it improves its interactions with neighboring Leu352, Phe518, Val523, Gly526, and Ala527. Finally, the sulphonamide group engages a bifurcated H-bond with Tyr355 and Arg120 side chains, and additional interaction energy can be gained from the relative closeness of the Val116 carbonyl group (Fig. 1).
The involvement of residues such as Arg120 and Tyr355 in the ligand binding assumes an important value in the light of experiments (15, 16). In line with our results that demonstrate the role of the polar interactions between SC-558 and the Tyr355 hydroxyl group, the mutation Tyr355Phe disfavors the binding of many ligands to COX (16). Moreover, Kurumbail et al. (6) have observed in many x-ray data that Arg120 is displaced from its usual position in the ligated enzyme. This is due to a weakening of the Arg120-Glu524 bond relative to the free form of the enzyme as also reflected by the large B factor. This has led these authors to suggest a temporary interaction of this residue with the ligand during its mechanism of biding to the enzyme.
An even stronger support to our suggestion that pose B is highly relevant comes from the comparison to the crystal structures of COX complexed with several nonselective inhibitors. For instance, comparing our unique pose of SC-558 into COX-2 to the binding mode of ibuprofen to COX-1 (PDB ID code 1eqg) (15), it clearly emerges that the main interactions with the protein are well conserved. In fact, the carboxylate group of ibuprofen takes part in a network of polar interactions involving Tyr355 and Arg120 similarly to what happens to the sulphonamide moiety (Fig. 3). In either case, the common pocket is occupied by groups that are similar in size, the phenyl in the case of ibuprofen and the pyrazole in SC-558. The similarity is even greater if inhibitors such as flurbiprofen or alclofenac are considered, where a halogen atom is substituted in the phenyl ring, thus enforcing the hydrophobic interactions with Leu352, Phe518, and Val523. It can be seen that a similar role is played by the trifluoromethyl group of SC-558 in pose B.
Fig. 3.
Comparison between (A) the alternative binding pose of SC-558 in COX-2 found during metadynamics simulations and (B) the x-ray binding conformation of ibuprofen in complex with COX-1 (PDB ID code 1eqg). The ligands and the interacting residues are represented as licorice, while the protein is represented as green cartoon with the α-helices forming the gate colored in orange. The hydrogens are not displayed for clarity.
The existence of two possible binding modes is fully compatible with the presence of an additional binding pose for many diarylheterocycles, which has been invoked by experimentalists to explain the time-dependent inhibition exhibited by this class of inhibitors (7–9). In fact, the slow tight-binding inhibition of compounds chemically similar to SC-558 is interpreted by these authors as due to the presence of an additional binding step, and in this sense a crucial role might be played by the rearrangement of the hydrogen bonding network formed by residues such as Arg120, Tyr355, and Glu524, which are critical for the transition from the relaxed to the tightened state of the enzyme (SI Text). The involvement of these key residues in the newly binding mode of SC-558 suggests that the time-dependent inhibition kinetic of SC-558 results from the ability of the ligand to bind in two distinct but equally strong ways. One can thus understand why COX-1 is resistant to the time-dependent inhibition of diarylheterocycles (6). In fact, the mutation in this region of Val523 in COX-2 to Ile523 in COX-1 reduces the space availability in COX-1 and in this isoform the transfer of SC-558 from the original crystallographic pose to the new one becomes more difficult.
Poses at the gate site.
Once all the minima inside the active site are filled, the ligand points toward the B–D helices facing the internal part of the helices that form the exit door of the enzyme (SI Text). Here, it can assume a variety of closely related conformations stabilized by several hydrophobic interactions in the site upper part with residues such as Ile91, Val98, Trp99, Val102, Ile112, Tyr115, Val116, Tyr355, and Phe357 (basin C in Fig. 1). Most conformations belonging to this site are characterized by the presence of a salt bridge between Arg120 and Glu524. Moreover, while the sulphonamide group of SC-558 engages stabilizing interactions with the surrounding water molecules, the phenyl ring of the sulphonamidephenyl moiety forms a π-π interaction with Tyr355. This corresponds to a local minimum whose stability was checked in 5 ns of nonbiased MD. During this time all these interactions were conserved as well as the salt bridge between Arg120 and Glu524, which keeps the protein in the tightened conformation.
The external pose.
Once the ligand has crossed the helices forming the gate of the enzyme, it reaches the outer part of the protein, where a further local minimum is found (SI Text). It is worth noting that this crossing event happens only when the following three phenomena occur concurrently: (i) the B–D helices breath, (ii) the gate formed by Tyr115 opens, and (iii) SC-558 forms a double H-bond with Tyr355 and Arg120. This can be clearly seen in our simulations approximately at 50 ns when the contact map CV has its maximum value for a relative large time interval (SI Text). At the external site, although the ligand is mostly solvated by water molecules, it continues interacting with a small number of surfacing hydrophobic residues such as Val98, Ile101, Val102, Ile105, Phe107, and Leu108 and with the His94 side chain through a H-bond (basin D in Fig. 1). Once again we checked the stability of this conformation via a 5-ns standard MD run (SI Text). This preliminary binding site just outside the enzyme (lobby-like site in Fig. 2), in the upper part of the door formed by the B–D helices, plays a pivotal role in the inhibitor/enzyme recognition process. Our hypothesis is complementary to the flourescence quenching experiments of Lanzo et al., who suggested for a SC-558 analogue an in and out movement from the selectivity pocket. They proposed that while in COX-1 the binding is a two-step process, in COX-2 a further not yet identified step takes place (8). Our calculations reveal the nature of this additional step, which is the reason of the slower dissociation rate of SC-558 from COX-2.
The part of the FES with the ligand inside the cavity is quantitatively well characterized and the free-energy differences are converged, while the determination of the relative energy of pose D with respect to the others is less accurate. In fact, while we have observed many recross events among A, B, and C, once out the ligand was not able to return inside. Thus, the free-energy difference between the bound (basin A) and the unbound state (basin D) of ≈-8.7 kcal/mol has a semiquantitative value.
SC-558 Dissociation Process in COX- 1.
Similar CVs have been used to study the undocking of SC-558 from COX-1 (SI Text). Interestingly, the FES exhibits only one energy minimum, and consequently a unique binding mode is found in the inner part of the cyclooxygenase site (Fig. 4). This corresponds to the most stable pose predicted by AutoDock (27, 28) and also to the starting point of our simulations. Interestingly, this conformation is very similar to the crystallographic pose of SC-558 in COX-2, although here the ligand is more weakly bound due to an only partial insertion of the sulphonamide moiety in the selectivity pocket, at variance with what happens to basin A of COX-2. This can be explained by the presence in COX-1 of the bulkier Ile523 in place of Val523 in COX-2 (29). The similarity of the inner poses found in COX-1 and COX-2 is supported by the experimental fluorescence quenching data indicating that SC-299, a close analogue of SC-558, occupies a very similar deep position in COX-1 and COX-2 (8). Moreover, the presence of the bulkier Ile523 in COX-1 might also be responsible for the absence in this isoform of the alternative binding pose found in COX-2, since the Ile side chain sensitively reduces the space available in that region to host a group such as the trifluoromethyl of SC-558 (Fig. 3).
Fig. 4.
The FES of the undocking process of SC-558 in COX-1 as a function of the distance and dihedral CVs using isosurfaces of 2 kcal/mol. Only an internal and an external energy minimum have been found. Once this inner minimum has been filled, SC-558 moves in the direction of the helices that form the exit door. Close to the minimum a shallow wide basin is to be found whose conformation differs very slightly from that of the lowest minimum. When the ligand is in between the two helices and the gate is in the open conformation, it leaves the binding site moving to the external pose.
Comparing COX-1 and COX-2 one sees that not only the alternative binding site is uniquely present in the latter but that the accessible free-energy surface inside the binding pocket is much larger. This explains the COX-2 selectivity of this compound and the higher residence time in COX-2 found for compounds similar to ours (8). In a study on a series of COX-2 selective inhibitors (9), the authors have advocated the possible existence of an additional binding step to explain their time-dependent inhibition kinetics. Our study validates their hypothesis and offers a microscopic description of this site.
Discussion
The clinical use of COX inhibitors necessitates that their binding mode at COX and, above all, their kinetics of inhibition are established, not only to fully understand their mechanism of inhibition, but also to provide precious insight for the rational improvements of specificity and inhibitory potency.
The full unbinding process of the potent and highly selective COX-2 inhibitor, SC-558, simulated by using metadynamics, revealed the presence of a binding mode alternative to that experimentally found in COX-2. The interest for the newly identified pose increases when a comparison with the x-ray binding conformation of some COX nonselective inhibitors is made. In fact, comparing this pose with the biding mode of ibuprofen or flurbiprofen, it emerges clearly that the overall interactions with the surrounding residues are conserved and, moreover, SC-558 engages additional hydrophobic contacts in a side pocket that further stabilize the newly found minimum. Many experimental data have suggested that some of the residues involved in the alternative binding pose of SC-558 play a key role in regulating the inhibition kinetics of many COX inhibitors (15, 16). As stated in the Introduction, we think that the inhibition kinetics and the selective behavior of COX inhibitors cannot be explained only in terms of the presence of different crucial residues such as Val434Ile, Arg513His, and Val523Ile, in the different isoforms. On the contrary, our results clearly show the ability of SC-558 to bind COX-2 in two different ways, supporting the theory of the presence of an additional binding pose for many diarylheterocycles, and consequently provide an explanation for the increased time of permanence and the slow binding rate in COX-2 for ligands structurally similar to SC-558.
Our results will be very helpful in the drug design using either automated techniques such as virtual screening, targeting both the x-ray and the alternative binding site, or using the classical rational drug design. In fact, modifications of the chemical structure of SC-558 can either improve or reduce the contacts with the residues present in either of the two possible binding sites. This would tune the time of permanence in the protein and consequently the kinetic profile and selectivity of the newly designed compounds. For instance, the addition of a polar substituent such as propionic acid at position 3 of the pyrazole ring in SC-558 would improve the selectivity and the affinity for the alternative binding site in COX-2, engaging H-bond interactions with the close Arg513 side chain, present univocally in this isoform. Basing the development of the newly designed compounds on the dynamical features of the ligand binding mechanism represents an innovative way of performing drug design that we are successfully experiencing designing new inhibitors that have shown promising COX-2 selective behavior in the preliminary results. This approach assumes an even greater value if specific properties of these compounds, such as their high selectivity, are responsible for their cardiotoxicity as some researchers have suggested (30). Certainly, much remains to be done to determine the binding mechanism of selective inhibitors in COXs, but if this relation between cardiotoxicity and selectivity is confirmed, by providing the molecular basis for the rational quest of novel COX-2 targeted therapeutic agents with lower toxicity, our study represents an innovative way of doing rational drug design since information captured by static experiments such as x-rays or mutagenesis data can now be assisted by information derived from the transient ligand/protein interactions engaged along the path that leads to the main binding site.
Methods
Metadynamics Simulations.
Before doing metadynamics simulations the binary complexes between COXs and SC-558 were equilibrated with a 5-ns MD under NPT conditions at 1 atm and 320 K using the parm99 version (31) of the all-atom Amber force field (32), as implemented in version 2.6 of the NAMD molecular dynamics simulation code, which was used throughout (33). All the simulations were carried out in explicit solvent and in cubic periodic boundary conditions.
The estimation F(s,t) at time t of the free-energy surfaces F(s) as a function of the CV s was determined by metadynamics (13) in its recently developed well-tempered variant (12), using the following formula:
 |
where V(s,t) is the bias potential added to the system and T is the temperature of the simulation. ΔT is the difference between the temperature of the CV and the temperature of the simulation. The bias potential is made up by the sum of the Gaussians deposited along the trajectories of the CVs.
Thanks to this new formalism, one can increase barrier crossing and facilitate the exploration in the CVs space by tuning ΔT. A Gaussian deposition rate of 1.1 kcal/mol per picosecond was initially used and gradually decreased on the basis of the adaptive bias with a ΔT of 2880 K.
The Path Collective Variables.
In order to determine the appropriate collective variables to describe the opening and closing of the three helices, we performed a preliminary set of calculations using the path CVs of Branduardi et al. (21), represented in the space of the contact map variable (CMAP) introduced by Bonomi et al. (22). The path method is extremely powerful whenever one wants to study a transition between states A and B. As state A we chose the x-ray conformation of the enzymes in which all the contacts contribute to have the enzymes in the closed state. State B represents the open form of the enzymes, and, due to the lack of experimental information, it was derived by first preliminary metadynamics trials. In these runs, carried out without path CVs, the ligand, pushed by the added bias, induced the opening of the helices and of the gates. Certainly, this is not the optimal way to choose the open conformation of the enzymes, but the path method itself, when used together with metadynamics, is able to find transition paths rather different from the initial one.
Let S(R) be a reduced representation of a generic configuration R. If the choice of S is appropriate, we would expect the reactive trajectories to be bundled in a narrow tube around the path. To trace this path, we follow the procedure of Branduardi et al. (21) introducing the two variables s(R) and z(R):
 |
 |
where P is the number of frames and s(R) and z(R) measure the intercept and distance of any microscopic configuration R from the path S(l). We described the transition between the closed and open conformations of the enzyme using five frames S(1)…S(5), with S(1) and S(5) being the closed (state A) and the open conformation (state B), respectively. Care must be taken that all the frames are equally spaced relative to the metric used. The λ value was set to 8.511 and 6.902 for COX-2 and COX-1, respectively.
Following ref. 22, we have used CMAP as reduced representation S(R), and we have measured the square distance ‖…‖2 of a generic state from a point belonging to the reference path using
 |
where C(R)i,j and C(l)i,j are the elements of the CMAP. A contact between atom i and j is defined as
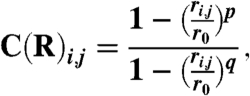 |
where ri,j is the distance between the two atoms and r0 is the typical distance at which the contact is formed (SI Text).
We performed metadynamics only in the space of s(R) while z(R) was constrained to z(R) < 0.15. This gives the possibility for the system to explore conformations different from the original path, while maintaining at the same time the system reasonably close to the chosen intermediate frames. In such a way, the loss of the alpha-helices folding is avoided.
In addition to s(R), we have also used a distance and a torsion CV to describe the different conformations of the ligand during the simulations. The former is defined as the distance between the center of mass of the ligand and of a group of atoms of the proteins. The latter is the dihedral angle defined by four atoms, two on the major inertia axes of the ligand and the other two chosen on a conserved secondary structure of the proteins (SI Text).
The VMD program (34) was used for visualization and data analysis while the figures were made using the PyMOL software (35).
Supplementary Material
Acknowledgments.
The authors thank Matteo Masetti, Davide Branduardi, and Giovanni Bussi for useful discussions. This work was supported by a grant from the Swiss National Supercomputing Centre—CSCS under project ID s233.
Note. During the review process, a 2.75 Å resolved x-ray structure of COX-1 complexed with celecoxib, an analogue of SC-558, was recently reported (36). This structure agrees remarkably well with our prediction of binding for SC-558 in COX-1. In fact, the binding conformation of SC-558 in COX-1 calculated through metadynamics is very similar to the crystallographic pose found for Celebrex in COX-1 with a low rmsd of 1.46 Å for the ligand heavy atoms (SI Text). This provides a further convincing evidence of the reliability of our model, which predicts a binding conformation of SC-558 in COX-1 similar to the crystallographic pose found in COX-2, although in COX-1 the ligand is more weakly bound due to an only partial insertion of the sulphonamide moiety in the selectivity pocket. This prediction has been now validated by the crystallographic structure (PDB ID code 3kk6).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0913377107/DCSupplemental.
References
- 1.Cuzick J, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: An international consensus statement. Lancet Oncol. 2009;10:501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 2.Smith W-L, Borgeat P, Fitzpatrick F-A. In: Biochemistry of Lipids, Lipoproteins and Membranes. Vance D-E, Vance J, editors. Amsterdam; London: Elsevier Science; 1991. pp. 297–3253. [Google Scholar]
- 3.Scheen A-J. [Withdrawal of rofecoxib (Vioxx): What about cardiovascular safety of COX-2 selective non-steroidal anti-inflammatory drugs?] Revue médicale de Liège. 2004;59:565–569. [PubMed] [Google Scholar]
- 4.Kearney P-M, et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006;332:1302–1308. doi: 10.1136/bmj.332.7553.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L-C, Ashcroft D-M. Risk of myocardial infarction associated with selective COX-2 inhibitors: Meta-analysis of randomised controlled trials. Pharmacoepidemiol Drug Saf. 2007;16:762–772. doi: 10.1002/pds.1409. [DOI] [PubMed] [Google Scholar]
- 6.Kurumbail R-G, et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature. 1996;384:644–648. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- 7.Copeland R-A, et al. Mechanism of selective inhibition of the inducible isoform of prostaglandin G/H synthase. Proc Natl Acad Sci USA. 1994;91:11202–11206. doi: 10.1073/pnas.91.23.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lanzo C-A, Sutin J, Rowlinson S, Talley J, Marnett L-J. Fluorescence quenching analysis of the association and dissociation of a diarylheterocycle to cyclooxygenase-1 and cyclooxygenase-2: Dynamic basis of cyclooxygenase-2 selectivity. Biochemistry-US. 2000;39:6228–6234. doi: 10.1021/bi992761o. [DOI] [PubMed] [Google Scholar]
- 9.Walker M-C, et al. A three-step kinetic mechanism for selective inhibition of cyclo-oxygenase-2 by diarylheterocyclic inhibitors. Biochem J. 2001;357:709–718. doi: 10.1042/0264-6021:3570709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan C, Rieke C-J, Rimon G, Wingerd B-A, Smith W-L. Partnering between monomers of cyclooxygenase-2 homodimers. Proc Natl Acad Sci USA. 2006;103:6142–6147. doi: 10.1073/pnas.0601805103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prusakiewicz J-J, Duggan K-C, Rouzer C-A, Marnett L-J. Differential sensitivity and mechanism of inhibition of COX-2 oxygenation of arachidonic acid and 2-arachidonoylglycerol by ibuprofen and mefenamic acid. Biochemistry-US. 2009;48:7353–7355. doi: 10.1021/bi900999z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barducci A, Bussi G, Parrinello M. Well-tempered metadynamics: A smoothly converging and tunable free-energy method. Phys Rev Lett. 2008;100:020603. doi: 10.1103/PhysRevLett.100.020603. [DOI] [PubMed] [Google Scholar]
- 13.Laio A, Parrinello M. Escaping free-energy minima. Proc Natl Acad Sci USA. 2002;99:12562–12566. doi: 10.1073/pnas.202427399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Picot D, Loll P-J, Garavito R-M. The x-ray crystal structure of the membrane protein prostaglandin H2 synthase-1. Nature. 1994;367:243–249. doi: 10.1038/367243a0. [DOI] [PubMed] [Google Scholar]
- 15.Selinsky B-S, Gupta K, Sharkey C-T, Loll P-J. Structural analysis of NSAID binding by prostaglandin H-2 synthase: Time-dependent and time-independent inhibitors elicit identical enzyme conformations. Biochemistry-US. 2001;40:5172–5180. doi: 10.1021/bi010045s. [DOI] [PubMed] [Google Scholar]
- 16.So O-Y, Scarafia L-E, Mak A-Y, Callan O-H, Swinney D-C. The dynamics of prostaglandin H synthases—Studies with prostaglandin H synthase 2 Y355F unmask mechanisms of time-dependent inhibition and allosteric activation. J Biol Chem. 1998;273:5801–5807. doi: 10.1074/jbc.273.10.5801. [DOI] [PubMed] [Google Scholar]
- 17.Gear C-W, Kevrekidis I-G, Theodoropoulos C. ‘Coarse’ integration/bifurcation analysis via microscopic simulators: Micro-Galerkin methods. Comput Chem Eng. 2002;26:941–963. [Google Scholar]
- 18.Hummer G, Kevrekidis I-G. Coarse molecular dynamics of a peptide fragment: Free energy, kinetics, and long-time dynamics computations. J Chem Phys. 2003;118:10762–10773. [Google Scholar]
- 19.Parrinello M. In: Physical Biology from Atoms to Medicine. Zewail AH, editor. London: Imperial College Press; 2008. pp. 247–265. [Google Scholar]
- 20.Luong C, et al. Flexibility of the NSAID binding site in the structure of human cyclooxygenase-2. Nat Struct Biol. 1996;3:927–933. doi: 10.1038/nsb1196-927. [DOI] [PubMed] [Google Scholar]
- 21.Branduardi D, Gervasio F-L, Parrinello M. From A to B in free energy space. J Chem Phys. 2007;126:054103. doi: 10.1063/1.2432340. [DOI] [PubMed] [Google Scholar]
- 22.Bonomi M, Branduardi D, Gervasio F-L, Parrinello M. The unfolded ensemble and folding mechanism of the C-terminal GB1 beta-hairpin. J Am Chem Soc. 2008;130:13938–13944. doi: 10.1021/ja803652f. [DOI] [PubMed] [Google Scholar]
- 23.Gervasio F-L, Laio A, Parrinello M. Flexible docking in solution using metadynamics. J Am Chem Soc. 2005;127:2600–2607. doi: 10.1021/ja0445950. [DOI] [PubMed] [Google Scholar]
- 24.Masetti M, Cavalli A, Recanatini M, Gervasio F-L. Exploring complex protein-ligand recognition mechanisms with coarse metadynamics. J Phys Chem B. 2009;113:4807–4816. doi: 10.1021/jp803936q. [DOI] [PubMed] [Google Scholar]
- 25.Soliva R, Almansa C, Kalko S-G, Luque F-J, Orozco M. Theoretical studies on the inhibition mechanism of cyclooxygenase-2. Is there a unique recognition site? J Med Chem. 2003;46:1372–1382. doi: 10.1021/jm0209376. [DOI] [PubMed] [Google Scholar]
- 26.Price M-L-P, Jorgensen W-L. Analysis of binding affinities for celecoxib analogues with COX-1 and COX-2 from combined docking and Monte Carlo simulations and insight into the COX-2/COX-1 selectivity. J Am Chem Soc. 2000;122:9455–9466. [Google Scholar]
- 27.Morris G-M, et al. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem. 1998;19:1639–1662. [Google Scholar]
- 28.Huey R, Morris G-M, Olson A-J, Goodsell D-S. A semiempirical free energy force field with charge-based desolvation. J Comput Chem. 2007;28:1145–1152. doi: 10.1002/jcc.20634. [DOI] [PubMed] [Google Scholar]
- 29.Gierse J-K, et al. A single amino acid difference between cyclooxygenase-1 (COX-1) and -2 (COX-2) reverses the selectivity of COX-2 specific inhibitors. J Biol Chem. 1996;271:15810–15814. doi: 10.1074/jbc.271.26.15810. [DOI] [PubMed] [Google Scholar]
- 30.Dogne J-M, Hanson J, Supuran C, Pratico D. Coxibs and cardiovascular side-effects: From light to shadow. Curr Pharm Des. 2006;12:971–975. doi: 10.2174/138161206776055949. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Cieplak P, Kollman P-A. How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? J Comput Chem. 2000;21:1049–1074. [Google Scholar]
- 32.Cornell W-D, et al. A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J Am Chem Soc. 1995;117:5179–5197. [Google Scholar]
- 33.Phillips J-C, et al. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Humphrey W, Dalke A, Schulten K. VMD: Visual molecular dynamics. J Mol Graphics. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 35.DeLano W-L. The PyMOL Molecular Graphics System. San Carlos, CA: DeLano Scientific; 2002. [Google Scholar]
- 36.Rimon G, et al. Coxibs interfere with the action of aspirin by binding tightly to one monomer of cyclooxygenase-1. Proc Natl Acad Sci USA. 2010;107:28–33. doi: 10.1073/pnas.0909765106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






