Abstract
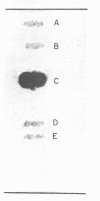
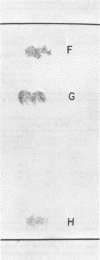
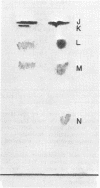
Cholesterol, free fatty acids, and phosphatidic acid are the predominant lipids of a T strain of Mycoplasma. The remaining neutral lipids are composed of cholesteryl esters, triglycerides, and diglycerides. Three glucose-containing glycolipids are present in trace amounts. In addition to phosphatidic acid, the phospholipids are comprised of phosphatidyl glycerol, diphosphatidyl glycerol, and phosphatidyl ethanolamine. Another polar lipid was found to be ninhydrin-positive and phosphate-free. It appears to be a diamino hydroxy compound containing adjacent fatty acid ester and N-acyl groups.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ford D. K. CULTURE OF HUMAN GENITAL "T-STRAIN" PLEUROPNEUMONIA-LIKE ORGANISMS. J Bacteriol. 1962 Nov;84(5):1028–1034. doi: 10.1128/jb.84.5.1028-1034.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford D. K., MacDonald J. Influence of urea on the growth of T-strain mycoplasmas. J Bacteriol. 1967 May;93(5):1509–1512. doi: 10.1128/jb.93.5.1509-1512.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins H. R. 2,6-Diamino-3-hydroxypimelic acid in microbial cell wall mucopeptide. Nature. 1965 Nov 27;208(5013):872–873. doi: 10.1038/208872a0. [DOI] [PubMed] [Google Scholar]
- Plackett P., Smith P. F., Mayberry W. R. Lipids of a sterol-nonrequiring Mycoplasma. J Bacteriol. 1970 Nov;104(2):798–807. doi: 10.1128/jb.104.2.798-807.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Pfendt E. A., Hayflick L. Sterol requirements of T-strain mycoplasmas. J Bacteriol. 1971 Jan;105(1):323–330. doi: 10.1128/jb.105.1.323-330.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPARD M. C., LUNCEFORD C. D. EFFECT OF PH ON HUMAN MYCOPLASMA STRAINS. J Bacteriol. 1965 Feb;89:265–270. doi: 10.1128/jb.89.2.265-270.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPARD M. C. The recovery of pleuropneumonia-like organisms from Negro men with and without nongonococcal urethritis. Am J Syph Gonorrhea Vener Dis. 1954 Mar;38(2):113–124. [PubMed] [Google Scholar]
- Shepard M. C., Lunceford C. D. Occurrence of urease in T strains of Mycoplasma. J Bacteriol. 1967 May;93(5):1513–1520. doi: 10.1128/jb.93.5.1513-1520.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. Replacement of Lysine by Hydroxylysine and Its Effects on Cell Lysis in Streptococcus faecalis. J Bacteriol. 1965 Sep;90(3):575–588. doi: 10.1128/jb.90.3.575-588.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. F., Koostra W. L. Phospholipids and glycolipids of sterol-requiring Mycoplasma. J Bacteriol. 1967 Jun;93(6):1853–1862. doi: 10.1128/jb.93.6.1853-1862.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. F. The lipids of mycoplasma. Adv Lipid Res. 1968;6:69–105. [PubMed] [Google Scholar]
- TSUNG C. M., SMITH W. G., LEACH F. R., HENDERSON L. M. Hydroxylysine metabolism in Streptococcus faecalis. J Biol Chem. 1962 Apr;237:1194–1197. [PubMed] [Google Scholar]
- WELLS M. A., DITTMER J. C. THE USE OF SEPHADEX FOR THE REMOVAL OF NONLIPID CONTAMINANTS FROM LIPID EXTRACTS. Biochemistry. 1963 Nov-Dec;2:1259–1263. doi: 10.1021/bi00906a015. [DOI] [PubMed] [Google Scholar]