Abstract
Vibrio cholerae is the causative agent of the diarrheal disease cholera. Many virulence factors contribute to intestinal colonization and disease including the Multifunctional Autoprocessing RTX toxin (MARTXVc). The Rho-inactivation domain (RID) of MARTXVc is responsible for inactivating the Rho-family of small GTPases, which leads to depolymerization of the actin cytoskeleton. Based on a deletion analysis of RID to determine the minimal functional domain, we have identified a subdomain at the N terminus of RID that is homologous to the membrane targeting C1 domain of Pasteurella multocida toxin. A GFP fusion to this subdomain from RID colocalized with a plasma membrane marker when transiently expressed within HeLa cells and can be found in the membrane fraction following subcellular fractionation. This C1-like subdomain is present in multiple families of bacterial toxins, including all of the clostridial glucosyltransferase toxins and various MARTX toxins. GFP-fusions to these homologous domains are also membrane associated, indicating that this is a conserved membrane localization domain (MLD). We have identified three residues (Y23, S68, R70) as necessary for proper localization of one but not all MLDs. In addition, we found that substitution of the RID MLD with the MLDs from two different effector domains from the Vibrio vulnificus MARTX toxin restored RID activity, indicating that there is functional overlap between these MLDs. This study describes the initial recognition of a family of conserved plasma membrane-targeting domains found in multiple large bacterial toxins.
Keywords: bacterial toxin, multifunctional autoprocessing RTX toxin, PMT, structural modeling, Vibrio cholerae
Bacterial pathogens use a wide array of secreted proteins to intoxicate mammalian cells and mediate disease. Many effectors target a specific organelle, necessitating strategies to traffic to certain subcellular locations after entry into the cytosol. For example, the cytolethal distending toxin active subunit, CdtB, uses a short N-terminal nuclear localization sequence attached to traffic to the nucleus (1). Similarly, many Type III secretion effectors contain distinct targeting signals: BteA uses a 130 aa N-terminal sequence to target lipid rafts (2); ExoS and YopE use internal 21 aa domains to associate with the host membrane (3); and ExoU uses a C-terminal sequence to localize to the membrane (4). For each of these effectors, subcellular localization is determined by a specific aa sequence separate from the catalytic site.
The multifunctional-autoprocessing RTX toxins (MARTX) are large bacterial toxins composed of multiple effector domains released by autoprocessing upon translocation into the host cell cytosol (5). The best characterized member of this family is the MARTX of Vibrio cholerae (MARTXVc), which is predicted to be 4545 aa in length (6, 7). MARTXVc carries three effector domains delivered by autoprocessing of which two function to disrupt the actin cytoskeleton. G-actin is covalently cross-linked into oligomers by the MARTXVc actin cross-linking domain (ACD) (8), whereas the Rho-inactivation domain (RID) reversibly inactivates host cell RhoGTPases (9). Transfection of cells with a plasmid expressing RID as a fusion with GFP induced cell rounding. In addition, when fused to the N-terminal portion of Anthrax toxin lethal factor (LFN) and delivered to cells by Anthrax toxin protective antigen (PA) (10), this domain was sufficient to induce cell rounding and Rho-inactivation (9).
Proteins with significant structural similarities to MARTXVc are present in other pathogenic bacteria such as Vibrio vulnificus, Aeromonas hydrophila, Yersinia pestis, Clostridium difficile, Xenorhabdus nematophila, and Photorhabdus luminescens. However, primary sequence comparisons indicate each toxin possesses a distinct collection of effector domains (reviewed in ref. 5). The V. vulnificus MARTX toxin (MARTXVv) contains four putative effector domains of unknown function (DUF) and a putative RID but no ACD (5). Three of the DUFs are homologous to other bacterial toxin domains; DUF3 with the α/β-hydrolase family of enzymes, DUF4 with the P. luminescens makes caterpillars floppy toxins (11), and DUF5 with the C2 domain of Pasteurella multocida toxin (PMT) (5).
In this work, we identify a conserved peptide sequence in 16 different bacterial toxins produced by both Gram-positive and Gram-negative bacteria. Functional analysis of four of these domains reveals that these toxins use this sequence in a shared strategy for plasma membrane targeting, despite the varied mechanisms of host cell intoxication employed by each toxin.
Results
Deletion Analysis of MARTXVc RID.
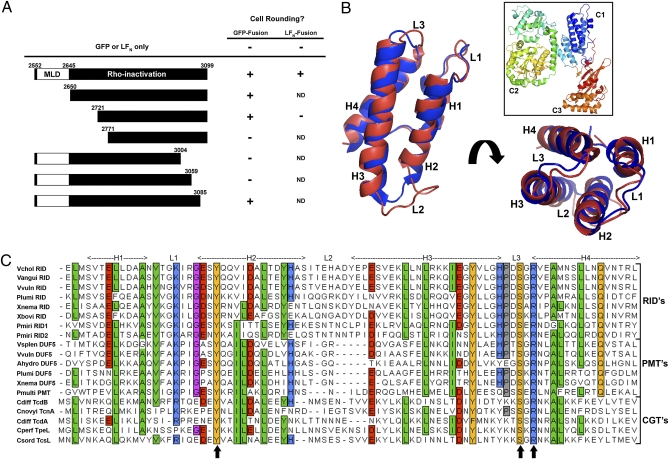
The MARTXVc RID (RIDVc) corresponds to aa 2552–3099 of the MARTXVc holotoxin (9). To establish the minimum functional size of RIDVc, a genetic deletion analysis on the domain was performed. Based on a CLUSTAL W alignment of the putative RID sequences of MARTX toxins from V. cholerae, V. vulnificus, Vibrio anguillarum, X. nematophila, and Xenorhabdus bovienii (Fig. S1), we generated three N-terminal and three C-terminal truncations in the eukaryotic expression plasmid pRID-GFP (Fig. 1A). The truncations were chosen to remove regions of significant (>60%) sequence divergence among the homologs, because these regions are likely not required for RID function. Transfection of pRID2650–3099-GFP, pRID2721–3099-GFP, or pRID2552–3085-GFP into HeLa cells caused cell rounding indistinguishable from pRID2552–3099-GFP but further deletions from either terminus of RIDVc abolished cell rounding (Fig. 1A and Fig. S2). Together, these data indicated that residues within aa 2721–3085 are necessary and sufficient for RIDVc activity when expressed in mammalian cells as a GFP-fusion protein.
Fig. 1.
Deletion analysis of RIDVc identifies a broadly conserved domain. (A) Portions of the N- and C-termini of RIDVc were deleted from pRID-GFP or LFN-RID. Each truncated RIDVc was tested for its ability to cause cell rounding after transfection (GFP-fusions) or delivery to the cell with PA (LFN-fusions). (B) Cartoon of the entire PMT toxin (boxed, PDB ID: 2EBF) with the PMT C1 domain (blue) overlaid with a model of MLDVcRID (red). (C) Alignment of 19 putative MLDs from various bacterial toxins: see Table S1 for accession numbers and protein details. Shading shows aa conservation and residue description: green = hydrophobic; orange = polar; blue = basic; red = acidic. Arrows indicate aa mutated to either Ala or a similar residue on MLDVcRID or MLDVvDUF5.
In an attempt to confirm that cell rounding was due to RhoA inactivation by the shorter RID fragment, an LFN-fusion to residues 2721–3099 of RIDVc was constructed and the amount of active GTP-bound RhoA within HeLa cells was monitored following PA-mediated delivery of purified LFN-RID2721–3099. Unlike the nearly 100% cell rounding caused by PA+LFN-RID2552–3099, incubation with PA+LFN-RID2721–3099 did not round HeLa cells even when added at a 1200× excess over the amount sufficient for LFN-RID2552–3099 toxicity (Fig. 1A and Fig. S3A). These results suggested that either the LFN fusion inhibits the functionality of RID2721–3099 or that the N terminus of RIDVc contains an essential element that is required for functional activity when RIDVc is delivered to the cell as a protein but is dispensable when the protein is overexpressed within the cell.
Residues 2561–2645 of MARTXVc Target the Plasma Membrane.
To further characterize the N-terminal sequence of RIDVc, the peptide sequence corresponding to aa 2552–2720 was analyzed using PSI-BLAST (12). This analysis showed that MARTXVc aa 2561–2645 shares homology with the N terminus of all known RIDs from other MARTX toxins as expected (Fig. S1), and also the N terminus of DUF5 from MARTXVv (aa 3591–3669, hereafter referred to as VvDUF5). The DUF5 effector domain is also present within MARTX toxins from Vibrio splendidus, A. hydrophila, P. luminescens, and X. nematophila (5, 13). The N terminus of DUF5 has been previously noted as sharing homology with the C1 domain of PMT (5). In turn, PMT C1 is known to share structural homology with the N terminus of C. difficile Toxin B (TcdB) and other closely related clostridial glucosyltransferase toxins (CGT) (14). A pair-wise alignment of the 19 identified sequences showed a high degree of similarity throughout the region (44–89%), indicating that these may be shared functional domains important for activity (Fig. 1C). All 19 of these domains were found just to the N-terminal side of three different classes of effector domains (RID, PMT, and CGT) within various large toxins.
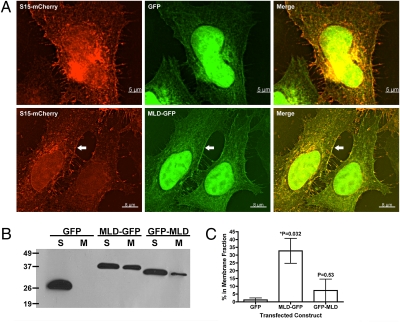
PMT C1 was previously shown to localize GFP to the plasma membrane of 293T cells when ectopically expressed (14). To test if the C1-related domain from RIDVc also facilitates membrane localization, aa 2561–2645 were transiently expressed in HeLa cells as a fusion to either the N or C terminus of GFP. A plasmid expressing the myristylation site and the polybasic membrane targeting sequence present within the first 15 aa of c-Src fused to mCherry (S15-mCherry) was used as a marker for the plasma membrane (15–18). This portion of c-Src mediates a direct association with host cell membrane components and has been shown in macrophages to colocalize with a biomarker for phosphatidylserine (PS) (15, 19, 20). When the plasmids expressing the N-terminal GFP-fusion (2561-2645-GFP) and S15-mCherry were cotransfected into HeLa cells, an overlap of both fluorescence signals at the cell periphery was observed, particularly compared to GFP alone where there was very little overlap with mCherry (Fig. 2A). Both GFP and 2561–2645-GFP showed fluorescence within the nucleus due to passive transport across the nuclear membrane; however, GFP alone was rarely observed at the membrane colocalized with S15-mCherry (Fig. 2A and Table 1). Transfection of a plasmid expressing MARTXVc aa 2561–2645 attached to the C terminus of GFP (GFP-2561-2645) did not display any distinct localization pattern within HeLa cells or overlap with S15-mCherry and was indistinguishable from GFP alone by microscopy (Table 1).
Fig. 2.
RIDVc aa2561-2645 is sufficient to drive GFP to the HeLa cell plasma membrane. (A) HeLa cells transfected with a plasma membrane marker (S15-mCherry) and either GFP or MARTXVc aa2561-2645-GFP (MLD-GFP) were imaged by deconvolution microscopy. Arrow shows overlap at the plasma membrane of mCherry and GFP signals. (B) Representative anti-GFP immunoblot of three separate experiments following membrane fractionation of HeLa cells transfected with the indicated constructs; S, soluble; M, membrane. (C) The average percentage of signal (+/− the standard deviation) in the membrane fraction following fractionation was determined by densitometry of immunoblots for each MLD-GFP construct (n = 3). Tabulated raw densitometry measurements are found in Table S2. A Student's t test was employed to determine the statistical significance of the difference between the amount of GFP versus each GFP-fusion in the membrane fractions; *, significantly different from GFP alone (P < 0.05).
Table 1.
Localization of each GFP fusion to HeLa cell–cell junctions after ectopic expression
| MLD construct | Bright junctions* | Total junctions | % Bright junctions |
| GFP | 3 | 40 | 8% |
| GFP-VcRID | 2 | 31 | 6% |
| VcRID-GFP | 34 | 38 | 89% |
| VvRID-GFP | 7 | 31 | 23% |
| VvDUF5-GFP | 43 | 43 | 100% |
| TcdB-GFP | 19 | 19 | 100% |
| VcRID-GFP mutants† | |||
| Y23F | 0 | 21 | 0% |
| Y23A | 2 | 29 | 7% |
| S68T | 2 | 21 | 10% |
| S68A | 1 | 25 | 4% |
| R70K | 4 | 49 | 8% |
| R70A | 3 | 40 | 8% |
| ΔH1-2 | 1 | 15 | 7% |
| VvDUF5-GFP mutants† | |||
| Y23F | 27 | 27 | 100% |
| Y23A | 0 | 34 | 0% |
| S62T | 11 | 71 | 15% |
| S62A | 7 | 56 | 13% |
| R64K | 0 | 36 | 0% |
* HeLa cell–cell junctions were scored for fluorescence intensity across a line bisecting the cell–cell junction. Bright junctions exhibited a single central peak of fluorescence intensity (Fig. 3B and SI Materials and Methods). Data are the aggregate counts of the total junctions scored for at least three independent experiments.
†Representative images for site-directed mutants can be viewed in Fig. S5.
In support of the microscopy, membrane fractionation showed 33 ± 8% of the 2561–2645-GFP fusion protein (MLD-GFP) was present in the membrane fraction, whereas GFP-2561–2645 (GFP-MLD) was rarely at the membrane and was not significantly different in localization from GFP alone (Fig. 2 B and C and Table S2). Immunoblotting with anti-GFP antibody confirmed stable fusion proteins were produced for all fusions tested and thus failure to localize to the membrane was not due to protein degradation. Altogether, these data support that aa 2561–2645 of MARTXVc is a peptide sequence required for plasma membrane targeting and thus represents a bacterial toxin membrane localization domain (MLD).
MLD Is a Functionally Conserved Domain Found in Many Bacterial Toxins.
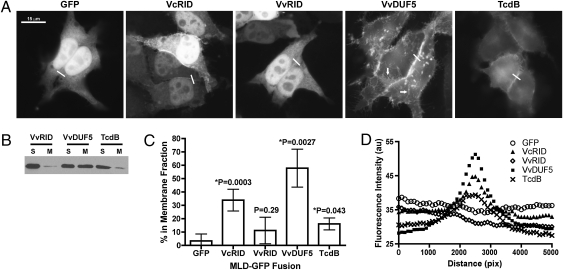
To test if conserved C1-like MLDs from other toxins likewise function in membrane targeting, HeLa cells were transfected with plasmids expressing the putative MLDs from VvRID (MLDVvRID), VvDUF5 (MLDVvDUF5), and TcdB (MLDTcdB) fused to GFP. Similar to MLDVcRID-GFP, cells ectopically expressing the MLDVvDUF5 and MLDTcdB fusions displayed clear membrane localization of GFP by epifluorescence microscopy (Fig. 3A) and were identified in the membrane by immunoblotting with anti-GFP antibody (Fig. 3 B and C). Fluorescence intensity distribution plotting for MLDVcRID, MLDVvDUF5, and MLDTcdB showed maximum GFP-signal at cell–cell junctions compared to GFP alone, which showed nearly uniform fluorescence throughout the cell with maximum fluorescence occurring randomly (Fig. 3D and Fig. S4). This biased localization suggests that these MLDs may preferentially target cell–cell junctions (Table 1 and Fig. S4). Intriguingly, even with 88% aa identity and 93% similarity with MLDVcRID, MLDVvRID showed only weak membrane localization and very little junction targeting (Fig. 3, Fig. S4, and Table 1). By contrast, MLDVvDUF5-GFP showed an apparent higher affinity for the plasma membrane (Fig. 3 C and D) and was also localized to vesicles throughout the cells (arrows in Fig. 3A). Despite its clear association with the plasma membrane by epifluorescence microscopy (Fig. 3 A and B and Table 1), the percentage of MLDTcdB found in the membrane upon fractionation was less than MLDVcRID-GFP or MLDVvDUF5-GFP but was statistically greater than GFP alone (Fig. 3 C and D). These data indicate that although each MLD has a function in targeting proteins to the plasma membrane, the relative amount of protein localized, the stability of each protein at the membrane upon fractionation, the specificity for cell–cell junctions, and the pattern of recycling varied among the four MLDs tested.
Fig. 3.
MLDVcRID homologs localize to the HeLa cell plasma membrane and at cell–cell junctions. (A) HeLa cells were transfected with the indicated MLD-GFP plasmid then fixed and imaged by epifluorescence microscopy 24 h later. (B) Representative anti-GFP immunoblot following membrane fractionation of HeLa cells transfected with the indicated constructs; S, soluble; M, membrane. (C) Densitometry was used to determine the average percentage of signal (+/− the standard deviation) (n ≥ 3) in the membrane fraction for the indicated construct as was done in Fig. 2C. Tabulated raw densitometry measurements are found in Table S3. (D) The average fluorescence intensity measurement of cell–cell junctions (white lines in A) was determined for each construct using ImageJ (SI Materials and Methods) (n ≥ 2 junctions from 3 independent transfection experiments).
Structural Requirements for Membrane Localization.
Analysis of the PMT (PDB ID: 2EBF), TcdB (PDB ID: 2BVL), and TcsL (PDB ID: 2VL8) structural models and a predicted model of MLDVcRID (Fig. 1B) shows the conserved MLD regions consist of four α-helices (designated H1-H4) connected by three short loop regions (designated L1-L3) (Fig. 1 B and C). To further test the structural requirements for proper membrane targeting, the first 30 aa of MLDVcRID, corresponding to H1 and most of H2, were deleted from MLDVcRID-GFP and the cellular location was monitored following transfection. This deletion prevented MLDVcRID-GFP localization to cell–cell junctions (Table 1), suggesting that all four helices are required for proper membrane targeting.
Fig. 1C shows that despite high divergence of sequence among the various MLDs, nine residues are conserved in at least 90% of the homologs with only three residues 100% identical. According to the PMT, TcdB, and TcsL structural models, Leu8, Ala12, Leu30, Leu54, Tyr61, and Ala73 (numbered according to their location on MLDVcRID) lie buried within the hydrophobic core formed by bundling of the four helices. These residues likely have conserved function to maintain structural integrity. The three 100% identical residues, Tyr23, Ser68, and Arg70, are near or within loops L1 and L3 and are oriented near each other on the same end of the helical bundle. Electrostatic potential predictions of the known and modeled MLD structures show that the interface formed by L1 and L3 appears to form a positively charged surface possibly involved in either protein or membrane interactions (Fig. 4).
Fig. 4.
The L1-L3 interface of each MLD displays a positive charge. (A) Cartoon representation of MLDPMT for orientation of the diagrams in B. (B) Electrostatic potential predictions for two faces of the crystallized MLDs (PMT, TcdB, and TcsL) and the modeled MLDs (VvDUF5, VcRID, and VvRID). All of the predictions indicate a net positive charge (blue) at the interface of L1 and L3 although the remainder of the helical bundles contain large patches of negative charge (red).
The potential importance of the three 100% identical residues for membrane localization was tested by altering each residue on MLDVcRID and MLDVvDUF5 to either an Ala or to a closely conserved residue (Tyr-Phe, Ser-Thr, Arg-Lys). Epifluorescence and confocal microscopy showed that substitution of any of these three residues on MLDVcRID abrogated GFP localization to cell–cell junctions or to the membrane (Table 1 and Fig. S5 B–G). Similarly, substitution of the homologous residues on MLDVvDUF5-GFP also abolished membrane localization (Table 1 and Fig. S5 H–M). However, MLDVvDUF5-GFP localization was decreased by mutation of Tyr23 to Phe (Fig. S5J). These results indicate that although plasma membrane localization is a conserved function of these domains, only the Ser and Arg residues within L3 are absolutely essential, suggesting that other requirements and specificities for localization are determined by nonconserved residues.
Functional Complementation Between the MLDs.
The variance of requirements for conserved residues and the extent of sequence divergence of exposed residues of the various MLDs suggest that each MLD may be uniquely paired with its cognate toxin. To test if one MLD can functionally complement another, chimeric toxins were generated in which MLDVcRID on LFN-RIDVc was replaced with either MLDVvRID or MLDVvDUF5, resulting in LFN-MLDVvRID-RIDVc or LFN-MLDVvDUF5-RIDVc, respectively. When applied along with PA, either chimera rounded HeLa cells indistinguishably from LFN-RIDVc after incubation for 3 h. (Fig. S3A).
A time course of cell rounding was then performed, comparing the percentage of HeLa cells that were round following incubation with varying concentrations of LFN-MLDVcRID-RIDVc, LFN-MLDVvRID-RIDVc, LFN-MLDVvDUF5-RIDVc, or LFN-ΔMLD-RIDVc. Both LFN-MLDVvRID-RIDVc and LFN-MLDVvDUF5-RIDVc were able to fully intoxicate cells similar to LFN-RIDVc at concentrations above 0.1 nM and the time required for cell rounding was not altered (Fig. S3B). Therefore, despite the differences in membrane localization of the individual MLD constructs when fused to GFP, replacing MLDVcRID with another MLD had a less than 10-fold effect on the dynamics of LFN-RIDVc-mediated cell rounding when delivered to HeLa cells using PA.
Discussion
The ability of a bacterial toxin to identify the location of its cellular target is essential for toxic activity. In this work, we describe the presence of a conserved peptide sequence within 16 large bacterial protein toxins capable of targeting known and putative effector domains within these toxins to the plasma membrane. Interestingly, the MLD is found associated with three distinct effectors: RID, typified by the RID of MARTXVc and also found in six other MARTX toxins; PMT C2, typified by PMT and present as DUF5 in five MARTX toxins; and CGT, typified by C. difficile TcdB and found in four other related toxins. Each of these effectors is known to specifically target proteins present at the membrane. The PMT C-terminal complex deamidates heterotrimeric G proteins via the C3 domain attached to C1/C2 (21), CGTs glucosylate Rho family GTPases (22), and RID inactivates Rho family GTPases although potentially by an indirect mechanism (9). This domain appears to be unique to these toxins and is different from the known membrane localization sequences of T3SS effectors (BteA, ExoS, YopE, ExoU) (2–4).
Functional analysis of representatives of each class of MLD identified in this study suggested that, although they show similar ability to localize GFP to the plasma membrane and MLDs can substitute for another in a chimeric toxin, the question that arises is whether an MLD is functional because it has specificity for lipids in membranes or because it targets host proteins that are present at the membrane. Previous studies support a mechanism of direct lipid association. Clostridium sordellii TcsL has been shown to bind liposomes enriched for PS (23), an anionic lipid preferentially associated with the cytosolic leaflet of the plasma membrane (19). Both binding to liposomes and Rac-glucosylation activity of TcsL were stimulated by PS-enriched liposomes, dependent upon the first 18 aa of the toxin, corresponding to H1 and L1 of the MLD. However, TcdB showed less selectivity for PS and was active also in liposomes enriched for phosphatidylglycerol. In our study, TcdB showed a weaker, but significant association with membranes such that localization observed in vivo following ectopic expression may have been dissociated from the membrane during sample preparation. These data indicate that although the MLDs may directly target lipids, they do so with varying affinity.
Further evidence supporting direct lipid association comes from analysis of the crystal structures of PMT, TcdB, and TcsL and structural modeling of MLDVcRID and MLDVvDUF5. The three 100% identical residues identified as essential for proper localization of MLDVcRID and MLDVvDUF5 are all found near each other, with their side chains directed inward rather than exposed to solvent. Our data suggest that the Tyr, Ser, and Arg residues may be directly involved in L1-L3 interactions required to maintain the overall structure of the four-helical bundle. The folding of MLDVvDUF5 may be less dependent on the Tyr residue itself but rather the size of the residue in its position, thereby allowing it to be substituted for Phe with little effect on membrane association. The defect in localization of the Ser68 and Arg70 mutants in both MLDVcRID and MLDVvDUF5, even when changed to Thr or Lys, further emphasizes that the interactions between L1 and L3 may be essential to proper localization. Assessment of the electrostatic potential of this region indicates that all of the MLDs with a known structure are positively charged at this end. Three-dimensional models constructed for MLDVcRID, MLDVvRID, and MLDVvDUF5 also suggest these MLDs are positively charged at the L1/L3 face (Fig. 4B). Thus, similar to the polybasic N terminus of Src, the positive surface of these MLDs may directly interact with the negatively charged acid lipids such as PS, at the plasma membrane (16, 19). As a further example, annexin, which is also known to specifically localize to PS lipids, is enriched in tight junctions suggesting there is an enrichment of these lipids at this site (24), providing justification for the observation that MLD localization is enhanced at cell–cell junctions.
An alternative model to explain the membrane localization is that the MLDs bind host membrane proteins. Because the different classes of MLDs show apparent distinct affinity for membrane, it is possible they target different proteins. Because the genetic requirement for localization may be limited to Ser68 and Arg70, it is possible that binding involves diverse residues at the L1/L3 loops or structural elements elsewhere on the helical bundle. The interface between the four helices includes a large hydrophobic patch. Similar patches in helical bundles have been shown to participate in protein–protein interactions, for example the ability of focal adhesion kinase to bind paxillin (25). Alternatively, differential affinity or binding to different proteins may depend upon the divergent L2-H3 region of each MLD. The amino acid composition of this region is highly variable among the homologs, with many of the MLDs lacking up to six residues from this region alone (Fig. 1C). The composition of the MLD in the L2-H3 region is more conserved within the subgroups of MLDs, an analysis that would suggest that interactions are effector related. However, this concept is contradicted by the finding that MLDVvDUF5 and MLDVvRID were able to complement the activity of MLDVcRID, suggesting that the MLDs are not matched to their cognate toxin. Thus, although it is possible that protein–protein interactions are important for function at the membrane, the proteins targeted are either similarly localized themselves or the interaction is with different faces of the same target protein.
Interactions between the toxin activity domains and the target membrane protein certainly also contribute to binding specificity. Our data shows that RID2721–3085 (without an MLD) was functional and able to round cells, but only when produced at high levels within the cell following transfection. Similarly, purified TcsLΔ18, although not toxic to cells after microinjection, was able to glucosylate soluble Rac in vitro (23). The toxic activity of PMT was also significantly decreased, but not completely abolished, when the C1 domain was deleted (14, 26). We believe that the high amount of RID2721–3099-GFP produced in the cell after transfection was sufficient to locate its cellular binding partner, despite not being directly targeted to the plasma membrane. However, the necessity of the individual MLDs for functionality of each full-length toxin remains to be determined.
The ability of the chimeric LFN toxins to fully mimic LFN-RIDVc activity suggests that the enzymatic activity of RIDVc could be the limiting factor in the dynamics of cell rounding. Accordingly, despite a possibility of MLDVvDUF5 to enhance the membrane association of RID, cell rounding did not occur more rapidly. Furthermore, although MLDVvRID did not significantly increase GFP membrane targeting, a sufficient amount of LFN-MLDVvRID-RIDVc required for RID-mediated cell rounding may have been properly localized to the membrane, thereby allowing intoxication to proceed indifferently from LFN-RIDVc.
Discovery of this conserved MLD advances our understanding of the biology of the MARTX toxins as a whole as well as the strategies many pathogenic Vibrios and emerging pathogens use to intoxicate host cells. Because this MLD is present in multiple families of bacterial toxins suggests a conserved mechanism for targeting protein toxins to the mammalian cell plasma membrane.
Materials and Methods
Construction of the Rho-Inactivation Domain (RID) Deletion Plasmids.
Fragments of V. cholerae rtxA encoding for the aa segments of RID indicated in Fig. 1A were amplified by PCR and inserted into pEGFP-N3 (Clontech) to yield the RID-GFP plasmids. Alignments were performed using either CLUSTAL W (27) and highlighted using TEXTSHADE through the Biology Workbench 3.2 server (http://workbench.sdsc.edu) or KAlign (http://www.ebi.ac.uk/Tools/kalign/) (28) and manually fitted to match secondary structure predictions.
Construction of Membrane Localization Domain GFP-Fusions.
The DNA corresponding to aa 2561–2645 of V. cholerae rtxA [according to the Lin sequence (7)], 2377–2461 and 3591–3669 of V. vulnificus rtxA, and 1–83 of C. difficile tcdB, were amplified by PCR and inserted into either pEGFP-N3 or pEGFP-C3 (Clontech). Point mutations on pMLDVcRID-GFP and pMLDVvDUF5-GFP were generated using the Quikchange XL II site-directed mutagenesis kit (Stratagene).
Purification and Application of LFN-Fusion Proteins.
PA, LFN-RIDVc, LFN-ΔMLD-RIDVc, LFN-MLDVvRID-RIDVc, and LFN-MLDVvDUF5-RIDVc were all purified as in Sheahan and Satchell (9) using an ÄKTA Purifier. Purity of each protein was assessed by SDS/PAGE. Intoxications were performed as in (29) with either PBS or 28 nmol PA and the indicated amount of LFN-fusion protein added to HeLa cells.
Microscopy.
HeLa cells were grown to approximately 70% confluence on acid-washed coverslips in 12-well dishes then transiently transfected using FuGene HD (Roche) following the manufacturer's protocol. Coverslips were washed with PBS and fixed in 3.7% formaldehyde in 100 mM Pipes pH 7.4 then mounted onto glass slides (18). Slides were imaged for epifluorescence (Fig. 3, and Figs. S2 and S3) with an inverted Leica DMIRE2 microscope with a CCD camera, for deconvolution (Fig. 2) with a Deltavision microscope and software (Applied Precision) (18), or for confocal microscopy (Fig. S5) with a Zeiss LSM510-Meta. Images were then processed and compiled using Adobe Photoshop CS3 Extended. Details for cell–cell junction quantification can be found in SI Text and Fig. S4.
Membrane Fractionation and Western Blotting.
Transfected HeLa cells grown in 6-well dishes were divided into cytosolic and membrane fractions as described previously (9). Equivalent volumes of each fraction were separated by 15% SDS/PAGE and transferred to nitrocellulose membranes. GFP and GFP-fusions were detected with GFP-specific antiserum conjugated to horseradish peroxidase (Miltenyi Biotec). Membranes were developed with Supersignal West Pico chemiluminescent substrate (Pierce). Band intensities were determined using National Institutes of Health ImageJ 1.40g. Raw data for densitometry measurements can be found in Tables S2 and S3.
Molecular Modeling.
The MLD sequences from VcRID, VvDUF5, and VvRID were input into the GENO3D server (http://geno3d-pbil.ibcp.fr) for 3D structure modeling (30). The structures of TcdB, PMT, and TcsL were used as target templates for all models. Structure alignments and electrostatic potential predictions were performed using MacPymol (Delano Scientific).
Supplementary Material
Acknowledgments
We thank K. Prochazkova and E. Campbell for technical assistance and T. Hope for the S15-mCherry plasmid and helpful insights in the initial stages of the project. This work was supported by National Institutes of Health award AI051490 and an Investigator in the Pathogenesis of Infectious Disease award from the Burroughs Wellcome Fund (to K.J.F.S.). B.G. was supported by an Institutional National Research Service Award Postdoctoral Research Fellowship T32-AI007476-11 and a National Research Service Award Postdoctoral Research Fellowship F32-AI075764-01A2.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908700107/DCSupplemental.
References
- 1.McSweeney LA, Dreyfus LA. Nuclear localization of the Escherichia coli cytolethal distending toxin CdtB subunit. Cell Microbiol. 2004;6:447–458. doi: 10.1111/j.1462-5822.2004.00373.x. [DOI] [PubMed] [Google Scholar]
- 2.French CT, et al. The Bordetella type III secretion system effector BteA contains a conserved N-terminal motif that guides bacterial virulence factors to lipid rafts. Cell Microbiol. 2009;11:1735–1749. doi: 10.1111/j.1462-5822.2009.01361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krall R, Zhang Y, Barbieri JT. Intracellular membrane localization of pseudomonas ExoS and Yersinia YopE in mammalian cells. J Biol Chem. 2004;279:2747–2753. doi: 10.1074/jbc.M301963200. [DOI] [PubMed] [Google Scholar]
- 4.Rabin SD, Hauser AR. Pseudomonas aeruginosa ExoU, a toxin transported by the type III secretion system, kills Saccharomyces cerevisiae. Infect Immun. 2003;71:4144–4150. doi: 10.1128/IAI.71.7.4144-4150.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satchell KJ. MARTX, multifunctional autoprocessing repeats-in-toxin toxins. Infect Immun. 2007;75:5079–5084. doi: 10.1128/IAI.00525-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heidelberg JF, et al. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature. 2000;406:477–483. doi: 10.1038/35020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin W, et al. Identification of a vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc Natl Acad Sci USA. 1999;96:1071–1076. doi: 10.1073/pnas.96.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheahan KL, Cordero CL, Satchell KJ. Identification of a domain within the multifunctional Vibrio cholerae RTX toxin that covalently cross-links actin. Proc Natl Acad Sci USA. 2004;101:9798–9803. doi: 10.1073/pnas.0401104101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheahan KL, Satchell KJ. Inactivation of small Rho GTPases by the multifunctional RTX toxin from Vibrio cholerae. Cell Microbiol. 2007;9:1324–1335. doi: 10.1111/j.1462-5822.2006.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spyres LM, et al. Cytosolic delivery and characterization of the TcdB glucosylating domain by using a heterologous protein fusion. Infect Immun. 2001;69:599–601. doi: 10.1128/IAI.69.1.599-601.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waterfield NR, et al. The insecticidal toxin makes caterpillars floppy 2 (Mcf2) shows similarity to HrmA, an avirulence protein from a plant pathogen. FEMS Microbiol Lett. 2003;229:265–270. doi: 10.1016/S0378-1097(03)00846-2. [DOI] [PubMed] [Google Scholar]
- 12.Altschul SF, et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldman B, et al. Xenorhabdus Genome Sequencing Project. Donald Danforth Plant Science Center. 2005. xenorhabdus.danforthcenter.org.
- 14.Kitadokoro K, et al. Crystal structures reveal a thiol protease-like catalytic triad in the C-terminal region of Pasteurella multocida toxin. Proc Natl Acad Sci USA. 2007;104:5139–5144. doi: 10.1073/pnas.0608197104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silverman L, Resh MD. Lysine residues form an integral component of a novel NH2-terminal membrane targeting motif for myristylated pp60v-src. J Cell Biol. 1992;119:415–425. doi: 10.1083/jcb.119.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwong J, Lublin DM. Amino-terminal palmitate or polybasic domain can provide required second signal to myristate for membrane binding of p56lck. Biochem Biophys Res Commun. 1995;207:868–876. doi: 10.1006/bbrc.1995.1266. [DOI] [PubMed] [Google Scholar]
- 17.Rodgers W. Making membranes green: Construction and characterization of GFP-fusion proteins targeted to discrete plasma membrane domains. Biotechniques. 2002;32:1044–1046. doi: 10.2144/02325st05. 1048, 1050-1041. [DOI] [PubMed] [Google Scholar]
- 18.Campbell EM, Perez O, Melar M, Hope TJ. Labeling HIV-1 virions with two fluorescent proteins allows identification of virions that have productively entered the target cell. Virology. 2007;360:286–293. doi: 10.1016/j.virol.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeung T, et al. Membrane phosphatidylserine regulates surface charge and protein localization. Science. 2008;319:210–213. doi: 10.1126/science.1152066. [DOI] [PubMed] [Google Scholar]
- 20.Yeung T, et al. Contribution of phosphatidylserine to membrane surface charge and protein targeting during phagosome maturation. J Cell Biol. 2009;185:917–928. doi: 10.1083/jcb.200903020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orth JH, et al. Pasteurella multocida toxin activation of heterotrimeric G proteins by deamidation. Proc Natl Acad Sci USA. 2009;106:7179–7184. doi: 10.1073/pnas.0900160106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Just I, et al. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 1995;375:500–503. doi: 10.1038/375500a0. [DOI] [PubMed] [Google Scholar]
- 23.Mesmin B, et al. A phosphatidylserine-binding site in the cytosolic fragment of Clostridium sordellii lethal toxin facilitates glucosylation of membrane-bound Rac and is required for cytotoxicity. J Biol Chem. 2004;279:49876–49882. doi: 10.1074/jbc.M406903200. [DOI] [PubMed] [Google Scholar]
- 24.Lee DB, Jamgotchian N, Allen SG, Kan FW, Hale IL. Annexin A2 heterotetramer: Role in tight junction assembly. Am J Physiol Renal Physiol. 2004;287:F481–F491. doi: 10.1152/ajprenal.00175.2003. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi I, Vuori K, Liddington RC. The focal adhesion targeting (FAT) region of focal adhesion kinase is a four-helix bundle that binds paxillin. Nat Struct Biol. 2002;9:101–106. doi: 10.1038/nsb755. [DOI] [PubMed] [Google Scholar]
- 26.Aminova LR, Luo S, Bannai Y, Ho M, Wilson BA. The C3 domain of Pasteurella multocida toxin is the minimal domain responsible for activation of Gq-dependent calcium and mitogenic signaling. Protein Sci. 2008;17:945–949. doi: 10.1110/ps.083445408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larkin MA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 28.Lassmann T, Sonnhammer EL. Kalign—an accurate and fast multiple sequence alignment algorithm. BMC Bioinformatics. 2005;6:298. doi: 10.1186/1471-2105-6-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cordero CL, Kudryashov DS, Reisler E, Satchell KJ. The Actin cross-linking domain of the Vibrio cholerae RTX toxin directly catalyzes the covalent cross-linking of actin. J Biol Chem. 2006;281:32366–32374. doi: 10.1074/jbc.M605275200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Combet C, Jambon M, Deléage G, Geourjon C. Geno3D: Automatic comparative molecular modelling of protein. Bioinformatics. 2002;18:213–214. doi: 10.1093/bioinformatics/18.1.213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.