Abstract
Oscillations of neural activity are ubiquitous in the brain and are critical for normal cognitive function. In the visual system, repetitive presentation of a stimulus results in the reduction of power elicited in the gamma frequency band. However, this reduction does not result in degradation of perception; on the contrary, perception is improved by prior experience with the stimulus. To explain how reduction of gamma frequency oscillations, observed in priming experiments, can lead to improvement in behavior, we assume that visual processing takes place in two distinct stages: representation sharpening in the early visual areas and competitive interaction among representations in the higher visual areas and the prefrontal cortex. Here, we present a network model of spiking neurons that demonstrates how stimulus repetition leads to a decrease in power of the local field potential oscillations in the gamma frequency range in the early layer and also improves network response by reducing the latency to reach a decision in the higher area.
Keywords: cerebral cortex, plasticity, representation sharpening, gamma oscillations, learning
Oscillations of neural activity are ubiquitous in the brain. There is evidence that neuronal oscillations are involved in cognitive functions (1–4). Recordings from cortical neurons suggest that synchronized activity of cortical neurons underlies oscillatory local field potentials (LFPs) in the cortex. Neurons encoding an attended object synchronize their spike times, which will distinguish them from other active neurons that might represent a different object (5). It has been observed in priming experiments that repeated presentation of the same visual stimulus results in reduction of the induced gamma activity (6). However, this reduction does not result in impairment of perception; surprisingly, perception is improved by prior experience with the object.
Priming is an unconscious form of memory that has been observed in perceptual, semantic, and conceptual domains (7–10). Although priming leads to more efficient processing of sensory stimuli, the neural activity in the cortex decreases with stimulus repetition. This was observed in single-cell recordings (11, 12) and in electroencephalographic (EEG) (6) and functional MRI experiments (13). This paradoxical improvement in behavior accompanied by reduction of neural activity in priming experiments was demonstrated to occur in a rate model (14) based on the representation sharpening hypothesis (15, 16). In this model, representation sharpening took place in the early visual areas and neural suppression was attributable to reduced populations of neurons representing the stimulus. This led to more selective activation of neurons representing objects in higher visual areas, thereby reducing ambiguity in visual perception.
Here, we used a spiking model to explore why gamma oscillations decreased during representation sharpening. Gamma activity is driven by synchronous neural activity representing a stimulus; therefore, stimulus repetition in priming experiments should lead to reduction of synchrony among neurons representing the stimulus, and thus to reductions of LFP oscillations and gamma power in EEG experiments (6). Representation sharpening could explain why reduction of oscillatory neural activity does not impair perception. We adapted the two-layer network architecture (Fig. 1A) of the rate model that previously explained suppression of neural activity in priming experiments (14) (Methods).
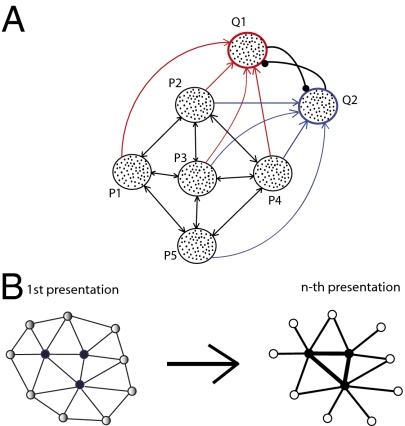
Fig. 1.
(A) Network model consisted of five populations of spiking neurons (P1–P5). Each population contained 100 excitatory and 25 inhibitory neurons. Recurrent excitatory connections between excitatory neurons in different populations were adjustable. Inhibitory interneurons received fixed excitatory connections from excitatory neurons from all five excitatory populations and sent inhibitory projections onto the neurons of the same population. A stimulus applied to the spiking neural network model led to representation sharpening with repetition, as observed in the rate model. After the first presentation, the strengths of connections between neurons in populations P1 and P2 increased but those between the other populations decreased (Lower Right). (B) Schematic illustration of representation sharpening. Initial presentation of a stimulus activated a pattern of units in early visual areas (Left). The units in the pattern were activated to varying degrees. Darker filled circles indicate higher activity levels. After several stimulus repetitions (Right), only the most active units continued to respond and weakly responding units were eliminated from the representation of the stimulus. Strengths of connections (indicated by thickness of lines connecting units) between strongly activated units were strengthened, and connections to weakly active units were eliminated.
Results
Representation Sharpening.
According to the representation sharpening hypothesis, neurons responding weakly to a stimulus are eliminated from the stimulus representation with repetition (15), a process that results in a sparser representation. The eliminated neurons, whose preferred features are farther from the feature of the stimulus, are less important for identifying that specific stimulus (17).
We previously showed in a rate-based model that neural suppression in priming experiments can be attributable to representation sharpening (14). This representation sharpening was assumed to take place in the early areas of the visual processing. Units in layer 1 (L1) of the rate model received external inputs with different strengths, which resulted in these units having different levels of activity (Fig. 1B). Hebbian learning (18) weakened synaptic connections from more active to less active units and strengthened connections in the opposite direction. As a consequence, weakly active units received less recurrent excitation, leading to a further decrease in the activity (Fig. 1B). In contrast, the excitation level of strongly activated units further increased. In this model, repetition of the stimulus eventually silenced initially weakly responding units. This process led to a smaller neural population representing the stimulus, consistent with the representation sharpening hypothesis (15).
In the rate model, we also have shown how the representation sharpening hypothesis could lead to improvement of perception of visual stimuli. A presented stimulus might partially activate representations of many objects in higher visual areas and in the prefrontal cortex (PFC), and those representations could compete with each other (14). Eventually, a representation of only one object should survive the competition and suppress the representations of other objects. We suggested that representation sharpening in the early visual areas led to more selective activation of representations in the higher visual areas; this facilitated competition between representations, and thereby improved perception of the primed objects.
Decreased Gamma Oscillations with Priming.
In EEG recordings, the presentation of a previously unexperienced visual stimulus induced oscillatory brain activity in the gamma frequency range (6), and repeated presentation of the same visual stimulus resulted in reduction of the gamma activity (Fig. 2A). Here, we suggest that the reduction of gamma power following repeated presentation of visual stimuli could be attributable to the same neural mechanisms that underlie representation sharpening.
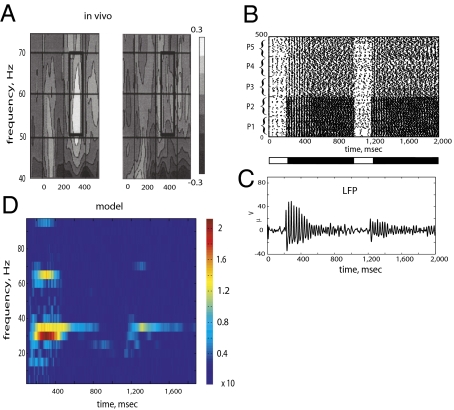
Fig. 2.
(A) Spectrogram of EEG signal in humans during repetition experiments [adapted with permission from Gruber and Muller (6) (Copyright 2002, Elsevier)]. The power of the EEG signal in the gamma frequency band (40–80 Hz) was stronger for the first presentation of the stimuli and decreased during the second presentation of the stimuli. (B) Rastergram of spike times of the excitatory neurons of P1–P5 populations. The first presentation of a stimulus started at 200 msec and terminated at 1,000 msec (black bar beneath the rastergram). The second time, the stimulus was presented at 1,200 msec and removed at 2,000 msec. Populations P1 and P2 received stronger external inputs than populations P3, P4, and P5. The first presentation of the stimulus induced spike time synchrony among neurons of all five populations; however, during the second presentation, the synchrony decreased in populations P3–P5 compared with that in populations P1 and P2. (C) LFP was measured as the total synaptic current to excitatory neurons of all five populations. Amplitude of the LFP was higher during the first presentation than during the second presentation. This was because all five populations synchronized their spikes during the first presentation, which resulted in high-amplitude LFP oscillations. During the second presentation, spike synchrony was observed mostly among neurons in populations P1 and P2, which resulted in a lower amplitude LFP. (D) Spectrogram of the LFP revealed a strong response in the gamma frequency range (30–40 Hz) during the first presentation, which weakened during the second presentation. Power was also found at the first harmonic at 60–70 Hz.
In the spiking neuron model, we assume that gamma band power reflects synchronized activity of cortical neurons representing a stimulus. An initial presentation of a stimulus resulted in synchronized firing among neurons of all five populations (Fig. 2B). On the second presentation of the same stimulus, the synchrony remained strong in populations P1 and P2 and weakened in populations P3, P4, and P5. The LFP was measured as synaptic currents to excitatory neurons. We observed that the amplitude of the LFP oscillations decreased from the first presentation of the stimulus to the second presentation (Fig. 2C). The LFP oscillations also lasted longer on the first stimulus presentation than on the second presentation. We measured power spectrum of the LFP oscillations and found that power of gamma band activity also decreased from the first presentation to the second presentation of a stimulus (Fig. 2D).
The difference in the neuronal synchronization during the first and second stimulus presentations was attributable to a decrease of synchronized firing among neurons in populations P3–P5. This decrease of synchrony among neurons in populations P3–P5 was attributable to the synaptic plasticity that underlies representation sharpening.
In the spiking neuron model, neurons in different populations had different average firing rates. Initially, this was attributable to neurons in populations P1 and P2 receiving stronger external inputs than neurons in populations P3, P4, and P5. The initial weights of the recurrent connections were randomly chosen (Fig. 3A). Higher firing rates of neurons in populations P1 and P2 and the competitive Hebbian learning rule led to strengthening of connections to the neurons in populations P1 and P2 and weakening connections to the neurons in populations P3, P4, and P5 (Fig. 3B). This resulted in the excitatory inputs to P1 and P2 neurons increasing and the excitatory inputs to P3–P5 neurons decreasing with stimulus presentation (Fig. 3C).
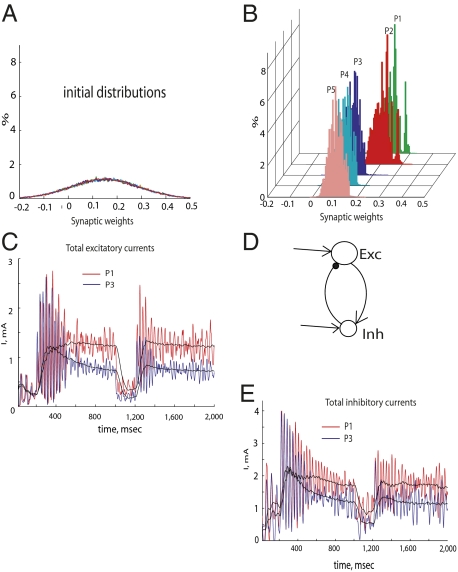
Fig. 3.
(A) Initial weights of excitatory connections between neurons in populations P1–P5 were randomly chosen according to Gaussian distribution with a mean of 0.15 and SD of 0.1. Different colors represent different excitatory populations (P1–P5). (B) Distributions of the excitatory weights after stimulus presentation. The distributions of the weights are shown at the end of the first presentation of a stimulus (at time 1,000 msec). The distributions were separated into two groups: Connections to more active neurons in populations P1 and P2 increased (red and green, mean = 0.2), whereas connections to less active neurons in populations P3, P4, and P5 decreased (mean = 0.1). Further presentations of the stimulus to the network did not qualitatively change this separation. (C) Dynamics of the total excitatory synaptic currents entering neurons in populations P1 (red) and P3 (blue) and low-pass-filtered versions (black). The total current in population P2 neurons was similar to that in population P1 neurons, and currents to P4 and P5 neurons were similar to those in P3 neurons. During the first stimulus, the currents in populations P1 and P2 increased with time, whereas the currents in populations P3, P4, and P5 decreased and remained lower during the second presentation. (D) Schematic diagram of connections among excitatory and inhibitory neurons that result in synchronized activity. Excitatory neurons drive activity in the adjacent inhibitory neurons, which, in turn, synchronize spike initiation in the excitatory neurons. (E) Dynamics of the total inhibitory synaptic currents into neurons in populations P1 (red) and P3 (blue) and low-pass-filtered versions (black). Currents in population P2 neurons were similar to those in population P1 neurons, and currents in P4 and P5 neurons were similar to those in P3 neurons. Because of the homeostatic plasticity of the inhibitory connections, the currents to populations P3, P4, and P5 decreased with priming.
Synchrony among neurons, as observed in the model, was generated by interaction between excitatory and inhibitory cells: Excitatory cells drive activity of inhibitory cells, which, in turn, synchronize excitatory cells (Fig. 3D). The balance between excitation and inhibition in the network was crucial for such synchrony to emerge (19–21). Synchrony among the excitatory neurons occurred when inputs from the excitatory neurons to the adjacent inhibitory neurons and the feedback inputs from the inhibitory neurons to the excitatory neurons were sufficiently strong. Weakening of the excitatory connections to neurons in populations P3–P5 resulted in a decrease of firing rates in those neurons. This triggered a homeostatic plasticity mechanism that maintained a steady level of activity in neural populations (22). According to the homeostatic learning rule, an increase or decrease of the total excitatory drive to an excitatory neuron will also lead to a proportional change of the inhibitory drive to that neuron. So, when excitatory inputs to the neurons in populations P3–P5 were reduced (Fig. 3C), the inhibitory interneurons, controlling the activity of the excitatory neurons in those populations, also weakened inhibitory inputs to those neurons (Fig. 3E). Because some minimal activity of excitatory neurons is required to establish synchrony by activating inhibitory interneurons (23), weakening the excitatory inputs to neurons in populations P3–P5 resulted in the weaker inhibitory inputs that could not synchronize the excitatory neurons in those populations.
In the model, the amplitude of the LFP oscillations, which contributes to the power of gamma activity in the EEG recordings, depends on both the synchrony among responding neurons and the number of responding neurons. Reduction of the population size of the responding neurons attributable to representation sharpening would therefore affect the amplitude of the LFP oscillations. In the model, however, we observed that the synchrony among neurons decreased earlier than the number of neurons representing stimuli changed. Thus, although both the number of the responding neurons and the synchrony among them may, in principle, contribute to the power of gamma activity, the reduction of synchrony among excitatory neurons was the main factor affecting the power of LFP oscillations in gamma frequency range.
The amplitude of the LFP oscillations also varied within each stimulus presentation: It was higher at the stimulus onset and then decreased toward stimulus termination. This variation of the LFP amplitude during the stimulus was a result of the firing rate adaptation in the excitatory neurons. Thus, different underlying mechanisms were responsible for the changes of LFP between the first and second presentations as well as within each presentation of the stimulus.
Competitive Interactions Among Representations in Higher Visual Areas.
In the previous rate model, a stimulus applied to the network activated representations of many different objects that competed with each other in layer 2 (L2) of a two-layer network (14). Because of a winner-take-all design of L2, a representation of one object in L2 should survive among representations of many competing objects. Representation sharpening in L1 led to selective activation of representations in L2, which facilitated competition between representations in L2, and thereby shortened reaction time of the network.
In the spiking network model, we also modeled L2 as a winner-take-all network that consisted of two populations of excitatory and inhibitory neurons, Q1 and Q2, representing two objects, respectively (Fig. 1A). There were excitatory-excitatory, excitatory-inhibitory, and inhibitory-excitatory connections within each population. There were also strong projections from the excitatory neurons of one population to the inhibitory neurons of the other population. Excitatory neurons in population Q1 of L2 received inputs from neurons in populations P1, P2, P3, and P4 of L1; excitatory neurons in population Q2 received inputs from neurons in populations P2, P3, P4, and P5 of L1.
A stimulus applied to L1 neurons in the first and the second presentations resulted in synchronized activity of L1 neurons (Fig. 4A). Activity of these L1 neurons triggered a response of L2 neurons. Because of a winner-take-all design of L2, this led to competition between populations Q1 and Q2 of L2, which resulted in the Q1 population suppressing the Q2 population during the first and second presentations of the stimulus (Fig. 4B). As in the rate model, stimulus repetition shortened reaction time of the network. During the first presentation of the stimulus, it took ≈400 msec for activity in population Q1 to suppress population Q2 (from 200 to 600 msec), and during the second presentation of the same stimulus, the suppression was faster and took only about 200 msec (from 1,200 to 1,400 msec).
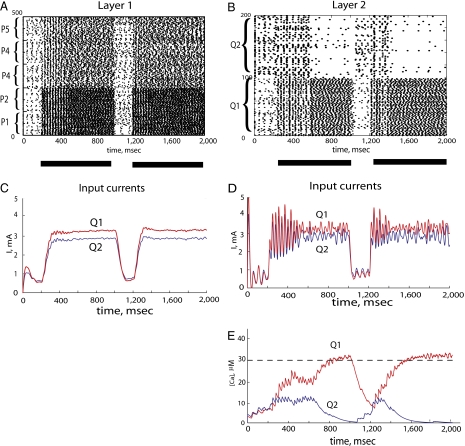
Fig. 4.
(A) Rastergram of the excitatory neurons in populations P1–P5. The first presentation of the stimulus started at 200 msec and terminated at 1,000 msec (black bar beneath the rastergram). The second presentation started at 1,200 msec and ended at 2,000 msec. As in Fig. 4, population P1 and P2 neurons received stronger inputs than population P3, P4, and P5 neurons; during the first presentation of the stimulus, spike time synchrony occurred among neurons of all five populations, and during the second presentation, synchrony was observed mainly among neurons in populations P1 and P2. (B) Rastergram of the excitatory neurons in populations Q1 and Q2. Competition between population Q1 and Q2 neurons (attributable to strong reciprocal inhibitory connections) led to population Q1 neurons suppressing the activity of population Q2 neurons. It took 400 msec (from 200 to 600 msec) for population Q1 neurons to suppress firing on population Q2 neurons in the first presentation of the stimulus, but it only took 200 msec (from 1,200 to 1,400 msec) for the second presentation of the stimulus. (C) Low-pass-filtered total synaptic currents to the excitatory neurons in population Q1 (red) and population Q2 (blue). On average, population Q1 neurons received stronger input than population Q2 neurons; however, amplitudes of the synaptic currents to both populations did not differ significantly during the first and second presentations. (D) Unfiltered total synaptic currents in the excitatory neurons in populations Q1 (red) and Q2 (blue) showed differences between the first and second presentations of the stimulus. Because the currents to the neurons in both populations originated from the synchronized neurons in P1–P5 populations, the currents in population Q1 and Q2 neurons were correlated. However, the correlation between the currents was weaker and shorter for the second presentation of the stimulus. (E) Temporal dynamics of the total calcium concentrations in population Q1 neurons (red) and population Q2 neurons (blue). When correlation between oscillatory inputs to neurons in populations Q1 and Q2 is strong (from 200 to 600 msec), neural activity in populations Q1 and Q2 coexists. Once correlation in inputs decreases, the winner-take-all nature of the network is observed. For the second presentation of the stimulus, the duration of strong correlation was much shorter (from 1,200 to 1,400 msec), which resulted in faster suppression of population Q2 neuron activity.
Winner-Take-All Network and Synchrony.
The Q1 population, which received inputs from populations P1–P4, had an advantage over the Q2 population, receiving inputs from populations P2–P5, because the activity of P1 and P2 neurons was higher than the activity of neurons in populations P3–P5. Therefore, on average, afferent synaptic currents to neurons in population Q1 were higher than currents to neurons in population Q2 (Fig. 4C). The difference between these two currents was slightly greater for the second presentation of the stimulus, but the change in the inputs was not as dramatic as the change in the reaction time—the time required by one population to suppress the other population (Fig. 4B). To explain this observation, we analyzed the difference between input currents to the L2 populations (Fig. 4D).
The sharply rising inputs from synchronized L1 neurons tended to synchronize spikes of L2 neurons. It was previously shown that synchrony in a population of spiking neurons prevents competition and winner-take-all behavior (24). Therefore, synchronous inputs to the L2 neurons effectively postponed the competition between L2 populations. Although a population receiving a stronger mean current eventually suppressed the other population (Fig. 4B), it occurred only when synchrony in driving inputs from L1 decreased (Fig. 4D). During the second presentation of the stimulus, the synchrony in L1 decreased more rapidly (Fig. 4D), thus accelerating competition in L2.
The temporal dynamics of the competition can be clearly observed if the calcium concentration is taken as an indicator of neural activity (Fig. 4E). When correlation between oscillatory inputs to neurons in populations Q1 and Q2 was strong (Fig. 4D), neural activity remained high in both populations of neurons (Fig. 4E). Once the correlation between inputs was decreased, the winner-take-all nature of the network was observed, with one population suppressing activity of the other.
Discussion
Cortical oscillations accompany normal cognitive functioning (1–5), and interfering with these oscillations could lead to cognitive impairment (25). However, perception was improved during priming experiments, in which the power of cortical EEG oscillations to repetitive stimuli presentations was reduced (6). Here, we studied the neural mechanisms behind such improvement.
In the spiking neuronal model, representation sharpening, previously explored in a rate model (14), was accompanied by a decrease in the power of the LFP oscillations in the gamma frequency range, as observed in priming experiments (6). The reduction of gamma activity in the priming experiments (6) was observed during induced gamma activity. The induced gamma activity is an active phenomenon that depends on perception of the presented stimuli. Evoked gamma activity is also present during visual stimuli presentation and is a reaction of the underlying recurrent networks to stimuli. In the model, we focused on the neural mechanisms that might underlie changes in the induced gamma activity with priming, because evoked gamma activity is not necessary for perception and does not change with priming (26).
In the model, reduction of the LFP oscillations in the first layer enhanced competition between populations of neurons in the second layer, connected through inhibition in a winner-take-all network. Neurons in these populations tended to synchronize because of recurrent inhibitory connections within and between populations, and synchrony among competing neural populations could impair competition (24). Competition can be facilitated by decreasing synchrony within L2 populations, such that they behave more like rate units. We accomplished this by adding slow NMDA currents to the excitatory synapses in the L2 neurons, consistent with the high expression levels of NMDA receptors in prefrontal cortical areas involved in decision making compared with sensory cortex (27). Competition in spiking neural networks can also be enhanced by slow inhibitory GABAB currents, which also decreases network synchrony (not implemented in the model). Decreased synchrony does not always facilitate competition in neural networks, and some degree of synchrony can improve competition (28, 29). How synchrony affects competition depends on many factors, such as the architecture and dynamics of a neural system.
Recordings of gamma band EEG responses may contain artifacts from eye movements (30), which could affect interpretation of the EEG study of object familiarity (6). However, in contrast to the broadband signal, ranging between 20 and 100 Hz, generated by the transient artifact (30), the EEG signal in the priming study had a narrow distribution with a peak at 50–60 Hz (6). Thus, the EEG signal observed in the priming experiments (6) matched the underlying neural activity better than that of the artifact. Similarly, the 30–40-Hz signal in the spectrogram from the model was based on many cycles of the LFP oscillations and was not attributable to spectral analysis of short transient activity. Nonetheless, this experiment should be repeated with better control of the eye movements.
In an experiment, when presented visual stimuli were meaningful and made sense to the subjects, there was a decrease of EEG power, but the power increased with stimulus repetition for meaningless stimuli, such as abstract drawings (31). One possible explanation is that the subjects lacked existing neural assemblies representing the meaningless drawings (31), and the observed increase in EEG power could be a consequence of establishing corresponding neural representations. In the model, presenting a meaningless stimulus to a subject would correspond to recruiting a network with weak recurrent connections. Once the lateral recurrent connections are strengthened with subsequent presentations and the total synaptic weight saturates, the competition among synapses can begin.
Methods
Model.
L1.
L1 in our model described processes taking place in early visual areas during priming. L1 consisted of five populations (P1–P5) of 100 excitatory and 25 inhibitory neurons in each population (Fig. 1A). We assumed that each L1 population responds differently to a particular feature of an object, with each one having a particular degree of preference for that feature. In area V1, for example, neurons in a column may respond to an oriented bar but how strongly they respond depends on each neuron's preferred orientation (32).
Hodgkin–Huxley equations with different parameters were applied to model excitatory and inhibitory neurons in L1 (SI). The connections among neurons were modeled as AMPA and GABA synapses (SI).
Excitatory neurons in each population were recurrently connected to excitatory neurons in other populations. These recurrent excitatory connections were adjustable and have been modified according to the Hebbian learning rule (18) augmented by competition among outgoing connections: Strengthening of some connections led to weakening of other connections (SI).
For simplicity, recurrent excitatory connections within each population were not included; they had relatively little impact on the network dynamics when they were included in the simulations. Neurons in the same population received random inputs with similar mean values; therefore, their average firing rates were nearly the same.
The inhibitory neurons received fixed (nonadjustable) connections with different weights from excitatory neurons of all populations and sent modifiable inhibitory projections to the excitatory neurons of the same population. These inhibitory connections were adjusted according to a homeostatic learning rule (SI).
Inputs to L1 neurons applied in two subsequent trials ranged from 200 to 1,000 msec and from 1,200 to 2,000 msec. Inputs to populations P1 and P2 were stronger (had higher mean values) than inputs to populations P3–P5.
The neural responses in the model adapted within a few hundred milliseconds. Therefore, if a single stimulus presentation was longer than the adaptation time, repeated presentations of the stimulus with equal total duration led to a greater total effect. The stimulus was repeatedly presented in the model with a 200-msec gap between the first and second presentations. This interval was necessary to allow the adaptation currents to relax to their basal levels.
L2.
L2 in the model described processes taking place in higher visual areas, such as the inferotemporal cortex (IT) or PFC, during priming. IT and PFC areas are important for object recognition (33). The architecture of the L2 network of the model was chosen so that (i) each population represented a distinct object and (ii) populations competed with each other, such that only one population could be active at a time (winner-take-all). The winner-take-all feature of L2 of the model predicts that neural representations in higher visual areas or the PFC are more competitive compared with representations in the earlier visual areas. Although we modeled this competition in L2 via strong inhibitory connections among L2 neurons, there might be other possible explanations of such competition.
L2 consisted of two populations (Q1 and Q2) of excitatory neurons (100 neurons in each population) and two populations of inhibitory neurons (25 neurons in each population) (Fig. 1A).
To model L2 neurons, we used Hodgkin–Huxley equations with the same parameters as those used for L1 neurons, except the excitatory neurons included NMDA receptors in addition to the AMPA receptors (SI). Inclusion of NMDA currents in L2 neurons served to reduce synchronization among the recurrently connected neurons within each excitatory population. It has been previously shown that synchrony in a population of spiking neurons prevents competition and winner-take-all behavior (24). Experimental evidence supporting this assumption comes from a study (26) that reported much higher expression of NMDA receptor subunit mRNAs in the PFC than in other cortical areas, including the primary visual cortex of the human brain.
The excitatory neurons in each L2 population were recurrently connected with neurons of the same population. Populations Q1 and Q2 competed with each other via reciprocal inhibition, such that one population should win the competition and suppress the other population.
Inhibitory neurons in each population received inputs from excitatory neurons of the other population and, in turn, sent projections to the excitatory neurons of the same population. The strengths of connections were randomly chosen from a uniform distribution between 0 and 0.5.
Neurons in population Q1 received inputs from neurons in populations P1–P4 of L1, and neurons in population Q2 received inputs from neurons in populations P2–P5 of L1.
Acknowledgments
This work was supported by the Howard Hughes Medical Institute (T.J.S.), the Swartz Foundation (S.M.), and grants from the National Institute on Deafness and Other Communication Disorders (M.B.) and the National Institute of Neurological Disorders and Stroke (M.B.).
Footnotes
*This Direct Submission article had a prearranged editor.
The authors declare no conflict of interest.
References
- 1.Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: Implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pesaran B, Pezaris JS, Sahani M, Mitra PP, Andersen RA. Temporal structure in neuronal activity during working memory in macaque parietal cortex. Nat Neurosci. 2002;5:805–811. doi: 10.1038/nn890. [DOI] [PubMed] [Google Scholar]
- 3.Tiesinga P, Fellous J-M, Sejnowski TJ. Regulation of spike timing in visual cortical circuits. Nat Rev Neurosci. 2008;9:97–107. doi: 10.1038/nrn2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tallon-Baudry C. Oscillatory synchrony and human visual cognition. J Physiol (Paris) 2003;97:355–363. doi: 10.1016/j.jphysparis.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- 6.Gruber T, Muller MM. Effects of picture repetition on induced gamma band responses, evoked potentials, and phase synchrony in the human EEG. Brain Res Cogn Brain Res. 2002;13:377–392. doi: 10.1016/s0926-6410(01)00130-6. [DOI] [PubMed] [Google Scholar]
- 7.Tulving E, Schacter DL. Priming and human memory systems. Science. 1990;247:301–306. doi: 10.1126/science.2296719. [DOI] [PubMed] [Google Scholar]
- 8.Schacter DL, Buckner RL. Priming and the brain. Neuron. 1998;20:185–195. doi: 10.1016/s0896-6273(00)80448-1. [DOI] [PubMed] [Google Scholar]
- 9.Wiggs CL, Martin A. Properties and mechanisms of perceptual priming. Curr Opin Neurobiol. 1998;8:227–233. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]
- 10.Schacter DL, Wig GS, Stevens WD. Reductions in cortical activity during priming. Curr Opin Neurobiol. 2007;17:171–176. doi: 10.1016/j.conb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Miller EK, Desimone R. The representation of stimulus familiarity in anterior inferior temporal cortex. J Neurophysiol. 1993;69:1918–1929. doi: 10.1152/jn.1993.69.6.1918. [DOI] [PubMed] [Google Scholar]
- 12.Miller EK, Desimone R. Parallel neuronal mechanisms for short-term memory. Science. 1994;263:520–522. doi: 10.1126/science.8290960. [DOI] [PubMed] [Google Scholar]
- 13.Wig GS, Grafton ST, Demos KE, Kelley WM. Reductions in neural activity underlie behavioral components of repetition priming. Nat Neurosci. 2005;8:1228–1233. doi: 10.1038/nn1515. [DOI] [PubMed] [Google Scholar]
- 14.Moldakarimov S, Bazhenov M, Sejnowski TJ. Representation sharpening can explain perceptual priming. Neural Comput. 2010;22:1312–1332. doi: 10.1162/neco.2009.04-09-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desimone R. Neural mechanisms for visual memory and their role in attention. Proc Natl Acad Sci USA. 1996;93:13494–13499. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grill-Spector K, Henson R, Martin A. Repetition and the brain: Neural models of stimulus-specific effects. Trends Cogn Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Ringo JL. Stimulus specific adaptation in inferior temporal and medial temporal cortex of the monkey. Behav Brain Res. 1996;76:191–197. doi: 10.1016/0166-4328(95)00197-2. [DOI] [PubMed] [Google Scholar]
- 18.Miller KD. Synaptic economics: Competition and cooperation in synaptic plasticity. Neuron. 1996;17:371–374. doi: 10.1016/s0896-6273(00)80169-5. [DOI] [PubMed] [Google Scholar]
- 19.Traub RD, Jefferys JG, Whittington MA. Simulation of gamma rhythms in networks of interneurons and pyramidal cells. J Comput Neurosci. 1997;4:141–150. doi: 10.1023/a:1008839312043. [DOI] [PubMed] [Google Scholar]
- 20.Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: Experimental and mathematical observations on network dynamics. Int J Psychophysiol. 2000;38:315–336. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- 21.Börgers C, Epstein S, Kopell NJ. Background gamma rhythmicity and attention in cortical local circuits: A computational study. Proc Natl Acad Sci USA. 2005;102:7002–7007. doi: 10.1073/pnas.0502366102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol. 2000;10:358–364. doi: 10.1016/s0959-4388(00)00091-x. [DOI] [PubMed] [Google Scholar]
- 23.Tiesinga P, Sejnowski TJ. Cortical enlightenment: Are attentional gamma oscillations driven by ING or PING? Neuron. 2009;63:727–732. doi: 10.1016/j.neuron.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lumer ED. Effects of spike timing on winner-take-all competition in model cortical circuits. Neural Comput. 2000;12:181–194. doi: 10.1162/089976600300015943. [DOI] [PubMed] [Google Scholar]
- 25.Thut G, Miniussi C. New insights into rhythmic brain activity from TMS-EEG studies. Trends Cogn Sci. 2009;13:182–189. doi: 10.1016/j.tics.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Bertrand O, Tallon-Baudry C. Oscillatory gamma activity in humans: A possible role for object representation. Int J Psychophysiol. 2000;38:211–223. doi: 10.1016/s0167-8760(00)00166-5. [DOI] [PubMed] [Google Scholar]
- 27.Scherzer CR, et al. Expression of N-methyl-D-aspartate receptor subunit mRNAs in the human brain: hippocampus and cortex. J Comp Neurol. 1998;390:75–90. doi: 10.1002/(sici)1096-9861(19980105)390:1<75::aid-cne7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 28.Burwick T. On the relevance of local synchronization for establishing a winner-take-all functionality of the gamma cycle. Neurocomputing. 2009;72:1525–1533. [Google Scholar]
- 29.Zeitler M, Fries P, Gielen S. Biased competition through variations in amplitude of gamma-oscillations. J Comput Neurosci. 2008;25:89–107. doi: 10.1007/s10827-007-0066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron. 2008;58:429–441. doi: 10.1016/j.neuron.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 31.Gruber T, Müller MM. Oscillatory brain activity dissociates between associative stimulus content in a repetition priming task in the human EEG. Cereb Cortex. 2005;15:109–116. doi: 10.1093/cercor/bhh113. [DOI] [PubMed] [Google Scholar]
- 32.Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968;195:215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riesenhuber M, Poggio T. Models of object recognition. Nat Neurosci. 2000;3(Suppl):1199–1204. doi: 10.1038/81479. [DOI] [PubMed] [Google Scholar]