Abstract
We have investigated the adaptation of the light-harvesting system of the photosynthetic bacterium Phaeospirillum molischianum (DSM120) to very low light conditions. This strain is able to respond to changing light conditions by differentially modulating the expression of a family of puc operons that encode for peripheral light-harvesting complex (LH2) polypeptides. This modulation can result in a complete shift between the production of LH2 complexes absorbing maximally near 850 nm to those absorbing near 820 nm. In contradiction to prevailing wisdom, analysis of the LH2 rings found in the photosynthetic membranes during light adaptation are shown to have intermediate spectral and electrostatic properties. By chemical cross-linking and mass-spectrometry we show that individual LH2 rings and subunits can contain a mixture of polypeptides derived from the different operons. These observations show that polypeptide synthesis and insertion into the membrane are not strongly coupled to LH2 assembly. We show that the light-harvesting complexes resulting from this mixing could be important in maintaining photosynthetic efficiency during adaptation.
Keywords: chromatic adaptation, membrane protein, photosynthetic bacteria, Rhodospirillum molischianum
Photosynthetic bacteria are able to efficiently convert incident light into chemical potential energy via a light-driven cyclic electron transfer pathway (1). This process depends on the efficient absorption of incident light energy and the transfer of this energy to the reaction center. Measurements of the quantum efficiency of the light-harvesting apparatus indicate that very little energy is lost during the migration of the exciton from the point of initial absorption to the reaction center (1). The light-harvesting system of many bacteria contains two different pigment–protein complexes. The core complex contains one or two reaction centers in close association with a light-harvesting system (LH1) and a smaller peripheral complex (LH2) that transfers the absorbed excitation energy to the core complex. Both types of light-harvesting complex, LH1 and LH2, are constructed from oligomers of a similar basic subunit containing 2 polypeptides (α and β) with associated bacteriochlorophyll and carotenoid pigments. Their structures are known from x-ray crystallography (2–4), electron crystallography (5), and more recently from atomic force microscopy (6–11).
The LH2 complexes are circular oligomers of typically 9 αβ subunits (3), in which 18 long wavelength-absorbing bacteriochlorophyll molecules are sandwiched between the inner ring of α-polypeptides and an outer-ring of β-polypeptides. A second series of 9 bacteriochlorohpyll molecules, closer to the cytoplasmic membrane surface, occupies gaps between the β-polypeptides. This general architecture is slightly variable, with in particular the number of monomeric units forming the circular architecture depending on the species and environmental conditions (2, 7, 12, 13). In the species studied in this work, Phaeospirillum (Ph.) molischianum, the LH2 usually contain 8 αβ subunits.
The structure of core complexes is somewhat more variable (14). The simplest are monomeric complexes, found in the Blastochloris, Rhodospirillum (Rsp.) (11, 15), and Phaeospirillum (16) species, in which a monomeric reaction center is completely surrounded by 16 αβ-subunits forming a closed ring. Two variations of this theme are known: Complexes containing a defect in ring are found in Rhodopseudomonas (Rps.) palustris (4, 7), and dimeric complexes, in which an s-shaped antenna array harbors two reaction centers, are found in Rhodobacter (Rb.) (16, 17).
The organization and composition of the photosynthetic apparatus is controlled by environmental conditions. In many species of bacteria, adaptation to low light intensities involves changing the proportions of core complexes and LH2. Extra LH2 are synthesized to increase the effective reaction center absorption cross section (18, 19). This modification is associated with the formation of large para-crystalline domains of LH2 in the photosynthetic membranes (20). Such domains have now been observed in several different bacteria including Ph. molischianum (6), Rsp. photometricum (11), and Rps. palustris (7).
In certain species of bacteria, notably Rps. palustris and some strains of Ph. molischianum and Rps. acidophila, adaptation to low light intensities is possible by a second mechanism. Differential expression of multiple different puc operons results in a modification of the spectral properties of the LH2 complexes present. The genes expressed under very low light conditions often give rise to light-harvesting complexes with having near-IR absorption maxima at 800 nm and 820 nm (21, 22) as opposed to the more standard complexes with absorption maxima at 800 nm and 850 nm. The molecular origin of the spectral difference between B800–820 and B800–850 type LH2 is well understood. Hydrogen bonds between the α- and β-polypeptides and the C2-acetyl groups of the bacteriochlorophyll (23–25), or rotation of the C2-acetyl groups (26) are responsible for tuning the energy of the bacteriochlorophyll Qy transition moment.
The current description of the light-harvesting system during this adaptation to very low light suggests that these complexes with blue-shifted absorption spectra [frequently called LH3 (22), or even LH4 (27)] form an ultraperipheral light-harvesting system that transfers energy to the more standard B800–850 absorbing LH2 and then to LH1 and the reaction center. The energetic funnel represented by this system with four levels (LH3, LH2, LH1, and the reaction center) assures the efficiency of light-harvesting. This description relies implicitly on a tight coupling between the biosynthesis of the individual polypeptides and the assembly of the 16 (or 18 in other species) polypeptides and 24 (or 27) bacteriochlorophylls into the LH2, to ensure that the complexes remain spectrally pure. Furthermore, this description suggests that LH3 function depends on the presence of LH2 to shuttle energy to the reaction center. Finally, the description depends on a specific organization of the different LH2-like complexes in the membrane. The assembly of the LH2 complexes depends on the activity of a specific protein PucC that is a member of the major facilitator superfamily. Because this protein has been shown to play a chaperone-like role (28) it could play a role in ensuring the coupling of synthesis and assembly. A coupling of assembly and polypeptide synthesis is attractive because it provides an explication for the lack of complexes intermediate between LH1 and LH2.
In this article, we show that this description of the light-harvesting system is incorrect. In Ph. molischianum, the light-harvesting system switches from a B800–850 type to a B800–820 type, and this switch can apparently be complete. Furthermore, under conditions where both types of complexes are synthesized there is not tight coupling between polypeptide synthesis and LH2 assembly. As a result, complexes with polypeptides from multiple operons are formed that have intermediate spectral and physico-chemical properties.
Results and Discussion
In response to low light intensity Ph. molischianum strain DSM120 progressively shifts from the production of B800–850 type LH2 to B800–820 type LH2 complexes (Fig. 1A). A culture initially grown at an intensity of approximately 20 Wm-2 shows an absorption spectrum typical of cells producing B800–850 type LH2 complexes (Red Line). In contrast, cells adapted to very low light intensities (< 1 Wm-2) show a spectrum associated with the production of B800–820 complexes (Blue Line). During adaptation to these low light intensities or at intermediate intensities cells with intermediate spectra can be observed (Green Line), indicating the presence of both types of LH2. It is worth noting that in the case of the very low light adapted cells (Blue Line), there is little evidence for B800–850 type LH2 complexes, the entire production having apparently shifted to the shorter wavelength absorbing form. This situation is somewhat different to that reported previously for another strain of Ph. molischianum (DSM119) in which production of the B800–820 type LH2 was only observed after deletion of the puc operon encoding the B800–850 type LH2 (29). However, Fig. 1A is in agreement with the spectra observed by Gibbs et al. (19). This shift of absorption is associated with a modification in the LH2 polypeptides synthesized through differential expression of the various puc operons. Analysis of genomic DNA from Ph. molischianum DSM120 by Southern blotting (Fig. S1) and genomic sequencing (SI Text) indicates the presence of six puc clusters in this strain. As in several other species (29, 30) several of the predicted polypeptides (PucA2 and PucA3) have the same amino acid sequence.
Fig. 1.
Low light, intermediate light, and high light LH2 types. (A) Spectra of cells grown under normal (approximately 20 Wm-2) and low light (< 1 Wm-2) conditions. Under normal conditions, the LH2 complexes give rise to peaks near 800 and 850 nm (Red Line), whereas under low light conditions the peaks are near 800 and 820 nm (Blue Line). A cell spectrum taken during adaptation in which both types of LH2 complex are found is shown in green. (B) Mass spectra of polypeptides present in LH2 isolated from normal (Red), low light (Blue), and intermediate (Green) conditions. The spectra have been offset vertically to aid the reader.
Examination of the polypeptides produced under the different conditions by mass spectrometry of isolated LH2 complexes (Fig. 1B) shows that under high light conditions 3 major polypeptide species can be distinguished (Red Line), with relative molecular masses of 5150.3 (β1), 5349.4 (β3), and 5994.5 (α1), corresponding to 2 β- and 1 α-polypeptide (Table S1). The β-polypeptide peaks appear as doublets with a difference of 14 Da at a mass about 14 Da above that expected from the sequence. This is attributed to mono- and dimethyl adducts. The α-polypeptide peak is again a doublet, here with 22 Da separating the 2 masses, suggesting H+ and Na+ adducts. Under low light conditions two major polypeptide species, with molecular masses of 5235.5 (β2) and 6109.0 Da (α2), are present corresponding to a single B800-820 type αβ-pair (Blue Line) that is assembled under these conditions. Once again there are additional peaks separated by 14 (β2) or 22 Da (α2). There also several minor peaks that can be attributed to the other polypeptides (α1, β1, and β3). The LH2 isolated from cells grown at intermediate conditions shows 5 peaks (Green Line) corresponding to those observed from high light and low light adapted cells. This indicated a progressive modification in the expression of the puc operons and the resulting polypeptide composition of the LH2 complexes during adaptation. There was no evidence of expression of any of the other Puc polypeptides under our growth conditions.
An important question that has not yet been directly addressed is how individual LH2 oligomers vary in cells producing a variety of different light-harvesting polypeptides. In the literature, it is generally assumed that LH2 complexes contain polypeptides from a single puc operon (22, 27). However, there is no direct evidence for this. As many species are able to produce multiple LH2 polypeptides, the formation of rings containing polypeptides from a single operon requires a tight coupling between membrane insertion and assembly, presumably via a specific assembly machinery capable of assuring homogeneity. This role is usually associated with the activity of the PucC chaperone (28).
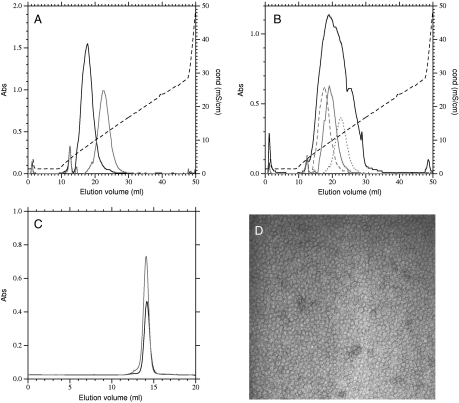
In Ph. molischianum adapted to high light or very low light, the different polypeptides have different charge distributions and elute differently from an anion-exchange column. In Fig. 2A we show typical elution profiles of B800–850 type LH2 complexes (Black Line) and B800–820 type complexes (Gray Line). The B800–820 complex elutes as a symmetric peak centered at a conductivity of 12.0 mS/cm whereas the B800–850 type elutes as a slightly less symmetric peak and centered at a conductivity of 9.0 mS/cm. This difference in elution is ample for resolving the peaks on this type of column.
Fig. 2.
Purification and analysis of LH2 complexes. (A) Elution profile of B800–850 type (Black) and B800–820 type (Gray) LH2 complexes from a resource Q anion-exchange column. Complexes were loaded onto the column, washed with 20 mM Tris pH8.0 0.05% DM, and separated with a 200–500 mM NaCl gradient in the same buffer. (B) Elution profile of LH2 complexes from cells synthesizing a mixture of B800–820 and B800–850 type complexes (Black), using the same conditions as above. Three of the colored fractions were rechromatographed (Gray) after desalting and all fractions were eluted as a symmetric peak centered on the original fraction. (C) Size-exclusion chromatography of B800–820 type (Dark Gray), B800–850 type (Black) and mixed-type LH2 (Light Gray) complexes. Complexes isolated by anion-exchange chromatography were concentrated and loaded onto a Superose 6 (GE Healthcare) column eluted at 0.2 ml/ min with 20 mM Tris pH8.0 containing 0.05% DM. All complexes eluted at the same volume, corresponding to monomeric complexes. (D) Transmission electron micrograph of negatively stained LH2 complexes purified by anion-exchange chromatography.
Attempts to isolate pure B800–850 and B800–820 type LH2 from cell expressing both types of polypeptide are frustrating. When LH2 complexes from cells expressing both types of peripheral antenna complex are purified on the same column they elute as a broad, poorly resolved peak (Fig. 2B, Black Line). This peak is centered at the intermediate conductivity of 10.5 mS/cm. Such chromatographic behavior could be due to poor resolution of complexes with intermediate properties. To test this, fractions from the initial chromatography were desalted and rechromatographed on the same column. In each case (Gray Lines) the sample eluted as a symmetric peak at the same position as in the first chromatography. Thus the apparent poor resolution is due to the presence of multiple unresolved forms with intermediate charge characteristics.
A second possible cause of such poor resolution is the presence of oligomeric stacks of LH2 complexes containing a mixture of B800–820 and B800–850 type complexes. Such aggregates have previously been observed by electron microscopy in several species including Rubruvivax gelatinosus (31). To exclude this possibility we subjected fractions from the ion-exchange chromatography to size-exclusion chromatography (Fig. 2C), all eluted at exactly the same position, and this corresponds to the result expected for one LH2 complex on the basis of hydrodynamic radius. There was no evidence for any dissociation into smaller complexes or aggregation into larger complexes of the LH2 in the buffer conditions that we use for chromatography. To further confirm this result, we have also observed purified LH2 samples by transmission electron microscopy after negative staining (Fig. 2D). Again, many individual rings can be observed, but not the characteristic chains of complexes that are observed in aggregated complexes (31).
A third possible cause of the poor resolution observed in Fig. 2B might be the presence of multiple charge isomers due to the differential trapping of charged phsopholipids. To search for phospholipids the LH2 samples where checked for their phospholipid content. Organic solvent extracts were concentrated and separated by thin layer chromatography prior to staining for phosphate, primary amines, and charring (Fig. S2). No phospholipids were detected in LH2 samples and the detection limit was estimated to be < 1 phospholipid molecule per LH2 ring. This is in contrast to the LH2 of Rps. acidophila in which associated phosphatidyl-ethanolamine can be detected after purification (32). Thus it seems unlikely that poor resolution on ion-exchange chromatography is due to the presence of heterogeneity caused by cosolubilized phospholipids.
This collection of chromatographic results provides strong evidence for the presence of LH2 containing mixtures of polypeptides from different operons. This suggests that there is only a weak coupling between polypeptide synthesis and LH2 assembly. Thus the LH2 rings can be assembled from polypeptides synthesized from different genes and operons.
Cross-Linking and Mass Spectrometry.
To confirm that the LH2 rings contain mixtures of polypeptides from different operons we analyzed (by MS) isolated LH2 samples after cross-linking. In dilute solution LH2 polypeptides can be cross-linked by intracomplex links. Thus by identifying the different cross-linked products we can identify the polypeptides initially present in a single LH2 complex. This is not possible in the membrane where the high protein density (6, 11) favors intercomplex cross-linking.
Two different cross-linking reagents were used (Fig. 3A): Dithio-bis-succinimidyl-propionate (DSP) and ethyleneglycol-bis-succinimidyl-succinate (EGS). Both these chemicals are able to cross-link primary amine groups, originating from a free polypeptide N-terminal or from lysine residues. DSP is able to cross-link groups that are 12.0 Å apart whereas EGS can span 16.1 Å. The cross-linked products were then analyzed by MALDI-TOF MS. The detection of products was particularly difficult, presumably because the reaction of the amine groups reduces the charge and increases the hydrophobicity of the polypeptides. Both these effects hinder their observation by MS.
Fig. 3.
Cross-linking of LH2 complexes. (A) Structures and reactions of the two cross-linking agents used (DSP and EGS). (B) αβ-heterodimers observed after DSP cross-linking of a low light adapting sample containing 5 different polypeptides (α1, α2, β1, β2, and β3). The numbered peaks 0–6 correspond to masses (see text and Table S2). (C) αα- and ββ-homodimers observed after EGS cross-linking of a sample containing three different polypeptides (α1, β1, and β2). The peaks are numbered 0–7 and in each case the pairs, labeled a and b, differ by the mass of one cross-link (Table S2).
Treatment with DSP allowed the detection of multiple αβ-heterodimers (Fig 3B, Table S2). The major adducts formed appear to be doubly cross-linked dimers containing one α- and one β-polypeptide, and two cross-links, with some minor contributions from species containing one cross-link, or that have reacted with the Tris used to quench the reaction. In Fig. 3B the spectrum from a sample containing a mixture of B800–820 and B800–850 type complexes is shown. The major peaks, 1 and 4, are attributed to the cross-links products α1β1 and α1β3, respectively containing two cross-links. Whereas more minor contributions are visible, though poorly resolved, and attributed to the products α1β2, α2β1, α2β2, α2β3 (peaks 2, 3, 5 and 6 respectively) again with two cross-links. When B800–850 type LH2 are cross-linked the three polypeptides present (α1, β1, and β3) give rise to the two possible αβ-dimers in the mass-spectrum (peaks 1 and 4).
In contrast to DSP, EGS favored the detection of αα- and ββ-homodimers (Fig. 3C, Table S2). An analysis is shown of B800–850 type complexes containing the polypeptides α1, β1, and β3. In this sample four different homodimers are observed: α1α1, peak 6; β1β1, peak 1; β1β3, peak 2; and β3β3, peak 3. The spectrum is somewhat complicated by the presence of cross-linked products containing one (series a–major) and two (series b–minor) cross-links, differing by a constant mass increment of 226.4 Da. The bands 4 and 5 are assigned to the hetero-dimeric species α1β1 and α1β3 respectively, again visible with one or two cross-links. In samples containing all 5 polypeptides, the 9 different homodimer species are present, though not always well resolved. Thus not only can the α- and β-polypeptides within an LH2 be derived from different operons, but the polypeptides that make up the octameric α- or β-rings in Ph. molischianum can derive from different operons. Importantly, the cross-linking results were not dependent on the protein concentration used with similar spectra obtained when cross-linking was performed at protein concentrations of 100 μ/ml and 3 mg/ml.
Because we are able to identify the various different αβ- αα- and ββ- pairs, the individual octameric rings cannot be formed uniquely from 8 copies of one α-polypeptide and 8 copies of one β-polypeptide. Rather, in agreement with the chromatographic results above, the LH2 rings appear to contain a mixture of α-polypeptides and a mixture of β-polypeptides from different operons. In particular, under certain conditions, the rings can contain a mixture of B800–850 and B800–820 type polypeptides. This result implies that synthesis of the different polypeptides and the assembly of the complexes are only weakly coupled. It is worth noting that in our mass spectrometric analysis there is no sign of complexes containing a mixture of LH1 and LH2 polypeptides. This implies that the properties of these polypeptides must be different enough to ensure little or no mixing even in the context of weak coupling between synthesis and assembly of LH2 complexes.
Functional Consequences.
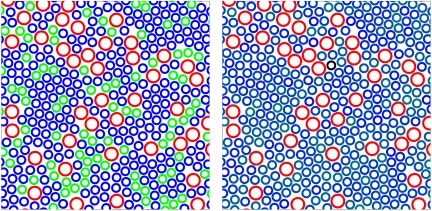
The presence of mixed LH2 complexes containing polypeptides of different types is expected to have consequences on the efficiency of light-harvesting by the photosynthetic membranes. In Fig. 4 we illustrate one of the consequences on the organization of LH1 (Larger Rings) and LH2 complexes (Smaller Rings) within a photosynthetic membrane of Ph. molischianum, as has been observed by atomic force microscopy (AFM) (6). Within these membranes the role of the LH2 complexes is to absorb incident radiation and transfer the energy to the reaction center situated at the center of the LH1 complexes.
Fig. 4.
Energy migration in light-harvesting arrays. (Left) An arrangement of complexes in a Ph. molischianum membrane derived from those observed by AFM (6). The LH2 complexes (Smaller Rings) have been colored blue to represent B800–820 complexes, except for a random 25% that have been colored green to represent B800–850 complexes. The LH1-reaction center contains core complexes, shown as larger red rings. Note that several groups of green rings are completely encircled by blue rings. (Right) The same arrangement of complexes as in the left panel, but in this case each LH2 is colored in a shade representing the number of B800–850 type polypeptides it contains. The absence of clear color contrast in this panel reflects the absence of energetic traps.
In the left panel we illustrate a membrane in which 25% of the LH2 complexes are were arbitrarily assigned to the B800–850 type (Green), whereas the other 75% are of B800–820 type (Blue). This corresponds to the proportions observed in some samples (Fig. 1A) investigated during this study. Some of the B800–850 type complexes (24 of 110) lack a connection to the core complexes, and are surrounded by 800–820 type complexes. Thus, for these complexes, energy transfer to the reaction center would involve a large uphill step from an 850 nm absorbing chromophore to an 820 nm absorbing chromophore, of approximately twice the available thermal energy. Such complexes are therefore expected to be able to act as traps within LH2 arrays. Excitons on such complexes have insufficient energy to be transferred efficiently to B800–820 complexes and then to the core complexes. B800–850 complexes could thus divert energy absorbed in the antenna array from photochemistry to fluorescence and heat, severely reducing the efficiency of light collection in such membranes.
In the second panel, we show the same arrangement in which each complex has been colored according to the mixture of B800–820 and B800–850 type polypeptides it is considered to have. A random 25% of polypeptides are taken as B800–850 type. The spectra of such complexes are thus intermediate between B800–820 and B800–850 type because the strong pigment coupling results in a delocalized exciton (1, 33). In contrast to the left panel, there are no traps formed in the antenna array and it is able to operate as an efficient light-harvesting system. Recently it was observed that LH2 complexes could form large domains under certain conditions (20) and it is suggested that this is partly to aid quinone diffusion through the crowded membrane (34). However, such domains could accumulate many B800–850 type traps, and this would reduce light-harvesting efficiency. We suggest that the observed weak coupling between polypeptide synthesis and membrane insertion is necessary to maintain light-harvesting efficiency during adaptation by the synthesis of different types of LH2.
Conclusions
It has been assumed for many years that the synthesis and assembly of light-harvesting complexes are tightly coupled. Such a coupling would prevent the mixing of LH1 and LH2 complexes to form heterogeneous or intermediate complexes (35) during synthesis but also assure that pure high light (B800–850) and pure low light (B800–820) LH2 complexes exist side by side. Such intermediate complexes have not, to our knowledge, been reported in the absence of strongly denaturing detergents. Here we observe heterogeneous LH2 complexes containing polypeptides from several different gene pairs, synthesized by different ribosomes from different messenger RNAs and therefore inserted independently into the membrane. This observation indicates that synthesis and assembly of LH2 complexes are not coupled. We suggest that the presence of such mixed complexes can be of considerable importance for the function of the photosynthetic system. Recently, while we were revising this article, such heterogeneous complexes were inferred on the basis of single molecule spectroscopic measurements of complexes isolated from Rps. acidophila (36). Interestingly. the specific organization proposed implies a thermodynamically controlled assembly process rather than a kinetically controlled one, in complete agreement with our observations. We believe that these observations can probably be generalized. Many membrane proteins are oligomers of one or several polypeptides (37), and frequently these polypeptides are members of closely related multigene families. The presence of such heterogenity will be a challenge for structural biology where ensemble techniques, such as crystallography or NMR, deal poorly with a stochastically mixed sample. In this context it is perhaps pertinent that only a very low resolution (8 Å) x-ray structure was obtained of the Rps. plaustris low light LH3 (22), possibly because these complexes contain a mixture of different polypeptides. However, such mixed systems may be of considerable importance n biology where the properties of mixed complexes can be different from either pure complex and possibly include nonlinear effects.
Materials and Methods
Complex Purification.
Cells were grown and membranes isolated as previously described (38) on modified Hutner medium (39) and harvested in late log phase (SI Text). For the purification of LH2 complexes isolated membranes were dialysed against Tris20 mM pH8 for 2 h to remove the majority of the sucrose. They were then solubilized by the addition of n-dodecyl-β-D-maltoside (DodM) to a final concentration of 1% and mixed gently for 1 h at 4 °C, protected from the light. The insolubilized material was removed by centrifugation for 45 min at 300,000 xg and the solubilized proteins loaded onto a discontinuous sucrose density gradient: 0.2, 0.4, 0.8, and 1.0 M sucrose in 20 mM Tris pH 8.0 with 0.05% DodM. After centrifugation overnight at 186,000 xg the red band at the interface between 0.4 and 0.8 M sucrose corresponds to the LH2 complexes. LH2 complexes were further purified and analyzed by ion-exchange chromatography, performed using a ResourceQ anion-exchange column (GE Healthcare) (SI Text). The complexes are very stable in DodM and showed no changes in pigment composition or spectrum during purification or after storage, indicating that dissociation and pigment loss are not a problem.
Cross-Linking.
For cross-linking with DSP, LH2 was transferred by dialysis to 20 mM Na phosphate buffer pH 7.3 containing 150 mM NaCl, 0.05% DodM. A 50 μl sample of LH2, adjusted in this buffer to a concentration of 1 mg/ml, was incubated at 37 °C for 10 min prior to the addition of 2.5 μl of 200 mM DSP (Pierce) in DMSO. The reaction was allowed to proceed for 20 min at 37 °C, and then the reaction stopped by the addition of 2.5 μl of 1 M Tris pH7.2 (40). Cross-linking with EGS (Pierce) was performed similarly, except that the buffer used throughout was 10 mM Na phosphate pH6.3, 0.05% DodM.
Mass-Spectrometry.
Mass spectra were obtained using standard methods on Ultraflex II and Microflex II MALDI-TOF mass spectrometers from Bruker Daltonics (see SI Text).
Models.
The Model membrane shown in Fig. 4 was constructed from images of Ph. molischianum membranes obtained by AFM as described previously (6) (SI Text).
Supplementary Material
Acknowledgments.
This work was supported by a grant from the French Ministry of Research (C.M.A.), the Centre National de la Recherche Scientifique (CNRS), and the Agence National de la Recherche Grant ANR-PNANO-06-0089.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914854107/DCSupplemental.
References
- 1.Hu X, Ritz T, Damjanović A, Autenrieth F, Schulten K. Photosynthetic apparatus of purple bacteria. Q Rev Biophys. 2002;35:1–62. doi: 10.1017/s0033583501003754. [DOI] [PubMed] [Google Scholar]
- 2.Koepke J, Hu X, Muenke C, Schulten K, Michel H. The crystal structure of the light-harvesting complex II (B800-850) from Rhodospirillum molischianum. Structure. 1996;4:581–97. doi: 10.1016/s0969-2126(96)00063-9. [DOI] [PubMed] [Google Scholar]
- 3.McDermott G, et al. Crystal structure of an integral membrane light-harvesting complex from photosynthetic bacteria. Nature. 1995;374:517–521. [Google Scholar]
- 4.Roszak AW, et al. Crystal structure of the RC-LH1 core complex from Rhodopseudomonas palustris. Science. 2003;302:1969–1972. doi: 10.1126/science.1088892. [DOI] [PubMed] [Google Scholar]
- 5.Karrasch S, Bullough PA, Ghosh R. The 85 A projection map of the light-harvesting complex I from Rhodospirillum rubrumreveals a ring composed of 16 subunits. EMBO J. 1995;14:631–8. doi: 10.1002/j.1460-2075.1995.tb07041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonçalves RP, Bernadac A, Sturgis JN, Scheuring S. Architecture of the native photosynthetic apparatus of Phaeospirillum molischianum. J Struct Biol. 2005;152:221–8. doi: 10.1016/j.jsb.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Scheuring S, Gonçalves RP, Prima V, Sturgis JN. The photosynthetic apparatus of Rhodopseudomonas palustris: Structures and organization. J Mol Biol. 2006;358:83–96. doi: 10.1016/j.jmb.2006.01.085. [DOI] [PubMed] [Google Scholar]
- 8.Scheuring S, Reiss-Husson F, Engel A, Rigaud J.-L, Ranck JL. High resolution topographs of the Rubrivivax gelatinosuslight-harvesting complex 2. EMBO J. 2001;20:3029–35. doi: 10.1093/emboj/20.12.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheuring S, et al. AFM characterization of tilt and intrinsic flexibility of Rhodobacter sphaeroideslight harvesting complex 2 (LH2) J Mol Biol. 2003;325:569–80. doi: 10.1016/s0022-2836(02)01241-x. [DOI] [PubMed] [Google Scholar]
- 10.Scheuring S, et al. Nanodissection and high-resolution imaging of the Rhodopseudomonas viridisphotosynthetic core complex in native membranes by AFM atomic force microscopy. Proc Natl Acad Sci USA. 2003;100:1690–3. doi: 10.1073/pnas.0437992100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheuring S, et al. Watching the photosynthetic apparatus in native membranes. Proc Natl Acad Sci USA. 2004;101:11293–7. doi: 10.1073/pnas.0404350101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kereche S, et al. The peripheral light-harvesting complexes from purple sulfur bacteria have different ‘ring’ sizes. FEBS Lett. 2008;582:3650–6. doi: 10.1016/j.febslet.2008.09.050. [DOI] [PubMed] [Google Scholar]
- 13.Scheuring S, Rigaud J.-L, Sturgis JN. Variable LH2 stoichiometry and core clustering in native membranes of Rhodospirillum photometricum. EMBO J. 2004;23:4127–33. doi: 10.1038/sj.emboj.7600429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sturgis J, Niederman R. Atomic force microscopy reveals multiple patterns of antenna organization in purple bacteria: Implications for energy transduction mechanisms and membrane modeling. Photosynth Res. 2008;95:269–278. doi: 10.1007/s11120-007-9239-0. [DOI] [PubMed] [Google Scholar]
- 15.Walz T, Ghosh R. Two-dimensional crystallization of the light-harvesting I-reaction center photounit from Rhodospirillum rubrum. J Mol Biol. 1997;265:107–11. doi: 10.1006/jmbi.1996.0714. [DOI] [PubMed] [Google Scholar]
- 16.Scheuring S. AFM studies of the supramolecular assembly of bacterial photosynthetic core-complexes. Curr Opin Chem Biol. 2006;10:387–93. doi: 10.1016/j.cbpa.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Bahatyrova S, et al. The native architecture of a photosynthetic membrane. Nature. 2004;430:1058–62. doi: 10.1038/nature02823. [DOI] [PubMed] [Google Scholar]
- 18.Aargaard J, Sistrom W. Control of synthesis of reaction center bacteriochlorophyll in photosynthetic bacteria. Photochem Photobiol. 1972;15:209–225. doi: 10.1111/j.1751-1097.1972.tb06240.x. [DOI] [PubMed] [Google Scholar]
- 19.Gibbs SP, Sistrom WR, Worden PB. The photosynthetic apparatus of Rhodospirillum molischianum. J Cell Biol. 1965;26:395–412. doi: 10.1083/jcb.26.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheuring S, Sturgis JN. Chromatic adaptation of photosynthetic membranes. Science. 2005;309:484–7. doi: 10.1126/science.1110879. [DOI] [PubMed] [Google Scholar]
- 21.Evans M, Hawthornthwaite AM, Cogdell RJ. Isolation and characterisation of the different B800-850 light-harvesting complexes from low- and high-light grown cells of Rhodopseudomonas palustris, strain. Biochim Biophys Acta. 1990;1016:71–76. [Google Scholar]
- 22.McLuskey K, Prince SM, Cogdell RJ, Isaacs NW. The crystallographic structure of the B800-820 LH3 light-harvesting complex from the purple bacteria Rhodopseudomonas acidophilastrain 7050. Biochemistry. 2001;40:8783–9. doi: 10.1021/bi010309a. [DOI] [PubMed] [Google Scholar]
- 23.Fowler GJ, Visschers RW, Grief GG, van Grondelle R, Hunter CN. Genetically modified photosynthetic antenna complexes with blueshifted absorbance bands. Nature. 1992;355:848–50. doi: 10.1038/355848a0. [DOI] [PubMed] [Google Scholar]
- 24.Sturgis JN, Jirsakova V, Reiss-Husson F, Cogdell RJ, Robert B. Structure and properties of the bacteriochlorophyll binding site in peripheral light-harvesting complexes of purple bacteria. Biochemistry. 1995;34:517–23. doi: 10.1021/bi00002a016. [DOI] [PubMed] [Google Scholar]
- 25.Sturgis JN, Robert B. Pigment binding-site and electronic properties in light-harvesting proteins of purple bacteria. J Phys Chem B. 1997;101:7227–7231. [Google Scholar]
- 26.Cogdell RJ, Howard TD, Isaacs NW, McLuskey K, Gardiner AT. Structural factors which control the position of the Q(y) absorption band of bacteriochlorophyll a in purple bacterial antenna complexes. Photosynth Res. 2002;74:135–41. doi: 10.1023/A:1020995224156. [DOI] [PubMed] [Google Scholar]
- 27.de Ruijter WPF, et al. Observation of the energy-level structure of the low-light adapted B800 LH4 complex by single-molecule spectroscopy. Biophys J. 2004;87:3413–20. doi: 10.1529/biophysj.104.044719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaschke PR, Leblanc HN, Lang AS, Beatty JT. The PucC protein of Rhodobacter capsulatusmitigates an inhibitory effect of light-harvesting 2 alpha and beta proteins on light-harvesting complex 1. Photosynth Res. 2008;95:279–84. doi: 10.1007/s11120-007-9258-x. [DOI] [PubMed] [Google Scholar]
- 29.Sauer PR, Lottspeich F, Unger E, Mentele R, Michel H. Deletion of a B800–850 light-harvesting complex in Rhodospirillum molischianumDSM119 leads to “revertants” expressing a B800–820 complex: Insights into pigment binding. Biochemistry. 1996;35:6500–6507. doi: 10.1021/bi9528255. [DOI] [PubMed] [Google Scholar]
- 30.Tadros MH, Waterkamp K. Multiple copies of the coding regions for the light-harvesting B800–850 alpha- and beta-polypeptides are present in the Rhodopseudomonas palustris genome. EMBO J. 1989;8:1303–8. doi: 10.1002/j.1460-2075.1989.tb03509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jirsakova V, Reiss-Husson F, Ranck JL. Oligomeric state of the light-harvesting complexes B800–850 and B875 from purple bacterium Rubrivivax gelatinosusin detergent solution. Biochim Biophys Acta. 1996;1277:150–160. [PubMed] [Google Scholar]
- 32.Russell NJ, Coleman JK, Howard TD, Johnston E, Cogdell RJ. Rhodopseudomonas acidophilastrain 10050 contains photosynthetic LH2 antenna complexes that are not enriched with phosphatidylglycerol, and the phospholipids have a fatty acyl composition that is unusual for purple non-sulfur bacteria. Biochim Biophys Acta. 2002;1556:247–53. doi: 10.1016/s0005-2728(02)00369-9. [DOI] [PubMed] [Google Scholar]
- 33.Sturgis JN, Robert B. The role of chromophore coupling in tuning the spectral properties of peripheral light-harvesting protein of purple bacteria. Photosynth Res. 1996;50:5–10. doi: 10.1007/BF00018216. [DOI] [PubMed] [Google Scholar]
- 34.Scheuring S, Sturgis JN. Dynamics and diffusion in photosynthetic membranes from Rhodospirillum photometricum. Biophys J. 2006;91:3707–17. doi: 10.1529/biophysj.106.083709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Broglie RM, et al. Isolation and characterization of the pigment–protein complexes of Rhodopseudomonas sphaeroides by lithium dodecyl sulfate/polyacrylamide gel electrophoresis. Proc Natl Acad Sci USA. 1980;77:87–91. doi: 10.1073/pnas.77.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brotosudarmo THP, et al. Single-molecule spectroscopy reveals that individual low-light LH2 complexes from Rhodopseudomonas palustris 21.6. have a heterogeneous polypeptide composition. Biophys J. 2009;97:1491–500. doi: 10.1016/j.bpj.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arce J, Sturgis JN, Duneau J-P. Dissecting membrane protein architecture: An annotation of structural complexity. Biopolymers. 2009;91:815–821. doi: 10.1002/bip.21255. [DOI] [PubMed] [Google Scholar]
- 38.Mascle-Allemand C, Lavergne J, Bernadac A, Sturgis JN. Organisation and function of Phaeospirillum molischianum photosynthetic apparatus. Biochim Biophys Acta. 2008;1777:1552–1559. doi: 10.1016/j.bbabio.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Clayton RK. Protein synthesis in the induced formation of catalase inRhodopseudomonas spheroides. J Biol Chem. 1960;235:405–7. [PubMed] [Google Scholar]
- 40.Moskalenko AA, Toropygina OA. Chemical crosslinking studies of the isolated light-harvesting B800–850 complex of Chromatium minutissimum. FEBS Lett. 1993;320:28–30. doi: 10.1016/0014-5793(93)81650-o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.