Abstract
Niemann-Pick type C disease (NPC) is a lysosomal storage disorder causing accumulation of unesterified cholesterol in lysosomal storage organelles. Recent studies have shown that hydroxypropyl-β-cyclodextrin injections in npc1−/− mice are partially effective in treating this disease. Using cultured fibroblasts, we have investigated the cellular mechanisms responsible for reduction of cholesterol accumulation. We show that decreased levels of cholesterol accumulation are maintained for several days after removal of cyclodextrin from the culture medium. This suggests that endocytosed cyclodextrin can reduce the cholesterol storage by acting from inside endocytic organelles rather than by removing cholesterol from the plasma membrane. To test this further, we incubated both NPC1 and NPC2 mutant cells with cholesterol-loaded cyclodextrin for 1 h, followed by chase in serum-containing medium. Although the cholesterol content of the treated cells increased after the 1-h incubation, the cholesterol levels in the storage organelles were later reduced significantly. We covalently coupled cyclodextrin to fluorescent dextran polymers. These cyclodextrin–dextran conjugates were delivered to cholesterol-enriched lysosomal storage organelles and were effective at reducing the cholesterol accumulation. We demonstrate that methyl-β-cyclodextrin is more potent than hydroxypropyl-β-cyclodextrin in reducing both cholesterol and bis(monoacylglycerol) phosphate accumulation in NPC mutant fibroblasts. Brief treatment of cells with cyclodextrins causes an increase in cholesterol esterification by acyl CoA:cholesterol acyl transferase, indicating increased cholesterol delivery to the endoplasmic reticulum. These findings suggest that cyclodextrin-mediated enhanced cholesterol transport from the endocytic system can reduce cholesterol accumulation in cells with defects in either NPC1 or NPC2.
Keywords: acyl CoA:cholesterol acyl transferase; cholesterol accumulation; lysosomal storage organelles; bis(monoacylgycerol)phosphate, pinocytosis
Niemann-Pick type C (NPC) disease is an autosomal recessive lysosomal storage disorder. Abnormal accumulations of unesterified cholesterol and other lipids [e.g., bis(monoacylglycerol)phosphate (BMP)] in late endosome/lysosome-like storage organelles (LSOs) are hallmarks of the disease (1, 2). Accumulation of cholesterol in the LSOs can be visualized by staining the cells with filipin, a fluorescent polyene antibiotic that binds unesterified cholesterol and can be used to assess cholesterol levels quantitatively (3–6). Clinical manifestations of NPC disease include neuronal degeneration, hepatosplenomegaly, and effects in other organs (1). No effective treatment is currently available for NPC disease (7), but recent studies involving administration of hydroxypropyl-β-cyclodextrin (HPβCD) in npc1−/− mice showed improvements in viability, hepatopathology, and neuropathology, including clearance of cholesterol and glycosphingolipids (8–12). These animal studies were not designed to address the molecular and cellular mechanisms by which cyclodextrins (CDs) could reduce the cholesterol accumulation in LSOs.
Studies using cultured cells have shown that various CDs can remove cholesterol from the plasma membrane (PM) (13–15). The cholesterol in LSOs of NPC-defective cells slowly exchanges with other pools of cellular cholesterol (16), so one possible mechanism would be that CD lowers PM cholesterol and slowly draws cholesterol from the LSOs. Removal of cholesterol from the PM is known to affect many signal transduction processes (17), and this could potentially be responsible for some of the beneficial effects seen in vivo. Alternatively, CDs, like other water-soluble molecules, are internalized by fluid phase pinocytosis and delivered to late endosomes/lysosomes (LE/LY) (18). Consistent with this, retention of CDs by cultured cells has been reported previously (15). Inside LE/LY, CDs could solubilize cholesterol so that it could either be delivered to the limiting membrane of the organelle or transported to other organelles.
Herein we present evidence that the reduction in cholesterol accumulation is due to CD action from inside LE/LY and not as a consequence of extraction of cholesterol from the PM. In agreement with a recent report (19), we also observed increased esterification of cholesterol by acyl CoA:cholesterol acyl transferase (ACAT) upon treatment of NPC mutant cells with CD. In addition, we show that methyl-β-cyclodextrin (MβCD) is more potent than HPβCD in reducing both cholesterol and BMP accumulation in NPC1 and NPC2 mutant fibroblasts.
Results
Effects of β-CDs on Human NPC Mutant Cells.
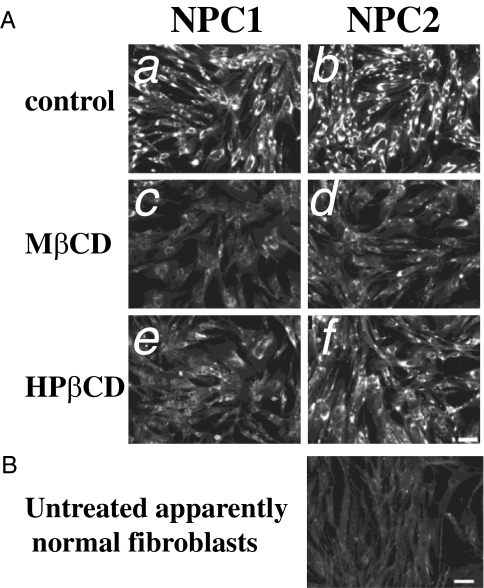
All of the studies showing CD efficacy in murine models of NPC1 used HPβCD for treatment (8–11). However, cell culture studies have shown that MβCD is more potent in extracting, delivering, and exchanging cholesterol than HPβCD (13–15). Therefore, we compared the efficacy of MβCD and HPβCD in reducing cholesterol accumulation in LSOs of NPC-defective cells. We examined the effects on NPC1 (GM03123) and NPC2 (GM18455) human fibroblasts after treatment with 300 μM CDs for 1 day. Treatment with either CD reduced the cholesterol accumulation, as detected by filipin labeling (Fig. 1).
Fig. 1.
Effect of CDs on cholesterol accumulation in NPC1- and NPC2-deficient cells. (A) Background and shading corrected images of untreated (a and b), MβCD-treated (c and d), or HPβCD-treated (e and f) GM03123 NPC1 cells (a, c, and e) or GM18455 NPC2 (b, d, and f) mutant cells. NPC mutant cells were treated with 300 μM CD for 1 day. (B) Untreated, apparently normal GM05659 cells, shown for comparison. Images were acquired as described previously (21). (Scale bars, 100 μm.)
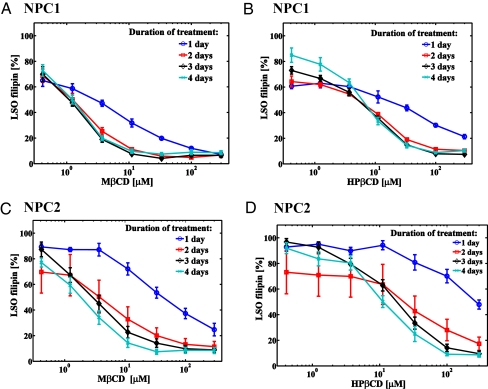
We used a previously described (20, 21) quantitative assay to measure filipin labeling of the LSOs in NPC-defective cells. The CD effects on LSO cholesterol accumulation in NPC1 and NPC2 mutant cells were dose and time dependent (Fig. 2). Both MβCD and HPβCD can reduce cholesterol accumulation in LSOs of NPC1 and NPC2 fibroblasts to near-normal levels using low micromolar concentrations. A comparison of LSO values for untreated NPC1, NPC2, and apparently normal cells is shown in Fig S1. Most of the decrease in LSO cholesterol occurred during the first 2 days of incubation. MβCD is more potent than HPβCD in eliciting this reduction (Table S1). This finding correlates well with previous studies, which showed that MβCD is more potent than HPβCD at extracting cholesterol from biological membranes (13–15).
Fig. 2.
Quantification of CD effects on cholesterol accumulation in NPC1 and NPC2 mutant cells. The filipin fluorescence in LSOs was measured in NPC1 (A and B) and NPC2 (C and D) mutant cells treated with either MβCD (A and C) or HPβCD (B and D) for 1–4 days. Data are presented as percentage of the average value for untreated controls in two independent experiments ± SEM (n = 6 for treated samples, n = 40 for control, where n is total number of wells per condition used for quantification).
We also used gas chromatography/mass spectrometry to show that overall cholesterol levels were reduced significantly upon CD treatment (Fig. S2).
Effects of β-CDs After Treatment: Withdrawal Studies.
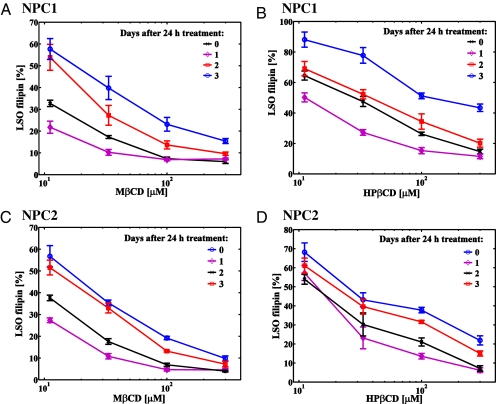
To determine whether the CD-mediated decrease in cholesterol accumulation would be sustained after the compound has been removed from the culture medium, we treated NPC1 and NPC2 mutant cells for 1 day with varying concentrations of MβCD or HPβCD (Fig. 3). The cells were then washed extensively to remove the compounds and returned to growth medium for up to 3 days. Cells that were allowed to grow for 1 more day after treatment with CD had a further decrease in LSO values when compared with cells that were treated for the same length of time but fixed immediately after treatment. After 2 and 3 days after treatment, cholesterol accumulation in LSOs of both NPC1 and NPC2 mutant cells started to increase, but there was still a significant reduction in the LSO cholesterol accumulation even after 3 days in serum-containing medium in the absence of extracellular CD.
Fig. 3.
Persistence of CD effects after treatment. NPC1 (A and B) and NPC2 (C and D) treated with various concentrations of MβCD (A and C) and HPβCD (B and D) for 1 day and then fixed (0 days after 24 h treatment), or rinsed extensively, and allowed to grow in normal growth medium for up to 3 additional days after CD removal. Quantification of LSO filipin fluorescence power is shown. Data are presented as percentage of the average value for untreated controls in two independent experiments ± SEM (n = 6 for treated samples, n = 24 for control, where n is total number of wells per condition used for quantification).
The delayed nature of CD effects (i.e., a further decrease in cholesterol accumulation after CD is removed from the medium) provided an initial indication that CD is acting to transfer cholesterol within the LSOs, as opposed to eliciting net efflux from LSOs by lowering cholesterol levels in the PM.
Acute MβCD Treatments of NPC-Defective Cells.
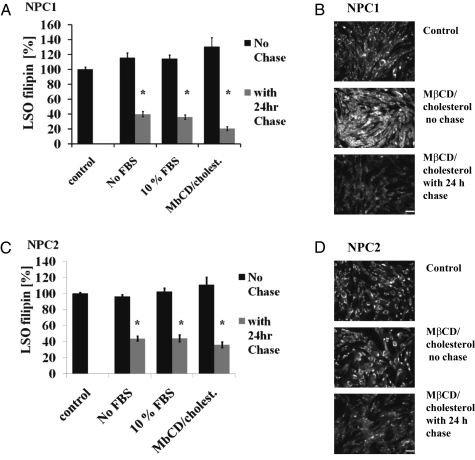
To determine whether the reduction of free cholesterol in LSOs of NPC-defective cells resulted from extraction of cholesterol by CD from the PM or from inside the LSOs, we acutely treated cells for 1 h with MβCD in medium without serum (to extract cholesterol from the PM), MβCD in medium plus 10% FBS [to exchange cholesterol between serum lipoproteins and the PM (13)], or MβCD loaded with cholesterol [≈5:1 (MβCD/cholesterol) ratio] to overload cells with cholesterol. Cells that were fixed immediately after treatment with MβCD (with or without serum) showed no marked decrease in LSO filipin staining (Fig S3). The cells that were treated with MβCD/cholesterol showed an overall increase in filipin staining (Fig. 4 B and D). Quantitative analysis of images taken 24 h after the treatment with CD revealed that regardless of the method of initial treatment there was a significant reduction in filipin staining of LSOs of treated cells as compared with untreated controls (Fig. 4). These findings are consistent with a mechanism in which CD is acting from the inside LY/LE to compensate for the lack of functional NPC1 or NPC2.
Fig. 4.
Short-term MβCD treatment. NPC1 (A and B) and NPC2 (C and D) mutant cells were treated for 1 h with 333 μM MβCD in growth media without serum, 333 μM MβCD in media plus 10% FBS, or 333 μM MβCD loaded with cholesterol [5:1 (MβCD/cholesterol) ratio]. Cells were either fixed immediately or rinsed extensively and returned to growth medium for 24 h. Quantification of LSO filipin fluorescence power is shown (A and C). Numeric data represent averages ± SEM of three independent experiments normalized to control (untreated) average value for each experiment. *P < 0.0001 vs. control (n = 12, where n is total number of wells used for quantification). Filipin images of NPC1 (B) or NPC2 (D) mutant cells treated with MβCD/cholesterol are shown. (Scale bars, 100 μm.)
MβCD–Dextran Conjugates Also Reduce Cholesterol Accumulation in NPC Mutant Cells.
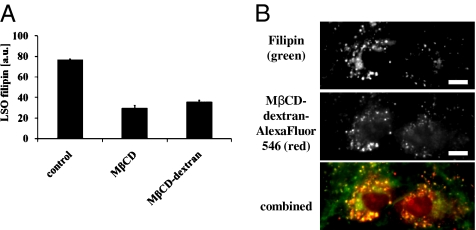
The endocytic uptake of dextran polymers and their delivery to the LE/LY has been characterized in many studies (18). To ensure CD delivery to LSOs, we covalently conjugated MβCD to dextran polymers via a polyethylene glycol linker. As shown in Fig. 5A, MβCD–dextran can also reverse cholesterol accumulation in the LSOs of NPC1-defective cells. To visualize CD trafficking we labeled MβCD–dextran conjugates with AlexaFluor546, and we observed that this conjugate was localized to intracellular organelles that were labeled with filipin (Fig. 5B). Thus, the MβCD–dextran–AlexaFluor546 polymer is in the LSOs.
Fig. 5.
MβCD–dextran localization and effects on LSO filipin in NPC1-defective cells. (A) Cells were treated for 2 days with 50 μM MβCD or 50 μM MβCD conjugated to dextran fixed with paraformaldehyde, stained, and imaged as described previously (21). Cholesterol accumulation in LSOs was measured by quantifying filipin fluorescence as described previously (20). Data represent averages ± SEM of one representative experiment (n = 8, where n is total number of wells per condition used for quantification). (B) Cells were incubated with AlexaFluor546–MβCD–dextran for 15 h followed by a 3-h chase in growth medium. The cells were fixed and imaged by epi-fluorescence microscopy. The MβCD concentration was 300 μM, with ≈2 MβCD per 72-kDa dextran chain. (Scale bars, 11 μm.)
Effects of Acute CD Treatments on Cholesterol Esterification.
To measure cholesterol levels within the endoplasmic reticulum in response to brief CD treatments, we measured cholesterol esterification by ACAT (Fig S4). We found increased esterification of cholesterol several hours after a short (1-h) CD treatment of NPC1- or NPC2-deficient cell lines. This increase was inhibited by compound S58-035, an ACAT inhibitor (22), demonstrating that the increase in esterification was mediated by ACAT. Corresponding reductions in cholesterol accumulation upon MβCD treatment, as measured by the LSO filipin assay, are shown in Fig. S5.
β-CD Effects on U18666A-Treated Fibroblasts.
To test whether the apparent EC50 for clearance of cholesterol from LSO depended on cholesterol levels accumulated and to examine this in an isogenic background, we used apparently normal human fibroblasts (GM05659) treated with various concentrations of the NPC phenotype-inducing compound U18666A (3-β-[(2-diethyl-amino)ethoxy]androst-5-en-17-one). U18666A is a class II amphipathic amine that has been reported to impair cholesterol efflux from LE/LY and create an NPC-like phenotype in treated cells (23). The extent of cholesterol accumulation in LSOs of U18666A-treated cells is dose dependent (Fig. S6A). This accumulation can be reversed in a dose-dependent manner by using either MβCD or HPβCD (Fig. S6 B and C). The EC50s for clearance of cholesterol from U18666A-treated cells are summarized in Table S2, and the dependence of EC50 on U18666A concentration is displayed in Fig. S6D. Higher levels of cholesterol in the cells require higher concentration of CD for clearance. The increased potency of MβCD vs. HPβCD is demonstrated once again, and it seems to be more prominent for higher levels of cholesterol accumulation.
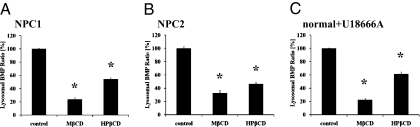
Effects of β-CDs on BMP Accumulation in NPC-Defective Cells.
Because levels of BMP are also elevated in the LSOs of NPC mutant cell (24), we examined the effects of CDs on BMP accumulation in NPC mutant cells. Both MβCD and HPβCD significantly reduced BMP accumulation, as measured by quantification of images of cells stained with anti-BMP antibody (Fig. 6). This indicates that secondary lipid accumulation was reduced along with cholesterol changes. Relative BMP levels in NPC1, NPC2, normal, and normal cells treated with U18666A are shown in Fig. S7.
Fig. 6.
CD effects on BMP accumulation in NPC mutant cells. BMP levels were quantified as described in SI Materials and Methods using fluorescence microscopy from images obtained with a 20x objective of cells stained with anti-BMP antibody and AlexaFluor488-conjugated secondary antibody in NPC1 (A), NPC2 (B) mutant, and 1 μM U18666A-treated normal (C) cells. Cells were incubated with 0.9 mM CD for ≈1 day. Data represent averages ± SEM of two independent experiments normalized to control (untreated) average value for each experiment. *P < 0.0001 vs. control (n = 16 for treated samples, n ≈ 64 for control, where n is total number of wells per condition used for quantification).
Discussion
Studies in npc1−/− mice have demonstrated that single injections of HPβCD can extend their lifespan (8–11). Subsequent studies with repeated injections of HPβCD have shown further prolongation of lifespan as well as clearance of various glycosphingolipids (12). These animal studies did not address the mechanism of action of CDs. A recent study (19) used cultured NPC1- and NPC2-deficient cells to show that HPβCD treatment increased ACAT-mediated esterification of cholesterol even in the absence of functional NPC proteins.
We have examined the role of CD in reducing cholesterol accumulation in NPC mutant cells. CDs are hydrophilic, membrane-impermeant molecules that can reach the inside of LE/LY via pinocytosis, similar to other membrane-impermeant molecules (18). Mammalian cells lack the enzymes for degradation of CDs (25), so CDs delivered to LE/LY should remain intact.
Several lines of evidence suggest that the major effect of CDs in reducing cholesterol accumulation is attributable to endocytosed CD. The effects on cholesterol reduction in LSOs continue for several days after extracellular CD has been removed from the medium (Fig. 3). The slow loss of effectiveness of CD would be consistent with reduced concentrations in the endocytic system as a consequence of cell division and vesicular transport out of lysosomes. We also found that brief (1-h) incubations with CD lead to a reduction in LSO cholesterol after ≈24 h in the absence of extracellular CD. This was true even when the initial incubation was with cholesterol-loaded CD, which caused an initial increase in cellular cholesterol levels (Fig. 4). We also conjugated MβCD to dextran polymers to ensure its delivery to the LSOs and found that this compound was able to correct NPC1 deficiency (Fig. 5A). Additionally, we observed that these conjugates were delivered to LSOs (Fig. 5B). These observations furnish further proof that CDs can act from inside the lumen of the LSO.
Both NPC1 and NPC2 mutant cells were tested in this study, and both cell types responded well to treatment by CDs. This indicates that CDs are capable of replacing the function of either NPC1 or NPC2. Because NPC2 is a small, soluble protein that has been shown to transfer cholesterol between membranes in vitro (26), its functional replacement by CD can be envisaged easily. The mechanism underlying the ability of a CD to bypass the requirement for functional NPC1 is less clear. NPC1 is a large transmembrane protein residing in the limiting membrane of LE/LY, and its role in cholesterol efflux is not well understood despite recent advances in understanding its cholesterol binding properties (27, 28). It is possible that to facilitate cholesterol egress from LE/LY, NPC2 has to interact with NPC1 to deliver cholesterol to the limiting membrane for egress (19, 27), whereas a CD can bypass that requirement and deliver cholesterol directly to the limiting membrane. Once in the limiting membrane, cholesterol could leave the LE/LY by vesicular or nonvesicular transport processes (29) to be delivered to other organelles.
To verify that the cholesterol liberated by CD treatments reaches the cytosolic compartment, we measured esterification of cholesterol by ACAT. We found that short treatments of either NPC1 or NPC2 cells with either CD, followed by ≈16-h chase, lead to an increase in cholesterol esterification by ACAT, indicating that more cholesterol is delivered to the endoplasmic reticulum in CD-treated cells (Fig. S5).
BMP is an unusual lipid that is present in high levels in late endosomes, and elevated levels of cholesterol associated with NPC mutations or treatment with U18666A lead to elevated levels of BMP in the LSOs (24, 30). To verify that the effects of CD treatment go beyond merely cholesterol reduction, we measured BMP levels in treated and untreated cells. We found that the BMP levels were reduced significantly by CD treatments (Fig. 6). This is consistent with the observation in npc1−/− mice that CD treatments reduce levels of storage of other lipids in addition to cholesterol (12).
Ultimately, our studies may have implications for development of CD-based therapies for treatment of NPC disease. First, we find that MβCD produces effects equivalent to those of HPβCD at approximately 3-fold lower concentrations. This is consistent with its better ability to extract cholesterol from biologic membranes (13–15). This observation may be important because relatively high amounts of HPβCD need to be injected to obtain a partial therapeutic effect (8–12). Additionally, the observation that CD works from inside LE/LY suggests that modifications that target CDs for endocytic uptake and retention might significantly enhance its potency. In conclusion, the studies presented in this article provide initial insights into the molecular and cellular mechanism of action of CD-mediated reduction of cholesterol and other lipids accumulations associated with NPC disease.
Materials and Methods
Materials and Reagents.
MEM, FBS, and HBSS were from Invitrogen. Anti-BMP, clone 6C4, antibody was from Echelon Biosciences. Other chemicals were from Sigma Chemicals.
Cell Lines.
Human fibroblast cell lines GM03123 (NPC1), GM18455 (NPC2), and GM05659 (apparently normal) were cultured as described previously (21).
Antibody Staining.
Anti-BMP immunofluorescence was performed as described previously (16).
Automated Fluorescence Microscopy and Image Analysis.
Filipin staining and automated image analysis (LSO filipin assay) were performed as described previously (21). Images were acquired using an ImageXpressMICRO imaging system from Molecular Devices (MDS Analytical Technologies) equipped with a 300-W xenon arc lamp. A CoolSnapHQ camera (Roper Scientific) was used to acquire images. The bright filipin intensity in the center of the NPC mutant cells was quantified using the same threshold value for all fields under all conditions in a given experiment.
MβCD-NH-(PEG)3-CONH-Aminodextran Synthesis.
Mono(6A-O-p-toluenesulfonyl) permethyl-β-cyclodextrin was added to a solution of NH2-(PEG)3-CONH-aminodextran in formamide. The resulting solution was heated to 100 °C and stirred for 48 h, at which point it was diluted with water and extensively dialyzed against water. After lyophilization (×2), the title compound was obtained as a white powder. If required, AlexaFluor546 was linked to the construct by addition of MβCD-NH-(PEG)3-CONH-aminodextran to a solution of PBS followed by the addition of AlexaFluor546 NHS ester. The resulting solution was stirred at ambient temperature for 48 h and was dialyzed extensively against water and lyophilized (×3) to provide the dye-labeled construct as a pink, cotton-like solid. Further details on the synthesis of this and other intermediates may be found in SI Materials and Methods.
Estimation of MβCD Substitution on Aminodextran.
Dextran derivitization with MβCD was estimated according to a method described previously (31). We used a SpectraMax M2 fluorometer (MDS Analytical Technologies) to detect changes in 1-naphthol fluorescence as the result of MβCD binding.
More details of materials and methods are supplied in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Harold Ralph for help with image acquisition and analysis. This work was supported by National Institutes of Health Grant R37-DK27083 and a grant from the Ara Parseghian Medical Research Foundation.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914309107/DCSupplemental.
References
- 1.Patterson MC, et al. Niemann-Pick disease type C: A lipid trafficking disorder. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Basis of Inherited Disease. 8th Ed. Vol. 3. New York: McGraw-Hill; 2001. pp. 3611–3633. [Google Scholar]
- 2.Mukherjee S, Maxfield FR. Lipid and cholesterol trafficking in NPC. Biochim Biophys Acta. 2004;1685:28–37. doi: 10.1016/j.bbalip.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Lefevre M. Localization of lipoprotein unesterified cholesterol in nondenaturing gradient gels with filipin. J Lipid Res. 1988;29:815–818. [PubMed] [Google Scholar]
- 4.Castanho MA, Coutinho A, Prieto MJ. Absorption and fluorescence spectra of polyene antibiotics in the presence of cholesterol. J Biol Chem. 1992;267:204–209. [PubMed] [Google Scholar]
- 5.Qin C, Nagao T, Grosheva I, Maxfield FR, Pierini LM. Elevated plasma membrane cholesterol content alters macrophage signaling and function. Arterioscler Thromb Vasc Biol. 2006;26:372–378. doi: 10.1161/01.ATV.0000197848.67999.e1. [DOI] [PubMed] [Google Scholar]
- 6.Bartz F, et al. Identification of cholesterol-regulating genes by targeted RNAi screening. Cell Metab. 2009;10:63–75. doi: 10.1016/j.cmet.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Patterson MC, Platt F. Therapy of Niemann-Pick disease, type C. Biochim Biophys Acta. 2004;1685:77–82. doi: 10.1016/j.bbalip.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Camargo F, et al. Cyclodextrins in the treatment of a mouse model of Niemann-Pick C disease. Life Sci. 2001;70:131–142. doi: 10.1016/s0024-3205(01)01384-4. [DOI] [PubMed] [Google Scholar]
- 9.Liu B, Li H, Repa JJ, Turley SD, Dietschy JM. Genetic variations and treatments that affect the lifespan of the NPC1 mouse. J Lipid Res. 2008;49:663–669. doi: 10.1194/jlr.M700525-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Liu B, et al. Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1−/− mouse. Proc Natl Acad Sci USA. 2009;106:2377–2382. doi: 10.1073/pnas.0810895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lope-Piedrafita S, Totenhagen JW, Hicks CM, Erickson RP, Trouard TP. MRI detects therapeutic effects in weanling Niemann-Pick type C mice. J Neurosci Res. 2008;86:2802–2807. doi: 10.1002/jnr.21707. [DOI] [PubMed] [Google Scholar]
- 12.Davidson CD, et al. Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS One. 2009;4:e6951. doi: 10.1371/journal.pone.0006951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atger VM, et al. Cyclodextrins as catalysts for the removal of cholesterol from macrophage foam cells. J Clin Invest. 1997;99:773–780. doi: 10.1172/JCI119223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christian AE, Haynes MP, Phillips MC, Rothblat GH. Use of cyclodextrins for manipulating cellular cholesterol content. J Lipid Res. 1997;38:2264–2272. [PubMed] [Google Scholar]
- 15.Kilsdonk EP, et al. Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem. 1995;270:17250–17256. doi: 10.1074/jbc.270.29.17250. [DOI] [PubMed] [Google Scholar]
- 16.Pipalia NH, Hao M, Mukherjee S, Maxfield FR. Sterol, protein and lipid trafficking in Chinese hamster ovary cells with Niemann-Pick type C1 defect. Traffic. 2007;8:130–141. doi: 10.1111/j.1600-0854.2006.00513.x. [DOI] [PubMed] [Google Scholar]
- 17.Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–621. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- 18.Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 19.Abi-Mosleh L, Infante RE, Radhakrishnan A, Goldstein JL, Brown MS. Cyclodextrin overcomes deficient lysosome-to-endoplasmic reticulum transport of cholesterol in Niemann-Pick type C cells. Proc Natl Acad Sci USA. 2009;106:19316–19321. doi: 10.1073/pnas.0910916106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pipalia NH, Huang A, Ralph H, Rujoi M, Maxfield FR. Automated microscopy screening for compounds that partially revert cholesterol accumulation in Niemann-Pick C cells. J Lipid Res. 2006;47:284–301. doi: 10.1194/jlr.M500388-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Rosenbaum AI, et al. Chemical screen to reduce sterol accumulation in Niemann-Pick C disease cells identifies novel lysosomal acid lipase inhibitors. Biochim Biophys Acta. 2009;1791:1155–1165. doi: 10.1016/j.bbalip.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross AC, Go KJ, Heider JG, Rothblat GH. Selective inhibition of acyl coenzyme A:cholesterol acyltransferase by compound 58-035. J Biol Chem. 1984;259:815–819. [PubMed] [Google Scholar]
- 23.Cenedella RJ. Cholesterol synthesis inhibitor U18666A and the role of sterol metabolism and trafficking in numerous pathophysiological processes. Lipids. 2009;44:477–487. doi: 10.1007/s11745-009-3305-7. [DOI] [PubMed] [Google Scholar]
- 24.Chevallier J, et al. Lysobisphosphatidic acid controls endosomal cholesterol levels. J Biol Chem. 2008;283:27871–27880. doi: 10.1074/jbc.M801463200. [DOI] [PubMed] [Google Scholar]
- 25.Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: Past, present and future. Nat Rev Drug Discov. 2004;3:1023–1035. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- 26.Storch J, Xu Z. Niemann-Pick C2 (NPC2) and intracellular cholesterol trafficking. Biochim Biophys Acta. 2009;1791:671–678. doi: 10.1016/j.bbalip.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon HJ, et al. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 2009;137:1213–1224. doi: 10.1016/j.cell.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Infante RE, et al. NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc Natl Acad Sci USA. 2008;105:15287–15292. doi: 10.1073/pnas.0807328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mesmin B, Maxfield FR. Intracellular sterol dynamics. Biochim Biophys Acta. 2009;1791:636–645. doi: 10.1016/j.bbalip.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi T, et al. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat Cell Biol. 1999;1:113–118. doi: 10.1038/10084. [DOI] [PubMed] [Google Scholar]
- 31.Grosse PY, Pinguet F, Joulia JM, Astre C, Bressolle F. High-performance liquid chromatographic assay for methyl-beta-cyclodextrin in plasma and cell lysate. J Chromatogr B Biomed Sci Appl. 1997;694:219–226. doi: 10.1016/s0378-4347(97)00103-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.