Abstract
Aging of the hematopoietic stem cell compartment is believed to contribute to the onset of a variety of age-dependent blood cell pathophysiologies. Mechanistic drivers of hematopoietic stem cell (HSC) aging include DNA damage accumulation and induction of tumor suppressor pathways that combine to reduce the regenerative capacity of aged HSCs. Such mechanisms do not however account for the change in lymphoid and myeloid lineage potential characteristic of HSC aging, which is believed to be central to the decline of immune competence and predisposition to myelogenous diseases in the elderly. Here we have prospectively isolated functionally distinct HSC clonal subtypes, based on cell surface phenotype, bearing intrinsically different capacities to differentiate toward lymphoid and myeloid effector cells mediated by quantitative differences in lineage priming. Finally, we present data supporting a model in which clonal expansion of a class of intrinsically myeloid-biased HSCs with robust self-renewal potential is a central component of hematopoietic aging.
Keywords: HSCs , leukemia, lineage specification
An outstanding issue in hematopoietic stem cell (HSC) biology is focused on understanding whether HSCs are intrinsically primed for commitment toward downstream lineages or if HSCs have a common “ground state” with respect to lineage priming potential, with all lineage fate decisions being orchestrated at the level of downstream progenitor cells (1). Embedded within this broader issue is the question of whether the stem cell pool is clonally diverse such that individual clones possess stable, functionally distinct properties. In principle, numerous HSC clonal subtypes that differ with respect to their downstream lineage potential and/or self-renewal capacity may exist. Several studies have recently provided evidence in support of such a model using limit dilution whole bone marrow (BM) (2, 3), or purified stem cell assays (4, 5). Indeed, an important study using extensive single-cell transplantation of highly purified stem cells demonstrated that the clonal contribution to the different blood cell lineages varies significantly and can be stably maintained through serial passaging, providing evidence that the pool of HSCs comprises at least two, and possibly more, distinct clonal subtypes imbued with differential lineage and self-renewal potential (4). Important clinical implications would emerge, should it happen that certain stem cell clones either come to predominate or are conversely extinguished from the stem cell pool, as has been suggested to underlie the development of a number of hematological disorders including myelodysplastic syndrome, acute myeloid leukemia (AML), and paroxysmal nocturnal hemoglobinuria (6).
Aging critically affects the function and composition of the mature blood cell compartments (7). This, combined with the discovery that the BM frequencies of stem cells (8–12), committed lymphoid (11, 13, 14), and myeloid progenitors (11) are altered with age, indicates profound changes in the homeostatic control of blood cell production at multiple levels of hematopoietic differentiation during aging. Studies from a number of groups have indicated that at least some of the homeostatic imbalances arising in the hematopoietic system with age are a direct result of age-dependent stem cell decline (15). Perhaps the most exemplary manifestation of this is the decline in lymphoid lineage potential that is driven, at least in part, by alterations in fate determination whereby stem cells become increasingly biased for myeloid lineage commitment at the expense of lymphoid lineage differentiation during aging (11, 12, 16–19). The differences in lineage potential characteristic of aged HSCs are manifest both in the steady state and upon transplantation into young irradiated recipients, indicating that this aspect of stem cell aging is cell autonomous. Global gene expression profiling of HSCs purified from young and old mice supports this interpretation with the demonstration that gene programs involved in specifying lymphoid fate are down-regulated with age, whereas genes associated with myeloid specification are up-regulated with age in the long-term repopulation stem cell compartment (11). However, as the epigenetic marks underlying these changes may be quite stable, it remains possible that age-associated changes in HSC lineage potential may still be guided by instructive signals emanating from the aged systemic or microenvironments, as is the case with other stem cell systems (20, 21).
To detail the differential lineage potential of HSCs, we examined the clonal composition of the HSC pool within the context of aging. We hypothesized that clonal heterogeneity within the HSC pool would allow the prospective isolation and characterization of functional distinct subsets of HSCs. In agree-ment with previous studies using limit dilution whole-BM trans-plants (2, 3) or purified stem cells (4, 5), a number of functionally diverse HSC subtypes in the BM of young mice were observed. One subtype, representing less than 25% of long-term multilineage reconstituting HSCs in young mice, was found to exhibit a preferential myeloid lineage potential (myeloid bias), a component that progressively increased following both serial transplantation and normal physiological aging. Lineage-biased HSC subsets could be prospectively isolated from young and old mice based on differential levels of Slamf1/CD150 expression within the long-term repopulating lineage−Sca1+c-kit+flt3−CD34− hematopoietic stem cell (HSC) compartment: myeloid-biased HSCs expressed high expression levels of Slamf1/CD150 (Slamf1high HSCs), whereas HSCs expressing lower levels of Slamf1 (Slamf1low HSCs) exhibited a balanced lineage output. Analysis of lineage specification genes provides evidence that the functional potential of these HSC subsets is underwritten by molecular differences in lineage priming. Transplantation experiments showed that Slamf1high HSCs have greater capacity than Slamf1low HSC to regenerate the primitive stem cell compartment, suggesting a more robust self-renewal potential. Consistent with this, Slamf1high HSCs progressively increase with time and predominate the stem cell pool with age. These findings provide evidence that hematopoietic lineage fate is largely regulated at the stem cell level by functionally distinct hematopoietic stem cell clones, and demonstrate that the selective expansion of a clonal subtype of intrinsically myeloid-biased HSCs is a central component of hematopoietic aging.
Results
Prospective Identification of Lineage-Biased Hematopoietic Stem Cells.
Single HSCs isolated using a highly selective marker panel have been shown to long-term reconstitute radio-ablated hosts with greater than 40% efficiency (22–24). Using that panel of markers, we purified BM lineage−Sca1+c-kit+flt3−CD34− cells (hereafter referred to as HSCs) from 3-month-old mice and competitively transplanted five cells into 65 lethally irradiated congenic recipients. Of the 45 mice (69%) that exhibited long-term multilineage engraftment, total donor chimerism varied substantially (Fig. S1), consistent with studies using purified stem cells (4, 25). We also observed considerable variation in the lineage output, which we classified into three categories: (i) myeloid-biased (>55% chimeric ratio of donor myeloid cells); (ii) balanced (chimeric ratio of donor myeloid cells between 5% and 55%); and (iii) lymphoid-biased (mice with <5% donor-derived myeloid chimeric ratios). These data are consistent with clonal diversity within the hematopoietic stem cell compartment, and are consistent with the findings of others using limit dilution BM transplantation (2, 4) or stem cells purified using different markers (2, 4).
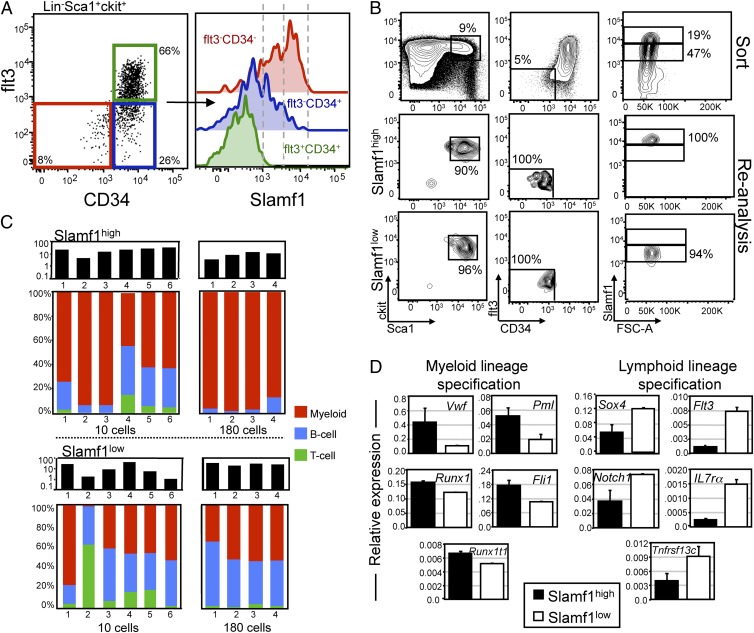
We sought to determine whether we could prospectively isolate lineage-biased HSCs based on differential expression of cell surface markers. We previously performed a comparative genome-wide expression profile of HSCs isolated from young and old mice (11) and determined that the homotypic self-receptor Slamf1 (CD150) was up-regulated in aged HSCs. This, combined with studies from Morrison et al. demonstrating that Slamf1 is a robust and reliable cell surface marker of HSCs (23), suggested that Slamf1 might be a useful marker to subfractionate the stem cell compartment. We therefore investigated the primitive lineage−Sca1+c-kit+ (LSK) BM compartment of young mice for Slamf1 expression and found that whereas the LSKCD34+flt3+ multipotent progenitor (MPPflt3+) population was uniformly negative for Slamf1 expression (Fig. 1A, green histogram), LSKCD34+flt3− multipotent progenitors (MPPflt3−) contained a mixture of Slamf1-positive and -negative cells (Fig. 1A, blue histogram). Within the HSC-containing LSKCD34−flt3− subset, however (Fig. 1A, red histogram), essentially all cells were Slamf1 positive, and expression could be observed in a bimodal distri-bution, with some cells expressing intermediate levels (Slamf1low) and others expressing higher levels (Slamf1high). All cells within the LSKCD34−flt3− compartment, including the Slamf1low and Slamf1high cells, were uniformly negative for CD48.
Fig. 1.
Prospective identification of lineage-biased hematopoietic stem cells. (A) Representative FACS plot of lineage−Sca1+c-kit+ cells separated based on CD34 and flt3 expression and subsequently investigated for cell surface expression of Slamf1. (B) FACS purification of Slamf1high and Slamf1low HSCs. (C) Slamf1high and Slamf1low HSCs were isolated and competitively transplanted at 10 cells or 180 cells per mouse. Bars (Upper) represent the overall donor chimerism. Bars (Lower) show lineage chimerism ratios of individual recipients of Slamf1high (Upper) and Slamf1low (Lower) HSCs 20 or 17 weeks, respectively, posttransplantation. (D) Quantitative real-time PCR data of a panel of myeloid and lymphoid lineage specification genes on FACS-sorted Slamf1high and Slamf1low HSCs. Expression is shown relative to the expression of beta actin. Results represent mean (SD) values from three independent experiments, with qRT-PCR analysis in each experiment performed in duplicate.
We next used differential Slamf1 expression to purify Slamf1low and Slamf1high cells from the LSKCD34−flt3− subset of young mice by FACS (Fig. 1B and Fig. S2) and competitively transplanted 10 or 180 cells of each subset into lethally irradiated congenic recipients. Long-term (>17 weeks) multilineage engraftment (B, T, and myeloid cells) was observed in both primary (Fig. 1C), and secondary recipients (Fig. S3) receiving Slamf1low cells or Slamf1high cells, demonstrating that both subsets contain HSC activity. Analysis of the in vivo lineage potential of these subsets revealed that while the Slamf1low cells gave rise to predominantly balanced reconstitution (eight of 10 recipients), the Slamf1high cells displayed a predominantly myeloid-biased reconstitution pattern (nine of 10 recipients) (Fig. 1C). Importantly, these lineage potentials were stable through serial transplantation (Fig. S3).
To explore the molecular basis for the differences in lineage potential, we performed quantitative real-time PCR (qRT-PCR) experiments on purified Slamf1low and Slamf1high HSCs for a set of genes involved in specifying myeloid and lymphoid fate and function. These experiments showed that, in all cases, the Slamf1high subset had elevated levels of expression of genes involved in specifying myeloid fate, whereas the Slamf1low HSCs uniformly expressed elevated levels of lymphoid specification transcripts (Fig. 1D). Taken together, these results show that lineage-biased HSCs can be prospectively isolated based on differential expression of Slamf1, and provide evidence that these cells’ lineage potential is primed by differential expression of factors involved in lineage specification.
Regenerative Capacity and Lineal Relationship of Slamf1high and Slamf1low Hematopoietic Stem Cells.
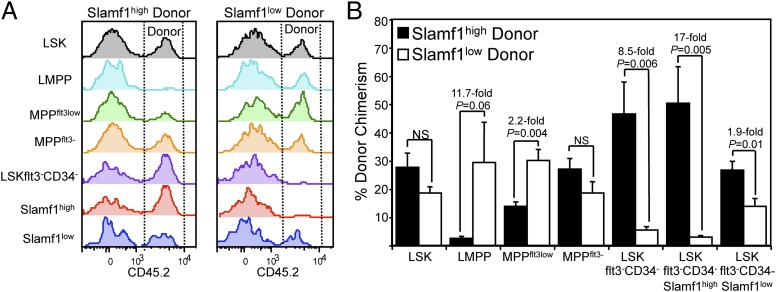
We next assayed the capacity of the Slamf1low and Slamf1high HSC subsets to regenerate stem and progenitor cell compartments. To this end, we competitively transplanted Slamf1low and Slamf1high HSCs into lethally irradiated recipients, and determined donor chimerism in the BM stem and progenitor cell compartment 5 months posttransplantation. These analyses revealed that whereas both HSC subsets exhibited an equal capacity to reconstitute the Lin−Sca1−c-kit+ myeloid progenitor compartment, Slamf1low HSCs were more efficient (4-fold) at reconstituting the CLPflt3+ lymphoid progenitor compartment (Fig. S4). Although both Slamf1low and Slamf1high HSCs shared an equal capacity to reconstitute the primitive LSK compartment, Slamf1low HSCs more readily regenerated the flt3high lymphoid-primed multipotent progenitor subset (LMPP) (26) and flt3low multipotent progenitor subsets (MPPflt3low). In contrast, Slamf1high HSCs exhibited much a greater capacity to regenerate the most primitive LSKCD34−flt3− HSC compartment, suggestive of a greater stem cell self-renewal capacity (Fig. 2 A and B). Consistent with this interpretation, Slamf1high HSCs regenerated both the Slamf1high and the Slamf1low LSKCD34−flt3− stem cell compartments to a higher degree than Slamf1low HSCs (Fig. 2 A and B). Examination of the lineal relationship between the HSC subsets showed that the Slamf1high HSCs preferentially gave rise to phenocopies of themselves, whereas Slamf1low HSCs predominantly gave rise to Slamf1low HSCs, and that these lineal relationships were stably maintained during serial transplantation (Fig. S5).
Fig. 2.
Slamf1high and Slamf1low HSCs differentially regenerate primitive hematopoietic subsets. (A) Representative histograms and (B) average donor contribution to the primitive BM stem and multipotent progenitor cell compartment of mice competitively transplanted with either 180 Slamf1high (Left) or Slamf1low HSCs (Right) analyzed 20 weeks posttransplantation. In all panels, n = 4 recipients for each of Slamf1high and Slamf1low HSC donors. Error bars denote SEM.
Aging Is Associated with Progressive Increase of Slamf1high Myeloid-Biased Hematopoietic Stem Cells.
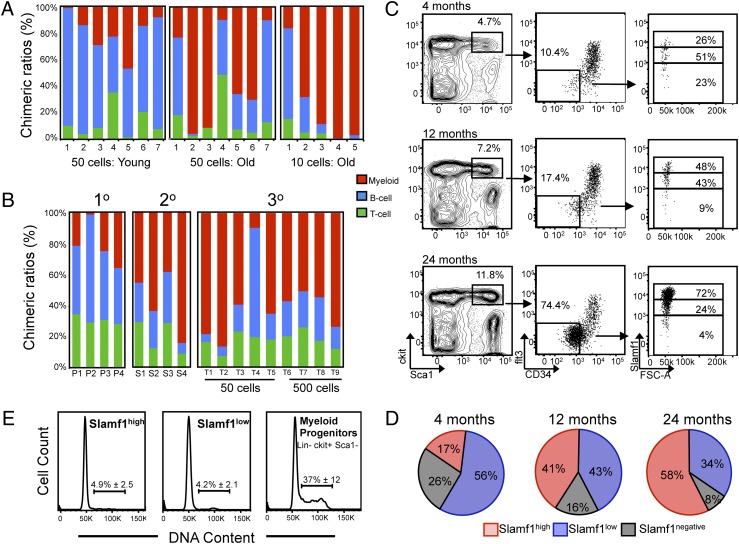
We and others have previously shown that aging of the primitive HSC compartment is accompanied by an intrinsic skewing of lineage potential, with HSCs from old mice preferentially reconstituting myeloid rather than lymphoid lineages (11, 12, 16, 18, 19). Consistent with this, recipient mice competitively transplanted with 10 or 50 HSCs purified from old mice predominantly showed myeloid lineage reconstitution in contrast to recipients transplanted with HSCs purified from young mice (Fig. 3A).
Fig. 3.
Slamf1high myeloid-biased HSCs predominate the stem cell pool with age. (A) Lineage potential of aged HSCs reveal myeloid bias in vivo. Either 10 or 50 LSKCD34−flt3− HSCs from 21- to 24-month-old donors, and 50 LSKCD34−flt3− HSCs from 3-month-old donors were competitively transplanted and peripheral blood chimeric ratios of B, T, and myeloid cells was determined 16–20 weeks posttransplantation. (B) Test-cell lineage chimerism ratios in serially transplanted mice in primary (1°), secondary (2°), and tertiary (3°) recipients. Columns depict individual recipients. (C) Representative FACS plots showing the primitive HSC compartment stained for Slamf1 expression in young adult (4 months), midaged (12 months), and old (24 months) mice. Cells were pregated on live (PI-negative), lineage-negative cells that were discriminated for doublets. (D) Pie charts showing average contribution to each of the Slamf1 populations within the LSKCD34−flt3− compartment (n = 3 for each age). (E) Representative FACS plots of cell cycle analysis on purified Slamf1high HSCs, Slamf1low HSCs, and Lin−Sca1−ckit+ myeloid progenitors with the average frequency of cells in S/G2/M shown with SD (n = 5 mice for each population).
Serially passaging HSCs through successive transplant recipients has been used as a means of modeling stem cell aging (27, 28); yet little is known about how serial transplantation affects HSC lineage output. To address this, we serially transplanted HSCs through primary (1°), secondary (2°), and tertiary (3°) recipients and analyzed peripheral blood chimerism throughout the course of the experiment (Fig. S6). Strikingly, we found that whereas primary recipients displayed donor chimeric ratios that were predominantly lineage balanced, the percentage of recipients showing myeloid-biased reconstitution increased with each successive round of transplantation (Fig. 3B). By the tertiary transplants, eight of nine recipients exhibited a myeloid-biased reconstitution pattern, similar to the reconstitution patterns seen in recipients transplanted with old HSCs (Fig. 3A). These data indicate that HSC reconstituting potential becomes increasingly myeloid biased during the normal physiological course of aging as well as serial transplantation.
These data, combined with the data regarding the lineage potential and self-renewal capacity of the Slamf1 HSC subsets (Fig. 1 and Fig. 2), raised the possibility that the clonal composition of the HSC pool might be altered in the relative frequencies of the lineage biased HSC subsets during aging. To investigate this we examined Slamf1 expression levels on LSKCD34−flt3− HSCs from young adult (4 months), midaged (12 months), and old mice (24 months) (Fig. 3C). This analysis showed that whereas the percentage of lineage balanced Slamf1low cells in the primitive HSC compartment gradually diminished with age, the proportion of myeloid-biased Slamf1high HSCs progressively increased such that by 24 months of age, Slamf1high HSCs predominated the stem cell pool (Fig. 3 C and D). Consistent with the large expansion of the phenotypic HSC compartment with age, the total BM frequency of the Slamf1high HSCs increased substantially with age, whereas the Slamf1low subset also expanded in numbers but to a lesser extent than the Slamf1high subset (Fig. S7). Cell cycle analysis was performed to address whether an increase in cell cycle rate might represent a selective force capable of propelling Slamf1high HSCs to predominate the stem over time. Consistent with studies detailing the deeply quiescent nature of adult HSCs (29–31), both HSC subsets were predominantly out of cycle, in contrast to downstream progenitors, which were rapidly cycling (Fig. 3E). Importantly, no significant differences were observed in the percentage of Slamf1high and Slamf1low HSCs in the S/G2/M phase of the cell cycle, suggesting that Slamf1high HSCs do not predominate the HSC compartment over time as a result of an increased cycling rate.
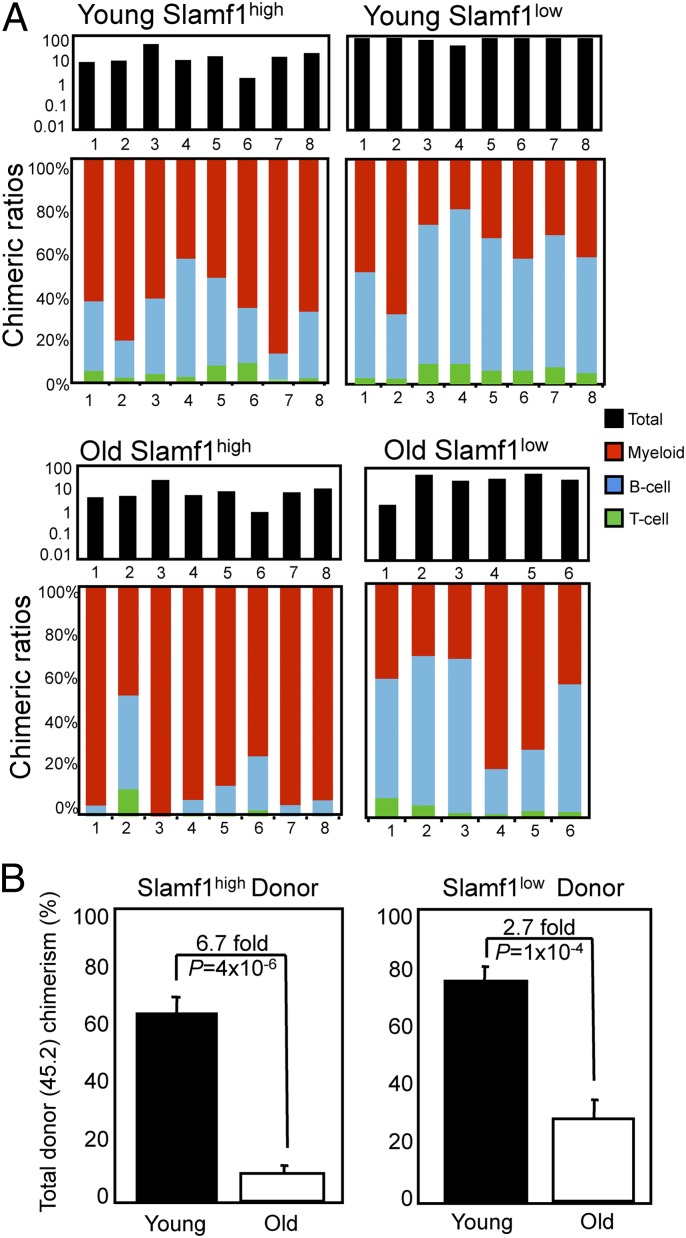
We next addressed whether differential expression of Slamf1 could be used to prospectively isolate lineage-biased HSCs from old mice. To this end, we purified Slamf1high and Slamf1low HSCs from young and old mice and competitively transplanted them into lethally irradiated congenic recipients. As expected, transplantation of Slamf1high HSCs from young mice led to myeloid-biased reconstitution (six of eight recipients), whereas Slamf1low HSCs transplants exhibited balanced (seven of eight recipients) reconstitution (Fig. 4A). Significantly, mice reconstituted with HSCs isolated from old mice showed a reconstitution pattern very similar to HSCs isolated from young mice: Slamf1high HSCs from old mice gave rise to myeloid-biased reconstitution (seven of eight recipients), and old Slamf1low HSCs exhibited predominantly balanced reconstitution (four of six) (Fig. 4A). These results demonstrate that the cell surface phenotype and lineage potential of the HSC clonal subtypes is maintained during aging. It is noteworthy, however, that the HSC subsets purified from old mice exhibited, on a per-cell basis, markedly diminished total reconstitution potential during both primary (Fig. 4B) and secondary transplantation (Fig. S3C), which is consistent with previous studies demonstrating a global repopulation deficit of HSCs during aging (11, 12, 32).
Fig. 4.
Slamf1high and Slamf1low HSCs maintain lineage potential during aging. (A) A total of 125 Slamf1high or Slamf1low HSCs were isolated from either young adult (4 months) or old (23 months) mice and competitively transplanted against 2 × 105 unfractionated BM cells. Peripheral blood analysis was performed 17 weeks posttransplantation. Black bars (Upper) represent total donor chimerism; colored bars (Lower) show lineage chimerism ratios in individual recipients. (B) Average total peripheral blood donor chimerism for the experiments presented in A is shown, with error bars denoting SEM.
Taken together, these data support a model in which clonal expansion of myeloid-biased HSCs imbued with robust self-renewal potential underlies the change in lympho-myeloid lineage commitment associated with aging in the hematopoietic system.
Discussion
Aging of the hematopoietic system is accompanied by declining immuno-competence, increased autoimmunity, diminished stress response, anemia, and increased predisposition to a spectrum of diseases including myelodysplastic syndrome and myeloid leukemia. Increasing evidence suggests that such pathophysiologies arise, at least in part, as a result of age-dependent stem cell decline. For example, hematopoietic aging is associated with a diminution of lymphoid lineage commitment, whereas myeloid lineage commitment is maintained or even slightly elevated. This age-associated lineage bias has been shown to result from age-dependent differences in the propensity of HSCs from young or old mice to commit and give rise to primitive oligo-potent myeloid or lymphoid progenitors (11, 14). This change in lineage commitment is believed to contribute to the decline of the adaptive immune system with age, and at the same time, provides a cellular rationale based on stem cell biology for the predisposition to myelogenous diseases in the elderly, and lymphoid malignancies in the young (15).
In addition to these lineage differences, HSCs from old mice also exhibit altered homing and mobilization properties (19), which likely contribute to the attenuated stress response and reduced regenerative capacity characteristic of the aged hematopoietic system. Molecular factors contributing to stem cell decline include age-dependent DNA damage accumulation (33) and epigenetic deregulation (32). However, humoral factors originating from the aged micro- or systemic environments that appear to be related to chronic inflammation might also be important in modulating HSC aging (32).
Two models have been put forth to account for the changing functional properties of the aging stem cell pool. In one model, the stem cell pool changes in the relative frequency and/or activity of distinct functional subtypes over time, such that the clonal composition of the functional stem cell pool in older individuals is divergent from that in young individuals. In an alternative model, the functional potential of most or all of the clones comprising the stem cell pool is more or less uniform at any given stage of life, yet the functional potential of the clones within the pool coordinately change over time. Consistent with data generated from whole-BM limit dilution experiments (16), the data presented herein provides evidence that the clonal selection model is the predominant mechanism contributing to aging of the HSC compartment. Our data show that the HSC pool becomes dominated during aging by the selective expansion of a subset of myeloid-biased Slamf1high HSC clones by virtue of a superior self-renewal capacity over lineage-balanced stem cells. This mechanism appears to adequately account for the well-documented change in lineage potential associated with stem cell aging (11, 12, 16, 18, 19). Our data do not, however, exclude the possibility that deficiencies within defined clonal subtypes also contribute to age-dependent stem cell decline. Indeed, the observation that total repopulating potential diminishes with age in both Slamf1high and Slamf1low HSCs, provides evidence for this concept. Age-dependent DNA damage accumulation seems to be a likely mechanism contributing to stem cell decline that may act independently of clonal selection. In support of this postulate, we have previously reported that HSCs bearing deficiencies in different genomic maintenance pathways exhibit dramatically reduced repopulating activity with age that is independent of lineage bias normally associated with aging (33).
The prospective isolation of phenotypically defined clonal subtypes of HSCs provides an unprecedented opportunity to dissect the functional, molecular, and epigenetic properties of distinct stem cell subtypes. Moreover, the observation that myeloid-biased HSCs predominate the stem cell pool during aging suggests that a better understanding of the molecular properties of this clonal subclass may provide insights into the onset of clinically important age-dependent diseases derived from stem cells such as AML and myelodysplastic syndrome.
Materials and Methods
Mice.
All mice used in this study were on the C57BL/6 background. Young mice were 12–14 weeks of age. Midaged (12 months) and old (22–24 months) mice were obtained from the National Institute of Aging (NIA).
HSC Purification and Transplantation.
HSCs were isolated, and competitive transplants were performed as previously described (33). Peripheral blood chimerism was determined by gating myeloid, B, or T cells and then determining the amount of donor cells contributing to each lineage. Chimeric ratios for each lineage were calculated by dividing individual lineage chimerism by the sum of all lineage chimerism values for individual mice. All flow cytometry and FACS data were analyzed using FlowJo software (Treestar).
Quantitative Real-Time PCR.
Total RNA was extracted from FACS sorted cells using TRIzol reagent (Invitrogen) and cDNA was generated using SuperScript Vilo (Invitrogen) cDNA synthesis kits according to manufacturer's instruction. RT-PCR primers spanning intron-exon boundaries were designed using Primer 3 (http://frodo.wi.mit.edu/primer3/input.htm). Real-time PCR was performed on the Eppendorf Mastercycler Realplex-2 using Perfecta (Quanta Biosciences) SYBR green reagent with 100-cell equivalent amounts of cDNA per reaction. β-Actin was used to normalize the expression.
Cell Cycle Analysis.
Slamhigh and Slamlow HSCs and Lin−Sca1−ckit+ myeloid progenitors from 15-week-old mice were sorted directly into cold 100% methanol. Cells were kept at −20°C for 12 h, and then resuspended in PI (50 μg/mL) with RNAseA (20 μg/mL) and analyzed by flow cytometry.
Supplementary Material
Acknowledgments
D.B. is supported by the Swedish Medical Research Council, the Swedish Strategic Research Foundation, the Swedish Cancer Society, the Crafoord foundation, and the Medical Faculty at Lund University. D.J.R. is supported by grants from the National Institutes of Aging and the Harvard Stem Cell Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/1000834107/DCSupplemental.
References
- 1.Miyamoto T, Akashi K. Lineage promiscuous expression of transcription factors in normal hematopoiesis. Int J Hematol. 2005;81:361–367. doi: 10.1532/ijh97.05003. [DOI] [PubMed] [Google Scholar]
- 2.Muller-Sieburg CE, Cho RH, Karlsson L, Huang JF, Sieburg HB. Myeloid-biased hematopoietic stem cells have extensive self-renewal capacity but generate diminished lymphoid progeny with impaired IL-7 responsiveness. Blood. 2004;103:4111–4118. doi: 10.1182/blood-2003-10-3448. [DOI] [PubMed] [Google Scholar]
- 3.Sieburg HB, et al. The hematopoietic stem compartment consists of a limited number of discrete stem cell subsets. Blood. 2006;107:2311–2316. doi: 10.1182/blood-2005-07-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dykstra B, et al. Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell. 2007;1:218–229. doi: 10.1016/j.stem.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Kent DG, et al. Prospective isolation and molecular characterization of hema-topoietic stem cells with durable self-renewal potential. Blood. 2009;113:6342–6350. doi: 10.1182/blood-2008-12-192054. [DOI] [PubMed] [Google Scholar]
- 6.Bagby GC, Meyers G. Bone marrow failure as a risk factor for clonal evolution: Prospects for leukemia prevention. Hematology. 2007;2007:40–46. doi: 10.1182/asheducation-2007.1.40. [DOI] [PubMed] [Google Scholar]
- 7.Berkahn L, Keating A. Hematopoiesis in the elderly. Hematology. 2004;9:159–163. doi: 10.1080/10245330410001701468. [DOI] [PubMed] [Google Scholar]
- 8.de Haan G, Nijhof W, Van Zant G. Mouse strain-dependent changes in frequency and proliferation of hematopoietic stem cells during aging: Correlation between lifespan and cycling activity. Blood. 1997;89:1543–1550. [PubMed] [Google Scholar]
- 9.de Haan G, Van Zant G. Dynamic changes in mouse hematopoietic stem cell numbers during aging. Blood. 1999;93:3294–3301. [PubMed] [Google Scholar]
- 10.Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 11.Rossi DJ, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci USA. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J Exp Med. 2000;192:1273–1280. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller JP, Allman D. The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. J Immunol. 2003;171:2326–2330. doi: 10.4049/jimmunol.171.5.2326. [DOI] [PubMed] [Google Scholar]
- 14.Min H, Montecino-Rodriguez E, Dorshkind K. Effects of aging on the common lymphoid progenitor to pro-B cell transition. J Immunol. 2006;176:1007–1012. doi: 10.4049/jimmunol.176.2.1007. [DOI] [PubMed] [Google Scholar]
- 15.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 16.Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: Aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111:5553–5561. doi: 10.1182/blood-2007-11-123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerrettaz LM, Johnson SA, Cambier JC. Acquired hematopoietic stem cell defects determine B-cell repertoire changes associated with aging. Proc Natl Acad Sci USA. 2008;105:11898–11902. doi: 10.1073/pnas.0805498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim M, Moon HB, Spangrude GJ. Major age-related changes of mouse hema-topoietic stem/progenitor cells. Ann N Y Acad Sci. 2003;996:195–208. doi: 10.1111/j.1749-6632.2003.tb03247.x. [DOI] [PubMed] [Google Scholar]
- 19.Liang Y, Van Zant G, Szilvassy SJ. Effects of aging on the homing and en-graftment of murine hematopoietic stem and progenitor cells. Blood. 2005;106:1479–1487. doi: 10.1182/blood-2004-11-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brack AS, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 21.Conboy IM, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 22.Ema H, Takano H, Sudo K, Nakauchi H. In vitro self-renewal division of hematopoietic stem cells. J Exp Med. 2000;192:1281–1288. doi: 10.1084/jem.192.9.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Takano H, Ema H, Sudo K, Nakauchi H. Asymmetric division and lineage commitment at the level of hematopoietic stem cells: Inference from differentiation in daughter cell and granddaughter cell pairs. J Exp Med. 2004;199:295–302. doi: 10.1084/jem.20030929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osawa M, et al. In vivo self-renewal of c-Kit+ Sca-1+ Lin(low/-) hemopoietic stem cells. J Immunol. 1996;156:3207–3214. [PubMed] [Google Scholar]
- 26.Adolfsson J, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Harrison DE, Astle CM. Loss of stem cell repopulating ability upon transplantation. Effects of donor age, cell number, and transplantation procedure. J Exp Med. 1982;156:1767–1779. doi: 10.1084/jem.156.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spangrude GJ, Brooks DM, Tumas DB. Long-term repopulation of irradiated mice with limiting numbers of purified hematopoietic stem cells: In vivo expansion of stem cell phenotype but not function. Blood. 1995;85:1006–1016. [PubMed] [Google Scholar]
- 29.Wilson A, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 30.Foudi A, et al. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat Biotechnol. 2008 doi: 10.1038/nbt.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossi DJ, et al. Hematopoietic stem cell quiescence attenuates DNA damage response and permits DNA damage accumulation during aging. Cell Cycle. 2007;6:2371–2376. doi: 10.4161/cc.6.19.4759. [DOI] [PubMed] [Google Scholar]
- 32.Chambers SM, et al. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5:e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossi DJ, et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.