Abstract
Deoxycytidine kinase (dCK) is a rate-limiting enzyme in deoxyribonucleoside salvage, a metabolic pathway that recycles products of DNA degradation. dCK phosphorylates and therefore activates nucleoside analog prodrugs frequently used in cancer, autoimmunity, and viral infections. In contrast to its well established therapeutic relevance, the biological function of dCK remains enigmatic. Highest levels of dCK expression are found in thymus and bone marrow, indicating a possible role in lymphopoiesis. To test this hypothesis we generated and analyzed dCK knockout (KO) mice. dCK inactivation selectively and profoundly affected T and B cell development. A 90-fold decrease in thymic cellularity was observed in the dCK KO mice relative to wild-type littermates. Lymphocyte numbers in the dCK KO mice were 5- to 13-fold below normal values. The severe impact of dCK inactivation on lymphopoiesis was unexpected given that nucleoside salvage has been thought to play a limited, "fine-tuning" role in regulating deoxyribonucleotide triphosphate pools produced by the de novo pathway. The dCK KO phenotype challenges this view and indicates that, in contrast to the great majority of other somatic cells, normal lymphocyte development critically requires the deoxyribonucleoside salvage pathway.
Keywords: combined immunodeficiency, deoxyribonucleoside salvage, dNTP metabolism, immune development, T and B lymphocytes
Genetic deficiencies in purine and pyrimidine metabolism are responsible for at least 14 different disorders with a broad spectrum of clinical manifestations (reviewed in ref. 1). Many of these disorders affect the immune system. Mutations in the adenosine deaminase and purine nucleoside phosphorylase genes lead to human immunodeficiency syndromes first described in the classic work of Giblett and colleagues (2, 3). Increased susceptibility to infections also accompanies disorders of pyrimidine metabolism such as orotic aciduria and the pyrimidine nucleotide depletion syndrome (1). Therefore, lymphocytes appear to be very sensitive to defects in deoxyribonucleoside metabolism.
Lymphocytes and other cells can produce deoxyribonucleotide triphosphates (dNTPs) for DNA replication and repair using two pathways: de novo synthesis and deoxyribonucleoside salvage. These pathways converge at the level of deoxyribonucleotide diphosphates (dNDP) (Fig. 1) and are further connected by complex feedback mechanisms (reviewed in ref. 4). Ribonucleotide reductase (RNR), the most important enzyme in the de novo pathway, produces all four dNDP precursors for DNA synthesis. Models explaining the production and maintenance of dNTP pools by the de novo pathway alone, without input from the deoxyribonucleoside salvage pathway, have been proposed (5). However, in tissues with active nucleoside salvage, recycling of extracellular dNs via the salvage pathway may also contribute to cellular dNTP pools (6).
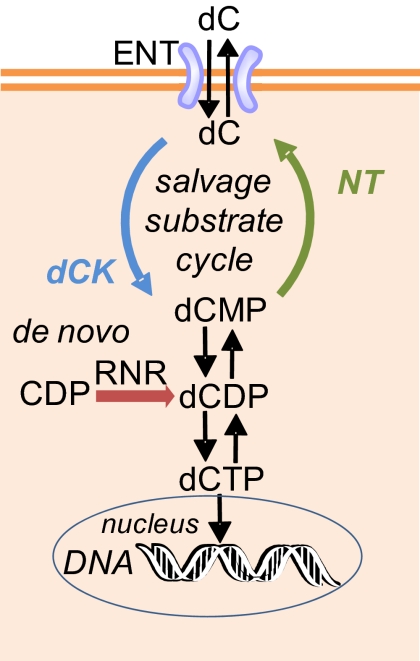
Fig. 1.
Model for dCK function in the context of cytosolic deoxycytidine metabolism. The two biochemical pathways (salvage and de novo) involved in deoxycytidine metabolism converge at the level of dCDP. The flux through the salvage pathway is bidirectional because of a substrate cycle in which deoxycytidine (dC) is continuously converted to (by dCK) and from (by NT) its corresponding monophosphate (dCMP). The rates of the phosphorylation and dephosphorylation reactions are regulated by feedback mechanisms and by substrate availability. Upstream of dCK, cellular and extracellular dC pools are in equilibrium via the nucleoside transporters (ENTs). Downstream of dCK, the dCMP pool is in equilibrium with the dCDP pool. The de novo pathway contributes to the dCDP pool by RNR-mediated reduction of CDP. ENT, equilibrative nucleoside transporters; NT, 5′ nucleotidase; dCDP, deoxycytidine diphosphate; CDP, cytidine diphosphate; RNR, ribonucleotide reductase.
The deoxyribonucleoside flux through the salvage pathway is controlled by specialized kinases (4). Mammalian genomes encode two cytosolic deoxyribonucleoside kinases with nonoverlapping substrate specificities: thymidine kinase 1 (TK1) and deoxycytidine kinase (dCK) (Fig. S1) (7). TK1 phosphorylates thymidine (dT) and deoxyuridine (dU) whereas dCK phosphorylates deoxycytidine (dC), deoxyadenosine (dA), and deoxyguanosine (dG); dCK can also contribute dTTP to the dNTP pool because the product dCMP can be converted to dTMP by dCMP deaminase and thymidylate synthase (8). Although dCK resembles RNR in terms of its ability to produce all four dNTPs, the biological function of this kinase is not yet defined. Several hypotheses have been formulated, including potential roles for dCK in DNA repair, in synthesis of liponucleotide precursors of membrane phospholipids, and in programmed cell death (reviewed in ref. 9). The evidence in favor or against these hypotheses is circumstantial. According to a model proposed by Reichard and colleagues (10–13), dCK may be responsible for “fine-tuning” dNTP pools in dividing cells through its involvement in a substrate (futile) cycle (Fig. 1). The cycle consists of a phosphorylation reaction catalyzed by dCK and a dephosphorylation reaction catalyzed by 5′ nucleotidase (5′NT). The rates of these opposing reactions are influenced by the rates of dNTP synthesis through the de novo pathway and by other factors. For example, in rapidly dividing cells, a decrease in the dCTP pool due to incorporation into DNA poises the substrate cycle toward anabolism by attenuating a dCTP-dependent negative feedback mechanism. dCK may be involved in a similar substrate cycle for deoxyadenosine (14).
Although the existence of salvage substrate cycles has been confirmed by biochemical studies in cultured cancer cells (10–13), their in vivo significance has yet to be tested experimentally. dCK and nucleotidase activities vary widely in different cell types (15). Lymphocytes express high levels of dCK (reviewed in ref. 9), and their nucleoside substrate cycles are geared toward anabolism (16). Furthermore, the importance of the deoxyribonucleoside substrate cycles may increase during developmental and functional events associated with proliferative stress, such as rapid clonal expansion of T and B cell precursors in thymus and bone marrow.
To elucidate the function of the deoxyribonucleoside salvage pathway, a genetic animal model of dCK deficiency would be of considerable interest. In the present study, we generated and analyzed dCK knockout (KO) mice. The analysis of this new genetic model demonstrated that loss of dCK function has a selective developmental impact. Although embryogenesis and the development of most organs and systems were not affected, dCK inactivation significantly impaired T and B lymphopoiesis. Beyond establishing the immunological relevance of an ancillary dNTP biosynthetic mechanism, the genetic model of dCK deficiency described herein may improve our understanding of the differential “wiring” of dNTP metabolic pathways in various cell types. In turn, this may lead to the identification of new therapeutic targets for immune disorders and lymphoid malignancies.
Results
Generation of the dCK KO Mice.
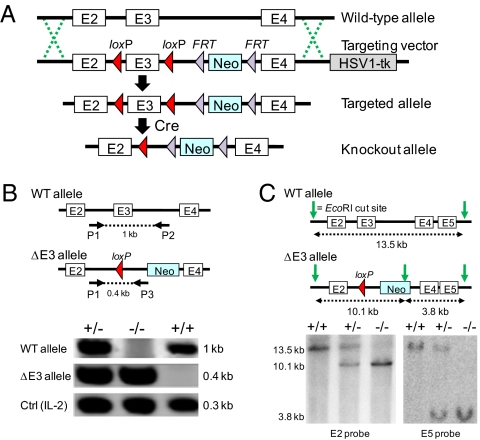
Given the lack of evidence in favor or against dCK being critically required during embryogenesis, organogenesis, or other essential developmental processes, we decided to use a conditional gene targeting strategy to avoid a lethal phenotype. A recombineering-based approach (for details, see Materials and Methods) was used to generate a dCK targeting construct in which exon 3 was flanked by loxP sites. Exon 3 deletion by Cre recombinase eliminates the dCK catalytic domain and also causes a frameshift mutation and early termination of dCK protein synthesis. Fig. 2 shows the design of the targeting vector, the strategy for homologous recombination in the dCK locus, and genotyping data confirming successful deletion of exon 3. The frequency of the ΔE3 allele among the progeny from heterozygote crosses followed the expected Mendelian ratio (23:44:26), indicating that the gene targeting strategy did not result in embryonic/perinatal lethality. Moreover, the developmental growth rates of mice carrying two copies of the ΔE3 allele were comparable to those of the wild-type (WT) littermates (Fig. S2).
Fig. 2.
Generation of dCK KO mice. (A) Strategy for homologous recombination in the dCK locus. (B) PCR genotyping using primers that amplify the WT allele or the KO allele that lacks exon 3. (C) Southern blot genotyping. See Materials and Methods for details.
Deletion of Exon 3 Inactivates dCK Function.
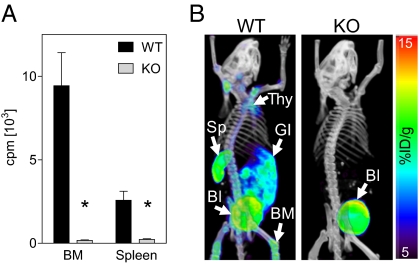
We employed two biochemical assays to validate our gene targeting strategy. These assays used radiolabeled 1-(2′-deoxy-2′-fluoroarabinofuranosyl) cytosine (FAC), a high-affinity substrate for dCK that closely resembles the endogenous dCK substrate deoxycytidine (17, 18). We first performed an in vitro dCK kinase assay on bone marrow and spleen cell lysates. The dCK KO lysates showed only background phosphorylation of [3H]FAC compared to lysates from the WT littermates (Fig. 3A).
Fig. 3.
Loss of deoxycytidine phosphorylation in the dCK KO mice. (A) In vitro dCK kinase assay using lysates from bone marrow and spleen, with [3H]FAC as substrate [*, P < 0.0001; n = 3 (WT) and 4 (KO)]. (B) [18F]FAC microPET/CT scans of the dCK WT and KO mice. Images are three-dimensional volume renderings. Thy, thymus; BM, bone marrow; Sp, spleen; GI, gastrointestinal tract; Bl, bladder.
As a corollary to the in vitro kinase assay, we imaged the dCK KO mice by positron emission tomography (PET) and computed tomography (CT) using 18F-labeled FAC {[18F]FAC (17)}. [18F]FAC PET/CT imaging allows noninvasive measurements of dCK activity throughout the body. There was a striking contrast between the [18F]FAC biodistribution in the WT mice compared to the dCK-deficient animals (Fig. 3B and Fig. S3). In the WT mice, [18F]FAC accumulated in hematopoietic and lymphoid tissues such as bone marrow, thymus, and spleen, reflecting dCK-dependent [18F]FAC trapping. None of these signals was visible in the dCK KO mice. The [18F]FAC uptake in the GI tract observed in the WT mice was absent in dCK KO mice, indicating that probe accumulation in this tissue is also dCK-specific.
Inactivation of dCK Induces a Partial Block in B Cell Development.
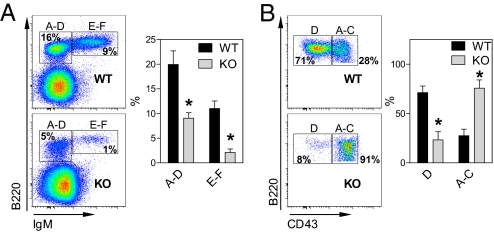
To determine whether dCK is required for normal lymphopoiesis, we first examined B cell development in the bone marrow (BM). Progress through B cell development is defined by the status of Ig gene rearrangements and by cell surface markers characteristic of various stages of lymphopoiesis. These markers are measured by flow cytometry (FACS) and are used to define subpopulations of B cell precursors according to the classification proposed by Hardy and colleagues (ref. 19, reviewed in ref. 20). Analysis of Hardy fractions E-F (B220+IgM+) and A-D cells (B220+IgM−) showed that dCK KO mice have a 2- to 3-fold deficit in both B cell precursor populations (Fig. 4A). Further subfractionation of the B220+IgM− population using the CD43 marker revealed a decrease in the percentage of pre-B late cells (Fraction D, CD43−), accompanied by an increase in the percentages of fraction A-C cells (CD43+), containing the pro-B populations (Fig. 4B).
Fig. 4.
Impaired B cell development in the dCK KO mice. (A) FACS analysis of B cell development in the bone marrow [*, P < 0.001; n = 5 (WT) and 6 (KO)]. (B) Immature B cells were further subfractionated based on CD43 expression to distinguish between fractions A–C (pro-B) and D (pre-B) populations in the BM (*, P < 0.002).
Inactivation of dCK Induces a Partial Block in Thymic T Cell Development.
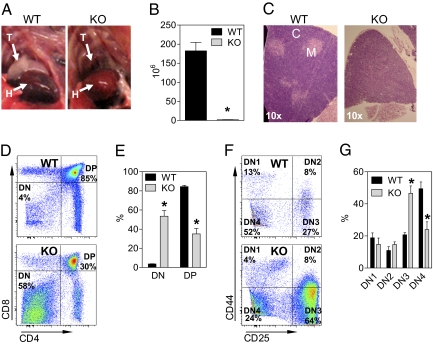
The most striking phenotype observed in the dCK KO mice concerns the size and morphology of the thymus. dCK KO mice analyzed at 6–8 weeks of age were severely microthymic. Thymi from the dCK KO mice averaged 90-fold fewer cells than those from the WT littermates (Fig. 5 A and B). Histologically, the dCK KO thymi lacked the normal corticomedullary organization and appeared less basophilic than WT thymi (Fig. 5C).
Fig. 5.
Impaired T cell development in the dCK KO mice. (A and B) Gross anatomy of thymic tissue and cellularity [*, P < 0.0001; n = 5 (WT) and 6 (KO)]. T, thymus; H, heart. (C) Histology of the dCK WT and KO thymi. C, cortex; M, medulla. (D and E) Flow cytometry (FACS) analysis of dCK WT and KO thymocytes. FACS plots are representative examples [*, P < 0.0001; n = 6 (WT) and 8 (KO)]. (F and G) DN thymocytes were further subfractionated into DN1–DN4 subsets by using the CD25 and CD44 markers [*, P < 0.002; n = 6 (WT) and 8 (KO)].
Similar to B cell development, T cell lymphopoiesis can also be subdivided by using cell surface markers such as CD4, CD8, CD44, and CD25. Proliferation of immature T cells in the thymus takes place at the CD4−/CD8− (double-negative or DN) stage (reviewed in ref. 21). The DN stage can be further subdivided based on the expression of CD44 and CD25 into four stages: DN1 (44+/25−), DN2 (44+/25+), DN3 (44lo/25+), and DN4 (44−/25−). Once the T cell receptor (TCR) β chain is produced, it combines with the pre-TCR α chain to form the pre-TCR. Signals from the pre-TCR trigger rapid clonal expansion followed by differentiation into CD4+/CD8+ double positive (DP) thymocytes. FACS analysis of dCK KO thymocytes showed a large reduction in the percentages of DP thymocytes (Fig. 5 D and E). On average, DP thymocytes constituted 85% of the live cell population in WT thymi whereas dCK KO thymi averaged only 37% DP thymocytes. The impaired production of dCK KO DP thymocytes appears even more dramatic when the 90-fold decrease in the total number of thymocytes is taken into account. The decrease in the percentages of DP cells was accompanied by a >10-fold increase in the DN fraction (Fig. 5 D and E). Further subfractionation of the DN thymocytes into the DN1-4 subpopulations showed a significant accumulation of dCK KO thymocytes at the DN3 stage (44lo/25+) and a reduction of the DN4 (44−/25−) population (Fig. 5 F and G).
Significant T and B Cell Lymphopenia and Abnormal Structure of Secondary Lymphoid Organs in the dCK KO Mice.
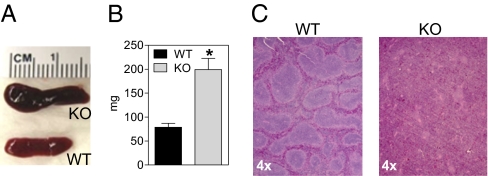
dCK KO mice have marked splenomegaly (Fig. 6A); their spleens weigh ≈3 times more than those from WT littermates (Fig. 6B). Histologically, the normal red and white pulp architecture is absent from the dCK KO spleens, typified by a distinct lack of white pulp (Fig. 6C). The increase in the spleen mass in the dCK KO mice may be due to extramedullary hematopoiesis, as indicated by an elevated percentage of reticulocytes (Fig. S4). Whether the mechanism by which dCK inactivation induces extramedullary hematopoiesis and splenic reticulocytosis is cell autonomous or not remains to be defined. The structure of peripheral lymph nodes from the dCK KO mice was also abnormal (Fig. S5). Overall, the absolute numbers of splenic and lymph node T and B cells were significantly lower in the dCK KO mice compared with the WT littermates (Table 1).
Fig. 6.
Splenomegaly and abnormal splenic histology in the dCK KO mice. (A and B) Spleen size and weight [*, P < 0.004; n = 5 (WT) and 6 (KO)]. (C) Spleen histology.
Table 1.
Absolute T and B cell numbers in peripheral lymphoid organs
| Genotype/lymphoid organ | CD3 (106) | CD19 (106) |
| WT spleen (n = 3) | 34.3 ± 1.9 | 64.7 ± 8.0 |
| KO spleen (n = 3) | 6.6 ± 1.1* | 18.1 ± 6.1* |
| WT lymph nodes (n = 3) | 9.7 ± 2.1 | 12.8 ± 2.5 |
| KO lymph nodes (n = 3) | 0.7 ± 0.1* | 0.7 ± 0.4* |
*, P < 0.02.
Discussion
Deoxycytidine Kinase Plays a Selective Role in Development.
The de novo and salvage pathways have been studied extensively. Recent in vivo studies of purine dNTP metabolism in various organs and cell types (22–24) and older studies in cell culture (reviewed in ref. 4) support a model in which the de novo pathway plays the dominant role in dNTP synthesis (4). Although the general applicability of this model awaits experimental confirmation, it is possible that dNTP metabolism in lymphocytes follows a distinct set of rules (6). To unequivocally determine the biological significance of the deoxyribonucleoside salvage pathway we generated and analyzed mice deficient for dCK, the salvage enzyme that phosphorylates pyrimidine and purine deoxyribonucleosides. dCK KO mice develop normally and are born at the expected Mendelian ratio. In mice, dCK is therefore dispensable for dNTP metabolism and DNA synthesis during embryogenesis, organogenesis, and other essential developmental processes. Because no other deoxyribonucleoside salvage enzyme can compensate for the loss of dCK function, it is likely that de novo dNTP production is sufficient to support rapid cell division during most developmental processes. The exception is lymphocyte development, which is affected by dCK inactivation. The severity of the defect in T cell development was surprising given the functional redundancy of dNTP biosynthetic pathways and the "fine-tuning" role in dNTP metabolism assigned to the deoxyribonucleoside salvage pathway by previous studies (reviewed in ref. 4). Our data suggest that, unlike other developmental programs, normal lymphopoiesis requires deoxyribonucleoside salvage and that defects in this pathway cannot be compensated by endogenously increased output of de novo synthesis.
Is dCK Required Postdevelopmentally for Normal Immune Function?
dCK KO mice have significant defects in the central production of T and B cells in the thymus and bone marrow. These mice also show reduced lymphocyte numbers in secondary lymphoid organs that appear structurally abnormal. However, none of the dCK KO mice generated so far have yet succumbed to opportunistic infections, despite being housed in conventional facilities from birth until ≈6 months of age. Pathogen challenge and allotransplantation studies will be necessary to precisely determine the degree of immunodeficiency in the dCK KO mice.
Why Do Developing Lymphocytes Depend on the Deoxyribonucleoside Salvage Pathway?
Thymocytes from the dCK KO mice display a partial block at the transition from the double negative (DN) to the double positive (DP) stage of development. A partial block is also present in B cell development at the pro-B to pre-B transition in the bone marrow. These T and B cell developmental stages follow critical checkpoints in lymphopoiesis where VDJ recombination occurs to generate productively rearranged TCR β and Ig heavy chains that become part of pre-T and B cell receptor complexes (21, 25). Signaling through these receptors halts further VDJ recombination, induces differentiation, and directs intense waves of clonal expansion. The rapid rate of cell division may be a proliferative stress that overwhelms the de novo dNTP biosynthetic machinery; it is estimated that immature T cell clones may expand 200- to 500-fold at this step (26). Analysis of thymocytes from dCK KO mice using the Ki67 marker suggests a defect in proliferation at the DN3 to DN4 transition (Fig. S6). It is possible that the absence of dCK may create a “bottleneck” in dNTP production that impairs the proliferation of DN thymocytes and their differentiation into DP cells. An analogous mechanism may explain the defects in the B cell lineage. This hypothesis merits further study by quantitative measurements of dNTP pools in dCK KO lymphocyte precursors.
The requirement for deoxyribonucleoside salvage in lymphopoiesis may be explained by microenvironmental and bioenergetic factors. The lymphopoietic niches in thymus and bone marrow contain lymphocyte precursors characterized by high rates of proliferation and death. The vast majority of normally developing thymocytes fail positive and negative selection checkpoints and undergo apoptosis (reviewed in ref. 27). DNA degradation and subsequent release of dNTPs from apoptotic thymocytes likely leads to increased extracellular concentrations of deoxyribonucleosides. By recycling these deoxyribonucleosides via the fast salvage pathway and reconverting them into dNTPs at a low ATP cost, rapidly dividing lymphocyte precursors may decrease their dependency on the slower and significantly more ATP demanding (4-fold) de novo synthesis. The analysis of the dCK KO mice shows that a similar requirement for active deoxyribonucleoside salvage does not seem to apply during embryogenesis, organogenesis, and tissue remodeling, processes that also involve high rates of proliferation and death (28). It remains to be determined whether dCK is required for the formation of germline cells.
The combined immunodeficiency syndromes noted in the dCK KO mice and in the adenosine deaminase and purine nucleoside phosphorylase genetic deficiencies in humans (2, 3) are not unique to genes involved in nucleotide metabolism. Deficiencies in various genes involved in VDJ recombination (e.g., RAG1 and 2, DNA-PKs, Ku70, Ku80, XRCC4, and ligase 4) quantitatively and qualitatively affect B and T cell development (reviewed in ref. 29). Some of these deficiencies highlight the well established fact that normal VDJ recombination requires the integrity of the DNA repair machinery. The combined immunodeficiency phenotype of the dCK KO mice together with previous studies showing that dCK activity is induced by DNA damage (reviewed in ref. 9) suggest that dCK-dependent dNTP production may also be required for DNA repair during VDJ recombination. On further analysis, the abnormal lymphocyte development observed in the dCK KO mice does not appear to be due to a severe impairment of VDJ recombination, as evidenced by positive CD3ε staining in DN4 thymocytes (Fig. S7) and by IgM expression on Hardy fraction E-F B cell progenitors (Fig. 4). Another speculated role of dCK in G1 thymocytes has been to provide pyrimidine nucleotides for terminal deoxynucleotidyl transferase (TdT)-mediated junctional diversity (6); however, in contrast to the dCK KO mice, TdT KO mice exhibit normal numbers of B and T cell precursors (29). Nonetheless, neither of these scenarios rules out the possibility that dCK deficiency may impair antigen receptor diversity by limiting substrate availability for TdT and DNA repair during VDJ recombination.
Other Genetic Deficiencies that Affect Lymphocyte Development by Altering dNTP Metabolism and Cell Proliferation.
Loss of adenosine deaminase leads to the accumulation of high intracellular levels of dATP that, by being toxic to developing lymphocytes, cause severe combined immunodeficiency in humans (2). Because dATP accumulation requires the enzymatic activity of dCK and adenosine kinase, small-molecule inhibitors of these kinases have been recently suggested as a new therapeutic approach for ADA-deficient patients (30). However, our findings suggest that complete inhibition of dCK activity may not be desirable, given the requirement for this kinase in normal lymphopoiesis.
From a biochemical function point of view, the enzyme that most closely resembles dCK is thymidine kinase 1 (TK1) (Fig. S1). A genetic model for TK1 deficiency has been reported (31). TK1 KO mice have a shortened life span due to sclerosis of the kidney glomeruli. Interestingly, the TK1 KO mice also display alterations in the lymphoid structure of the spleen and show lymph node atrophy. Direct comparisons of the immunological defects in the dCK and TK1 KO models and generation of mice lacking both cytosolic deoxyribonucleoside salvage kinases may shed additional light on the importance of deoxyribonucleoside recycling for normal lymphocyte development and function.
Defects in lymphopoiesis in dCK KO mice closely resemble the phenotype of mice deficient for cyclin D3, a key regulator of cell proliferation and the recipient of multiple oncogenic signals (32, 33). Given these similarities and the strict requirement for cyclin D3 in various murine and human T cell leukemias, future studies using dCK KO mice will help determine whether dCK is a downstream effector of cyclin D3 and whether active deoxyribonucleoside salvage is required for lymphoid oncogenesis.
Materials and Methods
Generation of the dCK Targeting Vector.
A recombineering-based approach (34) was used that employed the temperature sensitive λ prophage Red Recombinase and Arabinose inducible Cre recombinase in E. coli. This approach used two types of recombination sites: loxP sites (recognized by the bacteriophage P1-derived Cre recombinase) and FRT sites (recognized by the flippase recombinase - FLP). All reagents for recombineering were obtained from NCI-Frederick (http://recombineering.ncifcrf.gov). A genomic DNA sequence containing dCK retrieved from a BAC clone was transferred by homologous recombination into the pL253 vector to generate the pL253/dCK plasmid. A minitargeting vector containing a loxP flanked Neo cassette surrounded by regions homologous to an intronic site upstream of DCK exon 3 was electroporated into pL253/dCK-containing EL350 cells induced for λ prophage Red Recombinase. The Neo cassette was excised as described in ref. 34, resulting in a single loxP site upstream of exon 3. A second targeting step was then performed using a vector containing a FRT flanked Neo gene with an upstream loxP site. This final step yielded a genomic targeting construct containing dCK exon 3 flanked by loxP sites.
Generation of the global dCK KO mice is described in the SI Methods.
In Vitro and in Vivo Assays to Measure the Enzymatic Activity of dCK.
Splenocytes and bone marrow cells were lysed and dCK kinase activity was determined using selective binding of phosphorylated products of [3H]FAC (Moravek Biochemicals) to DE81 Whatman filter paper as previously described in ref. 18. [18F]FAC was synthesized and used for microPET/CT imaging studies as described in ref. 17. See SI Methods for a description of microPET/CT imaging studies.
Statistical Analyses.
Data are presented as means ± SEM. Group comparisons were performed by using the one-sample t test function in column statistics in Prism 5 software (GraphPad Software), using the observed mean value of WT samples as the theoretical mean of comparison. All P values are two-tailed, and P < 0.05 was considered to be statistically significant. Graphs were generated by using the Prism 5 software.
Supplementary Material
Acknowledgments
We thank Rachel Laing, Jason Lee, Amanda Armijo, and Andrew Tran for tissue processing and PET imaging acquisition and analysis; Felicia Codrea for FACS data acquisition and analysis; Greg Lawson for histology evaluation; Ken Dorshkind for insightful discussion; D. Stout, W. Ladno, B. Chun, and J. Edwards for microPET/CT imaging; and the cyclotron group for the production of PET probes. We acknowledge the use of the Jonsson Comprehensive Cancer Center Flow Cytometry and ES cell/Transgenic mice Facilities, and of the Broad Stem Cell Research Center Flow Cytometry Facility. This work was supported by National Cancer Institute/National Institutes of Health In Vivo Cellular and Molecular Imaging Center Award P50 CA86306 [to C.G.R., H.R.H. (P.I.), M.E.P., and D.O.C.], National Cancer Institute/National Institutes of Health Grants 5U54 CA119347 (to C.G.R.) and R24 CA92865, US Department of Energy Contract DE-FG02-06ER64249 (to M.E.P.), and the Dana Foundation (C.G.R.), California Institute for Regenerative Medicine Grant RT1-01126-1 (to M.E.P., O.N.W., and C.G.R.). A.S. is supported under an institutional T32 CA09297 Academic Training in Medical Oncology. O.N.W. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Conflict of interest statement: C.G.R., J.C., and O.N.W. are among the inventors of the national and PCT patent applications for the 18F-FAC technology referred to in this article. A group of UCLA faculty members including C.G.R., J.C., M.E.P., and O.N.W. are involved in Sofie Biosciences, a startup company that has licensed this intellectual property.
This article contains supporting information online at www.pnas.org/cgi/content/full/0913900107/DCSupplemental.
References
- 1.Nyhan WL. Disorders of purine and pyrimidine metabolism. Mol Genet Metab. 2005;86:25–33. doi: 10.1016/j.ymgme.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 2.Giblett ER, Anderson JE, Cohen F, Pollara B, Meuwissen HJ. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972;2:1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- 3.Giblett ER. ADA and PNP deficiencies: how it all began. Ann N Y Acad Sci. 1985;451:1–8. doi: 10.1111/j.1749-6632.1985.tb27090.x. [DOI] [PubMed] [Google Scholar]
- 4.Reichard P. Interactions between deoxyribonucleotide and DNA synthesis. Annu Rev Biochem. 1988;57:349–374. doi: 10.1146/annurev.bi.57.070188.002025. [DOI] [PubMed] [Google Scholar]
- 5.Thelander L, Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- 6.Cohen A, Barankiewicz J, Lederman HM, Gelfand EW. Purine and pyrimidine metabolism in human T lymphocytes. Regulation of deoxyribonucleotide metabolism. J Biol Chem. 1983;258:12334–12340. [PubMed] [Google Scholar]
- 7.Eriksson S, Munch-Petersen B, Johansson K, Eklund H. Structure and function of cellular deoxyribonucleoside kinases. Cell Mol Life Sci. 2002;59:1327–1346. doi: 10.1007/s00018-002-8511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staub M, Spasokukotskaja T, Benczur M, Antoni F. DNA synthesis and nucleoside metabolism in human tonsillar lymphocyte subpopulations. Acta Otolaryngol Suppl. 1988;454:118–124. doi: 10.3109/00016488809125014. [DOI] [PubMed] [Google Scholar]
- 9.Staub M, Eriksson S. The role of deoxycytidine kinase in DNA synthesis and nucleoside analog synthesis. In: Peters GJ, editor. Cancer Drug Discovery and Development: Deoxynucleoside Analogs in Cancer Therapy. Totowa, NJ: Humana; 2006. pp. 29–52. [Google Scholar]
- 10.Nicander B, Reichard P. Evidence for the involvement of substrate cycles in the regulation of deoxyribonucleoside triphosphate pools in 3T6 cells. J Biol Chem. 1985;260:9216–9222. [PubMed] [Google Scholar]
- 11.Bianchi V, Pontis E, Reichard P. Regulation of pyrimidine deoxyribonucleotide metabolism by substrate cycles in dCMP deaminase-deficient V79 hamster cells. Mol Cell Biol. 1987;7:4218–4224. doi: 10.1128/mcb.7.12.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bianchi V, Pontis E, Reichard P. Interrelations between substrate cycles and de novo synthesis of pyrimidine deoxyribonucleoside triphosphates in 3T6 cells. Proc Natl Acad Sci USA. 1986;83:986–990. doi: 10.1073/pnas.83.4.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Höglund L, Pontis E, Reichard P. Effects of deoxycytidine and thymidine kinase deficiency on substrate cycles between deoxyribonucleosides and their 5′-phosphates. Cancer Res. 1988;48:3681–3687. [PubMed] [Google Scholar]
- 14.Bianchi V, Ferraro P, Borella S, Bonvini P, Reichard P. Effects of mutational loss of nucleoside kinases on deoxyadenosine 5′-phosphate/deoxyadenosine substrate cycle in cultured CEM and V79 cells. J Biol Chem. 1994;269:16677–16683. [PubMed] [Google Scholar]
- 15.Carson DA, Kaye J, Seegmiller JE. Lymphospecific toxicity in adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency: possible role of nucleoside kinase(s) Proc Natl Acad Sci USA. 1977;74:5677–5681. doi: 10.1073/pnas.74.12.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carson DA, Kaye J, Wasson DB. The potential importance of soluble deoxynucleotidase activity in mediating deoxyadenosine toxicity in human lymphoblasts. J Immunol. 1981;126:348–352. [PubMed] [Google Scholar]
- 17.Radu CG, et al. Molecular imaging of lymphoid organs and immune activation by positron emission tomography with a new [18F]-labeled 2′-deoxycytidine analog. Nat Med. 2008;14:783–788. doi: 10.1038/nm1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laing RE, et al. Noninvasive prediction of tumor responses to gemcitabine using positron emission tomography. Proc Natl Acad Sci USA. 2009;106:2847–2852. doi: 10.1073/pnas.0812890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Epps HL. Bringing order to early B cell chaos. J Exp Med. 2006;203:1389. doi: 10.1084/jem.2036fta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciofani M, Zúñiga-Pflücker JC. The thymus as an inductive site for T lymphopoiesis. Annu Rev Cell Dev Biol. 2007;23:463–493. doi: 10.1146/annurev.cellbio.23.090506.123547. [DOI] [PubMed] [Google Scholar]
- 22.Macallan DC, et al. Measurement of cell proliferation by labeling of DNA with stable isotope-labeled glucose: studies in vitro, in animals, and in humans. Proc Natl Acad Sci USA. 1998;95:708–713. doi: 10.1073/pnas.95.2.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neese RA, et al. Measurement in vivo of proliferation rates of slow turnover cells by 2H2O labeling of the deoxyribose moiety of DNA. Proc Natl Acad Sci USA. 2002;99:15345–15350. doi: 10.1073/pnas.232551499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Busch R, Neese RA, Awada M, Hayes GM, Hellerstein MK. Measurement of cell proliferation by heavy water labeling. Nat Protoc. 2007;2:3045–3057. doi: 10.1038/nprot.2007.420. [DOI] [PubMed] [Google Scholar]
- 25.Herzog S, Reth M, Jumaa H. Regulation of B-cell proliferation and differentiation by pre-B-cell receptor signalling. Nat Rev Immunol. 2009;9:195–205. doi: 10.1038/nri2491. [DOI] [PubMed] [Google Scholar]
- 26.Falk I, Biro J, Kohler H, Eichmann K. Proliferation kinetics associated with T cell receptor-beta chain selection of fetal murine thymocytes. J Exp Med. 1996;184:2327–2339. doi: 10.1084/jem.184.6.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 28.Penaloza C, Lin L, Lockshin RA, Zakeri Z. Cell death in development: shaping the embryo. Histochem Cell Biol. 2006;126:149–158. doi: 10.1007/s00418-006-0214-1. [DOI] [PubMed] [Google Scholar]
- 29.Bassing CH, Swat W, Alt FW. The mechanism and regulation of chromosomal V(D)J recombination. Cell. 2002;109(Suppl):S45–S55. doi: 10.1016/s0092-8674(02)00675-x. [DOI] [PubMed] [Google Scholar]
- 30.Joachims ML, et al. Restoration of adenosine deaminase-deficient human thymocyte development in vitro by inhibition of deoxynucleoside kinases. J Immunol. 2008;181:8153–8161. doi: 10.4049/jimmunol.181.11.8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dobrovolsky VN, Bucci T, Heflich RH, Desjardins J, Richardson FC. Mice deficient for cytosolic thymidine kinase gene develop fatal kidney disease. Mol Genet Metab. 2003;78:1–10. doi: 10.1016/s1096-7192(02)00224-x. [DOI] [PubMed] [Google Scholar]
- 32.Sicinska E, et al. Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell. 2003;4:451–461. doi: 10.1016/s1535-6108(03)00301-5. [DOI] [PubMed] [Google Scholar]
- 33.Cooper AB, et al. A unique function for cyclin D3 in early B cell development. Nat Immunol. 2006;7:489–497. doi: 10.1038/ni1324. [DOI] [PubMed] [Google Scholar]
- 34.Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.