Abstract
Although membrane-bounded compartments are commonly considered a unique eukaryotic characteristic, many species of bacteria have organelles. Compartmentalization is well studied in eukaryotes; however, the molecular factors and processes leading to organelle formation in bacteria are poorly understood. We use the magnetosome compartments of magnetotactic bacteria as a model system to investigate organelle biogenesis in a prokaryotic system. The magnetosome is an invagination of the cell membrane that contains a specific set of proteins able to direct the synthesis of a nanometer-sized magnetite crystal. A well-conserved region called the magnetosome island (MAI) is known to be essential for magnetosome formation and contains most of the genes previously implicated in magnetosome formation. Here, we present a comprehensive functional analysis of the MAI genes in a magnetotactic bacterium, Magnetospirillum magneticum AMB-1. By characterizing MAI deletion mutants, we show that parts of its conserved core are not essential for magnetosome biogenesis and that nonconserved genes are important for crystal formation. Most importantly, we show that the mamAB gene cluster encodes for factors important for magnetosome membrane biogenesis, for targeting of proteins to this compartment and for several steps during magnetite production. Altogether, this genetic analysis defines the function of more than a dozen factors participating in magnetosome formation and shows that magnetosomes are assembled in a step-wise manner in which membrane biogenesis, magnetosome protein localization, and biomineralization are placed under discrete genetic control.
Keywords: bacterial organelle, biomineralization, compartmentalization, magnetosome, magnetotactic bacteria
The ability to form organelles and to organize the cytoplasm in several compartments is often considered a unique eukaryotic trait, one that is absent from simpler prokaryotic cells. However, microscopic studies have led to the identification of an increasing number of prokaryotic membrane-bounded organelles, suggesting that subcellular compartmentalization in eukaryotes and prokaryotes may share a common evolutionary origin, as reviewed elsewhere (1, 2). Although some prokaryotic organelles, such as the photosynthetic membranes of heterotrophic photosynthetic bacteria and the nucleus-like compartments found in some Planctomycete species, have been studied at the ultrastructural level, little is known about the molecular mechanisms of their assembly and maintenance. A thorough molecular understanding of intracellular compartmentalization in prokaryotes is necessary to draw meaningful mechanistic and evolutionary connections to the well-studied processes of organelle assembly in eukaryotes.
A particularly attractive system to characterize the cell biology of bacterial organelles is the magnetosome compartment of magnetotactic bacteria (MTB). The magnetosome organelle is a lipid-bounded invagination of the cytoplasmic membrane that directs the biomineralization of a single, highly ordered magnetic crystal of magnetite (Fe3O4) or greigite (Fe3S4). Individual magnetosomes are aligned in one or more chains that allow MTB to orient in geomagnetic field lines, which in turn facilitates their search for low-oxygen environments (3, 4). Magnetosomes have been largely used as a model to study biomineralization, the process by which living organisms build highly ordered three-dimensional structures out of inorganic molecules. MTB produce membrane-bounded magnetite crystals with a narrow and species-specific size and shape distribution under ambient conditions, unique properties that have made them a target for applications in biotechnology, nanotechnology, and medical sciences (5). In recent years, magnetosomes have also proved to be an excellent model to study the cell biology of bacterial organelle formation. To build a magnetosome, a cell must create and maintain a highly curved membrane compartment, sort the proper set of proteins to it, and organize individual magnetosomes into chains with the use of a dedicated cytoskeletal system (6, 7). Many of these processes resemble those implicated in the formation and maintenance of eukaryotic organelles; but at the moment, the molecular factors implicated in each one of these steps, or their chronology, remain for the most part unknown.
To date, the strategies to identify molecular factors important for magnetosome formation have been based on genetic screens for nonmagnetic mutants, comparative genomics of MTB, and proteomic analyses of purified magnetosome (8–13). These independent approaches have revealed that the majority of the genes potentially participating in magnetosome formation are grouped in four conserved gene clusters present within a large unstable genomic region called the magnetosome island (MAI) (8, 10, 14). This region appears to be conserved in all MTB analyzed thus far, although the size and gene content of the MAI vary significantly between species. Interestingly, the spontaneous loss of the MAI leads to a nonmagnetic phenotype, demonstrating its central role in magnetosome biogenesis (15, 16). In the magnetite-producing α-proteobacterium Magnetospirillum magneticum strain AMB-1 (AMB-1), MAI loss prevents both crystal and magnetosome compartment formation, indicating that at least some factors essential for magnetosome membrane biogenesis are present in that region (6).
This study presents a directed functional analysis of MAI genes in a magnetotactic bacterium with the goal of defining the molecular factors involved in magnetosome membrane biogenesis. The term magnetosome refers to both the magnetite crystal and its surrounding lipid bilayer. Accordingly, throughout this article, the term “magnetosome membrane” is used to describe only the lipid bilayer portion of the compartment. We show that two regions of the MAI play a crucial role in magnetite crystal formation and that the highly conserved mamAB gene cluster is essential for magnetosome membrane biogenesis in AMB-1. By independently disrupting each gene in this cluster, we demonstrate that this organelle is assembled in a step-wise manner such that magnetosome membrane biogenesis, magnetosome protein localization, and biomineralization are placed under discrete genetic control.
Results
The mamAB Gene Cluster Is Essential for Magnetosome Formation.
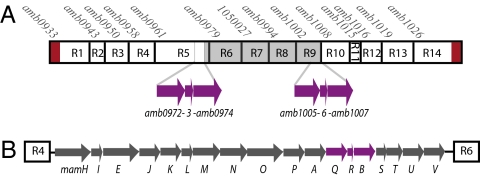
In AMB-1, the MAI contains 106 annotated ORFs, which represent ≈2% of the gene content of AMB-1. To determine parts of the MAI important for magnetosome formation, it was divided into 14 independent regions (named R1– R14) based on predicted operon structure and potential gene function (Fig. 1A and Table S1). To assess the importance of each region in the process of magnetosome formation, the magnetic properties of the mutants were quantified by measuring their ability to turn in an applied magnetic field in a spectrophotometric assay (9, 17) and by visualizing the magnetosome chains by transmission electron microscopy (TEM) (detail about the mutant characterization is provided in SI Text and Table S1). This analysis shows that the majority of MAI subdeletions retain a wild-type phenotype. However, deletions of regions R2 and R3 lead to severe defects in the size and morphology of the crystals and the magnetic properties of the cells (Fig. S1A). Most importantly, the deletion of region R5 is the only mutation that prevents the formation of magnetic minerals, as indicated by a null magnetic response and the lack of magnetite in the bacteria imaged by TEM.
Fig. 1.
Genomic organization of the MAI and the mamAB gene cluster in Magnetospirillum magneticum AMB-1. (A) Schematic representation of the 14 regions of the magnetosome island (labeled R1–R14) that were independently deleted in AMB-1. The name of the first gene of each region is indicated. Above R7, the genomic coordinate of beginning of the region is indicated. The 1,137-bp direct repeats flanking the MAI are represented by red rectangles. In purple, the three ORFs that constitute the perfect 1,957-bp duplication in the MAI of AMB-1 are represented (amb0972-3–4 and amb1005-6–7 respectively). The gray rectangle represents the region spontaneously deleted in SID25 (SI Text). (B) Organization of mamAB gene cluster (R5). mamQ, mamR, and mamB, corresponding to amb0972, amb0973, and amb0974 respectively, are shown in purple.
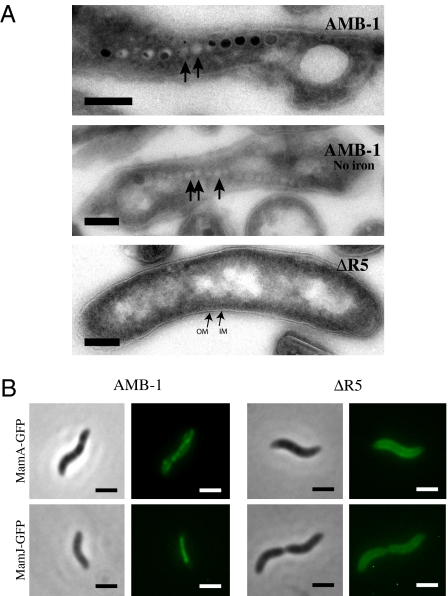
Because magnetosome membrane formation precedes magnetite formation (9), the nonmagnetic phenotype of the ΔR5 mutant could be explained either by the lack of magnetite or by the complete absence of magnetosome membranes. To determine the presence of magnetosome compartments in this strain, ultrathin cell sections obtained by cryo-ultramicrotomy were investigated by TEM (9). As shown in Fig. 2A, empty magnetosome compartments were observed in wild-type cells grown in the absence of iron. In contrast, no structures resembling magnetosomes were observed in the ΔMAI (6) (Fig. S1B) or the ΔR5 strains (Fig. 2A), suggesting that no magnetosomes are made in these mutants. In the absence of magnetosome membranes, magnetosome-associated proteins should be mislocalized within the cell. Thus, as an independent measure of magnetosome membrane formation, the localization of two magnetosome proteins, MamA and MamJ, tagged with the green fluorescent protein (GFP) was characterized in AMB-1 wild-type, ΔMAI, and ΔR5 cells. In wild-type cells (Fig. 2B), both tagged proteins localize as a line running along the inner curvature of the cell in a manner reminiscent of the subcellular position of the magnetosome chain. However, in the spontaneous ΔMAI mutant and the ΔR5 strain, MamA-GFP and MamJ-GFP are mislocalized; their fluorescent signals are mostly diffuse throughout the cytoplasm, with some enhanced accumulation around the cell membrane in a fraction of the population (Fig. S1C and Fig. 2B). The defects observed by combination of electron microcopy and localization study of GFP-tagged magnetosome proteins suggest that the region R5 encodes for one or several factors essential for magnetosome membrane invagination.
Fig. 2.
The mamAB gene cluster (R5) is essential for magnetosome membrane formation. (A) TEM images of ultrathin cryo-sections of AMB-1 and the ΔR5 strain reveal the presence of magnetosomes in wild-type cells and their absence from the mutant. Black arrows indicate the position of empty magnetosome compartments in wild-type cells. Electron-dense structures within the magnetosome compartments are magnetite crystals. (Scale bar, 100 nm.) IM, inner membrane; OM, outer membrane. (B) Magnetosome-associated proteins are mislocalized in the ΔR5 mutant. (Upper) Localization of MamA-GFP in AMB-1 wild-type and ΔR5 cells. (Lower) Localization of MamJ-GFP in AMB-1 wild-type and ΔR5 cells. Phase contrast (Left) and fluorescence images (Right). (Scale bar, 2 μm.)
Comprehensive Analysis of mamAB Genes.
ΔR5 carries an 18-kilobase deletion that encompasses the highly-conserved mamAB gene cluster (Fig. 1B), a region that contains several of the magnetosome formation factors found through genetic and proteomic studies (6, 7, 9, 11, 13, 18). Most of these genes are shared between AMB-1, Magnetospirillum gryphiswaldense MSR-1, Magnetospirillum magnetotacticum MS-1, Magnetococcus sp. MC-1 and the magnetotactic marine vibrio strain MV-1, and a subset have recently been found in the distantly related Desulfovibrio magneticus RS-1 (10, 19). Despite the apparent importance of this cluster, only three of its genes have been studied through direct genetic analysis. MamA is important for magnetosome activation (9) and MamK and MamJ are required for proper magnetosome chain organization (6, 7) leading us to hypothesize that these genes would not be necessary for the biogenesis of the magnetosome membrane. Thus, 14 single nonpolar deletions of the remaining genes of this cluster were generated and the last gene of the region, mamV, was disrupted by insertional mutagenesis. One potential complication in analyzing the function of mamAB genes is that three of its ORFs, mamQ, mamR, and mamB, are perfectly duplicated (100% identity at the nucleotide level) in the R9 gene cluster of the MAI (Fig. 1A). Therefore, these three genes were deleted in wild-type AMB-1 (leading to strains ΔmamQ, ΔmamR, and ΔmamB) and in the ΔR9 deletion strain (leading to strains ΔR9ΔmamQ, ΔR9ΔmamR, and ΔR9ΔmamB), a strain that synthesizes magnetosomes of wild-type appearance and is missing the repeat. Three mutants were nearly indistinguishable from wild-type cells (ΔmamH, ΔmamU, and ΔmamV), whereas the rest fell into two large classes of mutants: nonmagnetic mutants and mutants with altered magnetic phenotypes as a result of biomineralization defects (Table 1). The mutants that had severely decreased magnetic properties were systematically complemented (Table S2). In addition, as described in the following sections, they were subjected to a series of secondary screens to determine their specific role in magnetosome formation.
Table 1.
Phenotypic characterization of the mamAB mutants
| Mutant | Magnetic response (Cmag) | Presence of magnetosome membranes |
| ΔmamU | Wt | + |
| ΔmamV | Wt | + |
| ΔmamH | Intermediate | + |
| ΔmamQ | Intermediate | + |
| ΔmamR | Intermediate | + |
| ΔmamB | Intermediate | + |
| ΔmamP | Weak | + |
| ΔR9ΔmamR | Weak | + |
| ΔmamS | Weak | + |
| ΔmamT | Weak | + |
| ΔmamE | Null | + |
| ΔmamM | Null | + |
| ΔmamN | Null | + |
| ΔmamO | Null | + |
| ΔmamI | Null | — |
| ΔmamL | Null | — |
| ΔR9ΔmamQ | Null | — |
| ΔR9ΔmamB | Null | — |
Intermediate, 60–80% of wild-type magnetic response; Weak, less than 40% of wild-type magnetic response; Wt, not significantly different from the wild type. Presence of magnetosome membranes was assessed by cryo-ultramicrotomy and TEM.
Four Conserved Genes in the mamAB Cluster Are Essential, but Not Sufficient, for Biogenesis of the Magnetosome Membrane.
As evidenced by a null magnetic response, eight of the mutants generated above are unable to synthesize magnetic particles. This phenotype could be caused by the absence of magnetite crystals or by a complete block in magnetosome membrane formation. To distinguish between these two possibilities, the presence of empty magnetosome compartments in these mutants was investigated by cryo-ultramicrotomy followed by TEM (Table 1). This analysis revealed that four of the mutants, bearing deletions in mamE, mamM, mamN, or mamO, had chains of empty magnetosomes. In contrast the imaging of the ΔmamI and ΔmamL mutants showed that these strains do not form structures resembling empty magnetosome compartments. In addition, whereas ΔmamQ and ΔmamB mutants are magnetic, the ΔR9ΔmamQ and ΔR9ΔmamB double mutants fail to form magnetosome compartments. The phenotypes of the ΔR9ΔmamQ and ΔR9ΔmamB double deletion strains could be complemented in trans by the expression of mamQ or mamB, respectively, indicating that the two copies of these genes are functionally redundant.
mamI, mamL, mamQ, and mamB were the only genes found to be essential for magnetosome compartment formation in R5, suggesting that they may also be sufficient for inner membrane invagination in AMB-1. To test this, the ability of these four genes to restore membrane formation in the ΔR5 mutant strain was investigated. Using reverse transcription followed by PCR, it was first shown that under normal growth conditions amb1005 and amb1007, the duplicated versions of mamQ and mamB, respectively, are expressed from the R9 region of the MAI in the ΔR5 deletion strain. To provide mamI and mamL in trans, an integrational plasmid allowing for the expression of both genes (pAK397-IL) from a neutral chromosomal locus was constructed (Materials and Methods and SI Text). This plasmid allows for complementation in the single deletion strains ΔmamI and ΔmamL as well as in a strain where both genes were deleted (ΔmamLΔmamI), indicating that the construct can provide a sufficient amount of each gene product. When pAK397-IL was integrated in the ΔR5 strain, however, no structures resembling empty magnetosome membranes could be observed by TEM, indicating that mamI, mamL, mamQ, and mamB are not sufficient for magnetosome membrane formation. As no other single gene deletions within R5 led to the absence of magnetosome membranes, it is likely that a combination of additional factors located within the mamAB cluster is also required for magnetosome membrane biogenesis.
MamI Has a Magnetosome-Dependent Localization in AMB-1.
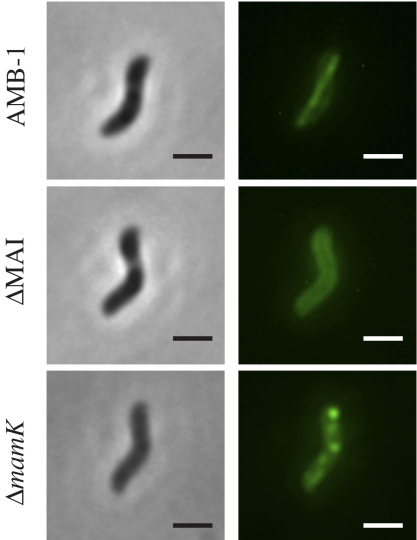
In contrast to MamQ and MamB, MamI and MamL have not previously been shown to be physically associated with magnetosomes in cell fractionation analyses of either AMB-1 or MSR-1 (11, 13, 20). As the genetic analysis indicates that they play a role in magnetosome membrane invagination, it might be expected that MamI and MamL would be, at least transiently, associated with the magnetosomes. To investigate the localization of these two small proteins, GFP fusions to MamI and MamL were visualized in AMB-1. These fusion proteins allow for partial complementation in the ΔmamI and ΔmamL mutants, respectively (Table S2). MamL-GFP mostly localizes around the cell membrane (SI Text and Fig. S2A) but can localize as aligned dots or very short lines running tangential to the inner curvature in ∼10% of the cells, suggesting that MamL-GFP may associate transiently with the magnetosomes (Fig. S2 B and C). In contrast, GFP-MamI localizes as a continuous straight line extending from pole to pole, running tangential to the inner curvature of the cell (Fig. 3), consistent with localization to the magnetosome chain. GFP-MamI is mislocalized in both the ΔMAI and ΔR5 mutants, where it appears all around the cell membrane (localization of GFP-MamI in the ΔMAI strain is shown in Fig. 3), also suggesting that it specifically associates with the magnetosomes in vivo. As further proof of the association of GFP-MamI with the magnetosome chain, its localization was investigated in a ΔmamK strain. MamK is a bacterial actin-like cytoskeletal protein required for proper alignment of the magnetosomes in a chain in AMB-1. In a ΔmamK mutant, the magnetosome chain is disorganized and individual magnetosomes can localize around the cell periphery (6). As illustrated in Fig. 3, a roughly linear pattern for GFP-MamI can still be observed in the ΔmamK mutant; however, the fluorescence is not homogeneously distributed, and intense foci of fluorescence outside the chain are present adjacent to the membrane, which is reminiscent of the clustering and uneven spacing of magnetosomes seen in the ΔmamK strain (6). The localization of GFP-MamI strongly suggests that MamI associates with the magnetosomes and that GFP-MamI can be used as a marker for the presence and positioning of magnetosome compartments.
Fig. 3.
GFP-MamI has a magnetosome-dependent localization in AMB-1. Phase contrast (Left) and fluorescence images (Right) of GFP-MamI in AMB-1 wild-type, ΔMAI, and ΔmamK mutants. (Scale bar, 2 μm.)
MamE Is Important for Localization of a Subset of Proteins to the Magnetosome Membrane.
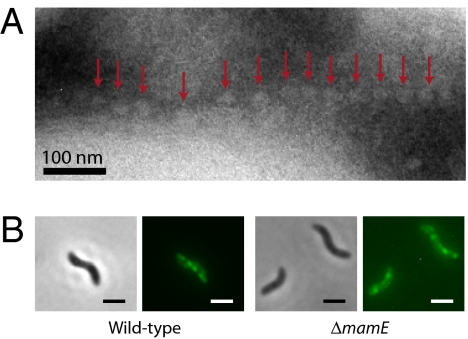
As described above, four nonmagnetic mutants carrying deletions of mamE, mamO, mamM, or mamN are able to form a chain of empty magnetosome membranes but cannot synthesize magnetite within these compartments (Fig. 4A). The absence of magnetite suggests that MamE, MamO, MamM, and MamN are potentially important for biomineralization and could be involved in iron transport, magnetite nucleation, or establishment of the proper chemical environment for magnetite synthesis in the magnetosome. However, this phenotype could also be a consequence of the inability of the mutant to properly localize magnetosome proteins. To test this possibility, the localization of the GFP-tagged magnetosome proteins MamA and MamJ was determined in these four mutants. Both MamA-GFP and MamJ-GFP are correctly localized in the ΔmamM, ΔmamN, and ΔmamO mutants, suggesting a potential role for MamM, MamN, and MamO in biomineralization. In the ΔmamE mutant, however, MamA-GFP is mislocalized and found at or in close proximity to the cell membrane as small foci that are randomly positioned as opposed to being organized as a line in wild-type AMB-1 cells (Fig. 4B). MamJ-GFP is also mislocalized in the ΔmamE mutant, although the defect is subtle (Fig. S3). These observations suggest that in the ΔmamE mutant, the absence of magnetite crystals could be a consequence of the mislocalization of at least a subset of magnetosome proteins.
Fig. 4.
The ΔmamE mutant forms empty magnetosome compartments and has a defect in magnetosome protein localization. (A) TEM image of an ultrathin cryo-section of a ΔmamE mutant cell. Empty magnetosomes are indicated by red arrows. (B) MamA-GFP is mislocalized in the ΔmamE strain. Phase contrast (Left) and fluorescence images (Right) of MamA-GFP in AMB-1 wild-type and ΔmamE cells. (Scale bar, 2 μm.)
Biomineralization Is Placed Under Discrete Genetic Control in AMB-1.
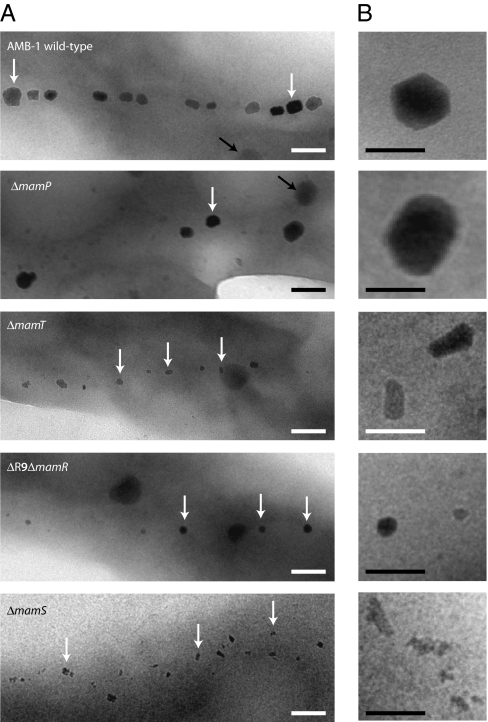
Finally, the deletion strains ΔmamP, ΔmamT, ΔmamR, and ΔmamS display drastically decreased magnetic properties and TEM shows that they each harbor a different biomineralization defect (Fig. 5). First, the ΔmamP mutant synthesizes fewer inclusions (up to four per cell compared with 15–25 per cell in the wild type) that resemble wild-type crystals in shape but are overall larger than those synthesized in wild-type AMB-1. All the particles measured were greater than 35 nm in length, and more than 70% are greater than 50 nm in length as compared with less than 30% in wild-type cells. This suggests that MamP could play a role in controlling crystal size and number in AMB-1. In the ΔmamT mutant, the chain of magnetosomes contains significantly smaller particles (15.9 ± 4.2 nm in width and 24.4 ± 8.3 nm in length compared with 32.3 ± 13.9 nm in width and 39.1 ± 16.1 nm in length for wild-type crystals). This phenotype suggests a role for MamT in magnetite crystal growth. mamR is the third gene, besides mamQ and mamB, that is present in the perfect 1,957–bp direct repeat, and it is identical to amb1006 (Fig. 1). Although the magnetic properties of the ΔmamR mutant are slightly lower than in the wild type (Table 1), no obvious defect in the magnetosome chain could be detected by TEM. In contrast, when both mamR and amb1006 are deleted (ΔR9ΔmamR), the cells retain the ability to produce magnetosomes, as indicated by the ability of cell pellets to be attracted to a bar magnet. However, the magnetic response could not be detected in the quantitative spectrophotometric assay. Electron microscopy shows that the ΔR9ΔmamR strain forms shorter chains (one to seven particles per cell) of significantly smaller-sized particles. More than 50% are between 10 and 20 nm in width, with an average size of 18.6 ± 7.3 nm in width and 21.2 ± 7.7 nm in length. Their morphology is similar to that of the wild type, indicating that MamR plays a role in controlling both particle number and size but does not participate in the control of crystal morphology. Finally, the ΔmamS mutant synthesizes a large majority of amorphous-looking particles (Fig. 5, white arrowheads) with few rounder crystals of wild-type appearance. They are significantly smaller than wild-type crystals (19.1 ± 5.7 nm in length), their spacing is irregular, and small clusters can be observed within the chain (Fig. 5B). This phenotype suggests that MamS plays a major role in controlling crystal morphology and size. However, the morphology defect in this mutant is different from that observed in the ΔmamT strain, suggesting that MamS and MamT participate in different steps during magnetite synthesis. It should be noted that the nature of the minerals observed in these mutants has not been determined and will require further investigation. These observations demonstrate that the number, size, and morphology of the magnetite crystals are placed under discrete genetic control in AMB-1.
Fig. 5.
Formation of magnetite is genetically controlled in AMB-1. (A) TEM images of whole cells of (top to bottom) AMB-1 wild-type, ΔmamP, ΔmamT, ΔR9ΔmamR, and ΔmamS mutants. White arrows indicate the position of magnetite crystals; the black arrows indicate unidentified storage granules. (Scale bar, 100 nm.) (B) Close-up views of crystals in the mutant strains shown in A. (Scale bar, 50 nm.)
Discussion
In this work, a comprehensive genetic approach was undertaken to characterize the steps and molecular factors controlling the biogenesis of a bacterial organelle, the magnetosome of magnetotactic bacteria. The magnetosome island, a conserved genomic region in MTB, was known to be essential for magnetosome biogenesis. However, the results of this study show that most of its genes are not essential for the assembly of a functional chain of magnetosomes. It is possible that some deletion strains would have a magnetosome defect under different growth conditions, or that the combination of several deletions would affect magnetosome formation in case of functional redundancy among the MAI genes. Interestingly, the degree of conservation of a region is not sufficient to predict its role in magnetosome formation. Indeed, at least one of the gene clusters conserved in MTB is not essential for magnetosome synthesis (mamXY gene cluster) (10) and, conversely, regions that are specific to AMB-1 are important for biomineralization and magnetosome membrane invagination (R2 and R9). Finally, the role in biomineralization of the mamCDF and mms6 gene clusters previously reported (11, 18, 21) was confirmed, as the ΔR3 mutant, a strain containing a deletion of both gene clusters, has a severe magnetite formation defect. Taken together, the deletion analysis of the MAI suggests that its size could be significantly reduced, facilitating further genetic manipulations to synthesize magnetosome-like compartments in heterologous systems. It should be emphasized, however, that genes outside of the MAI could also play important roles in the formation of this organelle.
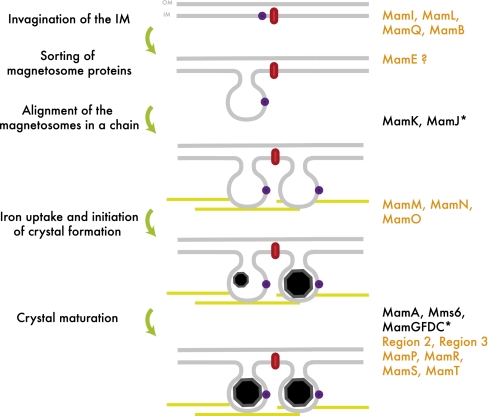
This global analysis reveals that magnetosome biogenesis relies on four major steps that can be genetically decoupled: inner membrane invagination, localization of the magnetosome proteins, positioning of the magnetosomes in the cell, and biomineralization. Interestingly, factors involved in each one of these steps are clustered within the conserved mamAB region (Fig. 6). First, the formation of a highly curved, membrane-bound compartment seems to rely on four conserved putative membrane proteins, MamB, MamQ, MamI, and MamL. With the possible exception of MamQ (discussed below), bioinformatic analyses of these proteins at the primary and secondary structure levels does not reveal any significant homology to eukaryotic proteins known to be involved in deformation of cellular membranes, such as the BAR domain-containing proteins and the Dynamin superfamily of GTPases (22) (SI Text). MamB is the only one of these four proteins that has a domain of known function. It is predicted to belong to the cation-diffusion facilitator superfamily, which includes a ferrous iron transport system (23). The homology of MamB with transporters suggests that it could have an indirect role in magnetosome membrane invagination or that it could potentially have a role in both magnetosome membrane invagination and biomineralization that would allow the cells to couple compartment and crystal formation. MamQ shares homology with the LemA protein, the function of which remains unknown (24). In addition, using its predicted secondary structure as a query, we discovered weak hits for MamQ to a number of eukaryotic proteins including Tropomyosin, Spectrin, and the EFC/BAR domain of Formin Binding Protein 17. We believe that this is mainly due to the high alpha-helical content of LemA-like proteins, which includes MamQ, and does not represent a true homology to BAR domains (SI Text). Finally, MamI and MamL are unique to MTB, although mamL was not annotated in a previous analysis of MC-1 (10). However, the protein encoded by the MC-1 gene Mmc1_2257 shares 32% identity with MamL of AMB-1, and its position downstream of mamK is conserved. Interestingly, mamQ and mamB, but not mamI and mamL, were found in the recently sequenced and distantly related Desulfovibrio magneticus RS-1, which belongs to the δ-proteobacteria (19). This suggests that magnetosome formation in RS-1 may rely on a different mechanism. Surprisingly, these four proteins do not seem to be sufficient to trigger magnetosome compartment formation in the absence of the mamAB gene cluster. Assuming that their expression in the engineered strain is optimal, this result would suggest that, in addition to MamB, MamQ, MamI, and MamL, a combination of other MamAB proteins that are not independently essential for inner membrane invagination are required for formation or maintenance of magnetosome membranes. Further genetic and biochemical studies are needed to elucidate the specific role of these proteins in membrane dynamics.
Fig. 6.
Model for step-wise assembly of magnetosomes in AMB-1. Steps leading to magnetosome formation are indicated on the left side of the model, and the factors known to play a role in each of these steps are indicated on the right side. Protein names in black indicate factors discovered in previous studies in AMB-1; in orange, proteins whose potential functions were defined in the present study. Asterisks indicate proteins characterized in MSR-1. Black octagons represent growing or mature, euhedral magnetite crystals. Red symbols indicate inner membrane proteins; purple dots indicate magnetosome-associated proteins; yellow lines represent the MamK cytoskeletal filaments. IM, inner membrane; OM, outer membrane.
Another key finding of this work is that magnetosome membrane biogenesis can occur independently and before the targeting of at least a subset of proteins to this compartment. MamE, a putative membrane-bound serine protease, is required for magnetite formation. In its absence, MamA and MamJ, which are not essential for biomineralization, are mislocalized, suggesting that MamE may also control the localization of other magnetosome proteins. Alternatively, MamE could play a direct role in biomineralization independent of its function in magnetosome protein localization.
After the magnetosome compartments are formed and positioned, the final step in magnetosome biogenesis is the biomineralization of magnetite. Three factors that are essential for crystal formation, as well as four factors that control the size, number, and morphology of the magnetite crystals, were also identified through this genetic analysis. The wide range of biomineralization phenotypes suggests a complex regulation of magnetite synthesis in AMB-1. A more thorough analysis of the crystals formed in these mutants may help to reveal intermediate minerals synthesized during magnetite formation.
In conclusion, the comprehensive genetic analysis of the conserved magnetosome island reveals a step-wise assembly of the magnetosome organelle in which membrane invagination, magnetosome protein localization, organelle positioning, and magnetite formation are independently regulated. This genetic study allowed the definition of the role of two large genomic regions and 12 conserved factors in the major steps of magnetosome formation. The fact that most factors investigated in this study do not share homology with proteins known to participate in organelle biogenesis in other systems may suggest a unique pathway for intracellular compartmentalization in MTB. However, it may be possible that, even in the absence of primary sequence conservation, the general mechanisms of compartmentalization are conserved across the various of domains of life. Finally, beyond the fundamental insights into potentially conserved processes in organelle-containing organisms, these results will also have an impact on efforts to manipulate and engineer magnetosome compartments for applications in nanotechnology and medical sciences.
Materials and Methods
General Microbiology and Molecular Biology.
Magnetospirillum magneticum strain AMB-1 was grown in microaerobic conditions in a slightly modified version of the media described previously (9) using 0.1 g sodium thiosulfate/L (9) (SI Text). Cmag measurements, conjugations, gene inactivations, and complementations were done as described previously (6) (SI Text).
mamI-mamL Two-Gene Operon.
A plasmid allowing for the expression of genes under the control of the tac promoter on the chromosome of AMB-1 was generated (pAK397; details in SI Text). A 1,106-bp fragment amplified from an intergenic region located between amb0397 and amb0398 was amplified and cloned in pAK0 (6). mamI and mamL were cloned in several steps as a two-gene operon in pAK22 (6) and then subcloned in the pAK397 vector leading to pAK397-IL. A ribosome binding site was provided for mamL.
TEM and Cryo-Ultramicrotomy.
TEM characterization and cryo-ultramicrotomy were performed using standard methods and as described previously (9) with slight modifications (SI Text). At least 200 sections of bacteria were analyzed for each strain and, in strains where present, 30–50% of the sections contained magnetosome chains. Mutants were designated as deficient in magnetosome membrane formation if none of the sections contained magnetosome-like structures.
Fluorescence Microscopy.
The GFP fusions were derived from the pAK22 plasmid and analyzed as described previously (6) (SI Text). For each construct, more than 200 cells were photographed and analyzed.
Supplementary Material
Acknowledgments
We thank the members of the Komeili laboratory for their support and valuable input into this work. We also thank J. Mui and Dr. S. K. Sears (both of the Facility for Electron Microscopy Research, McGill University) for assistance. A.K. was supported through grants from the Hellman Family Fund, the David and Lucille Packard Foundation and the National Institutes of Health (5R01GM084122-02). H.V. acknowledges financial support from the Natural Sciences and Engineering Research Council of Canada.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914439107/DCSupplemental.
References
- 1.Shively JM, editor. Microbiology Monographs Vol. 2., Complex Intracellular Structures in Prokaryotes. Heidelberg: Springer; 2006. [Google Scholar]
- 2.Fuerst JA. Intracellular compartmentation in planctomycetes. Annu Rev Microbiol. 2005;59:299–328. doi: 10.1146/annurev.micro.59.030804.121258. [DOI] [PubMed] [Google Scholar]
- 3.Komeili A. Molecular mechanisms of magnetosome formation. Annu Rev Biochem. 2007;76:351–366. doi: 10.1146/annurev.biochem.74.082803.133444. [DOI] [PubMed] [Google Scholar]
- 4.Smith MJ, et al. Quantifying the magnetic advantage in magnetotaxis. Biophys J. 2006;91:1098–1107. doi: 10.1529/biophysj.106.085167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faivre D, Schüler D. Magnetotactic bacteria and magnetosomes. Chem Rev. 2008;108:4875–4898. doi: 10.1021/cr078258w. [DOI] [PubMed] [Google Scholar]
- 6.Komeili A, Li Z, Newman DK, Jensen GJ. Magnetosomes are cell membrane invaginations organized by the actin-like protein MamK. Science. 2006;311:242–245. doi: 10.1126/science.1123231. [DOI] [PubMed] [Google Scholar]
- 7.Scheffel A, et al. An acidic protein aligns magnetosomes along a filamentous structure in magnetotactic bacteria. Nature. 2006;440:110–114. doi: 10.1038/nature04382. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda Y, Okamura Y, Takeyama H, Matsunaga T. Dynamic analysis of a genomic island in Magnetospirillum sp. strain AMB-1 reveals how magnetosome synthesis developed. FEBS Lett. 2006;580:801–812. doi: 10.1016/j.febslet.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Komeili A, Vali H, Beveridge TJ, Newman DK. Magnetosome vesicles are present before magnetite formation, and MamA is required for their activation. Proc Natl Acad Sci USA. 2004;101:3839–3844. doi: 10.1073/pnas.0400391101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richter M, et al. Comparative genome analysis of four magnetotactic bacteria reveals a complex set of group-specific genes implicated in magnetosome biomineralization and function. J Bacteriol. 2007;189:4899–4910. doi: 10.1128/JB.00119-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grünberg K, et al. Biochemical and proteomic analysis of the magnetosome membrane in Magnetospirillum gryphiswaldense. Appl Environ Microbiol. 2004;70:1040–1050. doi: 10.1128/AEM.70.2.1040-1050.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okuda Y, Denda K, Fukumori Y. Cloning and sequencing of a gene encoding a new member of the tetratricopeptide protein family from magnetosomes of Magnetospirillum magnetotacticum. Gene. 1996;171:99–102. doi: 10.1016/0378-1119(95)00008-9. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka M, et al. Origin of magnetosome membrane: Proteomic analysis of magnetosome membrane and comparison with cytoplasmic membrane. Proteomics. 2006;6:5234–5247. doi: 10.1002/pmic.200500887. [DOI] [PubMed] [Google Scholar]
- 14.Grünberg K, Wawer C, Tebo BM, Schüler D. A large gene cluster encoding several magnetosome proteins is conserved in different species of magnetotactic bacteria. Appl Environ Microbiol. 2001;67:4573–4582. doi: 10.1128/AEM.67.10.4573-4582.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsunaga T, et al. Complete genome sequence of the facultative anaerobic magnetotactic bacterium Magnetospirillum sp. strain AMB-1. DNA Res. 2005;12:157–166. doi: 10.1093/dnares/dsi002. [DOI] [PubMed] [Google Scholar]
- 16.Ullrich S, Kube M, Schübbe S, Reinhardt R, Schüler D. A hypervariable 130-kilobase genomic region of Magnetospirillum gryphiswaldense comprises a magnetosome island which undergoes frequent rearrangements during stationary growth. J Bacteriol. 2005;187:7176–7184. doi: 10.1128/JB.187.21.7176-7184.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuler D, Uhl R, Bauerlein E. A simple light scattering method to assay magnetism in Magnetospirillum gryphiswaldense. FEMS Microbiol Lett. 1995;132:139–145. [Google Scholar]
- 18.Scheffel A, Gärdes A, Grünberg K, Wanner G, Schüler D. The major magnetosome proteins MamGFDC are not essential for magnetite biomineralization in Mag-netospirillum gryphiswaldense but regulate the size of magnetosome crystals. J Bacteriol. 2008;190:377–386. doi: 10.1128/JB.01371-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakazawa H, et al. Whole genome sequence of Desulfovibrio magneticus strain RS-1 revealed common gene clusters in magnetotactic bacteria. Genome Res. 2009;19:1801–1808. doi: 10.1101/gr.088906.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schüler D. Molecular analysis of a subcellular compartment: The magnetosome membrane in Magnetospirillum gryphiswaldense. Arch Microbiol. 2004;181:1–7. doi: 10.1007/s00203-003-0631-7. [DOI] [PubMed] [Google Scholar]
- 21.Amemiya Y, Arakaki A, Staniland SS, Tanaka T, Matsunaga T. Controlled for-mation of magnetite crystal by partial oxidation of ferrous hydroxide in the presence of recombinant magnetotactic bacterial protein Mms6. Biomaterials. 2007;28:5381–5389. doi: 10.1016/j.biomaterials.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 22.McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–596. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- 23.Grass G, et al. FieF (YiiP) from Escherichia coli mediates decreased cellular accu-mulation of iron and relieves iron stress. Arch Microbiol. 2005;183:9–18. doi: 10.1007/s00203-004-0739-4. [DOI] [PubMed] [Google Scholar]
- 24.D'Orazio SE, Velasquez M, Roan NR, Naveiras-Torres O, Starnbach MN. The Listeria monocytogenes lemA gene product is not required for intracellular infection or to activate fMIGWII-specific T cells. Infect Immun. 2003;71:6721–6727. doi: 10.1128/IAI.71.12.6721-6727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.