Abstract
The intrinsic role of endogenous IL-17A in spontaneous intestinal tumorigenesis has not been addressed previously to our knowledge. Ablation of IL-17A significantly reduced tumor development in mice bearing a heterozygote mutation in the adenomatous polyposis coli (APC) gene (ApcMin/+ mice). There was also a decrease in inflammatory cytokines and proinflammatory mediators, reduced infiltration of lymphocytes including T cells, and preservation of intestinal architecture and the presence of APC protein in intestinal epithelial cells. Interestingly, IL-17A ablation also corrected immunological abnormalities such as splenomegaly and thymic atrophy in ApcMin/+ mice. CD4 T cells from ApcMin/+ mice showed hyperproliferative potential in vitro and in vivo and increased levels of IL-17A and IL-10. The effector CD4 T cells from ApcMin/+ mice were more resistant to regulatory T cell–mediated suppression. Finally, these CD4 T cells induced colitis in immunodeficient mice upon adoptive transfer, whereas the ablation of IL-17A in CD4 T cells in ApcMin/+ mice completely abolished this pathogenic potential in vivo. Taken together, our results show that CD4 T cell–derived IL-17A promotes spontaneous intestinal tumorigenesis with altered functions of CD4 T cells in ApcMin/+ mice.
Keywords: inflammation, T cells, colon cancer
The intestinal tract is subject to constant stimulation of immune cells and intestinal epithelial cells either by noninfectious antigens or infection. Recent studies identified the role of IL-17A in chronic inflammation in intestinal epithelium (1). Th17 cells (IL-17A–secreting T cells) are known to be involved in many autoimmune diseases, such as inflammatory bowel disease (IBD), experimental autoimmune encephalomyelitis, and rheumatoid arthritis (2, 3). It has been known that the level of IL-17A expression in the gut increases spontaneously over time in the pres-ence of ATP (4). IL-6 is a critical inducer of Th17 cell development via STAT3 and retinoid-related orphan receptor (ROR)–γt in conjunction with TGF-β (5, 6), whereas IL-23 is critical to terminally differentiate Th17 (IL-17–secreting) cells (3). Recent studies using a colitis model showed complex roles for IL-17A in the gut (7, 8), suggesting that IL-17A can be protective or proinflammatory in the intestinal environment.
The most extensively studied model of intestinal dysplasia in either humans or mice is familial adenomatous polyposis, which harbors a mutation in the gene encoding adenomatous polyposis coli (APC). Mutation in this gene is one of the most frequently detected mutations in intestinal dysplasia in human and animal models as well as other types of cancers (9). The ApcMin/+ mouse strain was generated by ethyl nitrosourea mutagenesis, resulting in a truncated form of APC (850 amino acids of 2,483) (10). WT APC plays a central role in normal colon to negatively regulate Wnt signaling, facilitating the proteosome-mediated degradation of β-catenin (11). The intestinal dysplasia actively develops from approximately week 11, with the complete loss of heterozygosity (LOH) of the APC gene, subsequently facilitating nuclear localization of phosphorylated β-catenin in colonic epithelial cells (12–14). These initial findings on the role of APC as a tumor suppressor gene in the Wnt–β-catenin pathway contributed to the understanding of intestinal tumorigenesis in colonic epithelial cells, but the role of the immune system to detect and eliminate these aberrant oncogenic cells has been in question based on its failure to prevent polyp growth. It has been suggested that the transfer of CD4+CD45RBhi cells can increase tumor numbers whereas the transfer of CD4+CD45RBlow cells can inhibit tumor growth in ApcMin/+ mice, suggesting that CD4 T cells may be an important cell subset to control intestinal tumorigenesis (15, 16). In addition, ApcMin/+ mice have been shown to undergo thymic atrophy and splenomegaly, suggesting abnormalities in the immune system of these mice (17).
To address the role of IL-17A in intestinal tumorigenesis, we ablated IL-17A in ApcMin/+ mice. We observed a significant decrease in intestinal tumorigenesis and the recovery of thymic and splenic cellularity. We also showed that CD4 T cells from ApcMin/+ mice are significantly altered to favor intestinal tumorigenesis and can induce colitis.
Results
IL-17A Ablation Significantly Decreases Intestinal Tumorigenesis.
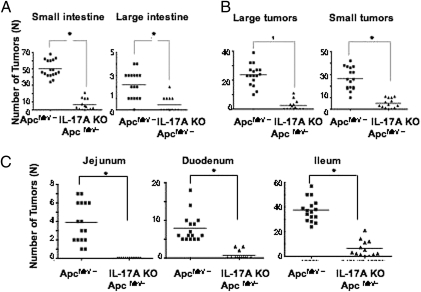
To address the intrinsic role of IL-17A in intestinal tumorigenesis, we bred ApcMin/+ mice to IL-17A–deficient mice (IL-17A KO- ApcMin/+). We observed a significant decrease in tumor numbers in both small (88.0%) and large intestine (80.3%; Fig. 1A). This marked decrease was evident both in the number of large tumors (>3.0 mm) and small tumors (<3.0 mm; Fig. 1B). The decrease in tumor numbers was consistently observed in each part of small intestine, whereas most tumors were found in the ileum in ApcMin/+ mice (Fig. 1C). The weight loss that occurred in ApcMin/+ mice is mostly a consequence of bowel obstruction, and the ablation of IL-17A largely restored mean body weight (Fig. S1). These results demonstrate that the ablation of IL-17A significantly decreased tumorigenesis in both the small and large intestinal tract.
Fig. 1.
IL-17A ablation significantly reduces intestinal tumorigenesis. (A) Tumor numbers of small and large intestine were counted in 20-week-old ApcMin/+ mice (n = 16) and IL-17A KO- ApcMin/+ mice (n = 13). (B) Tumors smaller than 3 mm and larger than 3 mm were counted separately. (C) Tumor numbers from each part of small intestine (duodenum, jejunum, and ileum). For both comparisons, *P < 0.002 using two-tailed t tests.
Lymphocyte Infiltration and Loss of APC Were Decreased by IL-17A Ablation.
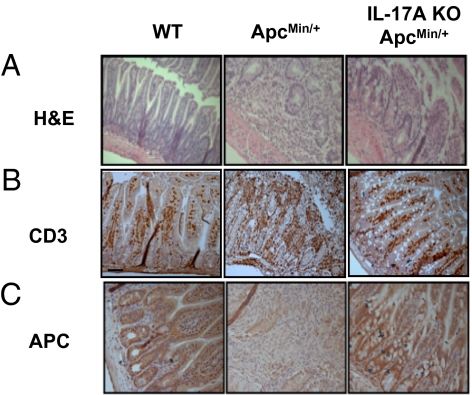
As intestinal inflammation accompanies lymphocyte infiltration, we examined whether lymphocytes infiltrated the small intestine. Infiltration of lymphocytes was more evident in ApcMin/+ mice compared with WT C57BL/6 mice and IL-17A KO- ApcMin/+ mice in H&E-stained sections (Fig. 2A). Also, CD3 T cells in IL-17A KO- ApcMin/+ mice were significantly reduced compared with ApcMin/+ mice (Fig. 2B). This increased infiltration of T cells included both CD4 and CD8 T cell populations in the lamina propria (Fig. S2). Furthermore, we showed that IL-17A+ CD4 T cells were increased whereas there was only a marginal increase of IL-6R expression (Fig. S3). To investigate whether the ablation of IL-17A can affect the canonical Wnt–β-catenin pathway with the loss of APC gene, we assessed the accumulation of APC protein in the small intestine of ApcMin/+ and IL-17A KO- ApcMin/+ mice. The level of APC protein was decreased in ApcMin/+ compared with WT or IL-17A KO- ApcMin/+ mice (Fig. 2C). Taken together, our results show that lymphocyte infiltration including CD4 and CD8 T cells does occur and this was inhibited by ablation of IL-17A in ApcMin/+ mice.
Fig. 2.
Ablation of IL-17A preserved small intestinal architecture in ApcMin/+ mice and reduces immune cell infiltration. Small intestines (ileum) of 20-week-old C57BL/6 (n = 5), ApcMin/+ (n = 4), and IL-17A KO- ApcMin/+ (n = 4) mice were harvested and stained with H&E (A) and murine anti-CD3 monoclonal antibody (B). Ileums of same group of mice were stained with anti-APC monoclonal antibody (C) with paraffin-embedded section.
Proinflammatory Cytokines and Mediators Are Significantly Decreased in the Tumors of IL-17A KO- ApcMin/+ Mice.
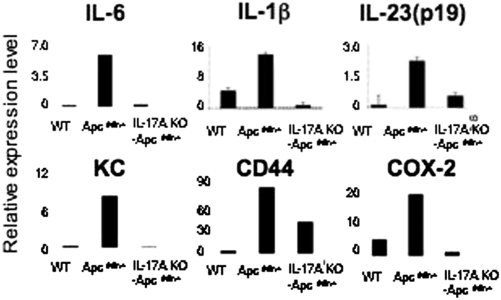
To investigate whether the ablation of IL-17A can down-regulate inflammation in the intestinal tract, we isolated tumors (3.0 mm in size) from small intestines of ApcMin/+ and IL-17A KO- ApcMin/+ mice and quantitated the mRNA expression of proinflammatory cytokines and mediators. There was a marked decrease in IL-6 and IL-23 (p19), as well as a decrease in IL-1β (Fig. 3), suggesting that the inflammatory microenvironment is significantly changed by ablating IL-17A. We also found a significant decrease in CD44 and proinflammatory mediators such as keratinocyte chemoattractant and Cox-2 (Fig. 3). This suggests that the deletion of IL-17A abrogated the initiation and stabilization of an IL-17A–mediated inflammatory environment.
Fig. 3.
IL-17A ablation reduced proinflammatory cytokines and mediators in tumors. Tumors (3 mm; one or two from each mouse) were dissected from 20-week-old ApcMin/+ mice (n = 5) and IL-17A KO- ApcMin/+ mice (n = 4). mRNA level was normalized against HPRT. For WT C57BL/6 mice, 5 mm of ileum was used. Values are means ± SD of all experiments.
IL-17A Ablation Corrected Immune Abnormalities.
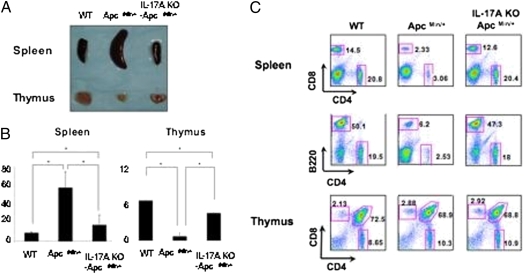
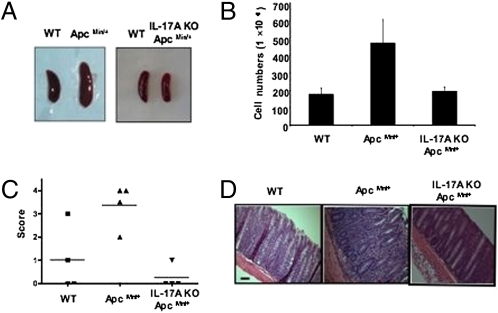
It has been dem-onstrated that ApcMin/+ mice undergo thymic atrophy and splenomegaly with lymphodepletion as well as loss of Peyer’s patches accompanying intestinal tumorigenesis (17, 18). It has also been reported that the rapid onset of tumors in the intestine coincides with the loss of thymus (15). Interestingly, thymic atrophy and splenomegaly were largely reversed in IL-17A KO- ApcMin/+ mice (Fig. 4). We further observed that the loss of CD4 and CD8 T cells as well as B cells from spleen in ApcMin/+ mice was also restored while maintaining the normal CD4/CD8 ratios by ablating IL-17A (Fig. 4C). However, the ratio of CD4 and CD8 thymocytes did not change in ApcMin/+ mice and it remained unchanged in IL-17A KO- ApcMin/+ mice (Fig. 4C). Of note, we did not observe significant lymphodepletion in mesenteric lymph nodes. Treatment with anti–IL-17A mAb substantially reduced the number of tumors whereas treatment with anti–IFN-γ had no significant effect (Fig. S4). Similar findings were made regarding reversal of thymic atrophy and splenomegaly (Fig. S4). Collectively, our data suggest that the thymus and spleen are the primary immune organs affected by ApcMin/+ in the presence of endogenous IL-17A in addition to intestinal tumorigenesis.
Fig. 4.
Splenomegaly and thymic atrophy of ApcMin/+ mice are corrected by IL-17A ablation. Spleens and thymuses from 20-week-old ApcMin/+ and IL-17A KO ApcMin/+ mice and littermate controls of ApcMin/+ were dissected (A) and counted for cell numbers (B), and subsequently analyzed by flow cytometry (C). Flow cytometry is a representative of five mice per group. *P ≤ 0.002; one-way analysis was used with Tukey correction at an α of 0.05.
CD4 T Cells from ApcMin/+ Mice Have Extensively Altered Functions.
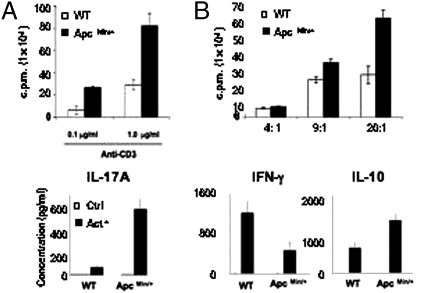
It has been demonstrated that CD4 T cells are a primary source of IL-17A. Based on our phenotype in ApcMin/+ mice and IL-17A KO ApcMin/+ mice, we questioned whether CD4 T cells from ApcMin/+ mice were significantly altered. First, we tested whether CD4 T cells from ApcMin/+ mice are more proliferative based on its role as a tumor suppressor gene, thereby regulating cell cycle. We observed that CD4 T cells from ApcMin/+ mice were hyperproliferative compared with their WT littermate controls upon T cell receptor stimulation (Fig. 5A). This hyperproliferation was more obvious following stronger stimulation through the T cell receptor. Interestingly, the activation status of naive T cells from ApcMin/+ mice and IL-17A KO ApcMin/+ mice did not show significant differences (Fig. S5). Next, we tested whether the effector CD4 T cells are more refractory to regulatory T cell (Treg)–mediated suppression. Upon coculture with WT Treg cells, ApcMin/+ CD4 effector T cells were more resistant to Treg-mediated suppression, especially at a high ratio of effectors to Treg, suggesting that the suppression on ApcMin/+ CD4 effector T cells are not as efficient as for WT CD4 effector T cells (Fig. 5B). We also tested Tregs from ApcMin/+ and littermate controls using an in vitro suppressor assay, but no significant differences were observed (Fig. S6). However, this suppression was not maintained in vivo (Fig. S7) in an IBD model. We further tested whether IL-17A acts on ApcMin/+ CD4 T cells in an autocrine manner by treatment with IL-17A, but it did not enhance proliferation (Fig. S8). Finally, we tested whether CD4 T cells are altered in their cytokine secretion. ApcMin/+ CD4 T cells indeed secreted significantly more IL-17A and IL-10 than WT CD4 T cells, whereas they produced less IFN-γ upon stimulation (Fig. 5C). We also observed preferential expression of IL-17A and IL-10 in Peyer’s patch CD4 T cells in ApcMin/+ mice (Fig. S9).
Fig. 5.
CD4 T cells from ApcMin/+ mice are functionally altered. (A) CD4 T cells from ApcMin/+ mice were isolated and activated with given amount of anti-CD3 and T cell–depleted irradiated splenocytes for 72 h. (B) Foxp3+ regulatory T cells were isolated from ApcMin/+ Foxp3-IRES-RFP mice and cocultured with Foxp3- CD4 effector T cells from either ApcMin/+ mice or their littermate control for 72 h for given ratios. (C) CD4 T cells from ApcMin/+ mice were isolated and activated for 96 h, and cytokines were measured by cytokine bead array. Data indicate means ± SD of three separate experiments as triplicates.
Ablation of IL-17A ApcMin/+ CD4 T Cells Corrects Their Aberrant Phenotype in Vivo.
Based on our results in vitro, we questioned whether ApcMin/+ CD4 T cells can be more proliferative and induce inflammation in the intestinal tract. To this end, we used adoptive transfer of CD4 T cells into Rag2-deficient mice. We measured CD4 and Foxp3 expression before transfer the transfer of CD4 T cells, and it showed no significant changes for each group of mice. First, we measured cell numbers in spleen of Rag2-deficient mice after 4 weeks of adoptive transfer (Fig. 6 A and B), and it showed that ApcMin/+ CD4 T cells proliferated more, whereas IL-17A KO ApcMin/+ CD4 T cell transfer did not show this hyperproliferation. This suggests that the ablation of IL-17A corrected the hyperproliferative potential of ApcMin/+ CD4 T cells. Next, we observed that ApcMin/+ CD4 T cells can cause more severe colitis than WT CD4 T cells whereas IL-17A ApcMin/+ CD4 T cells did not induce colitis (Fig. 6 C and D). This suggests that IL-17A ablation in ApcMin/+ CD4 T cells largely restored their altered functions in vivo. We further showed that regulatory T cells from ApcMin/+ mice could not prevent inflammatory bowel disease whereas those from IL-17A KO ApcMin/+ mice could prevent colitis (Fig. S7). Collectively, our data thus suggest that the altered functions of CD4 T cells from ApcMin/+ mice can be corrected by ablating IL-17A.
Fig. 6.
Ablation of IL-17A in CD4 T cells corrects altered functions in CD4 T cells from ApcMin/+ mice. (A) CD4 T cells from 11-week-old ApcMin/+ and IL-17A KO ApcMin/+ mice and littermate controls ApcMin/+ mice were isolated and transferred into Rag 2 KO mice. After 4 weeks of transfer, spleens were harvested and cell numbers were counted, and the ratio between CD4 effector T cells and Foxp3+ CD4 T cells was measured by flow cytometry (B). Colons were harvested and scored for their chronicity (C) and stained with H&E (D). The experiments are the representative of two independent experiments.
Discussion
Several studies demonstrated the role of IL-17A in autoimmune diseases as a proinflammatory cytokine. However, it has not been clearly addressed whether IL-17A does contribute clearly as a proinflammatory cytokine in spontaneous intestinal tumorigenesis. Controversial results regarding the role of IL-17A in different types of colitis models have been reported (7, 8). In addition, infection with enterotoxigenic bacteria caused intestinal tumorigenesis involving STAT3, perhaps through a Toll-like receptor (TLR)–mediated pathway by a specific bacterial pathogen (19, 20). Although these studies may imply that our intestinal tumorigenesis model is mainly contributed by a commensal TLR-mediated pathway, we cannot exclude the possibility there are other pathways that can contribute intestinal tumorigenesis significantly by Th17 cell generation. For instance, a previous report suggests that the complete ablation of TLR-mediated pathways in vivo by using MyD88-Trif DKO (i.e., double-KO) mice did not show any significant changes in the Th17 cell population in the gut. Our data suggest that the increase of IL-17A+CD4+ T cells drive intestinal tumorigenesis, but this may not be mediated by IL-6R. Indeed, IL-6–independent induction of Th17 cells has been reported (21). Furthermore, the use of antibiotics such as vancomycin and metronidazole to eliminate commensals reduced ATP level to generate Th17 cells in the gut, suggesting that the generation of Th17 cells and hence IL-17A secretion is not wholly dependent on bacterial pathogens through TLR-mediated pathways (4).
Our results clearly show that the role of IL-17A in spontaneous intestinal tumorigenesis is proinflammatory and very potent in a relatively early stage of tumor development. Many in vitro experiments to differentiate Th17 cells use the combination of TGF-β and IL-6 (22). Interestingly, the magnitude of inhibition by IL-17A ablation is much more potent than IL-6 ablation in ApcMin/+ mice (30% reduction in polyp numbers) (23). This suggests that IL-17A plays a central role in controlling intestinal tumorigenesis and is not solely dependent on the IL-6 and TGF-β–mediated Th17 cell pathway. Indeed the ablation of IL-17A did decrease the level of IL-6 significantly, but other proinflammatory mediators such as keratinocyte chemoattractant and Cox-2 were also reduced. The normal intestinal architecture of the small intestine in IL-17A KO ApcMin/+ mice suggested that IL-17A amplifies ApcMin/+–mediated intestinal tumorigenesis, and it is likely that it prevents LOH significantly based on our immunohistochemistry of Apc. Although a more thorough examination is required, it has been extensively demonstrated that LOH is an indicator of adenoma formation in our model (24). It has been suggested that the mutation of KRas can significantly increase adenocarcinoma formation when combined with ApcMin/+ genotype (25). As more than 50% of patients with colon cancer have these two mutations together, the ablation or elimination of IL-17A may abrogate this sequential pathway of adenocarcinoma development at an early point. Polyps were mainly found in the ileum of the small intestine, and we showed that Peyer’s patch CD4 T cells showed more IL-17A expression in ApcMin/+ mice.
Alongside with intestinal tumorigenesis, ApcMin/+ mice have splenomegaly and thymic atrophy at later times. Interestingly, the ablation of IL-17A has corrected these immune abnormalities that contribute to lymphodepletion, especially the complete reversion of thymic atrophy. It has been suggested that the polyp growth rate is accelerated when thymic atrophy appears, indicating that T cell–mediated responses may be important. In line with this, it has been suggested that effector CD4 T cells or regulatory T cells have the potential to control polyp numbers (15, 16), but the phenotype of CD4 T cells from ApcMin/+ mice has never been characterized to our knowledge. We showed the hyperproliferation of CD4 T cells in vitro and in vivo and showed that CD4 T cells from ApcMin/+ mice are altered in their control of proliferation. Interestingly, the ratio of effector T cells and regulatory T cells before and after transfer did not show significant changes, whereas the total numbers of CD4 T cells were increased in CD4 T cells from ApcMin/+ mice. This may be a result of the altered function of regulatory T cells or the resistance of CD4 effector T cells over regulatory T cell–mediated suppression. Indeed, we showed that the resistance of ApcMin/+ effector CD4 T cells was increased as the effector to Treg ratio is increased, which is closer to physiological conditions in vivo. Also, the inability of regulatory T cells from ApcMin/+ mice to block effector CD4 T cell–mediated IBD further suggests altered functions of regulatory T cells in vivo. The altered cytokine secretion from ApcMin/+ CD4 T cells indicates that there are qualitative alterations. Our result using IL-17A KO ApcMin/+ mouse CD4 T cells in vivo clearly shows that ablation of IL-17A in ApcMin/+ CD4 T cells abrogated hyperproliferation and colitogenic potential in immunodeficient mice, consistent with our observation of the phenotypic changes in intestinal tumorigenesis.
Taken together, our results suggest an important proinflammatory role of IL-17A and its correction of immune abnormalities in spontaneous intestinal tumorigenesis. We demonstrate altered CD4 T cell functions from ApcMin/+ mice, which favors intestinal tumorigenesis, and the ablation of IL-17A can abolish these altered function in Apc/Min+ mice, suggesting the importance of T cell mediated IL-17A in spontaneous intestinal tumorigenesis.
Materials and Methods
Mice.
ApcMin/+ mice (expressing a mutant gene encoding an adenomatous polyposis coli protein truncated at amino acid 850) were established on a C57BL/6 background. In all experiments, WT littermates of ApcMin/+ mice on C57BL/6 background were used. Rag2-deficient mice were housed in specific pathogen-free conditions. IL-17A–deficient (i.e., KO) mice were used under an agreement with Yoichiro Iwakura (Tokyo, Japan). For isolation of Foxp3+ CD4 T cells, ApcMin/+ mice were bred to Foxp3-IRES-RFP (FIR) mice on a C57BL/6 background (a gift from Richard Flavell, New Haven, CT). For all experiments, C57BL/6 mice were obtained as littermate controls of ApcMin/+ mice. For histopathology of C57BL/6 (littermate control of ApcMin/+ mice), ApcMin/+, and IL-17A KO- ApcMin/+ mice, ileum was dissected, fixed in 10% formalin, washed in PBS solution, paraffin-embedded, sectioned at 5 μm, and stained. All mouse protocols were approved by the Yale University Institutional Animal Care and Use Committee in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care International.
Colitis Model.
Splenic CD4 T cells (3 × 105) from 12-week-old ApcMin/+ mice and their littermate controls were isolated using a CD4+ T cell isolation kit (Miltenyi Biotech). The purity was higher than 97%. CD4 T cells were transferred into 7-week-old Rag2-deficient mice. Four weeks after transfer, spleens were harvested and cell numbers were counted. Splenocytes were stained with anti-CD4 (clone RM4-5) and anti-Foxp3 (clone FJK-16s) antibodies followed by CD16/CD32 (clone CD16/CD32) antibody. Colons were processed (four mice per group) and paraffin-embedded, and then stained with H&E. Colonic inflammation was scored for chronicity on a scale from 0 to 4 [0, normal mucosa; 1, minimally increased chronic inflammation; 2, mildly increased inflammation; 3, moderately increased inflammation (thickening of intestinal wall); and 4, severely increased inflammation (architecture distortion)].
Flow Cytometry.
Spleens and thymuses of 20-week-old ApcMin/+ mice and their littermate controls, and IL-17A KO- ApcMin/+ mice were harvested. After RBC lysis by RBC lysis buffer, cells were stained with CD16/CD32 antibody and then subsequently stained with anti-CD4 (clone RM4-5), anti-CD8α (clone 53–6.7), and anti-B220 (clone RA3-6B2) antibodies. For the stimulation of Peyer patch cells, PMA (100 ng/mL) and ionomycin (1μM) were used. Brefeldin A (1 μg/mL) was added for the last 4 h of culture. Cells were stained with anti-CD4 (clone RM4-5), anti–IL-17A (eBio17B7), anti–IL-10 (JES5-16E3) and isotype control antibodies. For lamina propria preparation, 13-week-old ApcMin/+ mice and their littermate control mice were killed, and the individual small intestines were processed as described (3). After Percoll gradient separation, lymphocytes were counted and stained with anti-CD4 (clone RM4-5) and anti-CD8α (clone 53–6.7) antibodies. FACSCalibur (BD Biosciences) was used for flow cytometry and data were analyzed by FlowJo software (Treestar).
Real-Time PCR.
Tumors (3 mm) from ApcMin/+ and IL-17A KO- ApcMin/+ mice were isolated and homogenized by Lysing Matrix D beads and homogenizer (MPBio). TRIzol (Invitrogen) was used to isolate mRNA and genomic DNA was further cleaned by genomic DNA removal kit (Qiagen). cDNA was synthesized with BDsprint cDNA synthesis kit (Clontech) and used with SYBR Green (Molecular Probes). Real-time PCR was performed with SYBR MX3000 bioanalyzer (Stratagene). Primers were synthesized by the Keck Biotechnology facility. We calculated relative gene expression by the ΔCT method. The mRNA expression was normalized against HPRT. Primer sequences are provided upon request.
In Vitro T Cell Activation and Cytokine Analysis.
For proliferation assays, 2 × 105 CD4 T cells from ApcMin/+ mice and their littermate controls were activated with 0.1 μg/mL or 1.0 μg/mL of murine anti-CD3 monoclonal antibody in the presence of T cell depleted irradiated splenocytes for 72 h. T cells were depleted with Thy1.1 microbeads (Miltenyi Biotec). Thymidine [H3] was added for the last 10 h of incubation. For cytokine analysis, 2 × 105 CD4 T cells were activated with plate bound anti-CD3 (clone 145.2C-11) and anti-CD28 (clone 37.51) for 96 h. Supernatants were harvested and analyzed by Luminex (Bio-Rad).
Statistical Analysis.
Significance tests were performed in SAS 9.1 software using either the Pearson χ2 statistic or one-way analysis of variance at significance levels of at least 0.05. Multiple comparisons were performed using a one-way analysis of variance in SAS with the Tukey correction for multiple comparisons keeping a type I experiment-wise error rate at 0.05. All comparisons were statistically significant at an α of 0.05 or more extreme.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (A.B.). W.-J.C. is supported by an Anna Fuller Foundation fellowship.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912675107/DCSupplemental.
References
- 1.Dubin PJ, Kolls JK. Th17 cytokines and mucosal immunity. Immunol Rev. 2008;226:160–171. doi: 10.1111/j.1600-065X.2008.00703.x. [DOI] [PubMed] [Google Scholar]
- 2.Ogura H, et al. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity. 2008;29:628–636. doi: 10.1016/j.immuni.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Abraham C, Cho J. Interleukin-23/Th17 pathways and inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1090–1100. doi: 10.1002/ibd.20894. [DOI] [PubMed] [Google Scholar]
- 4.Atarashi K, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 5.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 6.Ma CS, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205:1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Connor W, Jr., et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leppkes M, et al. RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 2009;136:257–267. doi: 10.1053/j.gastro.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Segditsas S, Tomlinson I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene. 2006;25:7531–7537. doi: 10.1038/sj.onc.1210059. [DOI] [PubMed] [Google Scholar]
- 10.Su LK, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 11.Näthke I. Cytoskeleton out of the cupboard: colon cancer and cytoskeletal changes induced by loss of APC. Nat Rev Cancer. 2006;6:967–974. doi: 10.1038/nrc2010. [DOI] [PubMed] [Google Scholar]
- 12.Aoki K, Taketo MM. Adenomatous polyposis coli (APC): a multi-functional tumor suppressor gene. J Cell Sci. 2007;120:3327–3335. doi: 10.1242/jcs.03485. [DOI] [PubMed] [Google Scholar]
- 13.Shitashige M, Hirohashi S, Yamada T. Wnt signaling inside the nucleus. Cancer Sci. 2008;99:631–637. doi: 10.1111/j.1349-7006.2007.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinen CD, et al. The APC tumor suppressor controls entry into S-phase through its ability to regulate the cyclin D/RB pathway. Gastroenterology. 2002;123:751–763. doi: 10.1053/gast.2002.35382. [DOI] [PubMed] [Google Scholar]
- 15.Rao VP, et al. Proinflammatory CD4+ CD45RB(hi) lymphocytes promote mammary and intestinal carcinogenesis in Apc(Min/+) mice. Cancer Res. 2006;66:57–61. doi: 10.1158/0008-5472.CAN-05-3445. [DOI] [PubMed] [Google Scholar]
- 16.Erdman SE, et al. CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res. 2005;65:3998–4004. doi: 10.1158/0008-5472.CAN-04-3104. [DOI] [PubMed] [Google Scholar]
- 17.Coletta PL, et al. Lymphodepletion in the ApcMin/+ mouse model of intestinal tumorigenesis. Blood. 2004;103:1050–1058. doi: 10.1182/blood-2003-03-0707. [DOI] [PubMed] [Google Scholar]
- 18.You S, et al. Developmental abnormalities in multiple proliferative tissues of Apc(Min/+) mice. Int J Exp Pathol. 2006;87:227–236. doi: 10.1111/j.1365-2613.2006.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin-Orozco N, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 21.Kimura A, Naka T, Kishimoto T. IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc Natl Acad Sci USA. 2007;104:12099–12104. doi: 10.1073/pnas.0705268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Baltgalvis KA, et al. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R393–R401. doi: 10.1152/ajpregu.00716.2007. [DOI] [PubMed] [Google Scholar]
- 24.Yamada Y, et al. Microadenomatous lesions involving loss of Apc heterozygosity in the colon of adult Apc(Min/+) mice. Cancer Res. 2002;62:6367–6370. [PubMed] [Google Scholar]
- 25.Haigis KM, et al. Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon. Nat Genet. 2008;40:600–608. doi: 10.1038/ngXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.