Abstract
The signaling pathway mediated by JNK transduces different types of signals, such as stress stimuli and cytokines, into functional responses that mediate apoptosis, as well as proliferation, differentiation, and inflammation. To better characterize the dynamic information flow and signal processing of this pathway in the cellular context, a genetically encoded, fluorescent protein-based biosensor was engineered to detect endogenous JNK activity. This biosensor, named JNKAR1 (for JNK activity reporter), specifically detects stress- (ribotoxic and osmotic) and cytokine- (TNF-α) induced JNK activity in living cells with a 15 to 30% increase in the yellow-to-cyan emission ratio because of a phosphorylation-dependent increase in FRET between two fluorescent proteins. JNK activity was detected not only in the cytoplasm, but also in the nucleus, mitochondria, and plasma membrane with similar kinetics after induction of ribotoxic stress by anisomycin, suggesting relatively rapid signal propagation to the nuclear, mitochondrial, and plasma membrane compartments. Furthermore, quantitative single-cell analysis revealed that anisomycin-induced JNK activity exhibited ultrasensitivity, sustainability, and bimodality, features that are consistent with behaviors of bistable systems. The development of JNKAR1, therefore, laid a foundation for evaluating the signaling properties and behaviors of the JNK cascade in single living cells.
Keywords: kinase, FRET, MAPK, subcellular, bistable
The c-Jun N-terminal kinases (JNKs), also known as stress-activated protein kinases, are a subfamily of MAPKs that is activated primarily by cytokines and environmental stress signals, such as UV irradiation, and oxidative and ribotoxic stresses (1, 2). The JNK subfamily consists of JNK1, JNK2, and JNK3, each with multiple isoforms generated through alternative splicing. Although a major function of JNK is to regulate apoptosis, it has also been shown to be involved in diverse biological responses, including cellular proliferation, differentiation, and inflammatory reaction.
JNKs, like other members of the MAPK family, are activated in a “three-tiered” cascade manner, where MAPKKKs phosphorylate and activate MAPKKs, which in turn phosphorylate and activate JNKs (1, 2). Although there are two identified MAPKKs, MKK4 and MKK7, which phosphorylate JNK at Thr-183 and Tyr-185, there is a far greater number of MAPKKKs, allowing JNK to respond to multiple types of signals. The different components of the cascade can bind to scaffold proteins, such as JNK interacting protein 1 (JIP1), which help to maintain pathway integrity and to permit coordinated and efficient activation of the pathway in response to specific signals (1, 2). In addition, various mechanisms exist in the JNK signaling pathway that might allow signal amplification without sacrificing specificity. For example, amplification of signals can be achieved by a functional module organized by sequential MAPKKK:MAPKK and MAPKK:JNK interactions, where each interaction is disrupted upon activation of a downstream kinase (3). Furthermore, JNK activation was shown to be autocatalytic in Xenopus laevis oocytes, as cytoplasm from stressed oocytes could promote full JNK activation in the recipient oocytes (4), suggesting JNK is embedded in a positive-feedback loop. Such feedback mechanisms might result in further amplification and control of the signal.
Although many components of the JNK cascade have been identified and the core functional modules and framework of the pathway topology are known, our understanding of the dynamic information flow and signal processing of this pathway in the live cellular context remains limited. What are the systems level behaviors of this pathway? For example, the JNK cascade, equipped with signaling amplification and feedback mechanisms, was shown to be able to process a graded signal into a switch-like response (5). Is this a characteristic behavior of the cascade? What are the molecular mechanisms responsible for maintaining the system-level behaviors? In addition, do spatial microdomains exist to compartmentalize JNK signaling? If so, how is JNK signaling differentially regulated in different compartments? Answering these questions requires characterizing the behaviors of the JNK signaling cascade in an intact cellular context. To perform such analyses, a method that can provide continuous quantitative measurement of JNK activity in local signaling compartments of single living cells is needed. Here, we report the development of a genetically encoded FRET-based biosensor for measuring JNK activity in living mammalian cells. Using this reporter, we characterized the JNK activity patterns in several subcellular locations in HeLa cells exposed to anisomycin, a potent inducer of JNK activity. We further examined the single-cell behaviors of JNK pathway in response to this ribotoxic stressor and discovered that individual HeLa cells exposed to anisomycin exhibited all-or-none behaviors at the level of JNK activity.
Results
Design of a JNK Activity Reporter.
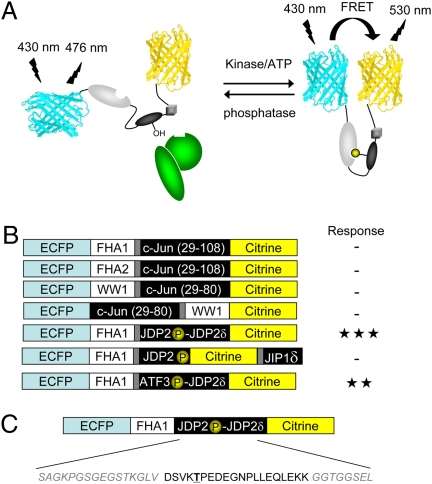
To develop a JNK activity reporter (JNKAR1), we used a general design of genetically encoded kinase activity reporters (6). The general design is based on a kinase activity-dependent molecular switch, which consists of a phosphoamino acid binding domain and a specific substrate for the kinase of interest. The molecular switch is sandwiched between two fluorescent proteins that act as a FRET pair. Once the substrate is phosphorylated by an active kinase of interest, the phosphoamino acid binding domain will bind to the phosphorylated form of the substrate, causing a change in the distance and relative orientation between the flanking FRET pair, and thus a change in FRET, which can be monitored by fluorescence microscopy (Fig. 1A).
Fig. 1.
Design of JNKAR1. (A) General schematic depicting a chimeric reporter consisting of ECFP, citrine, phosphoamino acid binding domain, substrate, and docking domain, with the kinase of interest (shown in green) recruited by the docking domain. (B) Domain structures of reporter designs that were tested. Each reporter used ECFP and citrine as the FRET pairs and used the phosphoamino acid binding domain, FHA1, FHA2, or WW1. The possible substrates included truncations of c-Jun or phosphoacceptor sites from JDP2 or ATF-3. For the JDP2- and ATF-3-based substrates, the docking domains (δ) from JDP2 or JIP1 were incorporated into the reporter. To test the different reporter designs, HeLa cells were transfected and treated with 5-μM anisomycin. -, < 5% response; ★★, 5 to 20% response; ★★★, > 20% response. (C) The construct that exhibited the greatest response is depicted showing the domain structure and amino acid sequence for the substrate, docking site and the linkers used. The phosphoacceptor, Thr, is boldface and underlined.
A specific JNK activity reporter calls for a selective JNK substrate. However, all MAPKs recognize similar phosphoacceptor sites composed of a serine or threonine followed by a proline (7). Substrate selectivity is often conferred by specific MAPK docking sites present in physiological substrates. Such docking interactions can bring a kinase and a substrate into close proximity, thus increasing the effective concentration of the substrate and enhancing both specificity and efficiency of substrate phosphorylation. Therefore, a docking motif for association with JNK needs to be incorporated into the reporter design. In this study, we tested three different docking motifs, including a JNK binding sequence located in the N-terminal transactivation domain of c-Jun at amino acids 33 to 43 (8), a unique binding motif immediately C-terminal to the phosphoacceptor site Thr-148 in Jun dimerization protein 2 (JDP2) (9), and a well-characterized docking domain from JIP1 (10).
Various combinations of substrates, docking domains, and phosphoamino acid binding domains were tested as the make-up of the molecular switch, with enhanced cyan fluorescent protein (ECFP) and citrine, a yellow fluorescent protein variant, serving as the FRET pair (Fig. 1B and Fig. S1). The combination that yielded the largest change in yellow-to-cyan emission ratio was the forkhead associated domain 1 (FHA1) paired with a substrate sequence based on JDP2 phosphorylation site, linked to the JDP2 docking domain (Fig. 1B). This particular combination is most likely responsive to both JNK1 and JNK2 because Thr-148 of JDP2 has been shown to be phosphorylated by both JNK isoforms (9, 11). As a part of the biosensor design, the serine at the +3 position with respect to the phosphoacceptor site of JDP2 in the substrate sequence was mutated to an aspartate (Fig. 1C), because the phospho-threonine binding FHA1 prefers an aspartate at the +3 position (12). The central linker between FHA1 and the substrate was identical to AKAR2, a previously designed reporter for PKA activity (13). This construct exhibited an up to 30% change in yellow-to-cyan emission ratio in HeLa cells treated with anisomycin (Fig. 1B) and was thus chosen for further examination.
Characterization of a JNK Activity Reporter.
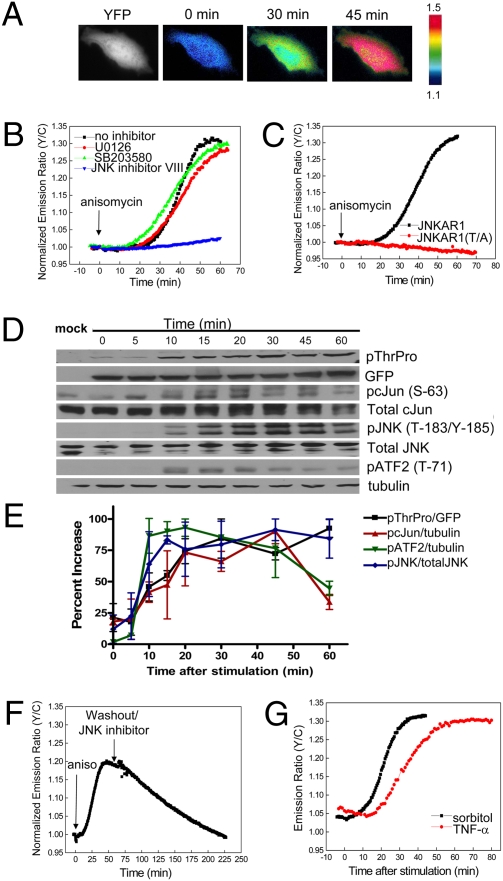
When the construct containing FHA1, JDP2-based substrate sequence, and JDP2 docking domain was expressed in HeLa cells, the biosensor was uniformly distributed throughout the cell as shown in the YFP fluorescence image (Fig. 2A). Treatment of the transfected HeLa cells with 5 μM anisomycin induced a 20.0 ± 6.6% (average ± STD, n = 57) increase in emission ratio of yellow-to-cyan over a period of 20 to 50 min (Fig. 2 A and B). The time needed to reach half maximum (t1/2) was 18.7 ± 3.6 min. Anisomycin at the concentration of 500 nM induced a similar response of 19.8 ± 8.7% with a t1/2 of 16.6 ± 3.8 min (n = 34).
Fig. 2.
Characterization of JNKAR1. (A) Pseudocolor images depict the FRET response of JNKAR1 to 5 μM anisomycin. YFP fluorescence image shows that JNKAR1 is localized throughout the cells. (B) HeLa cells expressing JNKAR1 were left untreated (n = 53) or pretreated with 10 μM U0126 (n = 12), 10 μM SB203580 (n = 19), or 10 μM JNK inhibitor VIII (n = 15) and then stimulated with 5 μM anisomycin. Representative normalized emission ratio time courses are shown. (C) Representative normalized emission ratio time courses for JNKAR1 or JNKAR1 (T/A) mutant. (D) Representative gels comparing the kinetics of phosphorylation of JNKAR1 with that of endogenous c-Jun (Ser-63), ATF-2 (Thr-71), and JNK (Thr-185/Tyr-185). (E) Averaged signals of phosphorylated JNKAR1 normalized to GFP, phosphorylated c-Jun normalized to tubulin, phosphorylated ATF-2 normalized to tubulin, and phosphorylated JNK normalized to total JNK are plotted. Error bars are the standard deviation of two independent runs. (F) Reversibility of the JNKAR1. JNKAR1-expressing HeLa cells were stimulated with anisomycin, washed five times with HBSS, and treated with 10 μM JNK inhibitor VIII. (G) Representative emission ratio time course of HeLa cells expressing JNKAR1 were treated with 500 mM sorbitol (n = 7) or 100 ng/mL TNF-α (n = 17).
To test if the biosensor responded specifically to JNK compared with other related MAPKs, HeLa cells were pretreated with 10 μM of U0126 or SB203580 to inhibit MEK, the upstream kinase of extracellular signal-regulated protein kinase (ERK) or p38 MAPK, respectively (Fig. 2B). Cells pretreated with U0126 or SB203580 displayed similar response profiles to anisomycin compared to control cells, 23.7 ± 5.2% (n = 12) and 24.2 ± 7.2% (n = 19), respectively. By contrast, pretreatment with 10 μM of JNK inhibitor VIII abolished the cellular response to anisomycin (n = 15) (Fig. 2B). In addition, this construct failed to respond to PDGF stimulation of serum-starved NIH 3T3 cells, a condition known to potently activate ERK, further suggesting that it does not respond to ERK (Fig. S2). All of these data, taken together, indicate that this biosensor, JNKAR1, responds specifically to JNK when compared with other related MAPKs, namely ERK and p38 MAPK.
To correlate the FRET response with the phosphorylation of JNKAR1, its phosphorylation was examined by Western blot analysis using an antiphospho-Thr-Pro antibody. Phosphorylation of JNKAR1 was shown to be enhanced upon anisomycin treatment and abolished by JNK inhibitor VIII pretreatment (Fig. S3). To confirm that changes in FRET result from phosphorylation of the reporter at the intended amino acid, we mutated the phosphoacceptor threonine in the JDP2 substrate sequence to alanine [JNKAR1 (T/A) mutant]. This mutation abolished the phosphorylation at this threonine (Fig. S3), as well as the change in yellow-to-cyan emission ratio upon anisomycin treatment (Fig. 2C), indicating that phosphorylation of the predetermined threonine is responsible for the FRET change.
We next compared the kinetics of JNKAR1 with time courses of JNK activation indicated by its phosphorylation, kinetics of phosphorylation of endogenous JNK substrates, c-Jun, and activating transcription factor 2 (ATF-2), as well as the temporal changes in JNK activity detected by an in vitro assay (5), all from anisomycin-treated HeLa cells. As shown in Fig. 2 D and E, phosphorylation of JNKAR1 reached a plateau at ≈20 min after stimulation. Similarly, phosphorylation of ATF-2 (Thr-71), c-Jun (Ser-63), and JNK (Thr-183/Tyr-185) itself reached maximum at ≈10, 20, and 15 min after stimulation, respectively. These data suggest that the kinetics of JNKAR1 are similar to that of JNK activation and endogenous substrate phosphorylation, and is also consistent with the temporal changes of JNK activity, as previously shown (5).
To test the reversibility of the reporter, anisomycin was removed and 10 μM of JNK inhibitor VIII was added to the cells after the anisomycin-induced response reached a plateau. This treatment led to a gradual decrease in emission ratio of yellow-to-cyan (Fig. 2F), which corresponded with a decrease in phosphorylation of JNKAR1 (Fig. S4A). Dephosphorylation of JNKAR1 showed slower kinetics compared to that of ATF-2 under the same condition, with complete reversal of the signal to baseline level occurring in 60 to 90 min (Fig. S4A). Upon removal of JNK inhibitor VIII, rechallenge by another dose of anisomycin could induce a second episode of phosphorylation (Fig. S4B). These data suggest that the response of this biosensor is reversible, although with slower kinetics of dephosphorylation compared with ATF-2.
Because JNK has been shown to be activated by a number of diverse stimuli, ranging from stress to cytokines, JNKAR1 was tested against other known activators of JNK. As shown in Fig. 2G, HeLa cells expressing this construct that were exposed to osmotic stress by 500 mM sorbitol showed an increase in yellow-to-cyan emission ratio of 18.4 ± 4.2% (n = 5), whereas an inflammatory cytokine TNF-α at the concentration of 100 ng/mL induced a response of 20.4 ± 3.4% (n = 17). These data suggest that JNKAR1 can specifically report JNK activity stimulated by different types of signals in living cells.
Visualization of Subcellular JNK Activity.
With a functional reporter, we set out to characterize spatiotemporal dynamics of JNK activity in living cells by using anisomycin as the stimulant. Anisomycin can cause ribotoxic stress responses, thereby leading to activation of JNK. Such responses require the presence of translationally activated ribosomes at the time of exposure to the drug, suggesting that JNK activation originates from damage to the active ribosomes (14). JNKs can phosphorylate a variety of physiological substrates that reside at distinct subcellular locations (7). However, JNK activity patterns at different subcellular locations have not been directly analyzed and compared, and it is not clear how a signal from damaged ribosomes affect JNK activity in different cellular organelles, such as the nucleus, mitochondria, and plasma membrane.
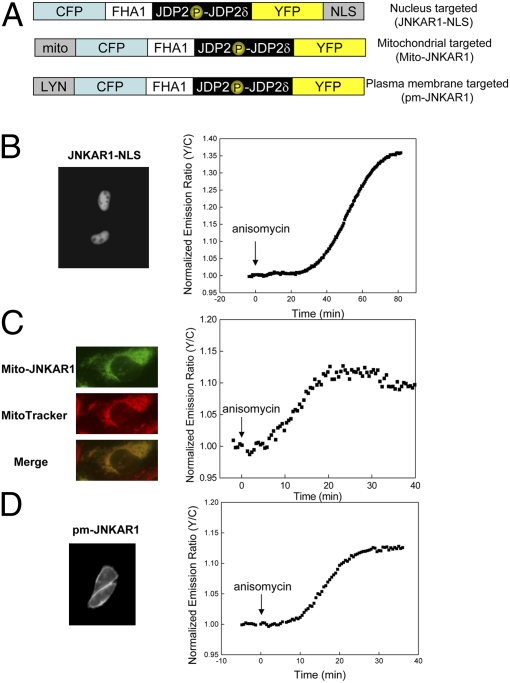
To visualize JNK activity at different subcellular locations, we prepared several fusions of JNKAR1 to various specific targeting motifs (Fig. 3A). When fused to a nuclear localization signal (NLS), JNKAR1 was appropriately targeted to the nucleus (Fig. 3B). Addition of anisomycin elicited a robust change in yellow-to-cyan emission ratio [30.8 ± 5.0% (n = 4)], indicating strong nuclear JNK activity.
Fig. 3.
Visualization of subcellular JNK activity. (A) Domain structures of nuclear, mitochondria, and plasma membrane targeted JNKAR1. (B) (Left) YFP fluorescence image of nuclear targeted JNKAR1. (Right) Representative emission ratio time course of JNKAR1-NLS with anisomycin stimulation (n = 4). (C) (Left, Top to Bottom) YFP fluorescence image of mitochondrial targeted JNKAR1, MitoTracker Red 580 to stain mitochondria, and overlay of the two images. (Right) Representative emission ratio time course of Mito-JNKAR1 with anisomycin stimulation (n = 22). (D) (Left) YFP fluorescence image of plasma membrane targeted JNKAR1. (Right) Representative emission ratio time course of pm-JNKAR1 with anisomycin stimulation (n = 3).
A mitochondria targeted JNKAR1 (Mito-JNKAR1) was constructed by introducing a targeting motif taken from the N-terminal sequence of DAKAP1a, which is responsible for targeting DAKAP1a to mitochondria (15). As shown in Fig. 3C, Mito-JNKAR1 was effectively targeted to mitochondria, where the anisomycin stimulation induced a relatively weak increase in yellow-to-cyan emission ratio [11.0 ± 3.5% (n = 22)], but with similar kinetics (t1/2 = 18.1 ± 5.5 min) to those observed in the cytosol and nucleus. Addition of 10 μM JNK inhibitor VIII decreased the resting starting ratio (Fig. S5), suggesting that basal JNK activity might exist at the mitochondria and contribute to the weak signal detected. This observation is in agreement with a previous report showing the presence of phosphorylated JNK in the outer mitochondrial membrane in untreated primary cortical neurons (16).
To localize the reporter to the plasma membrane, we fused a plasma membrane-targeting motif from the N-terminal portion of Lyn kinase (17) to JNKAR1. The N-terminal portion of Lyn kinase is known to provide targeting to plasma membrane through myristoylation and palmitoylation. As shown in Fig. 3D, pm-JNKAR1 was properly targeted to the plasma membrane, and responded to anisomycin stimulation with a 13.4 ± 0.3% (n = 3) increase in yellow-to cyan emission ratio. These data suggest that an internal stress, such as ribotoxic stress induced by anisomycin, can lead to JNK-mediated phosphorylation of substrates that reside in the plasma membrane.
Visualization of All-or-None Behaviors of Mammalian JNK Signaling Cascade.
Several characteristic features of the JNK pathway, including an activation cascade that allows signal amplification, potential feedback loops, and dual-site phosphorylation of JNK itself, make it potentially suitable to serve as a signal processor to convert graded inputs into switch-like outputs. Thus the JNK pathway may behave as a bistable system that toggles between two alternative steady-states (18–20). Indeed, it has been shown that in individual Xenopus oocytes, JNK responds to progesterone and sorbitol in an all-or-none manner, although at the level of a population of oocytes, the response of JNK becomes graded (4), suggesting that JNK was part of a “digital” bistable signaling system in this cell type. However, these studies could not be easily extrapolated into mammalian cells because of the difference in cell size and difficulty in obtaining quantitative JNK activation data by phospho-JNK staining and flow cytometry (5). In one specific case, the existence of subpopulations of Jurkat cells based on phorbol myristate acetate-induced expression levels of CD69, a T-cell surface-activation marker, allowed the analysis of JNK activity in presorted Jurkat subpopulations, which led to the demonstration of all-or-none responses of both JNK and CD69 in phorbol myristate acetate -treated Jurkat cells (5). However, it is not clear how JNK responds to different stimuli in mammalian cells and whether a binary response is a general characteristic of the JNK pathway.
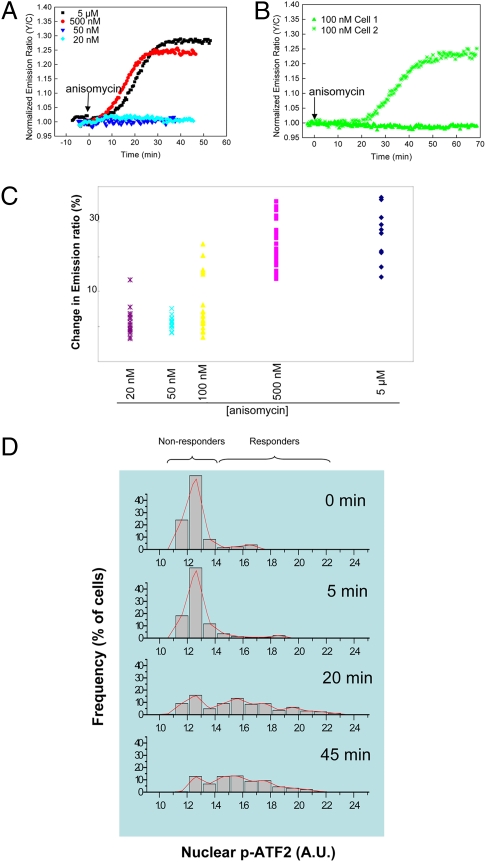
The development of JNKAR1 provided us with a unique tool to examine the system-level behaviors of the JNK cascade. Quantitative single-cell analysis of JNK activity was performed by exposing HeLa cells to varying concentrations of anisomycin, ranging from 20 nM to 5 μM, followed by live-cell imaging. As shown in Fig. 4A and Fig. S6, at the doses of 20 and 50 nM, the majority of cells failed to respond to anisomycin stimulation; at 500 nM and 5 μM all cells responded, suggesting presence of ultrasensitivity in response. Importantly, at an intermediate concentration (100 nM), anisomycin elicited two distinct types of responses from HeLa cells (Fig. 4B and Fig. S6). A subpopulation of HeLa cells showed essentially no response to 100 nM anisomycin (≤ 5%), whereas other cells responded well (≥ 10%). The difference between these two populations was statistically significant (P < 0.001) (Fig. 4 B and C). When the averaged responses from all cells at each dose were calculated and plotted as a population dose-response curve, the ultrasensitive characteristics of the JNK response to anisomycin became more evident, with a Hill coefficient as high as 9 (Fig. S7A). Of note, although the average response from all cells at each dose varied, the level of JNK activity in the “responder” populations was not correlated with the dose of anisomycin. The average response of the responders to 100 nM anisomycin stimulation (17.7 ± 3.5%, n = 6) was of the same amplitude, compared with the response stimulated by 500 nM anisomycin (19.8 ± 8.7%, n = 34), and to that stimulated by 5 μM anisomycin (20.0 ± 6.6%, n = 57).
Fig. 4.
JNK exhibits all-or-none response to anisomycin. (A) Normalized emission ratio time courses from representative experiments of HeLa cells expressing JNKAR1 that were treated with different doses of anisomycin (5 μM, 500 nM, 50 nM, and 20 nM). (B) Normalized emission-ratio time courses of two JNKAR1 expressing cells that were treated with 100 nM anisomycin. (C) Scatter plot of JNKAR1 responses, shown in percentage-change of emission ratio of yellow-to-cyan, in individual HeLa cells, in response to various doses of anisomycin. (D) Imstain analysis of nuclear phospho-ATF-2 level, as detected by an anti-phospho-ATF-2 antibody, in HeLa cells stimulated with an intermediate concentration (70 nM) of anisomycin for different durations.
To corroborate the live-cell imaging data, we also used a microfluidic device, known as Imstain, to perform automated in-chip immunostaining to evaluate JNK-mediated phosphorylation of endogenous substrates at the single-cell level. This approach has been successfully used for high-content quantitative analysis of cell signaling pathways, including the JNK pathway (21). Fig. 4D shows the results from these experiments, where phospho-ATF-2 was detected after treatment of HeLa cells with 70 nM anisomycin for 0, 5, 20, and 45 min. At both the 20 and 45 min time points, bimodal distributions of the signal were observed, with one population remaining unresponsive and the other responding by increasing phosphorylation of ATF-2 (Fig. S8). Ultrasensitivity was also observed at the phospho-ATF-2 level when different doses of anisomycin were applied with a Hill coefficient of 17.2 (Fig. S7B). These data, taken together, suggest that the mammalian JNK signaling cascade exhibits switch-like responses to the stress signal anisomycin and that such a binary response may be a general characteristic of JNK-mediated stress responses.
Discussion
Cellular function requires accurate translation of extracellular cues into functional responses via intracellular signaling cascades. The JNK signaling cascade mediates a variety of cellular functions in response to various types of stimuli and stressors via intricate regulatory mechanisms. The core module of the signaling cascade has been identified to contain a three-tiered activation relay. However, a better understanding about how the signaling specificity, efficiency, and fidelity are achieved requires characterization of the cellular- and systems-level behaviors of the signaling cascade and identification of wiring of the circuitry that is crucial to maintain the appropriate behaviors in the context of the entire signaling network, as well as in cellular time and space. This task presents a significant challenge to the existing methods for monitoring JNK activity. We therefore engineered a genetically-encoded FRET-based JNKAR1 for quantitative measurement of JNK activity in single mammalian cells with high spatiotemporal resolution.
The development of JNKAR1 is based on a generalizable design of kinase activity reporters that couples a FRET pair with a kinase-dependent molecular switch consisting of a phosphoamino acid binding domain and a kinase substrate. Incorporation of a kinase docking domain into the reporter design (6) to enhance phosphorylation efficiency and specificity is likely to be an important consideration for engineering such reporters for MAPKs (22, 23) and other kinases that use substrate recruitment strategies for their physiological substrates. As a surrogate substrate of JNK, JNKAR1 can be specifically phosphorylated by JNK, but not by other related MAPKs. However, the phosphatases that dephosphorylate JNKAR1 are not known. In fact, the kinetics of dephophorylation of JNKAR1 is slower than that of an endogenous JNK substrate, ATF-2, suggesting that JNKAR1 may be a relatively poor substrate for cellular phosphatases, at least in HeLa cells. Slow dephophorylation may limit the ability of the biosensor to report down-regulation of JNK activity. Future experiments will aim at improving the reversibility of JNKAR1 by modifying the substrate sequences, altering the FHA1 domain or incorporating sequences for recruiting phosphatases.
In this study, we applied JNKAR1 to characterize the activity patterns of JNK in response to the ribotoxic stress anisomycin at various subcellular locations. Nuclear-targeted JNKAR1 showed a robust response to anisomycin, which exhibited a similar pattern to the cytosolic response (Fig. 3B vs. 2B). This is in accordance with anisomycin causing changes in gene expression through phosphorylation of a number of transcription factors, such as c-Jun, c-Myc, FOXO, and p53 (7). Furthermore, anisomycin-induced JNK activity was readily detected at two other cellular locations, mitochondria and plasma membrane, and similar kinetics to the cytosolic response was observed. Mitochondria has been shown to be a site for active JNK upon stress stimulation, which contains known JNK substrates, such as the antiapoptotic Bcl-2 family members, Bcl-2 and Bcl-xL (24). JNK has also been shown to translocate to mitochondria upon stress signals, such as anisomycin (16) or 6-hydroxydopamine (25). The observed similar kinetics between cytosolic and mitochondrial responses suggests that the signal propagation to the mitochondrial loci occurs without significant delay despite the involvement of translocation of activated JNK to the mitochondria. On the other hand, it is clear from these experiments that plasma membrane-tethered substrates can also gain access to active JNK induced by ribotoxic stress. Such signal propagation may be important for TNF-α-mediated inhibition on insulin action through insulin receptor substrate-1 (26), and may also have implications in the regulation of cell migration by JNK and its plasma membrane substrate paxillin (27).
In addition to providing spatiotemporal information of JNK activity in a live-cell context, JNKAR1 revealed several key features of the systems-level behaviors of the JNK signaling cascade. First, the response of JNK to anisomycin in HeLa cells was shown to be ultrasensitive, suggesting a switch-like nonlinear response behavior of the JNK cascade (19). The observed ultrasensitivity is consistent with a previous study evaluating JNK responses using populations of HeLa, HEK293, and Jurkat cells (5), although differences in sensitivities of the assays may exist. Second, an intermediate concentration of anisomycin (100 nM) caused full activation of JNK in some cells and no activation in others. The existence of bimodal populations at intermediate levels of the stimulating signal, which were either unresponsive or fully responsive, is most consistent with behaviors of bistable systems. Finally, some bistable systems can generate irreversible responses. At all doses, the anisomycin-induced responses are sustained for as long as we observed, although the slow dephosphorylation of JNKAR1 may also contribute to the observed apparent irreversibility. In this regard, it is notable that phosphorylated JNK also showed signs of apparent sustainability, maintaining its phosphorylation to a high degree after 60 min of anisomycin stimulation (Fig. 2 D and E).
Although switch-like behaviors have emerged as fairly common characteristics of signaling molecules, only a few kinases, including ERK (28), JNK (4, 5) and Cdc2 (29) have been shown to elicit this type of response. Interestingly, ERK has been shown to exhibit all-or-none behaviors in one signaling system (28) but not another (30), suggesting that such behaviors are context-dependent. Our results showed that HeLa cells are also capable of converting a graded ribotoxic stress signal to a binary response, suggesting that the all-or-none behavior may be a general characteristic of stress-induced JNK signaling. However, the responses of JNK to other types of stimuli have not been systematically analyzed and the mechanisms underlying the graded-to-binary signal processing of JNK cascade are not well understood. In this regard, the development of JNKAR1 has laid a foundation for evaluating the behaviors of the JNK signaling cascade in a variety of signaling systems under different conditions, which should lead to a better understanding of the feedback mechanisms that are critically involved in orchestrating such responses. On a broader scale, systems behaviors, such as the all-or-none behaviors demonstrated here, have significant implications for understanding the logic of signal transduction and can only be effectively studied in the cellular context. FRET-based activity reporters such as JNKAR1 will, therefore, serve as powerful tools for characterizing the wiring and functioning of signaling circuitry toward a comprehensive understanding of signal transduction networks.
Materials and Methods
Reporter Construction.
JNKAR1 was constructed by PCR using citrine as a template with DSVKTPEDEGNPLLEQLEKKGGTGGSEL added immediately N-terminal to citrine. The PCR product was inserted into a modified AKAR vector backbone that includes ECFP, FHA1, and the linker, SAGKPGSGEGSTKGLV. For the c-Jun-based candidate constructs, truncated c-Jun was amplified by PCR from c-Jun cDNA and WW1 domain was amplified from Pin1 cDNA. Truncated c-Jun and WW1 domain were then cloned in between ECFP and citrine. Alternatively, truncated c-Jun was cloned C-terminal to FHA1 or FHA2. For the ATF-3-JDP2δ-based reporter, the peptide QNGRTPEDESEGNPLLEQLEKKGGTGGS was added immediately N-terminal to citrine in a similar fashion to JNKAR1. Finally, for JIP1δ containing reporter, DSVKTPEDEGNPGGTGGS was added N-terminal to citrine and GGTGGSRPKRPTTLNLF was added C-terminal to citrine. The constructs were first cloned into pRSET B (Invitrogen) and then subcloned into pcDNA 3.1 (Invitrogen) behind a Kozak sequence for mammalian expression. For plasma membrane targeting, KKKKKSKTKCVIM was added N-terminal to ECFP. For nuclear targeting, PKKKRKVEDA was added C-terminal to citrine. For targeting to mitochondria, MAIQLRSLFPLALPGMLALLGWWWFFSRKK was added N-terminal to ECFP. All constructs were sequence-verified after cloning into pRSET B and after subcloning into pcDNA 3.1.
Cell Culture.
HeLa cells were cultured in DMEM supplemented with 10% FBS and plated onto sterilized glass coverslips mounted in 35-mm tissue culture dishes. Cells were then transfected using Lipofectamine 2000 (Invitrogen) and imaged 18 to 24 h later. Cells with a passage number less than 50 were used in imaging, as higher-passage cells may introduce additional variations at the kinetics level. For Western blot analyses, the cells were transfected 24 to 48 h before collected for lysis.
Imaging.
Cells were washed twice with HBSS buffer and imaged in HBSS in the dark room on a heated stage prewarmed to 37° C with addition of anisomycin (Sigma), sorbitol (Sigma), or TNF-α (Biomol). Cells may be pretreated with the indicated doses of U0126 (Sigma), SB203580 (Biomol), or JNK inhibitor VIII (EMD Chemicals) in DMEM supplemented with 10% FBS at 37° C, 5% CO2. Then the cells were washed with HBSS and imaged in HBSS supplemented with the indicated inhibitor. Cells were imaged on a Zeiss Axiovert 200M microscope with a cooled charge-coupled device camera (Roper Scientific) controlled by METAFLUOR 6.2 software. Dual-emission ratio imaging used a 420DF20 excitation filter, a 450DRLP dichroic mirror and two emission filters (475DF40 for ECFP and 535DF25 for citrine) alternated by a filter-changer Lambda 10–2 (Sutter Instruments). Exposure time was 50 to 500 ms and images were acquired every 30–60 s. Fluorescence images were background-corrected by subtracting the autofluorescence of nontransfected cells (or background with no cells) from the emission intensities of cells expressing reporters. The ratios of yellow-to-cyan emissions were then calculated at different time-points and normalized by dividing all ratios by the emission ratio before stimulation.
Western Blotting.
The cells were lysed with RIPA lysis buffer containing EDTA-free protease inhibitor mixture (Roche Applied Science). Protein concentration in the lysate was determined using BCA protein quantitation assay (Pierce). For Western blotting, 30 to 50 μg of total protein was solubilized in SDS sample buffer by boiling, and fractionated in a polyacrylamide gel. Protein was transferred to a nitrocellulose membrane and subjected to primary and secondary antibody incubation. Antibodies used in Western blotting were anti-phospho-c-Jun (Ser-63), anti-c-Jun, anti-phospho-Thr-Pro, anti-phospho-JNK (Thr-183/Tyr-185), anti-JNK, anti-β-tubulin (Cell Signaling Technology); anti-GFP, anti-phospho-ATF-2 (Thr-71) (Santa Cruz Biotechnology); and horseradish peroxidase-labeled goat anti-rabbit or anti-mouse IgG secondary antibody (Pierce). The membranes were developed using horseradish peroxidase-based chemiluminescent substrate (Pierce).
Microfluidic Device Operation.
Devices were flushed with 10 μg/mL fibronectin for 30 min to coat the glass substratum, and then the fibronectin was flushed away with medium. HeLa cells were seeded at a concentration of 9 × 106 cells/mL and then allowed to attach for at least 4 h. After treatment of cells, the devices were run similarly as described in ref. 21.
Immunocytochemistry.
Cells were fixed in 4% paraformaldehyde for 20 min, permeabilized in 0.1% Triton X-100 for 5 min, blocked in 10% goat serum (Invitrogen) for 60 min, stained with 1:100 dilution of phospho-ATF-2 antibody (Santa Cruz Biotechnology) plus 2 μg/mL Hoechst 33258 (Sigma) in 10% goat serum for 60 min, and then stained with 1:200 Alexa Fluor 594 conjugated to highly cross-absorbed goat anti-mouse IgG (Invitrogen) for 60 min with PBS washes in between each step. Devices were imaged under nonsaturating conditions on a Zeiss Axiovert 200M microscope using Slidebook 4.1 (Intelligent Imaging Innovations). Data analyses of fluorescence intensity were performed as previously described (21).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants R01 DK073368 and DP1 OD006419 (to J.Z.); and GM072024 and RR020839 (to A.L.).
Footnotes
*This Direct Submission article had a prearranged editor.
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909671107/DCSupplemental.
References
- 1.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 3.Xia Y, Wu Z, Su B, Murray B, Karin M. JNKK1 organizes a MAP kinase module through specific and sequential interactions with upstream and downstream components mediated by its amino-terminal extension. Genes Dev. 1998;12:3369–3381. doi: 10.1101/gad.12.21.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagowski CP, Ferrell JE., Jr Bistability in the JNK cascade. Curr Biol. 2001;11:1176–1182. doi: 10.1016/s0960-9822(01)00330-x. [DOI] [PubMed] [Google Scholar]
- 5.Bagowski CP, Besser J, Frey CR, Ferrell JE., Jr The JNK cascade as a biochemical switch in mammalian cells: ultrasensitive and all-or-none responses. Curr Biol. 2003;13:315–320. doi: 10.1016/s0960-9822(03)00083-6. [DOI] [PubMed] [Google Scholar]
- 6.Ni Q, Titov DV, Zhang J. Analyzing protein kinase dynamics in living cells with FRET reporters. Methods. 2006;40:279–286. doi: 10.1016/j.ymeth.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol Mol Biol Rev. 2006;70:1061–1095. doi: 10.1128/MMBR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dérijard B, et al. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 9.Katz S, Heinrich R, Aronheim A. The AP-1 repressor, JDP2, is a bona fide substrate for the c-Jun N-terminal kinase. FEBS Lett. 2001;506:196–200. doi: 10.1016/s0014-5793(01)02907-6. [DOI] [PubMed] [Google Scholar]
- 10.Barr RK, Boehm I, Attwood PV, Watt PM, Bogoyevitch MA. The critical features and the mechanism of inhibition of a kinase interaction motif-based peptide inhibitor of JNK. J Biol Chem. 2004;279:36327–36338. doi: 10.1074/jbc.M402181200. [DOI] [PubMed] [Google Scholar]
- 11.Katz S, Aronheim A. Differential targeting of the stress mitogen-activated protein kinases to the c-Jun dimerization protein 2. Biochem J. 2002;368:939–945. doi: 10.1042/BJ20021127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durocher D, et al. The molecular basis of FHA domain:phosphopeptide binding specificity and implications for phospho-dependent signaling mechanisms. Mol Cell. 2000;6:1169–1182. doi: 10.1016/s1097-2765(00)00114-3. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Hupfeld CJ, Taylor SS, Olefsky JM, Tsien RY. Insulin disrupts beta-adrenergic signalling to protein kinase A in adipocytes. Nature. 2005;437:569–573. doi: 10.1038/nature04140. [DOI] [PubMed] [Google Scholar]
- 14.Ouyang DY, Wang YY, Zheng YT. Activation of c-Jun N-terminal kinases by ribotoxic stresses. Cell Mol Immunol. 2005;2:419–425. [PubMed] [Google Scholar]
- 15.Ma Y, Taylor S. A 15-residue bifunctional element in D-AKAP1 is required for both endoplasmic reticulum and mitochondrial targeting. J Biol Chem. 2002;277:27328–27336. doi: 10.1074/jbc.M201421200. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Q, Lam PY, Han D, Cadenas E. c-Jun N-terminal kinase regulates mitochondrial bioenergetics by modulating pyruvate dehydrogenase activity in primary cortical neurons. J Neurochem. 2008;104:325–335. doi: 10.1111/j.1471-4159.2007.04957.x. [DOI] [PubMed] [Google Scholar]
- 17.Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- 18.Ferrell JE., Jr Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr Opin Cell Biol. 2002;14:140–148. doi: 10.1016/s0955-0674(02)00314-9. [DOI] [PubMed] [Google Scholar]
- 19.Ninfa AJ, Mayo AE. Hysteresis vs. graded responses: the connections make all the difference. Sci STKE. 2004;2004:pe20. doi: 10.1126/stke.2322004pe20. [DOI] [PubMed] [Google Scholar]
- 20.Pomerening JR. Uncovering mechanisms of bistability in biological systems. Curr Opin Biotechnol. 2008;19:381–388. doi: 10.1016/j.copbio.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Cheong R, Wang CJ, Levchenko A. High content cell screening in a microfluidic device. Mol Cell Proteomics. 2009;8:433–442. doi: 10.1074/mcp.M800291-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato M, Kawai Y, Umezawa Y. Genetically encoded fluorescent indicators to visualize protein phosphorylation by extracellular signal-regulated kinase in single living cells. Anal Chem. 2007;79:2570–2575. doi: 10.1021/ac062171d. [DOI] [PubMed] [Google Scholar]
- 23.Harvey CD, et al. A genetically encoded fluorescent sensor of ERK activity. Proc Natl Acad Sci USA. 2008;105:19264–19269. doi: 10.1073/pnas.0804598105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Lin A. Role of JNK activation in apoptosis: a double-edged sword. Cell Res. 2005;15:36–42. doi: 10.1038/sj.cr.7290262. [DOI] [PubMed] [Google Scholar]
- 25.Eminel S, Klettner A, Roemer L, Herdegen T, Waetzig V. JNK2 translocates to the mitochondria and mediates cytochrome c release in PC12 cells in response to 6-hydroxydopamine. J Biol Chem. 2004;279:55385–55392. doi: 10.1074/jbc.M405858200. [DOI] [PubMed] [Google Scholar]
- 26.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J Biol Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 27.Huang C, Rajfur Z, Borchers C, Schaller MD, Jacobson K. JNK phosphorylates paxillin and regulates cell migration. Nature. 2003;424:219–223. doi: 10.1038/nature01745. [DOI] [PubMed] [Google Scholar]
- 28.Ferrell JE, Jr, Machleder EM. The biochemical basis of an all-or-none cell fate switch in Xenopus oocytes. Science. 1998;280:895–898. doi: 10.1126/science.280.5365.895. [DOI] [PubMed] [Google Scholar]
- 29.Pomerening JR, Sontag ED, Ferrell JE., Jr Building a cell cycle oscillator: hysteresis and bistability in the activation of Cdc2. Nat Cell Biol. 2003;5:346–351. doi: 10.1038/ncb954. [DOI] [PubMed] [Google Scholar]
- 30.Whitehurst A, Cobb MH, White MA. Stimulus-coupled spatial restriction of extracellular signal-regulated kinase 1/2 activity contributes to the specificity of signal-response pathways. Mol Cell Biol. 2004;24:10145–10150. doi: 10.1128/MCB.24.23.10145-10150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.