Abstract
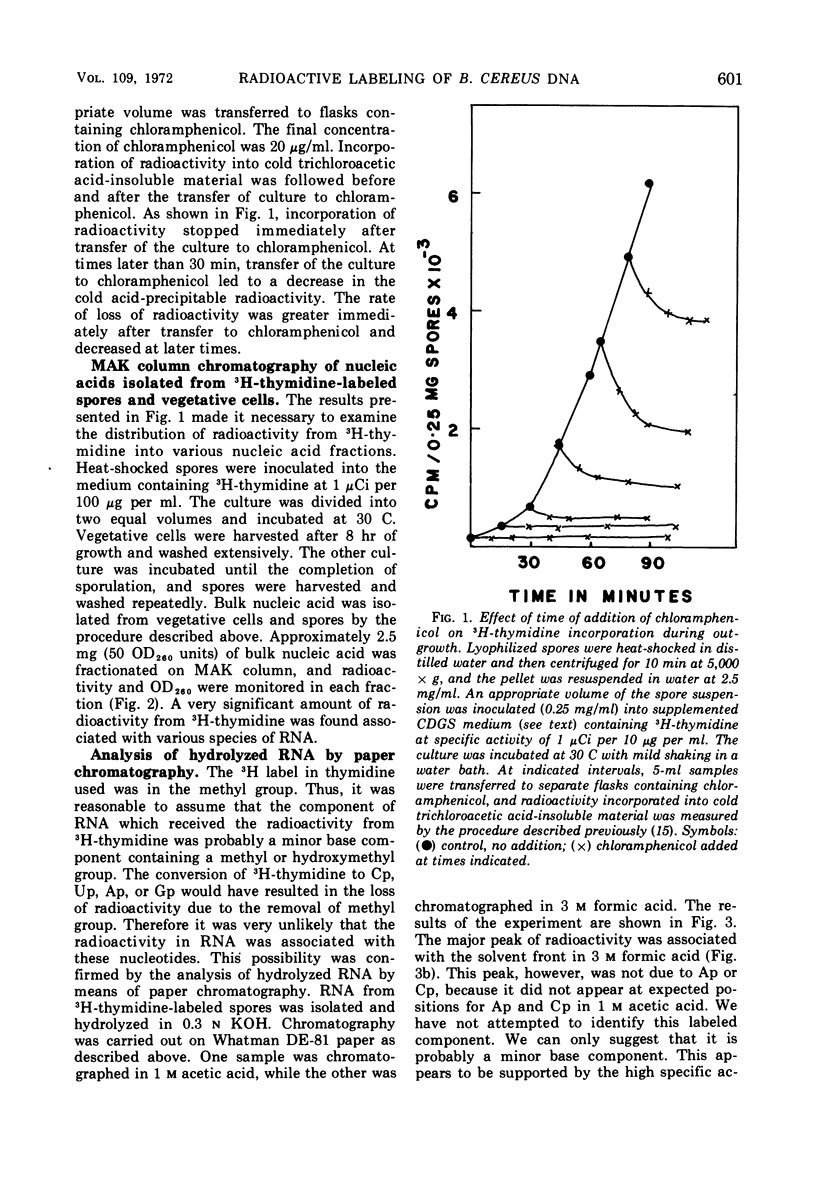
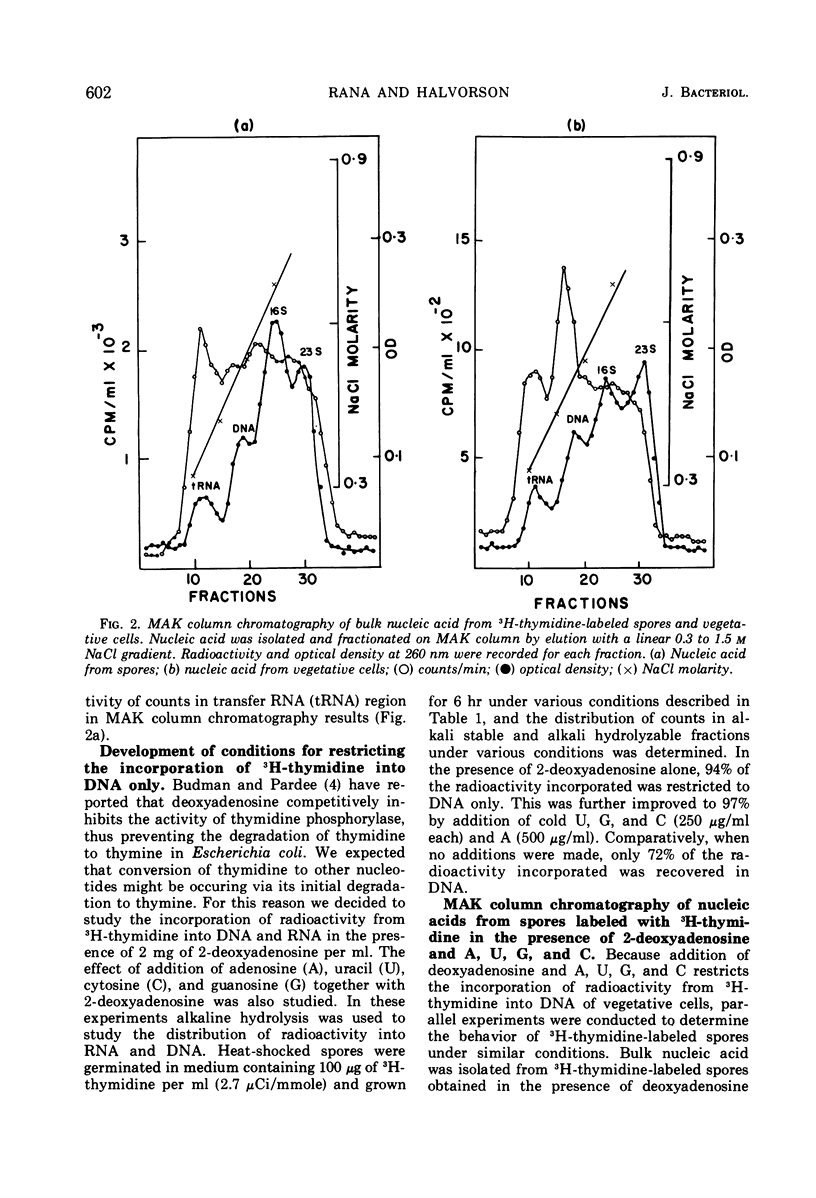
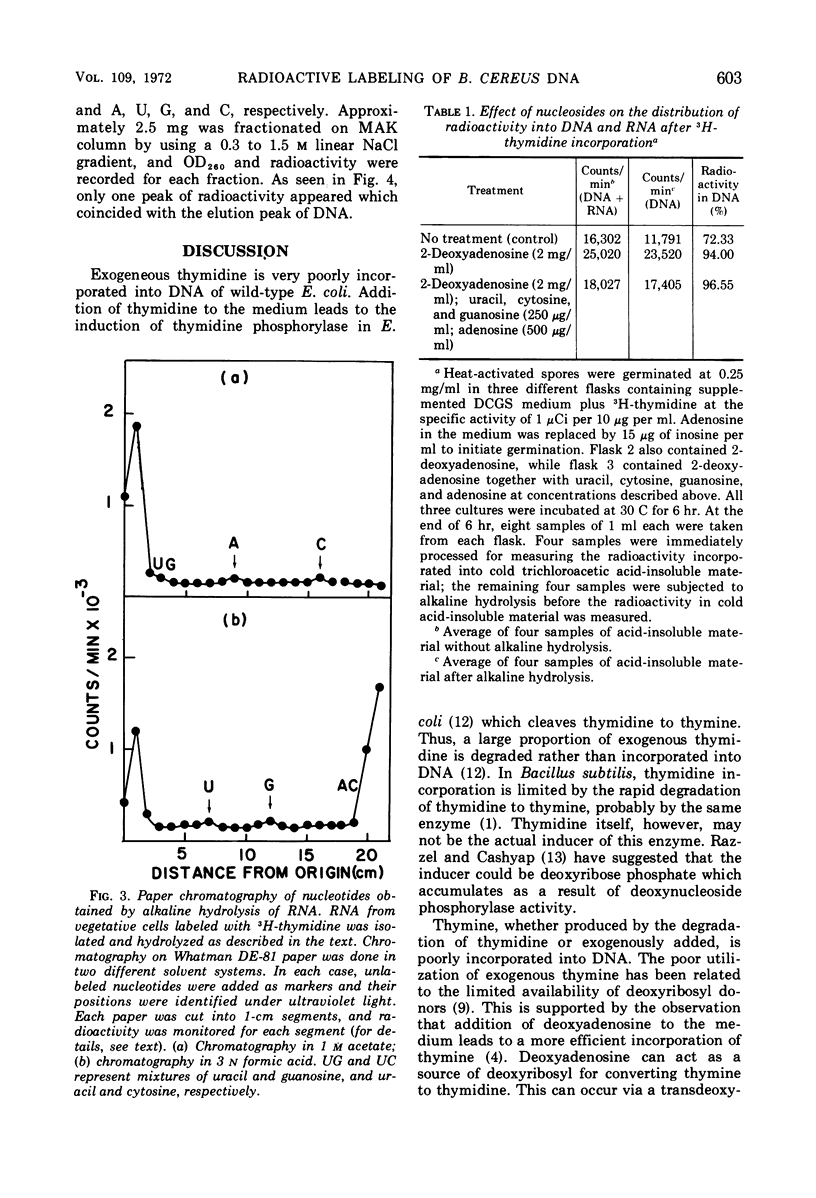
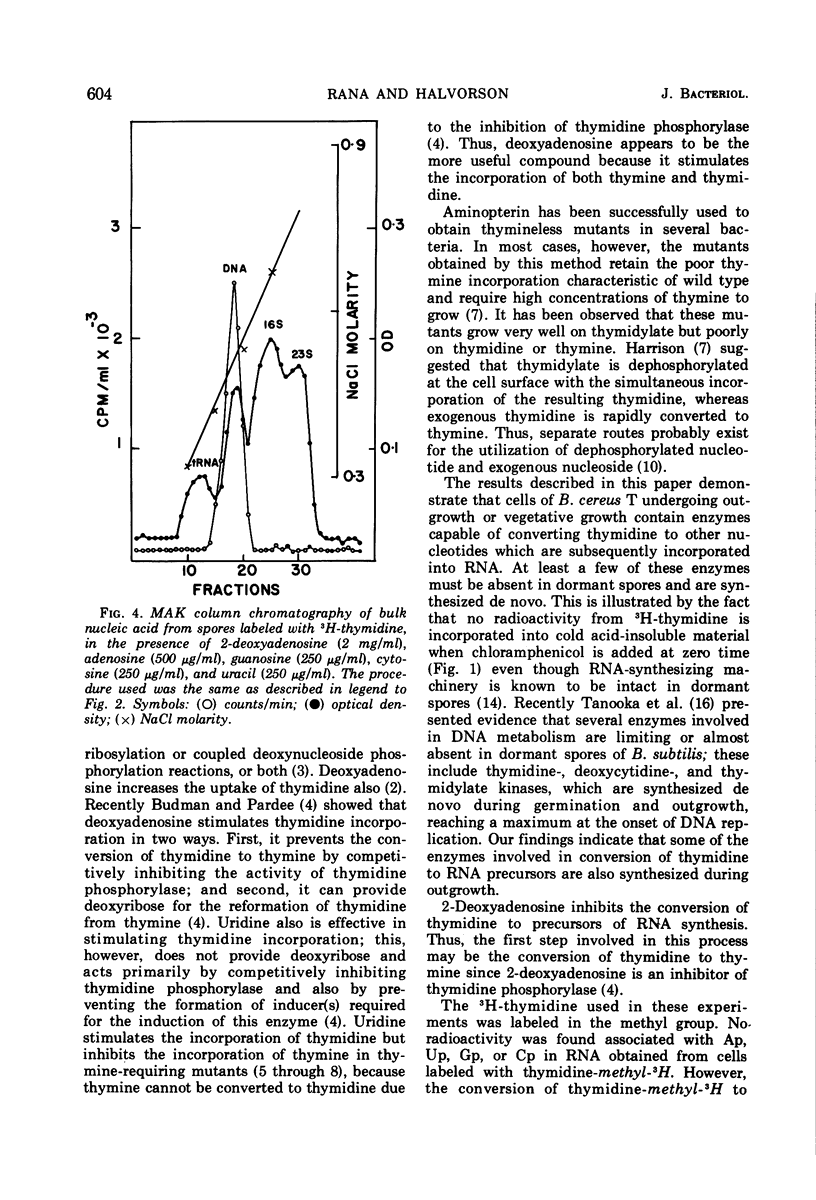
When heat-activated spores of Bacillus cereus T (thy−) were germinated and grown in medium containing 3H-thymidine, a significant amount of radioactivity was incorporated into ribonucleic acid and deoxyribonucleic acid (DNA). A method was developed to restrict the incorporation of radioactivity from 3H-thymidine into DNA only. This was accomplished by labeling the cells with 3H-thymidine in the presence of 2 mg of 2-deoxyadenosine per ml, 250 μg each of uracil, cytosine, and guanosine per ml, and 500 μg of adenosine per ml. Under these conditions, 97% of the radioactivity incorporated into cold trichloroacetic acid-insoluble material was associated with DNA only. In the absence of these compounds, DNA contained only 72% of the total radioactivity incorporated into cold acid-insoluble material.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BODMER W. F., GRETHER S. UPTAKE AND INCORPORATION OF THYMINE, THYMIDINE, URACIL, URIDINE, AND 5-FLUOROURACIL INTO THE NUCLEIC ACIDS OF BACILLUS SUBTILIS. J Bacteriol. 1965 Apr;89:1011–1014. doi: 10.1128/jb.89.4.1011-1014.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budman D. R., Pardee A. B. Thymidine and thymine incorporation into deoxyribonucleic acid: inhibition and repression by uridine of thymidine phosphorylase of Escherichia coli. J Bacteriol. 1967 Nov;94(5):1546–1550. doi: 10.1128/jb.94.5.1546-1550.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN S. S., BARNER H. D. Studies on the induction of thymine deficiency and on the effects of thymine and thymidine analogues in Escherichia coli. J Bacteriol. 1956 May;71(5):588–597. doi: 10.1128/jb.71.5.588-597.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. Technique for starvation of Escherichia coli of thymine. J Bacteriol. 1965 Oct;90(4):1153–1154. doi: 10.1128/jb.90.4.1153-1154.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A. P., Jr Thymine incorporation and metabolism by various classes of thymine-less bacteria. J Gen Microbiol. 1965 Dec;41(3):321–333. doi: 10.1099/00221287-41-3-321. [DOI] [PubMed] [Google Scholar]
- Jayaraman K., Müller-Hill B., Rickenberg H. V. Inhibition of the synthesis of beta-galactosidase in Escherichia coli by 2-nitrophenyl-beta-D-fucoside. J Mol Biol. 1966 Jul;18(2):339–343. doi: 10.1016/s0022-2836(66)80251-6. [DOI] [PubMed] [Google Scholar]
- LICHTENSTEIN J., BARNER H. D., COHEN S. S. The metabolism of exogenously supplied nucleotides by Escherichia coli. J Biol Chem. 1960 Feb;235:457–465. [PubMed] [Google Scholar]
- NAKATA H. M. ORGANIC NUTRIENTS REQUIRED FOR GROWTH AND SPORULATION OF BACILLUS CEREUS. J Bacteriol. 1964 Nov;88:1522–1524. doi: 10.1128/jb.88.5.1522-1524.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACHMELER M., GERHART J., ROSNER J. Limited thymidine uptake in Escherichia coli due to an inducible thymidine phosphorylase. Biochim Biophys Acta. 1961 Apr 29;49:222–225. doi: 10.1016/0006-3002(61)90888-5. [DOI] [PubMed] [Google Scholar]
- RAZZELL W. E., CASSHYAP P. SUBSTRATE SPECIFICITY AND INDUCTION OF THYMIDINE PHOSPHORYLASE IN ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1789–1793. [PubMed] [Google Scholar]
- Steinberg W., Halvorson H. O. Timing of enzyme synthesis during outgrowth of spores of Bacillus cereus. II. Relationship between ordered enzyme synthesis and deoxyribonucleic acid replication. J Bacteriol. 1968 Feb;95(2):479–489. doi: 10.1128/jb.95.2.479-489.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanooka H., Terano H., Otsuka H. Increase of thymidine, thymidylate and deoxycytidine kinase activites during germination of bacterial spores. Biochim Biophys Acta. 1971 Jan 1;228(1):26–37. doi: 10.1016/0005-2787(71)90543-0. [DOI] [PubMed] [Google Scholar]



