Abstract
The traceless Staudinger ligation is an effective means to synthesize an amide bond between two groups of otherwise orthogonal reactivity: a phosphinothioester and an azide. An important application of the Staudinger ligation is in the ligation of peptides at a variety of residues. Here, we demonstrate that the traceless Staudinger ligation can be achieved in water with a water-soluble reagent. Those reagents that provide a high yield of amide product discourage protonation of the nitrogen in the key iminophosphorane intermediate. The most efficacious reagent, bis(p-dimethylaminoethylphenyl)phosphinomethanethiol, mediates the rapid ligation of equimolar substrates in water. This reagent is also able to perform a transthioesterification reaction with the thioester intermediate formed during intein-mediated protein splicing. Hence, the traceless Staudinger ligation can be integrated with expressed protein ligation, extending the reach of modern protein chemistry.
Introduction
Proteins are becoming accessible targets for synthetic chemistry.1 This development could enhance access to natural and nonnatural proteins, as well as accelerate the use of proteins as chemotherapeutic agents.2,3 Still, hurdles exist. Solid-phase peptide synthesis4 is limited to peptides of ≤40 residues,2,5 and thus cannot be used to mimic protein biosynthesis, which occurs by a sequential route. The convergent synthesis of proteins by the coupling of protected peptides is compromised by low yields due to the limited solubility and aggregation of the fragments.2
Efforts to overcome these obstacles have achieved notable success. An especially powerful approach is “native chemical ligation”, which employs a transthioesterification reaction between two deprotected peptides, one with a C-terminal thioester and the other with an N-terminal cysteine residue, to form a native amide bond.6 An extension of this strategy, “expressed protein ligation”, employs recombinant DNA technology to produce the C-terminal thioester.7-9 Although these methods enable the convergent synthesis of proteins, they rely on a cysteine residue being at the N terminus of a reactant and retain that cysteine at each junction of the product. Cysteine is the second least common amino acid, comprising only 1.7% of all residues in proteins.10 This requirement limits access to both synthetic and semisynthetic proteins.
Recently, the “traceless Staudinger ligation” has emerged as a new peptide ligation strategy for the chemical synthesis of proteins.1,11,12 This method is based on the Staudinger reduction, wherein a phosphine reduces an azide via an iminophosphorane intermediate.13 The iminophosphorane can be acylated to yield, ultimately, an amide (Scheme 1).14 An important feature of this reaction is its potential to mediate the nonengrammic ligation of peptides at virtually any residue.1,15 The Staudinger ligation is chemoselective (especially towards the azide),16,17 retains stereochemistry,18 and has been used in the orthogonal assembly of a protein19 and for the site-specific immobilization of peptides and proteins to a surface.20
Scheme 1.
Putative Mechanism of the Traceless Staudinger Ligation Mediated by Phosphinothiol 1
(Diphenylphosphino)methanethiol (1) is the most efficient known phosphinothiol for ligating peptides to form a junction at a glycine residue.12,17 Recently, the p-methoxy-substituted phosphinothiol 2 was shown to afford high yields at non-glycyl residues.15 Though having desirable attributes, neither of these phosphinothiols is soluble in water. Indeed, all traceless Staudinger ligations have been performed in organic solvents or organic/aqueous mixtures.
The ability to effect the traceless Staudinger ligation in water could expand the utility of the Staudinger ligation. In particular, we envisioned that transthioesterification with a water-soluble phosphinothiol could enable the generation of phosphinothioesters at the C terminus of proteins generated by expressed protein ligation. That ability would overcome the requirement for a cysteine residue at the ligation junction.21
Here, we describe the development of water-soluble phosphinothiol reagents that enable the first traceless Staudinger ligations in water. Doing so requires careful consideration of the mechanism of the ligation reaction. We go on to demonstrate that this water-soluble phosphinothiol can be integrated with expressed protein ligation to generate an intact protein with a C-terminal phosphinothioester, poised for Staudinger ligation.
Results and Discussion
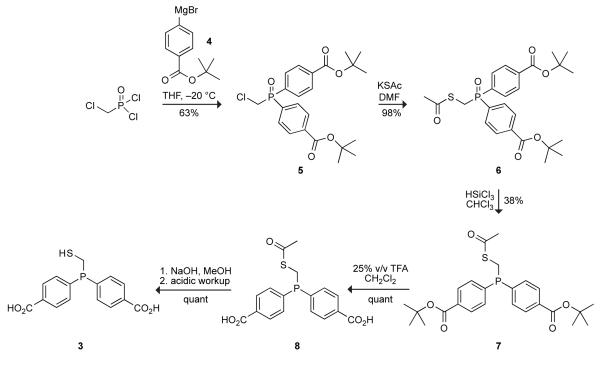
We first explored bis(p-carboxyphenyl)phosphinomethanethiol 3 as a water-soluble phosphinothiol. Our notion was to retain the aryl groups of phosphinothiols 1 and 2, which increase the yield of peptide ligations,22 while adding two hydrophilic carboxyl groups.23 The synthetic route to phosphinothiol 3 is shown in Scheme 2. Briefly, Grignard reagent 4 was added to (chloromethyl)phosphonic dichloride to yield diester 5. Potassium thioacetate displacement of the chloro group gave thioester 6 in high yield. Its phosphine oxide group was reduced with excess trichlorosilane, and its two carboxyl groups were unmasked with trifluoroacetic acid. Finally, deprotection of the thiol group was achieved in basic MeOH to give phosphinothiol 3 in 23% overall yield.
Scheme 2.
Route for the Synthesis of Acidic Phosphinothiol 3
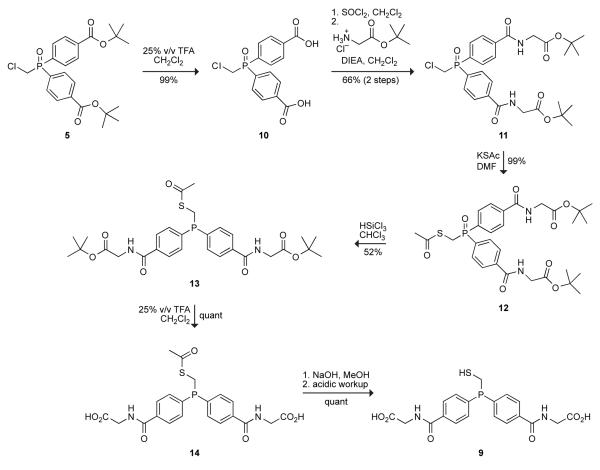
Unfortunately, phosphinothiol 3 was barely soluble in water, remaining as a viscous residue that was unable to mediate a traceless Staudinger ligation (data not shown). Inspired by hippuric acid being more soluble than benzoic acid,24 we sought to increase the water-solubility of phosphinothiol 3 by condensing glycine with its two carboxyl groups. The synthetic route to phosphinothiol 9 is shown in Scheme 3. Briefly, cleavage of the esters in diester 5, followed by treatment with excess thionyl chloride generated the diacid chloride. Reaction with glycine t-butyl ester gave diamide 11, which was carried on as in Scheme 2 to give phosphinothiol 9 in 33% overall yield.
Scheme 3.
Route for the Synthesis of Acidic Phosphinothiol 9
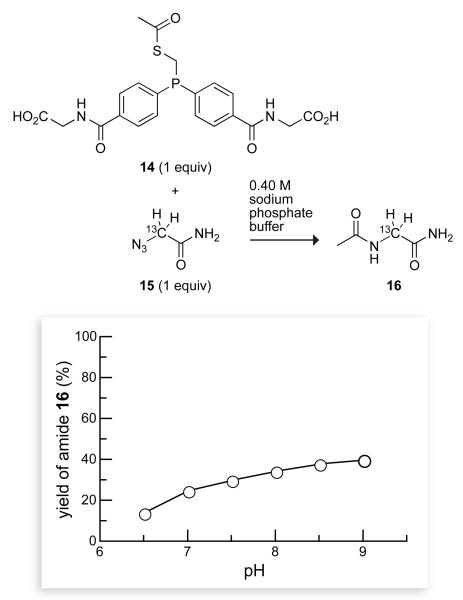
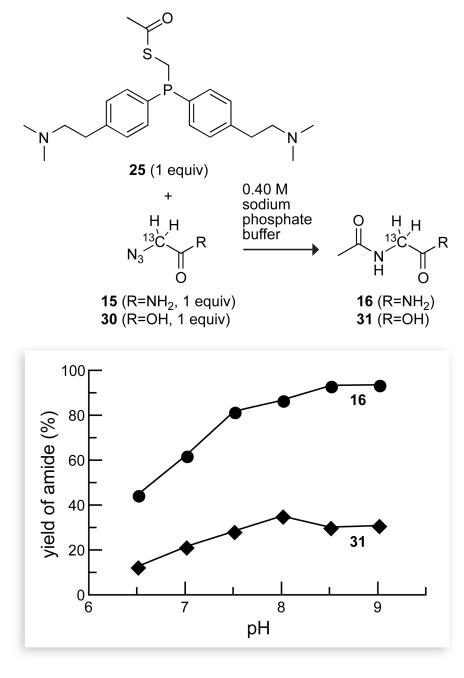
The solubility of phosphinothiol 9 was greater than that of phosphinothiol 3, approaching 50 mM in 0.4 M sodium phosphate buffer (pH 7.8). The utility of phosphinothiol 9 was determined by monitoring its ability to mediate a traceless Staudinger ligation in water with a 13C NMR assay developed in our laboratory.17 The major product of the reaction of phosphinothioester 14 with azide 15 was the amine byproduct, glycinamide, which is formed by the reduction of the azido group. The yield of the desired product, amide 16, was strongly dependent on the pH of the reaction mixture, increasing at high pH (Figure 1). Accordingly, a means of achieving a high yield would be to perform reactions with phosphinothiol 9 at high pH. Unfortunately, the hydrolysis of thioesters is rapid at high pH.25 In addition, it would be desirable to perform traceless Staudinger ligations near neutral pH, where proteins themselves tend to be most stable.
Figure 1.
Dependence of the yield of amide 16 on the solution pH in a traceless Staudinger ligation mediated by an acidic phosphinothiol. Reactions were performed with equimolar amounts of 14 and 15 (0.16 M) in 0.40 M sodium phosphate buffers of varying pH. 13C-Labeled glycinamide ([13Cα]GlyNH2) is the major product of this reaction at each pH. Data are the mean values (SE = ±2%) from two or three experiments.
The pH dependence of amide formation in the reaction of phosphinothioester 14 with azide 15 suggested to us that a key atom must be unprotonated to effect the traceless Staudinger ligation in water. We reasoned that this atom is the nitrogen of the iminophosphorane. Path A in Scheme 4 (like Scheme 1) shows the putative mechanism for a typical Staudinger ligation to form an amide product. In a protic solvent, the nitrogen of the iminophosphorane could become protonated, as in Path B. That protonation would prevent the desired S→N acyl transfer, as well as make the adjacent phosphorous more electrophilic. The attack of water on that phosphorous would ultimately lead to the amine byproduct (Path B).26
Scheme 4.
Putative Mechanism of the Traceless Staudinger Ligation Mediated a Phosphinothiol in Aqueous Solution
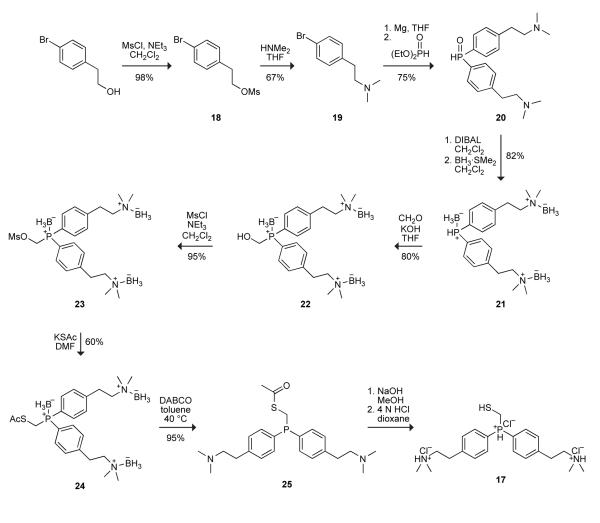
We tested our hypothesis by using intramolecular Coulombic interactions to modulate the acidity of the iminophosphorane intermediate.27 Specifically, we synthesized bis(p-dimethylaminoethylphenyl)phosphinomethanethiol (17), which has dimethyl amino groups that could not only impart water solubility but also (when protonated) serve to discourage protonation of the iminophosphorane nitrogen. We chose tertiary amino groups, rather than primary or secondary ones, to obviate intramolecular S→N acyl transfer in ensuing thioesters.
The synthetic route to phosphinothiol 17 is shown in Scheme 5. Briefly, 4-bromophenethyl alcohol was converted to mesylate 18, and then amine 19. Addition of its Grignard reagent to diethyl phosphite gave phosphine oxide 20 in moderate yields. Phosphine oxide 20 was reduced by treatment with diisobutyl aluminum hydride (DIBAl-H)28 and then reacted with borane to protect the phosphino group from oxidation. (Three equivalents of borane were required due to its high affinity for the two amino groups.) Complexation with borane was otherwise beneficial, making compounds 21–24 less polar and hence easier to purify. Phosphine–borane complex 21 was reacted with formaldehyde to generate alcohol 22, which was converted to mesylate 23 and then reacted with potassium thioacetate to yield protected phosphinothioester 24. 1,4-Diazabicyclo[2.2.2]octane (DABCO; 3 equiv) was used to remove the borane groups. Deprotection of the thiol group was achieved in basic MeOH, followed by acidification in 4 N HCl/dioxanes to give phosphinothiol 17 in 17% overall yield. As a chloride salt, phosphinothiol 17 was an easy-to-handle, odor-free white solid that was stable in air for several days and soluble at >1 M in 0.4 M sodium phosphate buffer (pH 7.8).
Scheme 5.
Route for the Synthesis of Basic Phosphinothiol 17
We evaluated the ability of phosphinothiol 17 to mediate a traceless Staudinger ligation in water. As with phosphinothiol 9, phosphinothiol 17 provided amide 16 with a yield that was dependent on the pH of the reaction mixture, increasing at high pH (Figure 2). At each pH, however, the ligation yield with basic phosphinothiol 17 was much greater than that with acidic phosphinothiol 9, as expected from a consideration of the relevant Coulombic interactions. It is noteworthy that the yields with phosphinothiol 17 reached 94% at high pH and exceeded 80% in all solutions with pH ≥ 7.5. Moreover, these yields are for reactions performed with equimolar reactants, which contrasts with the auspicious excess of nucleophile typically used in solid-phase peptide synthesis, native chemical ligation, and expressed protein ligation.
Figure 2.
Dependence of amide yield on the solution pH in a traceless Staudinger ligation mediated by a basic phosphinothiol. Reactions were performed with equimolar amounts of 25 and 15 (or 30) (0.16 M) in 0.40 M sodium phosphate buffers of varying pH. Data are the mean values (SE = ±2%) from two or three experiments.
Phosphinothiol 17 is not only cationic rather than anionic, but also has a different type of substituent in the para position than does phosphinothiol 9. The Hammett constants for a secondary amide and a protonated dimethylaminoethyl group are σp = 0.36 and 0.14, respectively.29 A higher σp value (like a positive charge) would serve to decrease the pKa of the iminophosphorane nitrogen. Despite this favorable attribute, phosphinothiol 9 is still an inferior reagent than is phosphinothiol 17, indicating that Coulombic effects are more important than inductive and resonance effects in the ability of phosphinothiols 9 and 17 to mediate the traceless Staudinger ligation in water.
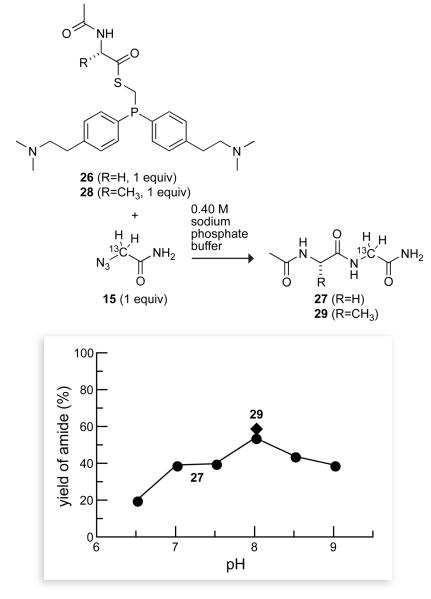
We then determined the efficacy of phosphinothiol 17 in effecting the Staudinger ligation to form peptides in water. Phosphiniothiol 17 was condensed with N-acetyl glycine by standard methods using DIC and HOBt. Yields for the ligation of the resulting phosphinothioester 26 and azide 15 exceeded 50% at pH 8, but diminished somewhat at higher pH (Figure 3). This decrease and the concomitant increase in the formation of the glycinamide byproduct could arise from hydrolysis of the thioester group of 26 to regenerate phosphinothiol 17, which could reduce azide 15.
Figure 3.
Dependence of peptide yield on the solution pH in a traceless Staudinger ligation mediated by a basic phosphinothiol. Reactions were performed with equimolar amounts of 26 (or 28) and 15 (0.16 M) in 0.40 M sodium phosphate buffers of varying pH. Data are the mean values (SE = ±2%) from two or three experiments.
The efficacy of the traceless Staudinger ligation in water using phosphinothiol 17 is not limited to the ligation of two glycyl residues. Phosphinothiol 17 was condensed with N-acetyl alanine by standard methods using DIC and HOBt to give phosphinothioester 28 and then reacted with azide 15 in 0.40 M sodium phosphate buffer at pH 8.0, which produced a high yield in the Gly + Gly coupling. The yield of amide 29 in this Ala + Gly coupling was 59%, which is similar to that observed in the Gly + Gly coupling (Figure 3). Thus, the yield of the traceless Staudinger ligation in water was not diminished by the additional steric encumbrance imposed by a non-glycyl residue.
The traceless Staudinger ligation mediated by phosphinothiol 17 is expeditious in water. An NMR-based assay15,17 was used to monitor the rate of the reaction of phosphinothioester 28 and azide 15. This Ala + Gly coupling was found to proceed with a second-order rate constant of k2 = 7.4 × 10−3 M−1s−1 in water. This rate constant, which is similar to that reported previously for a Gly + Gly coupling mediated by phosphinothiol 1 in DMF/D2O (6:1) (k2 = 7.7 × 10−3 M−1s−1),17 corresponds to t1/2 = 10 min for a reaction with 0.16 M azide.
Having a proximal negative charge on the azide rather than the phosphinothioester also has deleterious consequences. We found that the Staudinger ligation with azide 30, which has a carboxyl group (rather than the carboxyamide group of azide 15) provides a low yield of amide 31 at each pH (Figure 2).30 Again, these data are consistent with protonation of the iminophosphorane nitrogen being undesirable.
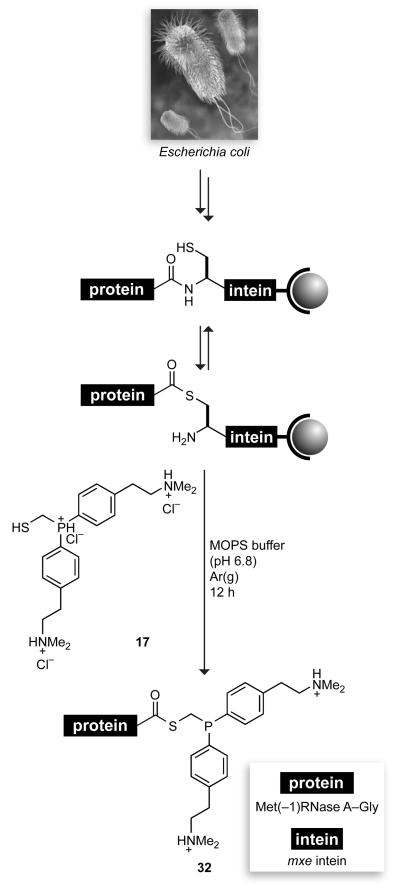
Finally, we determined whether our water-soluble phosphinothiol could be integrated with expressed protein ligation. This ability would highlight an intrinsic advantage of using a phosphinothiol11 rather than a phosphinoalcohol31 to mediate the Staudinger ligation, as only a phosphinothiol can effect the requisite acyl transfer from a protein–intein thioester.9 To do so, we used a model protein, bovine pancreatic ribonuclease (RNase A), which has been the object of much seminal work in protein chemistry32 and has been manipulated previously with expressed protein ligation.8,19,33,34 The C-terminal residue of RNase A is valine, which is known to diminish the cleavage efficiency of protein–intein thioesters.35 To avert this problem, we used recombinant DNA technology to insert a glycine residue between the C terminus of RNase A and the N terminus of the mxe intein. The resulting Met(−1)RNase A–Gly–mxe intein–chitin-binding domain fusion protein was produced in Escherichia coli, and the cell lysate was loaded onto a chitin resin (Scheme 6), as described previously.34 The chitin resin was then incubated with phosphinothiol 17 (40 mM). The column eluate contained the desired product, phosphinothioester 32, as verified by MALDI–TOF mass spectrometry (m/z 14225, expected: 14224). It is noteworthy that phosphinothiol 17 was able to perform the transthioesterification directly—without the need for a catalytic small-molecule thiol.36 The lowering of the thiol pKa of phosphinothiol 17 upon protonation of its two dimethylamino groups could enhance its reactivity.
Scheme 6.
Route for the Integration of the Traceless Staudinger Ligation and Expressed Protein Ligation
Conclusions
A water-soluble phosphinothiol has been developed that enables efficient traceless Staudinger ligations. The use of dimethylamino groups on the phosphinothiol apparently serves not only to facilitate its water-solubility but also to discourage protonation of the nitrogen in the iminophosphorane intermediate, which enables S→N acyl transfer to compete with hydrolysis. As a proof-of-principle for its utility in the integration of Staudinger ligation and expressed protein ligation, the water-soluble phosphinothiol and recombinant DNA technology were used to generate a protein with a C-terminal phosphinothioester, which is poised for undergoing Staudinger ligation. Studies are ongoing in our laboratory to further apply such phosphinothioesters in the semisynthesis of enzymes.
Experimental Methods
General
Reagent chemicals were obtained from commercial suppliers, and reagent grade solvents were used without further purification. Procedures were performed at room temperature (<23 °C) unless indicated otherwise. Reactions were monitored by thin-layer chromatography with visualization by ultraviolet light or staining with KMnO4, ninhydrin, PMA, or I2. Compound purification was carried out with flash chromatography on silica gel, which had a mesh of 230–400 (ASTM) and a pore size of 60 Å. The removal of solvents and other volatile materials “under reduced pressure” refers to the use of a rotary evaporator at water-aspirator pressure (<20 torr) and a water bath of <40 °C.
Instrumentation
NMR spectra were acquired at ambient temperature with a Bruker DMX-400 Avance spectrometer (1H, 400 MHz; 13C, 100.6 MHz; 31P, 161 MHz) or Bruker Avance DMX-500 spectrometer (1H, 500 MHz; 13C, 125.7 MHz; 31P, 202 MHz) at the National Magnetic Resonance Facility at Madison (NMRFAM) or a Varian Inova 500 (1H, 500 MHz; 13C, 125.7 MHz; 31P, 202 MHz) spectrometer at the University of Wisconsin Nuclear Magnetic Resonance Facility. Carbon-13 and phosphorus-31 spectra were proton-decoupled, and phosphorus-31 spectra were referenced against an external standard of deuterated phosphoric acid (0 ppm).
Mass spectrometry was performed with a Micromass LCT (electrospray ionization, ESI) in the Mass Spectrometry Facility in the Department of Chemistry or a Voyager DE-Pro mass spectrometer (matrix-assisted laser desorption–time-of-flight, MALDI–TOF) in the Biophysics Instrumentation Facility.
Phosphine oxide 5
4-Iodo-t-butyl benzoate (6.98 g, 22.9 mmol) was dissolved in dry THF (45 mL), and cooled to −20 °C with an isopropanol/dry ice bath. Freshly prepared isopropylmagnesium bromide (1.0 M solution in THF, 25 mL, 25 mmol) was added to this solution dropwise.37 After stirring for 1.5 h at −20 °C, the reaction mixture containing Grignard reagent 4 was added dropwise slowly to a cooled solution of chloromethylphosphonic dichloride (1.57 g, 11.5 mmol) in THF (45 mL) at −20 °C. The resulting solution was allowed to warm to room temperature overnight. After quenching with 2 mL of water, the solvent was removed under reduced pressure. The resulting oil was dissolved in CH2Cl2, and this solution was washed with brine. The combined organic extracts were dried over anhydrous MgSO4(s) and filtered, and the filtrate was concentrated to yield an orange oil that was purified by flash chromatography (silica gel, 2% v/v MeOH in CH2Cl2) to give phosphine oxide 5 as a white solid in 63% yield. Spectral data. 1H NMR (CDCl3, 400 MHz) δ 8.15–8.11 (m, 4H), 7.92–7.85 (m, 4H), 4.12 (d, J = 6.7 Hz, 2H), 1.61 (s, 18H) ppm; 13C NMR (CDCl3, 125 MHz) δ 164.71, 136.20, 133.09, 131.71 (d, J = 10.8 Hz), 129.79 (d, J = 13.2 Hz), 82.31, 37.48 (d, J = 74.4 Hz), 28.33 ppm; 31P NMR (CDCl3, 161 MHz) δ 27.88 ppm; MS (ESI) m/z 473.1273 (MNa+ [C23H28ClO5PNa+] = 473.1261).
Phosphine oxide 6
Potassium thioacetate (432 mg, 3.78 mmol) was added to a solution of phosphine oxide 5 (1.42 g, 3.15 mmol) in anhydrous DMF (25 mL) under Ar(g). The reaction mixture was stirred overnight, and the solvent was removed under reduced pressure. The residue was dissolved in ethyl acetate (15 mL) and the resulting solution was washed with water and brine. The combined organic extracts were dried over anhydrous MgSO4(s) and filtered, and the solvent was removed under reduced pressure. The residue was purified by flash chromatography (silica gel, 50% v/v ethyl acetate in hexanes) to give phosphine oxide 6 as a light amber solid in 98% yield. Spectral data. 1H NMR (CDCl3, 400 MHz) δ 8.09–8.07 (m, 4H), 7.84–7.80 (m, 4H), 3.80 (d, J = 8.3 Hz, 2H), 2.28 (s, 3H), 1.59 (s, 18H) ppm; 13C NMR (CDCl3, 125 MHz) δ 193.07, 164.84, 135.88, 134.82, 131.25 (d, J = 9.3 Hz), 129.26 (d, J = 11.8 Hz), 82.24, 30.35, 28.37, 27.24 (d, J = 71.3 Hz) ppm; 31P NMR (CDCl3, 161 MHz) δ 28.36 ppm; MS (ESI) m/z 513.1487 (MNa+ [C25H31O6PSNa+] = 513.1477).
Phosphinothioester 7
Phosphine oxide 6 (1.52 g, 3.09 mmol) was dissolved in anhydrous chloroform (25 mL) under Ar(g). Trichlorosilane (4.7 mL, 46.3 mmol) was added to this solution, and the resulting solution was stirred under Ar(g) for 72 h. The solvent was removed under reduced pressure. (CAUTION: Excess trichlorosilane in the removed solvent was quenched by the slow addition of saturated sodium bicarbonate in a well-ventilated hood.) The residue was purified by flash chromatography (silica gel, 50% v/v ethyl acetate in hexanes) to give phosphinothioester 7 as a pale yellow oil in 38% yield. Spectral data. 1H NMR (CDCl3, 400 MHz) δ 7.97–7.95 (m, 4H), 7.46–7.43 (m, 4H), 3.53 (d, J = 3.6 Hz, 2H), 2.30 (s, 3H), 1.59 (s, 18H) ppm; 13C NMR (CDCl3, 125 MHz) δ 194.12, 165.21, 141.78 (d, J = 17.8 Hz) 132.77, 132.72 (d, J = 18.7 Hz), 129.42 (d, J = 5.6 Hz), 81.77, 30.32, 25.30 (d, J = 24.7 Hz) 20.12 ppm; 31P NMR (CDCl3, 161 MHz) δ –14.19 ppm; MS (ESI) m/z 497.1508 (MNa+ [C25H34BO5PSNa+] = 497.1528).
Phosphinothioester 8
Phosphinothioester 7 (100 mg, 0.21 mmol) was dissolved in 1:3 trifluoroacetic acid/dichloromethane (2.5 mL) under Ar(g), and the resulting solution was stirred for 4 h or until the reaction was judged to be complete by TLC. The solvent was removed under reduced pressure as an azeotrope with toluene to give phosphinothioester 8 as an off-white solid in quantitative yield. Spectral data. 1H NMR (DMSO-d6, 400 MHz) δ 7.94–7.92 (m, 4H), 7.56–7.52 (m, 4H), 3.67 (d, J = 3.1 Hz, 2H), 2.30 (s, 3H) ppm; 13C NMR (DMSO-d6, 125 MHz) δ 193.66, 166.48, 141.54 (d, J = 17.6 Hz), 132.26 (d, J = 18.6 Hz), 130.95, 128.84 (d, J = 7.0 Hz), 29.92, 23.49 (d, J = 21.4 Hz) ppm; 31P NMR (DMSO-d6, 161 MHz) δ –13.52 ppm; MS (ESI) m/z 361.0293 (MH− [C17H15O5PSH−] = 361.0300).
Phosphinothiol 3
Phosphinothioester 8 (100 mg, 0.21 mmol) was dissolved in degassed MeOH (1 mL), and degassed 2 N NaOH (1.5 mL) was added to this solution under Ar(g). The resulting solution was stirred for 1.5 h. The solvent was removed under reduced pressure, and the residue was acidified with 4 N HCl and extracted into EtOAc (3 × 5 mL). The combined organic extracts were dried over anhydrous MgSO4(s) and filtered, and the solvent was removed under reduced pressure to give phosphinothiol 3 a pale yellow oil in 76% yield. Spectral data. 1H NMR (DMSO-d6, 400 MHz) δ 7.93–7.90 (m, 4H), 7.56–7.52 (m, 4H), 3.30 (d, J = 5.7 Hz, 2H) ppm; 13C NMR (DMSO-d6, 125 MHz) δ 166.97, 142.53 (d, J = 17.5 Hz), 132.74 (d, J = 18.0 Hz), 131.26, 129.25 (d, J = 5.1 Hz), 18.55 (d, J = 13.1 Hz) ppm; 31P NMR (DMSO-d6, 161 MHz) δ −8.48 ppm; MS (ESI) m/z 319.0181 (MH− [C15H13O4PSH−] = 319.0194).
Phosphine oxide 10
Phosphine oxide 5 (2.0 g, 4.44 mmol) was dissolved in CH2Cl2 (35 mL), and TFA (8.75 mL) was added to this solution. The resulting solution was stirred for 4 h. The solvent was removed under reduced pressure to give phosphine oxide 10 as an off-white solid in quantitative yield. Spectral data. 1H NMR (CD3OD, 400 MHz) δ 8.22–8.20 (m, 4H), 8.00–7.95 (m, 4H), 4.53 (d, J = 5.3 Hz, 2H) ppm; 13C NMR (CD3OD, 125 MHz) δ 168.48, 136.35, 135.38, 132.77 (d, J = 8.4 Hz), 131.18 (d, J = 12.9 Hz), 38.19 (d, J = 73.8 Hz) ppm; 31P NMR (CD3OD, 161 MHz) δ 31.60 ppm; MS (ESI) m/z 337.0020 (MNa+ [C25H34BO5PSNa+] = 337.0033).
Phosphine oxide 11
Phosphine oxide 10 (2.54 g, 7.5 mmol) was dissolved in anhydrous CH2Cl2 (65 mL) and anhydrous DMF (5 mL) under Ar(g). Thionyl chloride (3.28 mL, 45 mmol) was added dropwise to this solution, and the resulting solution was stirred for 3 h. The solvent and all traces of excess thionyl chloride were removed under reduced pressure. In a separate flask, glycine t-butyl ester–HCl (4.88 g, 29.1 mmol) was dissolved in anhydrous CH2Cl2 (65 mL) and N,N-diisoprolylethylamine (DIEA, 10.0 mL, 58.2 mmol) was added to this solution. The resulting solution was cooled to 0 °C with an ice bath. The incipient acid chloride was added dropwise slowly and with stirring, and the reaction mixture was allowed to warm to room temperature and stirred for 4 h. The reaction mixture was washed with 1 N HCl (35 ml) and brine, dried over anhydrous MgSO4(s), and filtered. The solvent was removed under reduced pressure, and the residue was purified by flash chromatography (silica gel, 4% v/v MeOH in CH2Cl2) to give phosphine oxide 11 as a white solid in 66% yield. Spectral data. 1H NMR (CDCl3, 400 MHz) δ 9.05–9.02 (m, 2H), 8.01–7.95 (m, 8H), 4.72 (d, J = 5.3 Hz, 2H), 3.91 (d, J = 6.1 Hz, 4H), 1.42 (s, 18H) ppm; 13C NMR (CDCl3, 125 MHz) δ 169.23, 166.42, 138.31, 132.99, 131.99, 127.80, 82.84, 42.72, 37.39 (d, J = 75.1 Hz), 28.25 ppm; 31P NMR (CDCl3, 161 MHz) δ 27.94 ppm; MS (ESI) m/z 587.1675 (MNa+ [C27H34ClN2O7PNa+] = 587.1690).
Phosphine oxide 12
Potassium thioacetate (677 mg, 5.93 mmol) was added to a solution of phosphine oxide 11 (2.79 g, 4.94 mmol) in anhydrous DMF (45 mL) under Ar(g). The resulting solution was stirred overnight, after which the solvent was removed under reduced pressure. The residue was dissolved in ethyl acetate (25 mL), and the resulting solution was washed with water and brine. The combined organic extracts were dried over anhydrous MgSO4(s) and filtered, and the solvent was removed under reduced pressure. The residue was purified by flash chromatography (silica gel, 5% v/v MeOH in CH2Cl2) to give phosphine oxide 12 as a yellow oil in 85% yield. Spectral data. 1H NMR (CDCl3, 400 MHz) δ 7.90–7.79 (m, 4H), 7.71–7.65 (m, 4H), 4.12 (d, J = 5.0 Hz, 4H), 3.75 (d, J = 8.1 Hz, 2H), 2.25 (s, 3H), 1.41 (s, 18H) ppm; 13C NMR (CDCl3, 125 MHz) δ 192.69, 169.19, 166.58, 137.90, 134.30, 130.76, 127.68, 82.51, 42.61, 30.16, 28.16, 26.84 ppm; 31P NMR (CDCl3, 161 MHz) δ 29.04 ppm; MS (ESI) m/z 627.1923 (MNa+ [C29H37N2O8PSNa+] = 627.1906).
Phosphinothioester 13
Phosphine oxide 12 (2.54 g, 4.2 mmol) was dissolved in anhydrous chloroform (38 mL) under Ar(g). Trichlorosilane (6.35 mL, 62.9 mmol) was added to this solution, and the resulting solution was stirred under Ar(g) for 72 h. The solvent was removed under reduced pressure. (CAUTION: Excess trichlorosilane in the removed solvent was quenched by the slow addition of saturated sodium bicarbonate in a well-ventilated hood.) The residue was purified by flash chromatography (silica gel, 2% v/v MeOH in CH2Cl2) to give phosphinothioester 13 as a white solid in 52% yield. Spectral data. 1H NMR (CDCl3, 400 MHz) δ 7.80–7.78 (m, 4H), 7.48–7.44 (m, 4H), 6.73 (bs, 2H), 4.13 (d, J = 5.0 Hz, 4H), 3.53 (d, J = 4.3 Hz, 2H), 2.30 (s, 3H), 1.50 (s, 18H) ppm; 13C NMR (CDCl3, 125 MHz) δ 194.39, 169.29, 166.84, 140.94 (d, J = 17.4 Hz), 134.84, 133.10 (d, J = 20.3 Hz), 127.30 (d, J = 6.2 Hz), 82.84, 42.69, 30.50, 20.25, 25.45 (d, J = 24.4 Hz) ppm; 31P NMR (CDCl3, 161 MHz) δ −14.42 ppm; MS (ESI) m/z 611.1937 (MNa+ [C29H37N2O7PSNa+] = 611.1957).
Phosphinothioester 14
Phosphinothioester 13 (100 mg, 0.17 mmol) was dissolved in 25% v/v trifluoroacetic acid in dichloromethane (2 mL) under Ar(g), and the resulting solution was stirred for 4 h. The solvent was removed under reduced pressure as an azeotrope with toluene to give di-acid 14 as an off-white solid in quantitative yield. Spectral data. 1H NMR (DMSO-d6, 400 MHz) δ 8.92–8.88 (m, 2H), 7.87–7.85 (m, 4H), 7.55–7.51 (m, 4H), 3.91 (d, J = 5.4 Hz, 4H), 3.66 (d, J = 12.7 Hz, 2H), 2.30 (s, 3H) ppm; 13C NMR (DMSO-d6, 125 MHz) δ 194.02, 171.26, 166.05, 140.34 (d, J = 17.2 Hz), 134.51, 132.56 (d, J = 19.3 Hz), 127.39 (d, J = 4.8 Hz), 41.25, 30.26, 24.01 (d, J = 23 Hz) ppm; 31P NMR (DMSO-d6, 161 MHz) δ −14.55 ppm.
Phosphinothiol 9
Phosphinothioester 14 (100 mg, 0.21 mmol) was dissolved in degassed MeOH (1 mL), and degassed 2 N NaOH (1.5 mL) was added to this solution under Ar(g). The resulting solution was stirred for 1.5 h. The solvent was removed under reduced pressure, and the residue was acidified with 4 N HCl and extracted into EtOAc (3 × 5 mL). The combined organic extracts were dried over anhydrous MgSO4(s) and filtered, and the solvent was removed under reduced pressure to give phosphinothiol 9 as an off-white solid quantitative yield. Spectral data. 1H NMR (DMSO-d6, 400 MHz) δ 8.98 (bs, 2H), 7.97–7.85 (m, 4H), 7.59–7.46 (m, 4H), 3.91 (d, J = 5.6 Hz, 4H), 3.50 (bs, 2H) ppm; 13C NMR (DMSO-d6, 125 MHz) δ 171.19, 166.05, 134.33, 132.47, 131.09, 127.43, 41.27, 18.78 (d, J = 21.5 Hz) ppm; 31P NMR (DMSO-d6, 161 MHz) δ – 14.47 ppm.
[2-13C]-2-Azido-acetamide (15)
[2-13C]-2-Azido-acetic acid was synthesized from [13Cα]glycine by a procedure described previously.38 [2-13C]-2-Azido-acetic acid (300 mg, 2.94 mmol) was dissolved in CH2Cl2 (10 ml). 1,1′-Carbonyldiimidazole (477 mg, 2.94 mmol) was added to this solution, and the resulting mixture was stirred for 20 min. Ammonia (25 ml, 0.5 M in dioxane, 14.7 mmol) was then added, and the resulting solution was stirred for 2 h. The solvent was removed under reduced pressure, and the amber residue was purified by flash chromatography (silica gel, 10% v/v MeOH in CH2Cl2) to give [2-13C]-2-azido-acetamide (15) as a white solid in 76% yield. Spectral data. 1H NMR (CDCl3, 400 MHz) δ 6.20 (bs, 1H), 5.40 (bs, 1H), 4.00 (d, J = 143.7 Hz, 2H) ppm; 13C NMR (CDCl3, 125 MHz) δ 170.13, 52.55 ppm; MS (ESI) m/z 101.0417 (MH+ [C2H4N4OH+] = 101.0419).
Ac[13Cα]GlyNH2 (16)
AcSCH2PPh2 (ref 17; 258 mg, 0.62 mmol) and azide 15 (63 mg, 0.62 mmol) were dissolved in THF (6.5 mL). This solution was allowed to stir for 12 h. The white precipitate that formed was isolated by filtration, and judged to be identical to commercial AcGlyNH2 by NMR spectroscopy.
Mesylate 18
Triethylamine (5.2 mL, 37.3 mmol) was added to a solution of 4-bromophenethyl alcohol (5.0 g, 24.9 mmol) in CH2Cl2 (200 mL), and the resulting solution was cooled to 0 °C with an ice bath. Methanesulfonyl chloride (2.7 mL, 34.8 mmol) was added dropwise to the reaction mixture, and the resulting solution was allowed to warm slowly to room temperature overnight. The solution was washed with 0.1 N HCl and brine, and the combined organic extracts were dried over anhydrous MgSO4(s) and filtered, and the solvent was removed under reduced pressure. The crude yellow solid was purified by flash chromatography (silica gel, 70% v/v hexanes in CH2Cl2) to give mesylate 18 as a white solid in 98% yield. Spectral data. 1H NMR (CDCl3, 400 MHz) δ 7.46 (d, J = 8.5 Hz, 2H), 7.12 (d, J = 8.6 Hz, 2H), 4.39 (t, J = 6.9 Hz, 2H), 3.02 (t, J = 6.7 Hz, 2H), 2.89 (s, 3H) ppm; 13C NMR (CDCl3, 125 MHz) δ 135.55, 132.52, 130.92, 121.70, 69.83, 37.68, 35.31 ppm.
Dimethylamine 19
Mesylate 18 (6.82 g, 24.4 mmol) was dissolved in anhydrous THF (150 mL) under Ar(g), and dimethylamine (2 M in THF, 50 mL, 100 mmol) was added to this solution. The resulting solution was heated at 45 °C for 16 h. The white solid that formed was removed by filtration, and the solvent was removed under reduced pressure. The residue was purified by flash chromatography (silica gel, 10% v/v MeOH in CH2Cl2) to give dimethylamine 19 as a pale yellow oil in 66% yield. Spectral data. 1H NMR (CDCl3, 400 MHz) δ 7.40 (d, J = 8.4 Hz, 2H), 7.08 (d, J = 8.0 Hz, 2H), 2.75 (t, J = 7.9 Hz, 2H), 2.53 (t, J = 7.9 Hz, 2H), 2.31 (s, 6H) ppm; 13C NMR (CD3OD, 125 MHz) δ 139.35, 132.87, 131.85, 121.40, 61.34, 44.94, 33.23 ppm; MS (ESI) m/z 228.0387 (MNa+ [C10H14BrNNa+] = 228.0388).
Phosphine oxide 20
Dimethylamine 19 (7.66 g, 33.6 mmol) was dissolved in anhydrous THF (72 mL) under Ar(g) in a flame-dried roundbottom flask equipped with a reflux condenser. To facilitate generation of the Grignard reagent, a catalytic amount of I2 was added to the solution. Crushed magnesium turnings (971 mg, 40.3 mmol) were then added to this solution, and the resulting solution was heated at reflux for 2 h to generate the Grignard reagent.
In a separate flamed-dried flask, diethyl phosphite (1.3 mL, 10.1 mmol) was dissolved in anhydrous THF (30 mL), and cooled to 0 °C with an ice bath. The solution of Grignard reagent was added dropwise to this solution, and the resulting solution was allowed to warm to room temperature and stirred overnight. The reaction mixture was then quenched with water (2 mL), and the solvent was removed under reduced pressure. The residue was dissolved in CH2Cl2, and the resulting solution was washed with water and brine. The combined organic extracts were dried over anhydrous MgSO4(s) and filtered, and the solvent was removed under reduced pressure. The residue was purified by flash chromatography (silica gel, 20% v/v MeOH in CH2Cl2) to give phosphine oxide 20 as a colorless oil in 75% yield. Spectral data. 1H NMR (CDCl3, 400 MHz) δ 8.03 (d, J = 1.2 ppm, 1H), 7.64–7.59 (m, 4H), 7.35–7.33 (m, 4H), 2.83 (t, J = 7.5 Hz, 4H), 2.54 (t, J = 8.2 Hz, 4H), 2.29 (s, 12H) ppm; 13C NMR (CDCl3, 125 MHz) δ 145.72, 131.08 (d, J = 11.5 Hz), 129.41 (J = 12.4 Hz), 128.71, 61.11, 45.64, 34.56 ppm; 31P NMR (CDCl3, 161 MHz) δ 21.75 ppm; MS (ESI) m/z 345.2090 (MNa+ [C20H29N2OPNa+] = 345.2096).
Phosphine–borane Complex 21
A solution of phosphine oxide 20 (3.18 g, 9.24 mmol) in anhydrous CH2Cl2 (25 mL) was added dropwise slowly to a solution of DIBAL (1 M in CH2Cl2, 46.2 mL, 46 mmol) under Ar(g) in a flame-dried three-neck round-bottom flask. The resulting solution was stirred for 20 min, then cooled to 0 °C with an ice bath. The solution was then diluted with CH2Cl2 (20 mL), and a sparge needle of Ar(g) was allowed to blow through the solution for 5 min. A solution of 2 N NaOH (20 mL) was added dropwise slowly to the reaction mixture (CAUTION: Gas evolution!) followed by a saturated solution of Rochelle's salt (20 mL) to dissipate the emulsion that forms. The resulting biphasic solution was transferred to a separatory funnel, and the organic layer was separated, dried over anhydrous MgSO4(s), filtered, and concentrated under reduced pressure to ~75 mL. The resulting solution was cooled to 0 °C with an ice bath under Ar(g), and borane·dimethyl sulfide complex (10 M, 2.96 mL, 29.6 mmol) was added dropwise. The resulting reaction mixture was allowed to warm slowly to room temperature overnight. The solvent was removed under reduced pressure, and the crude oil was purified by flash chromatography (silica gel, CH2Cl2) to give phosphine–borane complex 21 as a white solid in 82% yield. Spectral data. 1H NMR (CDCl3, 400 MHz) δ 7.62–7.57 (m, 4H), 7.31–7.29 (m, 4H), 6.30 (d, J = 378.8 Hz, 1H), 3.13–3.09 (m, 4H), 2.95–2.91 (m, 4H), 2.67 (s, 12H), 2.30–0.70 (m, 9H) ppm; 13C NMR (CDCl3, 125 MHz) δ 142.42, 133.54 (d, J = 10.7 Hz), 129.79 (d, J = 10.0 Hz), 124.66 (d, J = 58.7 Hz), 65.83, 52.12, 31.53 ppm; 31P NMR (CDCl3, 161 MHz) δ 0.00 ppm; MS (ESI) m/z 343.2474 (MH+ −2BH3 [C20H32BN2PH+] = 343.2474).
Phosphine–borane Complex 22
Phosphine–borane complex 21 (2.32 g, 6.27 mmol) was dissolved in 1:1 THF/CH2Cl2 (60 mL). Formaldehyde (37% v/v in H2O; 3.83 mL) was added to this solution, followed by potassium hydroxide (358 mg, 6.39 mmol). The resulting biphasic solution was stirred overnight at room temperature, after which the organic solvent was removed under reduced pressure. The residue was dissolved in ethyl acetate (30 mL), and the organic layer was dried over anhydrous MgSO4(s), filtered, and concentrated under reduced pressure. The residue was purified by flash chromatography (silica gel, 5% v/v ethyl acetate in CH2Cl2) to give phosphine–borane complex 22 as a white solid in 80% yield. Spectral data. 1H NMR (CDCl3, 400 MHz) δ 7.69–7.64 (m, 4H), 7.34–7.32 (m, 4H), 4.42 (d, J = 6.5 Hz, 2H), 3.15–3.11 (m, 4H), 2.97–2.93 (m, 4H), 2.67 (s, 12H), 2.20–0.50 (m, 9H) ppm; 13C NMR (CDCl3, 125 MHz) δ 142.46, 133.38 (d, J = 8.7 Hz), 129.72 (d, J = 10.6 Hz), 125.16 (d, J = 56.1 Hz) 65.90, 60.60 (d, J = 42.1 Hz), 52.18, 31.11 ppm; 31P NMR (CDCl3, 161 MHz) δ 16.92 ppm; MS (ESI) m/z 423.3047 (MNa+ [C21H40B3N2OPNa+] = 423.3055).
Phosphine–borane Complex 23
Triethylamine (650 μL, 4.67 mmol) was added to a solution of phosphine–borane complex 22 (1.25 g, 3.11 mmol) in CH2Cl2 (30 mL), and this solution was cooled to 0 °C with an ice bath. Methanesulfonyl chloride (337 μL, 4.36 mmol) was added dropwise, and the resulting solution was allowed to warm slowly to room temperature overnight. The solution was washed with 0.1 N HCl and brine, and the combined organic extracts were dried over anhydrous MgSO4(s), filtered, and concentrated under reduced pressure. The residue was purified by flash chromatography (silica gel, 2% v/v ethyl acetate in CH2Cl2) to give phosphine–borane complex 23 as a white solid in 95% yield. Spectral data. 1H NMR (CDCl3, 400 MHz) δ 7.69–7.64 (m, 4H), 7.37–7.27 (m, 4H), 4.87 (d, J = 2.0 Hz, 2H), 3.17–3.13 (m, 4H), 2.98–2.94 (m, 4H), 2.95 (s, 3H), 2.67 (s, 12H), 2.20–0.50 (m, 9H) ppm; 13C NMR (CDCl3, 125 MHz) δ 143.20, 133.51 (d, J = 10.4 Hz), 129.88 (d, J = 10.8 Hz), 123.51 (d, J = 57.8 Hz) 65.76, 64.58 (d, J = 37.4 Hz), 52.19, 37.71, 31.11 ppm; 31P NMR (CDCl3, 161 MHz) δ 17.82 ppm; MS (ESI) m/z 501.2811 (MNa+ [C22H42B3N2O3PSNa+] = 501.2831).
Phosphine–borane Complex 24
Potassium thioacetate (404 mg, 3.54 mmol) was added to a solution of phosphine–borane complex 23 (1.41 g, 2.95 mmol) in anhydrous DMF (29 mL) under Ar(g). The resulting solution was stirred overnight at room temperature, after which the solvent was removed under reduced pressure. The residue was dissolved in ethyl acetate (20 mL), and the resulting solution was washed with water and brine. The combined organic extracts were dried over anhydrous MgSO4(s), filtered, and concentrated under reduced pressure. The residue was purified by flash chromatography (silica gel, 2% v/v ethyl acetate and 28% hexanes in CH2Cl2) to give phosphine–borane complex 24 as a white solid in 60% yield. Spectral data. 1H NMR (CDCl3, 400 MHz) δ 7.65–7.60 (m, 4H), 7.33–7.30 (m, 4H), 3.68 (d, J = 7.0 Hz, 2H), 3.14–3.10 (m, 4H), 2.97–2.93 (m, 4H), 2.67 (s, 12H), 2.27 (s, 3H), 2.20–0.60 (m, 9H) ppm; 13C NMR (CDCl3, 125 MHz) δ 193.32, 142.50, 133.00 (d, J = 10.5 Hz), 129.55 (d, J = 12.3 Hz), 126.03 (d, J = 56.2 Hz), 65.75, 54.09, 30.98, 30.27, 23.88 (d, J = 35.6 Hz) ppm; 31P NMR (CDCl3, 161 MHz) δ 18.69 ppm; MS (ESI) m/z 481.2929 (MNa+ [C23H42B3N2OPSNa+] = 481.2932).
Phosphinothioester 25
Phosphine–borane complex 24 (250 mg, 0.55 mmol) was dissolved in toluene (5 mL) under Ar(g). DABCO (190 mg, 1.69 mmol) was added, and the resulting solution was heated to 40 °C for 4 h. The solvent was removed under reduced pressure, and the residue was purified by flash chromatography (silica gel, 20% v/v MeOH in CH2Cl2) to give phosphinothioester 25 as a colorless oil in 86% yield. Spectral data. 1H NMR (CDCl3, 400 MHz) δ 7.36–7.33 (m, 4H), 7.20–7.18 (m, 4H), 3.47 (d, J = 3.5 Hz, 2H), 2.79–2.75 (m, 4H), 2.55–2.51 (m, 4H), 2.29 (s, 15H) ppm; 13C NMR (CDCl3, 125 MHz) δ 194.89, 141.76, 134.25 (d, J = 12.9 Hz), 132.95 (d, J = 19.5 Hz), 129.03 (d, J = 6.1 Hz), 61.36, 45.61, 34.29, 30.46, 26.13 (d, J = 21.1 Hz) ppm; 31P NMR (CDCl3, 161 MHz) δ −16.84 ppm; MS (ESI) m/z 417.2135 (MH+ [C23H33N2OPSH+] = 417.2129).
Phosphinothiol 17
Phosphinothioester 25 (323 mg, 0.78 mmol) was dissolved in degassed MeOH (8 mL), and NaOH (31 mg) was added to this solution under Ar(g). The resulting solution was stirred at room temperature for 1.5 h. The solvent was removed under reduced pressure, and the residue was acidified with 4 N HCl in dioxane and filtered to give phophosphinothiol 17 as a white solid in quantitative yield. Spectral data. 1H NMR (DMSO-d6, 400 MHz) δ 11.05 (bs, 1H), 7.40 (t, J = 7.3 Hz, 4H), 7.30 (t, J = 8.2 Hz, 4H), 4.25 (bs, 2H), 3.26–3.22 (m, 4H), 3.20–3.18 (m, 2H), 3.05–3.01 (m, 4H), 2.77 (s, 12H), 1.20 (t, J = 7.0 Hz, 1H) ppm; 13C NMR (DMSO-d6, 125 MHz) δ 138.12, 135.50, 132.93, 128.97, 56.83, 41.87, 45.22, 29.47 ppm; 31P NMR (DMSO-d6, 161 MHz) δ −10.60 ppm; MS (ESI) m/z 375.2039 (MH+ [C21H31N2PSH+] = 375.2024).
Phosphinothioester 26
N-Acetyl glycine (95.3 mg, 0.81 mmol) was dissolved in anhydrous DMF (6 mL) under Ar(g). Hydroxybenzotriazole (105 mg, 0.78 mmol) was then added to the solution, followed by N,N′-diisopropylcarbodiimide (DIC, 121 μL, 0.78 mmol). After allowing the reaction mixture to stir for 20 min, a solution of phosphinothiol 17 (293 mg, 0.78 mmol) was added, followed by DIEA (564 μL, 3.24 mmol). The resulting solution was stirred for 4 h at room temperature under Ar(g). The solvent was removed under reduced pressure, and the resulting crude oil was purified by flash chromatography (silica gel, 20% v/v MeOH in CH2Cl2) to give phosphinothioester 26 as a colorless oil in 75% yield. Spectral data. 1H NMR (CDCl3, 400 Hz) δ 7.35–7.31 (m, 4H), 7.20–7.18 (m, 4H), 6.04 (s, 1H), 4.16 (d, J = 5.4 Hz, 2H), 3.49 (d, J = 3.9 Hz, 2H), 2.81–2.77 (m, 4H), 2.59–2.55 (m, 4H), 2.32 (s, 12H), 2.03 (s, 3H) ppm; 13C NMR (CDCl3, 125 MHz) δ 196.45, 170.38, 141.79, 133.99, 132.99, 129.11, 61.29, 49.25, 45.58, 34.18, 25.60, 23.14 ppm; 31P NMR (CDCl3, 161 MHz) δ −16.04 ppm; MS (ESI) m/z 474.2335 (MH+ [C25H36N3O2PSH+] = 474.2344).
AcGly[13Cα]GlyNH2 (27)
AcGlySCH2PPh2 (ref 17; 207 mg, 0.62 mmol) and [2-13C]-2-azido-acetamide (15) (63 mg, 0.62 mmol) were dissolved in THF (6.5 mL). The resulting solution was allowed to stir for 12 h. The white precipitate that formed was isolated by filtration to give AcGly[13Cα]GlyNH2 (27) as a white solid in 61% yield. Spectral data. 1H NMR (DMSO-d6, 400 MHz) δ 8.17 (bs, 1H), 8.07 (bs, 1H), 7.21 (bs, 1H), 7.08 (bs, 1H), 3.67 (d, J = 5.7 Hz, 2H), 3.61 (dd, J = 138.6 Hz, 5.9 Hz, 1H), 1.85 (s, 3H) ppm; 13C NMR (DMSO-d6, 125 MHz) δ 171.19, 170.68, 169.90, 169.29, 50.52, 41.83, 22.50 ppm; MS (ESI) m/z 197.0728 (MNa+ [C6H11N3O3Na+] = 197.0732).
Phosphinothioester 28
N-Acetyl alanine (20.6 mg, 0.16 mmol) was dissolved in anhydrous DMF (2 mL) under Ar(g). Hydroxybenzotriazole (20.3 mg, 0.15 mmol) was then added to the solution, followed by N,N′-diisopropylcarbodiimide (DIC, 23.5 μL, 0.15 mmol). After allowing the reaction mixture to stir for 20 min, a solution of phosphinothiol 17 (62.4 mg, 0.15 mmol) was added, followed by DIEA (104 μL, 0.6 mmol). The resulting solution was stirred for 4 h at room temperature under Ar(g). The solvent was removed under reduced pressure, and the resulting crude oil was purified by flash chromatography (silica gel, 20% v/v MeOH in CH2Cl2) to give phosphinothioester 28 as a colorless oil in 78% yield. Spectral data. 1H NMR (CDCl3, 400 Hz) δ 7.33 (t, J = 7.6 Hz, 4H), 7.19 (t, J = 7.5 Hz, 4H), 6.10 (d, J = 7.6 Hz, 1H), 4.66 (q, J = 7.6 Hz, 1H), 3.47–3.44 (m, 2H), 2.81–2.77 (m, 4H), 2.57–2.53 (m, 4H), 2.31 (s, 12H), 1.99 (s, 3H), 1.29 (d, J = 7.1 Hz, 3H) ppm; 13C NMR (CDCl3, 125 MHz) δ 200.05, 169.74, 141.68, 134.26, 133.03 (d, J = 18.4 Hz), 129.11, 61.25, 55.04, 45.51, 34.11, 25.74 (d, J = 23.6 Hz), 23.29, 19.04 ppm; 31P NMR (CDCl3, 161 MHz) δ −15.93 ppm.
AcAlaGlyNH2 (unlabeled 29)
N-Acetyl-alanyl-pentafluorophenol ester (100 mg, 0.34 mmol) and glycinamide–HCl salt (37 mg, 0.34 mmol) were dissolved in anhydrous THF (3 mL). DIEA (146 μL, 0.84 mmol) was added to this solution, and the resulting solution was allowed to stir for 12 h. The solution was filtered to give AcAlaGlyNH2 (unlabeled 29) as a white solid in 50% yield. AcAla[13Cα]GlyNH2 (29) was prepared in a similar maner. Spectral data. 1H NMR (DMSO-d6, 400 MHz) δ 8.16–8.13 (m, 1H), 7.16 (bs, 1H), 7.08 (bs, 1H), 4.17–4.14 (q, J = 6.1 Hz, 1H), 3.64–3.52 (m, 2H), 1.84 (s, 3H), 1.19 (d, J = 7.0 Hz, 3H) ppm; 13C NMR (DMSO-d6, 125 MHz) δ 172.65, 171.03, 169.60, 48.71, 41.95, 22.49, 17.65 ppm.
Staudinger Ligations of 14 + 15, 25 + 15, 26 + 15, 28 + 15, and 30 + 25
The respective phosphinothioester (0.1 mmol) and azide (10.7 mg, 0.1 mmol) were dissolved in 0.40 M sodium phosphate buffers (0.6 mL) of various pH. The resulting reaction mixtures were stirred for 16 h and the reaction was monitored with our 13C NMR assay.17
Production of Met(−1)RNase A–Gly–mxe Intein–Chitin-binding Domain
Plasmid pJK01, which directs the expression of the Met(−1)RNase A–Gly–mxe intein–chitin-binding domain fusion protein,34 was transformed into E. coli BL21(DE3) cells. Luria–Bertani (LB) medium (5 mL) containing ampicillin (0.10 mg/mL) was inoculated with a single colony, and the resulting culture was grown for 16 h at 37 °C. Cells were collected by centrifugation at 2000g for 2 min and resuspended in LB medium (4 mL). Four 4-L flasks, each containing 1 L of LB medium with ampicillin (0.10 mg/mL), were each inoculated with 1 mL of the resuspended cells. The resulting cultures were grown with shaking at 37 °C until OD = 0.5 at 600 nm. Gene expression was then induced by the addition of isopropyl β-d-thiogalactopyranoside (IPTG, to 0.5 mM), and the cultures were grown for an additional 3–4 h at 25 °C. Cells were collected by centrifugation, and stored at −20 °C.
Loading of Fusion Protein onto Chitin
Frozen cells (0.25 g from 0.1 L of E. coli culture) were thawed and suspended in lysis and column buffer (LCB, 5 mL), which was 20 mM 3-(N-morpholino)propanesulfonic acid (MOPS)–NaOH buffer (pH 6.8) containing NaCl (0.5 M), ethylenediaminetetraacetic acid (EDTA; 0.1 mM), and Triton X-100 (0.1% w/w). Cells were lysed by sonication, and the cell lysate was clarified by centrifugation at 15,000g for 30 min. Chitin resin (New England Biolabs, Ipswich, MA; 1-mL bed volume) was loaded into a column, and equilibrated with degassed LCB (5 mL). The clarified cell lysate was applied slowly to the column of chitin resin. The loaded resin was washed thoroughly with LCB (8 mL), and then LCB containing 0.5 M NaCl (2 mL).
Protein Phosphinothioester 32
The chitin column was washed with degassed cleavage buffer (CB, 5 mL), which was 50 mM MOPS–NaOH buffer (pH 6.8) containing NaCl (0.50 M) and EDTA (0.10 mM). A solution of CB (2.5 mL) containing phosphinothiol 17 (45 mg, 40 mM) was added to the chitin resin, and 2 mL of buffer was allowed to elute by gravity. The column was sealed with a stopper, and left under Ar(g) overnight. Protein phosphinothioester 32 was eluted from the column by the addition of 2 mL of a solution of NaCl (0.5 M), and precipitated by the addition of 300 μL of a solution of sodium deoxycholate (1% w/v) followed by 60 μL of a solution of trichloroacetic acid (50% w/v). The precipitate was collected by centrifugation at 5,000g for 5 min, resuspended in acetone to extract small molecules including excess phosphinothiol 17, and subjected to centrifugation again. Analysis of the precipitate by MALDI–TOF mass spectrometry showed the mass of protein phosphinothioester 32 to be m/z = 14225 (expected for Met(−1)RNase A–Gly–SCH2(P(C6H4-p-CH2CH2NMe2)2), C603H950N175O194PS14, 14224).
Supplementary Material
Acknowledgements
We are grateful to Dr. L. L. Kiessling for contributive discussions, Dr. B. L. Nilsson for initial studies relevant to this work, J. Kalia for plasmid pJK01, and R. J. Johnson and Dr. M. T. Borra for advice on protein purification. M.B.S. was supported by an ACS Division of Organic Chemistry Fellowship, sponsored by Abbott Laboratories. This work was supported by Grant GM44783 (NIH). NMRFAM was supported by Grant P41RR02301 (NIH); the University of Wisconsin Nuclear Magnetic Resonance Facility was supported by Grants CHE-9208463 (NSF) and RR08389 (NIH).
Footnotes
Supporting Information Available: NMR spectra of the novel compounds reported herein. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Nilsson BL, Soellner MB, Raines RT. Annu. Rev. Biophys. Biomol. Struct. 2005;34:91–118. doi: 10.1146/annurev.biophys.34.040204.144700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray BL. Nat. Rev. Drug Discov. 2003;2:587–593. doi: 10.1038/nrd1133. [DOI] [PubMed] [Google Scholar]
- 3.(a) Niazi SK. Handbook of Biogeneric Therapeutic Proteins. CRC Press; Boca Raton, FL: 2005. [Google Scholar]; (b) Banga AK. Therapeutic Peptides and Proteins. CRC Press; Boca Raton, FL: 2005. [Google Scholar]
- 4.Merrifield B. Science. 1986;232:341–347. doi: 10.1126/science.3961484. [DOI] [PubMed] [Google Scholar]; Merrifield B. Protein Sci. 1996;5:1947–1951. doi: 10.1002/pro.5560050925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albericio F. Curr. Opin. Chem. Biol. 2004;8:211–221. doi: 10.1016/j.cbpa.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Kent S. J. Peptide Sci. 2003;9:574–593. doi: 10.1002/psc.475. [DOI] [PubMed] [Google Scholar]
- 7.Muir TW, Sondhi D, Cole PA. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6705–6710. doi: 10.1073/pnas.95.12.6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans TC, Benner J, Xu MQ. Protein Sci. 1998;7:2256–2264. doi: 10.1002/pro.5560071103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muir TW. Annu. Rev. Biochem. 2003;72:249–289. doi: 10.1146/annurev.biochem.72.121801.161900. [DOI] [PubMed] [Google Scholar]
- 10.McCaldon P, Argos P. Proteins. 1988;4:99–122. doi: 10.1002/prot.340040204. [DOI] [PubMed] [Google Scholar]
- 11.Nilsson BL, Kiessling LL, Raines RT. Org. Lett. 2000;2:1939–1941. doi: 10.1021/ol0060174. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson BL, Kiessling LL, Raines RT. Org. Lett. 2001;3:9–12. doi: 10.1021/ol006739v. [DOI] [PubMed] [Google Scholar]
- 13.Meyer J, Staudinger H. Helv. Chim. Acta. 1919;2:635–646. [Google Scholar]
- 14.Köhn M, Breinbauer R. Angew. Chem. Int. Ed. Engl. 2004;43:3106–3116. doi: 10.1002/anie.200401744. [DOI] [PubMed] [Google Scholar]
- 15.Soellner MB, Tam A, Raines RT. J. Org. Chem. 2006;71:9824–9830. doi: 10.1021/jo0620056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Bräse S, Gil C, Knepper K, Zimmermann V. Angew. Chem. Int. Ed. Engl. 2005;44:5188–5240. doi: 10.1002/anie.200400657. [DOI] [PubMed] [Google Scholar]; (b) Agard NJ, Baskin JM, Prescher JA, Lo A, Bertozzi CR. ACS Chem. Biol. 2006;1:644–648. doi: 10.1021/cb6003228. [DOI] [PubMed] [Google Scholar]
- 17.Soellner MB, Nilsson BL, Raines RT. J. Am. Chem. Soc. 2006;128:8820–8828. doi: 10.1021/ja060484k. [DOI] [PubMed] [Google Scholar]
- 18.Soellner MB, Nilsson BL, Raines RT. J. Org. Chem. 2002;67:4993–4996. doi: 10.1021/jo025631l. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson BL, Hondal RJ, Soellner MB, Raines RT. J. Am. Chem. Soc. 2003;125:5268–5269. doi: 10.1021/ja029752e. [DOI] [PubMed] [Google Scholar]
- 20.(a) Soellner MB, Dickson KA, Nilsson BL, Raines RT. J. Am. Chem. Soc. 2003;125:11790–11791. doi: 10.1021/ja036712h. [DOI] [PubMed] [Google Scholar]; (b) Watzke A, Kohn M, Gutierrez-Rodriguez M, Wacker R, Schroder H, Breinbauer R, Kuhlmann J, Alexandrov K, Niemeyer CM, Goody RS, Waldmann H. Angew. Chem. Int. Ed. Engl. 2006;45:1408–1412. doi: 10.1002/anie.200502057. [DOI] [PubMed] [Google Scholar]
- 21.Liu L, Hong ZY, Wong CH. ChemBioChem. 2006;7:429–432. doi: 10.1002/cbic.200500437. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson BL, Soellner MB, Raines RT. In: Chemical Probes in Biology (NATO ASI Series) Schneider MP, editor. Kluwer Academic; Boston, MA: 2003. pp. 359–369. [Google Scholar]
- 23.We were inspired initially by the common water-soluble disulfide reducing agent, tris(2-carboxyethyl) phosphine (TCEP) (Houk J, Whitesides GM. J. Am. Chem. Soc. 1987;109:6825–6836.). Accordingly, we synthesized the water-soluble bis(2-carboxyethyl) thiomethyl phosphine but found its mediation of traceless Staundinger ligations in water to yield a preponderance of amine byproduct (data not shown).
- 24.Windholz M, Budavari S, Blumetti RF, Otterbein ES, editors. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. (10th ed.) 1983 entry 4611. [Google Scholar]
- 25.Noda LH, Kuby SA, Lardy HA. J. Am. Chem. Soc. 1953;75:913–917. [Google Scholar]
- 26.Like the iminophosphorane phosphorus, the thioester carbon becomes more electrophilic and hence susceptible to hydrolysis upon protonation of the iminophosphorane. Thioester hydrolysis would decrease the yield of amide 16 in a pH-dependent manner and is thus consistent with the data in Figure 1.
- 27.(a) Kirkwood JG, Westheimer FH. J. Chem. Phys. 1938;6:506–512. [Google Scholar]; (b) Kirkwood JG, Westheimer FH. J. Chem. Phys. 1938;6:513–517. [Google Scholar]
- 28.Busacca CA, Lorenz JC, Grinberg N, Haddad N, Hrapchak M, Latli B, Lee H, Sabila P, Saha A, Sarvestani M, Shen S, Varsolona R, Wei XD, Senanayake CH. Org. Lett. 2005;7:4277–4280. doi: 10.1021/ol0517832. [DOI] [PubMed] [Google Scholar]
- 29.(a) Hammett LP. Chem. Rev. Vol. 17. 1935. pp. 125–136. [Google Scholar]; Hammett LP. Physical Organic Chemistry: Reaction Rates, Equilibria, and Mechanisms. McGraw-Hill; New York: 1940. [Google Scholar]; (b) Hansch C, Leo A, Taft RW. Chem. Rev. 1991;91:165–195. [Google Scholar]
- 30.Lee and co-workers have reported that couplings of azide 30 as well as 14 similar azido acids mediated by (diphenylphosphino) methanethiol proceed in “quantitative yield” (Kim H, Cho JK, Aimoto S, Lee Y-S. Org. Lett. 2006;8:1149–1151. doi: 10.1021/ol0530629.). These results could not be reproduced in our laboratory.
- 31.Saxon E, Bertozzi CR. Science. 2000;287:2007–2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- 32.Raines RT. Chem. Rev. 1998;98:1045–1066. doi: 10.1021/cr960427h. [DOI] [PubMed] [Google Scholar]
- 33.(a) Hondal RJ, Nilsson BL, Raines RT. J. Am. Chem. Soc. 2001;123:5140–5141. doi: 10.1021/ja005885t. [DOI] [PubMed] [Google Scholar]; (b) Arnold U, Hinderaker MP, Nilsson BL, Huck BR, Gellman SH, Raines RT. J. Am. Chem. Soc. 2002;124:8522–8523. doi: 10.1021/ja026114n. [DOI] [PubMed] [Google Scholar]; (c) Arnold U, Hinderaker MP, Köditz J, Golbik R, Ulbrich-Hoffmann R, Raines RT. J. Am. Chem. Soc. 2003;125:7500–7501. doi: 10.1021/ja0351239. [DOI] [PubMed] [Google Scholar]
- 34.Kalia J, Raines RT. ChemBioChem. 2006;7:1375–1383. doi: 10.1002/cbic.200600150. [DOI] [PubMed] [Google Scholar]
- 35.(a) Yee CS, Seyedsayamdost MR, Chang MC, Nocera DG, Stubbe J. Biochemistry. 2003;42:14541–14552. doi: 10.1021/bi0352365. [DOI] [PubMed] [Google Scholar]; (b) Lue RY, Chen GY, Hu Y, Zhu Q, Yao SQ. J. Am. Chem. Soc. 2004;126:1055–1062. doi: 10.1021/ja037914g. [DOI] [PubMed] [Google Scholar]
- 36.Johnson ECB, Kent SBH. J. Am. Chem. Soc. 2006;128:6640–6646. doi: 10.1021/ja058344i. [DOI] [PubMed] [Google Scholar]
- 37.Dohle W, Lindsay DM, Knochel P. Org. Lett. 2001;3:2871–2873. doi: 10.1021/ol0163272. [DOI] [PubMed] [Google Scholar]
- 38.Lundquist JT, Pelletier JC. Org. Lett. 2001;3:781–783. doi: 10.1021/ol0155485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.