Abstract
Recent studies suggest that glutaredoxin-1 (Glrx-1) may serve as therapeutic target for diabetic hearts. Since the level of reactive oxygen species (ROS) is increased in the pathologic hearts including ischemia/reperfusion (I/R) and diabetes, we assumed that upregulation of Glrx-1 could reduce the cardiac risk factors associated with I/R and/or diabetes. Diabetes was induced in mice by i.p. injection of streptozotocin (150 mg/kg). Eight days after when the blood glucose was elevated to 400 mg/dL, the animals were randomly assigned to one of the following three groups, which received either empty vector, or LacZ or Glrx-1 adenoviral construct. Four days later, isolated working hearts were subjected to 30 min ischemia followed by 2 h reperfusion. Glrx-1 gene therapy significantly enhanced the Glrx-1 level, which prevented I/R-mediated reduction of ventricular recovery, increased myocardial infarct size and cardiomyocyte apoptosis in diabetic myocardium. In concert, Glrx-1 prevented diabetes and ischemia-reperfusion induced reduction of cardioprotective proteins including Akt, FoxO-1, and hemeoxygenase-1 and abolished the death signal triggered by Jnk, p38 mitogen-activated protein kinase, and c-Src. Glrx-1 gene therapy appears to prevent cardiac complications in diabetic heart due to the I/R by switching the death signal into survival signal by activating Akt-FoxO-signaling network.
Keywords: Glutaredoxin-1, Glrx-1 gene therapy, diabetes, Akt-FoxO-signaling network, Ischemia/reperfusion
Introduction
Glutaredoxins (Glrx) belong to the members of the thioredoxin superfamily of thiol/disulfide exchange catalysts, and hence known as thiol transferase, which serves as reductants of protein-SG mixed disulfides that provide reducing equivalents to ribonucleotide reductase1. In mammalian system, Glrx exist in two major forms – Glrx-1 localizes in the cytosol and Glrx-2 localizes primarily in the mitochondria, but can also localize in the nucleus2. Both Glrx-1 and Glrx-2 play a crucial role in redox regulation and protect cells against apoptosis2. A recent study demonstrated that overexpression of Glrx-1 by ischemic preconditioning and transgenic mice overexpressing Glrx-1 were resistant to myocardial ischemic reperfusion injury3.
Glutathione together with glutathione enzyme system and thioltransferase play a crucial role in defense against reactive oxygen species (ROS) that are potential hazards against a variety of degenerative diseases including ischemic heart disease, cardiomyopathy, hypertension, atherosclerosis, diabetes, and heart failure4. Reduced levels of glutathione, glutathione utilizing enzymes, and thioltransferase were found in the platelets of diabetic patients5. Glrx-1 level was reported to be reduced after brain ischemia6. Glrx and Trx were found to be enhanced in human coronary arteries, where they were suggested to protect vascular cells in normal conditions as well as against elevated oxidative stress in atherosclerotic lesions7. An increased level of Glrx-1 was seen in the hearts of diabetic rats8, where it was shown that K+ channels underlying Ito are regulated in a redox sensitive manner via the thioredoxin (Trx) system. In addition to that an upregulation of Glrx-1 was seen in the eye of diabetic rats9. High amount of Glrx mRNA and protein was found in pancreatic β-cells, where NADPH/Glrx/Trx redox regulation was shown to mediate nutrient-induced insulin secretion10. Upregulation of Glrx-1 in the diabetic animals denotes an adaptive response to mitigate the challenge of redox unbalance during the initial phase of diabetes. During the advanced phase, however, the antioxidants including Glrx-1 fall indicating a failed adaptive response.
The above reasons indicate that Trx/Glrx system not only does play a crucial role in the redox regulation in the ischemic heart, but also helps maintaining the redox homeostasis in diabetic hearts. The present study was designed to examine the effects of Glrx-1 gene therapy in the diabetic myocardium whether it could ameliorate the cardiovascular risks associated with diabetes and ischemia-reperfusion. The results of this study documented that upregulation of Glrx-1 through Glrx-1 gene therapy could reduce cardiac complications in diabetic heart associated with ischemia-reperfusion.
Results
Glrx-1 Gene Therapy enhanced the Expression of Glrx-1 Protein
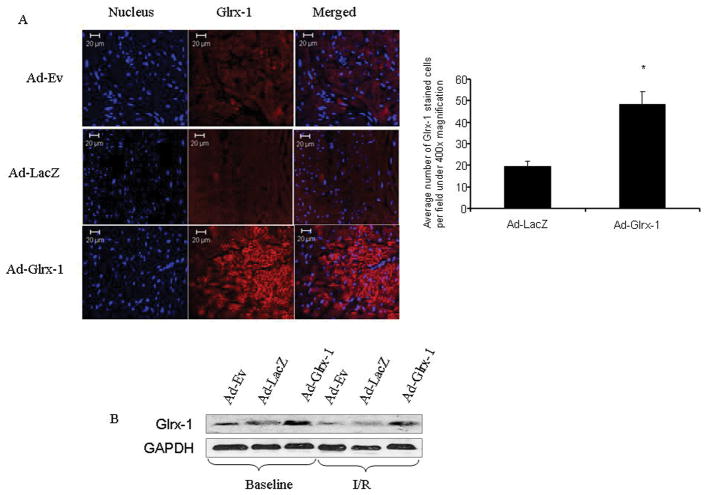
First, we evaluated if our gene therapy was successful by examining the expression of Glrx-1 in the heart by Immunohistochemistry. As shown in Figure 1A, Glrx-1 was highly expressed in the heart after the Ad-Glrx-1 gene therapy, while only a low level of Glrx-1 was detected in the Ad-Ev and Ad-LacZ groups indicating the success of gene delivery. To further confirm the success of the gene delivery, Western blot analysis was performed as shown in Figure 1B. Thus, the expression was higher in Ad-Glrx-1 injected group, and it remains higher also after ischemia reperfusion compared to the Ad-Ev or Ad-LacZ group.
Figure 1.
(A)- Confocal microscopic images of left ventricular tissue sections showing the staining of nucleus (blue with Topro-3-iodide) and Glrx-1 (Red). (B)- Western immunoblot analysis of Glrx-1, the hearts were obtained from diabetic animals injected with Ad-Ev or Ad-LacZ or Ad-Glrx-1 with or without subjecting to 30 min of global ischemia and 120 min of reperfusion.
Effect of Glrx-1 Gene Therapy on Diabetic Mouse Heart Function Determined by M-mode Echocardiography
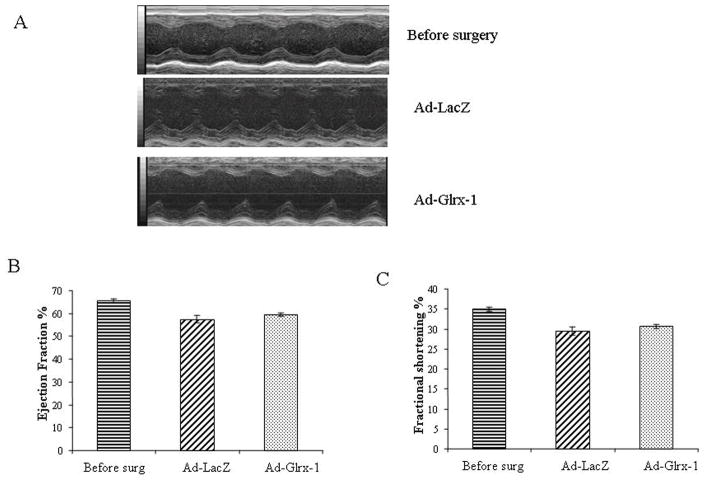
M-mode Echocardiography was performed both before and after gene delivery in randomly assigned groups. Animals from each group underwent successive echocardiography after proper induction of anesthesia before surgery and after 4 days of delivering Ad-EV, Ad-LacZ or Ad-Glrx-1. No significant difference was found between pre and post surgery groups with any of the vectors (Figure 2).
Figure 2.
Effect of Ad-LacZ, and Ad-Glrx-1 injection on cardic function. Representative M-mode images (A), Ejection fraction (B), Fractional shortening (C). Values are Mean ± SEM of 3 animals in each group.
Glrx-1 Gene Therapy Improved Ventricular Recovery in Isolated Diabetic Mouse Hearts
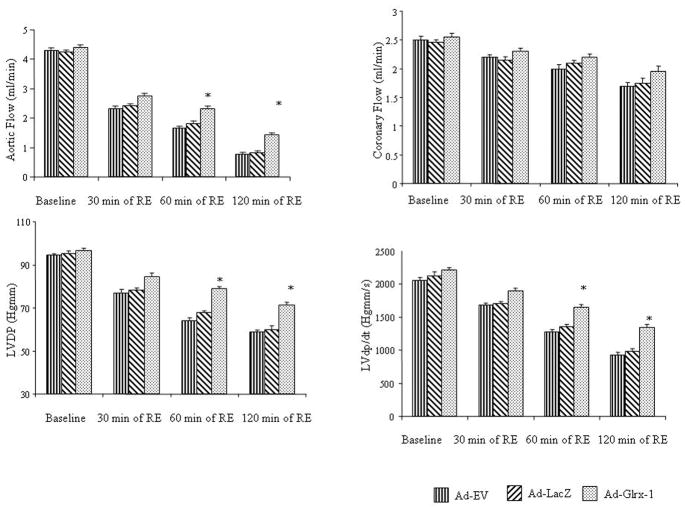
To assess whether Glrx-1 gene therapy helps the diabetic hearts against ischemia reperfusion injury, the isolated hearts obtained from the diabetic animals injected with Ad-Ev, Ad-LacZ or Ad-Glrx-1, were subjected to 30 min of global ischemia followed by 120 min of reperfusion. At baseline, there were no significant differences in left ventricular functional parameters between groups (Figure 3). After I/R, the Glrx-1 hearts showed improved left ventricular function, including improved aortic flow, LVDP and LVdp/dt compared the Ad-Ev and Ad-LacZ groups. Coronary flow did not show any significant differences.
Figure 3.
Cardiac function in ischemic/reperfused heart obtained from diabetic animals after Ad-Ev, or Ad-LacZ or Ad-Glrx-1 treatment, after isolation hearts were subjected to 30 min of global ischemia followed by 120 min of reperfusion. Values are Means ± SEM; n = 6 in each group. *p<0.05 vs Ad-LacZ.
Glrx-1 Gene Therapy Reduced the I/R-mediated Myocardial Infarct Size and Cardiomyocyte Apoptosis in Diabetic Heart
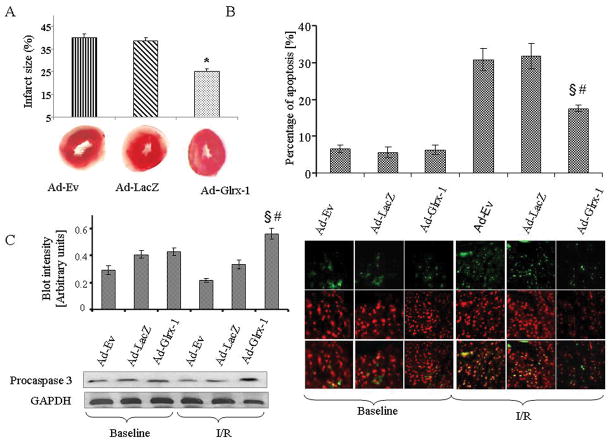
As shown in Figure 4A, after 30 min of global ischemia and 120 of reperfusion there were no significant differences in infarct size measured by TTC method between Ad-EV and Ad-LacZ group, but Ad-Glrx-1 treated group exhibited significantly less infarct. Figure 4B shows the cardiomyocyte apoptosis assessed by TUNEL staining. At baseline there were no significant differences in between the groups; however after I/R in Ad-Glrx-1 group, the numbers of the apoptotic cells were significantly lower in comparison with Ad-EV and Ad-LacZ groups. As shown in Figure 4C, procaspase 3 level was similar among the groups in baseline condition. After I/R, procaspase 3 level was significantly reduced in case of empty vector or LacZ treated diabetic hearts, whereas in Glrx-1 treated group, the procaspase 3 level remained higher.
Figure 4.
After 30 min of ischemia and 120 min of reperfusion, TTC in phosphate buffer was infused into the hearts. The hearts were then sliced, and infarct size was calculated as a ratio of the infracted area/risk area. The effects of the Ad-Ev, Ad-LacZ or Ad-Glrx-1 treatment on myocardial infarct size are shown in panel A. The effect of the different treatments on the myocardial apoptosis assessed by TUNEL assay as shown in panel B. Panel C shows the level of procaspase-3 in diabetic hearts after injecting with Ad-Ev or Ad-LacZ or Ad-Glrx-1 with or without subjecting them to 30 min of global ischemia and 120 min of reperfusion (C). Results are expressed as mean ± SEM of 3 animals in each group. §p<0.05 vs. Ad-Ev- I/R and #p< 0.05 vs Ad-LacZ-I/R.
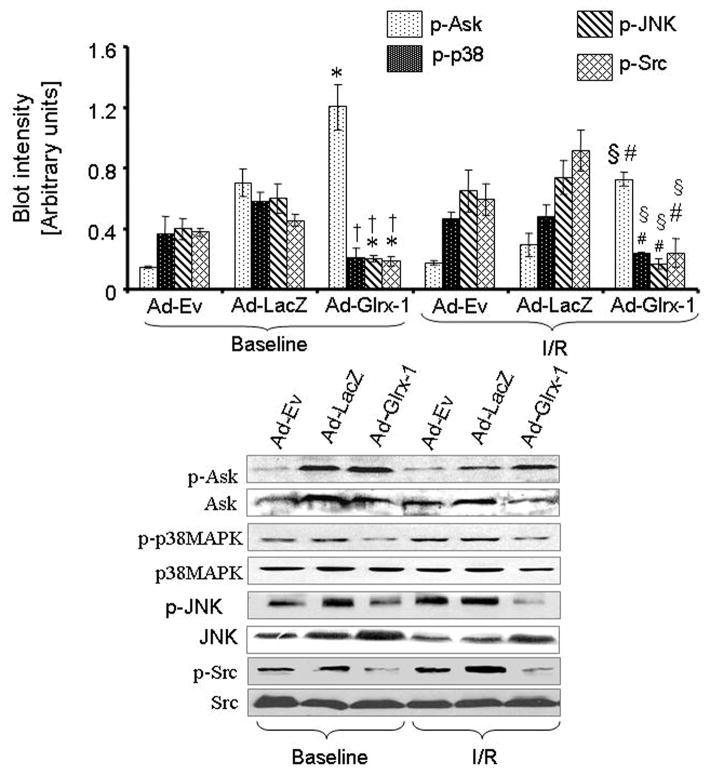
Glrx-1 Gene Therapy Inhibited I/R-mediated Apoptotic Signaling in Diabetic Myocardium
To determine the mechanisms by which Glrx-1 gene could inhibit the death signal generated in the diabetic heart after I/R, we monitored apoptotic signaling proteins such as Ask-1, Jnk, c-Src and p38 mitogen-activated protein kinase (MAPK). Figure 5 shows the results. Ask-1, p38 MAPK, Jnk and c-Src – all were phosphorylated even at the baseline level. Among the groups, the level of the p-Ask in the Ad-Glrx-1 group was the highest at the baseline level, which remained at the same level even after ischemia, indicateing the inhibition of the Ask. The level of p-Jnk before and after ischemia was decreased in Glrx-1 group compared to the other two groups. Simmilar to p-Jnk, the p-p38 MAPK was also lower in the Ad-Glrx-1 group at baseline and after ischemia/reperfusion in comparison with both Ad-Ev and Ad-LacZ groups. The level of p-c-Src also showed the same pattern as was observed in case of p-Jnk.
Figure 5.
Western blot analysis of Ask-1, p-Ask-1, p38 MAPK, p-p38 MAPK, Jnk, p-Jnk, c-Src and p-c-Src. Proteins were isolated from the hearts obtained from the diabetic animals injected with Ad-Ev, Ad-LacZ or Ad-Glrx-1 with or without subjecting them to ischemia/reperfusion. Figures are representative images of three different groups and each experiment was repeated at least three times. Results are expressed as mean ± SEM of 3 animals in each group and each experiment were done atleast thrice. *p<0.05 vs.Ad-Ev -BL and †p< 0.05 vs Ad-LacZ-BL §p<0.05 vs.Ad-Ev-I/R and #p< 0.05 vs Ad-LacZ-I/R.
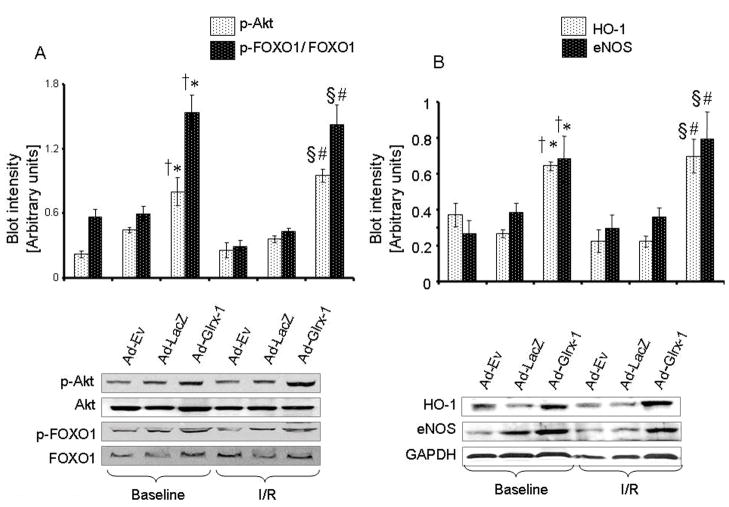
Glrx-1 Gene Therapy Induced the Activation of Survival Proteins in Diabetic Myocardium
As a measure of Glrx-1-induced survival proteins, we monitored the activation of Akt and FoxO-1 as well as endothelial nitric oxide synthase (e-NOS) and HO-1. As depicted in Figure 6, the level of p-Akt was higher in Ad-Glrx-1 group both at baseline and after ischemia reperfusion compared to the other two groups. Extensive phosphorylation of FoxO-1 occurred after Glrx-1 gene therapy as compared to Ad-Ev and Ad-LacZ groups both at baseline and after ischemia/reperfusion (Figure 6A). The activation of e-NOS and HO-1 is shown at the bottom of figure 6. Ad-Glrx-1 treatment enhanced the level of e-NOS and HO-1 both at baseline and after ischemia reperfusion compared to Ad-Ev or Ad-LacZ groups
Figure 6.
Western blot analysis of Akt, p-Akt, FoxO-1, p-FoxO-1 are sown in panel A, and Western blot analysis of HO-1 and e-NOS are sown in panel B. GAPDH was used as loading control. Proteins were isolated from the hearts obtained from the diabetic animals injected with Ad-Ev, Ad-LacZ or Ad-Glrx-1 with or without subjecting them to ischemia/reperfusion. Figures are representative images of three different groups, and each experiment was repeated at least three times. Results are expressed as mean ± SEM of experiments. *p<0.05 vs.Ad-Ev -BL and †p< 0.05 vs Ad-LacZ-BL §p<0.05 vs.Ad-Ev-I/R and #p< 0.05 vs Ad-LacZ-I/R.
Discussion
The salient features of the present study include that Glrx-1 gene therapy in diabetic hearts (i) improved ventricular recovery, and reduced myocardial infarct size and cardiomyocyte apoptosis indicating that Glrx-1 treatment reduces the ischemia reperfusion induced injury in diabetic heart, (ii) attenuated death signaling molecules including c-Src, p38 MAPK, Jnk, (iii) enhanced survival signaling molecules including activation of Akt, FoxO-1, HO-1 a cardioprotctive phase II enzyme, and e-NOS.
Glrxs belong to the thioredoxin superfamily of thiol/disulfide exchange catalysist, and therefore known as thiotransferases. Four different Glrxs have been identified in the mammalian cells including Glrx-1, Glrx-2, monothiol Glrx-3 (PICOT) and Glrx-52,11,12. Glrx1 is the most well characterized gene of the glutaredoxin family proteins. It is a 12 kDa protein that mainly resides in cytosol, but can be translocated to the nucleus after certain stimuli. Glrx-1 is ubiquitously present in the mammalian tissues including heart. A recent study also showed the existence of Glrx-1 in mitochondrial intermembrane space13. Glrx-1 appears to play a role in cardioprotection primarily functioning as an antioxidant. A recent study showed that the level of myocardial Glrx-1 was not affected by ischemia/reperfusion, but it was significantly increased by preconditioning of the heart to ischemic stress3. Moreover, the hearts of the Glrx-1Tg/+ transgenic animals exhibited more resistance against I/R injury. Blocking the effects of Glrx-1 with cadmium made the preconditioned hearts more susceptible to I/R injury, suggesting that the overexpression of Glrx-1 could reduce the ischemic injury3.
The involvement of ROS in concert with the deficit of antioxidant reserve14 is a well known etiology for the deterioration of the diabetic myocardium. One of the consequences of the ROS is oxidative modification of proteins that involve the reactivity of the free SH group of the cysteine residues15. The active site of Glrx-1 has a Cys-Pro-Tyr-Cys- motif, and it can catalyze the reduction of protein-mixed disulfides and can regulate the SH repair mechanisms thereby controlling the function of the proteins susceptible for oxidation2. Thus, it is not surprising that Glrx-1 can protect the heart presumably by protecting the SH group preserving cysteine residues.
Glrx-1 inhibits apoptosis by functioning as a negative regulator of Ask-1 through its ability to inhibit Ask-1 and its subsequent signal transduction pathway16,17 involving Jnk, and p38 MAPK, leading to apoptosis. Moreover, the phosphorylation of Ask-1 at Ser 83 by Akt suppress Ask-1 activity and Ask-1 mediated apoptosis18,19. Consistent with the previous reports, our results show that Glrx-1 therapy could also inhibit the Ask activation as well as the activation of Jnk and p38 MAPK and supressed the apoptosis.
Glrx-1 protects the cells against apoptosis through the regulation of Akt redox status20. The activated Akt on one hand phosphorylates Ask-1 at the Ser 83 site to inhibit its activation, and on the other hand it phosphorylates FoxO. Phosphorylation of FoxO by Akt decreases its DNA binding capacity and translocates it from the nucleus to the cytosol21, where it gets degraded after ubiquitinylation, leading to cell survival. Our results indicate that Glrx-1 gene therapy activates the Akt-FoxO1 pathway simultaneously supressesing the Ask-Jnk-p38 pathway.
In summary, adenovirus mediated Glrx-1 gene therapy in diabetic hearts enhanced the amount of Glrx-1. In concert, these hearts restored the reduced left ventricular function associated with diabetes and I/R. Glrx-1 gene therapy attenuated myocardial infarction, apoptosis and death signals medited through Ask-1/Jnk/p38 MAPK pathway associated with diabetes and ischemia/reperfusion, and enhanced the survival signals including the phosphorylation of Akt, FoxO-1 and induced e-NOS and cardioprotective phase II enzyme HO-1. Our results suggest that Glrx-1 gene therapy protects the heart via redox regulation of Akt and appears to form a signaling network involving Ask-1/Jnk/p38 MAPK and Akt-FoxO-1, which switch diabetes and/or ischemia-reperfusion induced death signal into a survival signal. The extrapolation of our findings obtained in isolated diabetic mouse hearts to an actual clinical situation should be viewed with caution due to its nature and experimental circumstances of streptozotocin induced diabetic animal model. However, further studies are necessary to investigate the long term effect of Glrx-1 gene therapy in ischemic and diabetic myocardium.
Materials and methods
Animals
All animals in this study received humane care in agreement with “The Principles of Laboratory Animal Care” established by the National Society for Medical Research and The Guide for the Care and Use of Laboratory Animals devised by the National Academy of Sciences and published by the National Institutes of Health (Publication Number NIH 85-23, revised 1996). Male C57B6J mice (The Jackson Laboratory, Bar Harbor, ME, USA) between 23–28 gm were fed ad libitum regular mice chow with free access to water until the start of the experiment.
Generation of Recombinant Adenoviruses Carrying a myc-tagged Human Glrx-1 cDNA
The entire cDNA fragment coding for the human Glrx-1 (IMAGE clone 346606, NCBI accession #W79548) was initially released from the plasmid vector pT7T3 by digestion with enzymes EcoRI and NotI and then ligated into the corresponding restriction sites in vector pcDNA3.1(+) (Invitrogen, Carlsbad, CA). The resultant plasmid was used as a template for polymerase chain reaction (PCR) using forward primer 5′-GTTTAAACTTAAGCTTG GTACCGAGCTCGG-3′ [corresponding to the multiple cloning site of pcDNA3.1(+)] and reverse primer 5′-CTAGTCGACCTGCAGAGCTCCAATCTGCTTTAGC-3′ (for insertion of a SalI restriction site after the last amino acid of the human Glrx-1 protein). The PCR amplified DNA fragment was digested with enzymes BamHI and SalI, followed by cloning into the corresponding restriction sites in vector pCMV-Tag 1 (Stratagene, La Jolla, CA). This allows fusion of a myc peptide to the C-terminus of the human Glrx-1 protein. The entire cDNA fragment encoding the myc-tagged human Glrx-1 was subsequently isolated from the vector by digestion with enzymes BamHI and KpnI, and then cloned into the corresponding sites in vector pUC19. The cDNA fragment in the pUC19 vector, which is flanked by two EcoRI restriction sites, was finally inserted into the EcoRI site in vector pENTR2B (Invitrogen, Carlsbad, CA), resulting in a plasmid named pENTR-TGlrx-1. An adenoviral vector with Ad5 backbone, in which the myc-tagged human Glrx-1 cDNA is driven by the human cytomegalovirus (CMV) immediate early promoter, was then generated by the LR Clonase-catalyzed site-specific recombination reaction between plasmids pENTR-TGlrx-1 and pAD/CMV/V5-DEST following the protocol recommended by the manufacturer (Invitrogen, Carlsbad, CA). The entire recombinant adenoviral sequence was finally released from the plasmid by digestion with enzyme PacI and then transfected into 293A cells for production of recombinant viruses. Recombinant adenoviruses were also generated from the same adenoviral vector without the inserted myc-tagged human Glrx-1 cDNA (pAd/CMV/V5-DEST) and with the insertion of the E. coli β-galactosidase gene (pAd/CMV/V5-GW/LacZ) for the use as controls.
Induction of Diabetes
After 4 hours of fasting period, streptozotocin dissolved in citrate buffer (pH 4.5), was injected intraperitoneally with a dose of 150 mg/kg bodyweight. After 8 days the diabetes was verified by markedly increased blood sugar level >400 mg/dL (457±15 in Ad-Ev, 468 ± 19 in Ad-LacZ, and 449 ± 17 in Ad-Glrx-1, respectively).
Surgical Procedure
Survival surgery in mice was performed as mentioned in our previous study22. To induce anesthesia, ketamine (100 mg/kg) and xylazine (2mg/kg) were injected intraperitoneally. Bupernorphine hydrochloride (0.1 mg/kg) was used as analgesic agent and gentamycin (1mg/kg) were used as antibiotic to prevent infections. After intubation, mice were artificially ventilated using a small animal ventilator (MINIVENT mouse ventilator model 845, Harvard Aparatus). Small thorachotomy were performed lateral to the midsternal line in the fourth intercostal place, and vectors either empty or LacZ or Glrx-1 encoded, were injected into three different places into the anterior wall of the left ventricle between the left anterior descending artery and the left arterial branch, using a 30 gauge needle, (2×1010-PFU/ml, final volume was 10μl). After the delivery of the vector, the chest was closed with 6-0 suture layer by layer, and mice were allowed to recover.
M-Mode Echocardiography
After confirming diabetes, the mice were anesthetized with isoflurene, shaved and placed on a heated pad. Transthoracic two-dimensionally guided M-mode echocardiography was performed using a Vevo 770 ultrasound system equipped with a 25-MHz transducer (Vevo 770, Visual-Sonics Inc., Toronto, ON, Canada) to visualize the left ventricle. The functional parameter measured: were as follows: percentage of left ventricular (LV) fractional shortening (FS), LV ejection fraction (EF), and heart rate (HR). The process was done four days after gene delivery23.
Isolated Heart Preparation and Cardiac Function Assessment
After 4 days of gene delivery, the mice were anesthetized with pentobarbital (80 mg/kg b/w i.p. injection) (Abbott Laboratories, North Chicago, IL) and heparin sodium (500 IU/kg b/w i.p. injection) (Elkin-Sinn Inc., Cherry Hill, NJ) was given as anticoagulant. After sufficient deep anesthesia, thorachotomy was performed; hearts were excised, and perfused with modified Krebs-Henseleit bicarbonate buffer (millimolar concentration: sodium chloride 118, potassium chloride 4.7, calcium chloride 1.7, sodium bicarbonate 25, potassium biphosphate 0.36, magnesium sulfate 1.2 and glucose 10, and after its oxygenization pH was 7.4 at 37 °C) according to Langendorff method without pacing. After 10 min of wash out period, the preparation was switched to the working mode for 10 min. At the end of the 10 min in working mode, baseline cardiac parameters (aortic flow (AF), coronary flow (CF), left ventricle develop pressure (LVDP) and first derivative of developed pressure LVdp/dt)) were recorded, and ischemia was initiated. After 30 min of global ischemia the hearts were reperfused with KHB buffer for 120 minutes, and cardiac functions were recorded after 30-, 60-, and 120 min of reperfusion. Aortic and coronary flows were measured by time collecting effluents dripping from the heart. Other parameters including LVDP and LVdp/dt were assessed from the continuously obtained pressure signal using a Gould 6600 series signal conditioner (Gould Instrument Systems Inc, Valley View, OH, USA) and monitored on a CORDAT II real-time data acquisition and analysis system (Triton Technologies, San Diego, CA, USA)24.
Infarct Size Estimation
At the end of the experiments, 1 % (w/v) solution of triphenyl tetrazolium chloride (TTC) in phosphate buffer was infused through the aortic cannula at 37°C, after the hearts were stored at −70°C for subsequent analysis. The hearts were sliced into 1.2 mm thickness of cross-sectional pieces, and were fixed in 2% Paraformaldehyde, placed between two cover slips and digitally imaged using a Microtek ScanMaker 600z. To quantitate the areas of infarct in pixels, a NIH image 5.1 (a public-domain software package) were used. The infarct size was quantified and expressed in pixels22.
Immunofluorescence Analysis
Deparaffinized heart tissue sections were incubated with Glrx-1 antibody (1:25 dilution) for 2 h at room temperature. Alexa Fluor 594 (2 μg/ml) was used as secondary antibody and the nuclear staining was done with Topro-3-iodide (100 μM). Images were obtained in a confocal fluorescence microscope using appropriate wavelengths22. Glrx-1 antibody-stained cells were quantified using a confocal fluorescence microscope under 400x magnification. Random five fields were selected from each tissue section, and at least five tissue sections were examined for each group. *p < 0.01 vs. vector treatment.
TUNEL Assay for Assessment of Apoptotic Cell Death
Immunohistochemical detection of apoptotic cells was carried out using the TUNEL method (Promega, Madison, WI). The TUNEL staining was performed according to the manufacturer’s instructions, as reported previously25.
Western Blot Analysis
Western blot was performed as described previously25. The following primary antibodies were obtained from Cell Signaling Technology (Boston, MA, USA): Ask1, p-Ask1 (Ser 83), FoxO-1, p-FoxO-1, caspase 3, Jnk, p-Jnk, p38MAPK, p-p38 MAPK, c-Src, and p-c-Src. The following primary antibodies: p-Akt, Akt, e-NOS, HO-1, and glyceraldehyde-6-phosphate dehydrogenase (GAPDH) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Glrx-1 antibody was obtained from Abcam (Cambridge, MA, USA).
Statistical Analysis
Data are expressed as the mean ± standard error of mean (SEM). Analysis of variance test followed by Bonferroni’s was carried out to test for any differences between mean values of all groups. If differencesbetween groups were established, values of each treated group were compared with those of the control group by a modified t-test. Western blot data are expressed as the mean ± standard error of mean (SEM). Kruskal-Wallis test was carried out to test for any differences between mean values of all groups. If differences between groups were established, values of each treated group were compared with those of the control group by a modified t-test.
Acknowledgments
This study was supported by National Heart, Lung, and Blood Institute Grants NIH HL-22559, HL-33889, HL-34360, OTKA 72315 and TAMOP-4.2.2-08/1-2008-0007.
References
- 1.Holmgren A. Thioredoxin and glutaredoxin system. J Biol Chem. 1989;264:13963–13966. [PubMed] [Google Scholar]
- 2.Berndt C, Lillig CH, Holmgren A. Thiol-based mechanisms of the thioredoxin and glutaredoxin systems: implications for diseases in the cardiovascular system. Am J Physiol Heart Circ Physiol. 2007;292:H1227–H1236. doi: 10.1152/ajpheart.01162.2006. [DOI] [PubMed] [Google Scholar]
- 3.Malik G, Nagy N, Ho Y-S, Maulik N, Das DK. Role of glutaredoxin-1 in cardioprotection: An insight with Glrx-1 transgenic and knockout animals. J Mol Cell Cardiol. 2008;44:261–269. doi: 10.1016/j.yjmcc.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Maulik N, Das DK. Emerging potential of thioredoxin and thioredoxin interacting proteins in various disease conditions. Biochim Biophys Acta. 2008;1780:1368–82. doi: 10.1016/j.bbagen.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Di Simplicio P, de Glorgio LA, Cardaioli E, Lecis R, Micell M, Rossi R, et al. Glutathione, glutathione utilizing enzymes and thioltransferase in platelets of insulin-dependent diabetic patients: relation with platelet aggregation and with microangiopatic complications. Eur J Clin Invest. 1995;25:665–669. doi: 10.1111/j.1365-2362.1995.tb01983.x. [DOI] [PubMed] [Google Scholar]
- 6.Takagi Y, Nakamura T, Nishiyama A, Nozaki K, Tanaka T, Hashimoto N, et al. Localization of Glutaredoxin (Thioltransferase) in the Rat Brain and Possible Functional Implications during Focal Ischemia. Biochem Biophys Res Commun. 1999;258:390–394. doi: 10.1006/bbrc.1999.0646. [DOI] [PubMed] [Google Scholar]
- 7.Okuda M, Inoue N, Azumi H, Seno T, Sumi Y, Hirata KI, et al. Expession of glutaredoxin in human coronary arteries. Its potential role in antioxidant protection against atherosclerosis. Arterioscler Thromb Vasc Bol. 2001;21:1483–1487. doi: 10.1161/hq0901.095550. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Xu Z, Li S, Rozanski GJ. Redox regulation of Ito remodeling in diabetic rat heart. Am J Physiol Heart Circ Physiol. 2005;288:H1417–H1424. doi: 10.1152/ajpheart.00559.2004. [DOI] [PubMed] [Google Scholar]
- 9.Shelton MD, Kern TS, Mieyal JJ. Glutaredoxin regulates nuclear factor kappa-B and intracellular adhesion molecule in muller cells, model of diabetic retinopathy. J Biol Chem. 2007;282:12467–12474. doi: 10.1074/jbc.M610863200. [DOI] [PubMed] [Google Scholar]
- 10.Ivarsson R, Quintens R, Dejonghe S, Tsukamoto K, Veld P, Renstrom E, et al. Rodox control of exocytosis. Regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes. 2005;54:2132–2142. doi: 10.2337/diabetes.54.7.2132. [DOI] [PubMed] [Google Scholar]
- 11.Lundstrom-Ljung J, Holmgren A. Glutaredoxin accelerates glutathione-dependent folding of reduced ribonuclease A together with protein disulfide-isomerase. J Biol Chem. 1995;270:7822–7828. doi: 10.1074/jbc.270.14.7822. [DOI] [PubMed] [Google Scholar]
- 12.Rozell B, Barcena JA, Martinez-Galisteo E, Padilla CA, Holmgren A. Immunochemical characterization and tissue distribution of glutaredoxin (thioltransferase) from calf. Eur J Cell Biol. 1993;62:314–323. [PubMed] [Google Scholar]
- 13.Pai HV, Starke DW, Lesnefsky EJ, Hoppel CL, Mieyal JJ. What is the functional significance of the unique location of glutaredoxin 1 (GRx1) in the intermembrane space of mitochondria? Antioxidants & Redox Signaling. 2007;9:2027–2034. doi: 10.1089/ars.2007.1642. [DOI] [PubMed] [Google Scholar]
- 14.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxico. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol. 1997;15:351–69. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- 16.Song JJ, Rhee JG, Suntharalingam M, Walsh SA, Spitz DR, Lee YJ. Role of glutaredoxin in metabolic oxidative stress. Glutaredoxin as a sensor of oxidative stress mediated by H2O2. J Biol Chem. 2002;277:46566–46577. doi: 10.1074/jbc.M206826200. [DOI] [PubMed] [Google Scholar]
- 17.Song JJ, Lee YJ. Differential role of glutatredoxin and thioredoxin in metabolic oxidative stress- induced activation of apoptosis signal-regulating kinase I. Biochem J. 2003;373:845–885. doi: 10.1042/BJ20030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim AH, Khursigara G, Sun X, Franke TF, Chao MV. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol. 2001;21:893–901. doi: 10.1128/MCB.21.3.893-901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satoh M, Matter CM, Ogita H, Takeshita K, Wang CY, Dorn GW, et al. Inhibition of Apoptosis- Regulated Signaling Kinase-1 and Prevention of Congestive Heart Failure by Estrogen. Circulation. 2007;115:3197–3204. doi: 10.1161/CIRCULATIONAHA.106.657981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murata H, Ihara Y, Nakamura H, Yodoi J, Sumikawa K, Kondo T. Glutaredoxin exerts an antiapoptotic effect by regulating the redox state of Akt. J Biol Chem. 2003;278:50226–33. doi: 10.1074/jbc.M310171200. [DOI] [PubMed] [Google Scholar]
- 21.Burgering BM, Kops GJ. Cell cycle and death control: long live Forkheads. Trends Biochem Sci. 2002;27:352–360. doi: 10.1016/s0968-0004(02)02113-8. [DOI] [PubMed] [Google Scholar]
- 22.Gurusamy N, Lekli I, Gorbunov N, Gherghiceanu M, Popescu LM, Das DK. Cardioprotection by adaptation to ischemia augments autophagy in association with BAG-1 protein. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00495.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juric D, Wojciechowski P, Das DK, Netticadan T. Prevention of concentric hypertrophy and diastolic impairment in aortic-banded rats treated with resveratrol. Am J Physiol Heart Circ Physiol. 2007;292:H2138–43. doi: 10.1152/ajpheart.00852.2006. [DOI] [PubMed] [Google Scholar]
- 24.Engelman DT, Watanabe M, Engelman RM, Rousou JA, Kisin E, Kagan VE, et al. Hypoxic preconditioning preserves antioxidant reserve in the working rat heart. Cardiovascular Res. 1995;29:133–140. [PubMed] [Google Scholar]
- 25.Mukherjee S, Lekli I, Das M, Azzi A, Das DK. Cardioprotection with alpha-tocopheryl phosphate: amelioration of myocardial ischemia reperfusion injury is linked with its ability to generate a survival signal through Akt activation. Biochim Biophys Acta. 2008;1782:498–503. doi: 10.1016/j.bbadis.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]