Abstract
Previous work indicated that BDNF, through the trkB receptor, increases DNA synthesis in oligodendrocyte progenitor cells (OPCs) and differentiation of post-mitotic oligodendrocytes (OLGs) of the basal forebrain (BF). In the present studies, BDNF knockout animals were used to investigate BDNF’s effects on OLG lineage cells (OLCs) in vivo. OLCs of the BF were found to express the trkB receptor, suggesting they are responsive to BDNF. Immunohistochemistry using NG2 and CC1 antibodies was utilized to examine numbers of NG2+ OPCs and CC1+ post-mitotic BF OLGs. In the embryo (E17), BDNF −/− animals display reduced NG2+ cells. This reduction was also observed in BDNF +/− mice at E17 and at postnatal day 1 (P1), P14 and adult, suggesting that BDNF plays a role in OPC development. BDNF +/− mice do not exhibit deficits in numbers of CC1+ OLGs. However, myelin basic protein (MBP), myelin associated glycoprotein (MAG), and proteolipid protein (PLP) are reduced in BDNF +/− mice, suggesting that BDNF plays a role in differentiation. These data indicate that progenitor cells and myelin proteins may be affected in vivo by a decrease in BDNF.
Keywords: Glia, Neurotrophins, Growth Factors, Glial Development, MBP, NG2
Introduction
Several growth factors and cytokines influence oligodendrocyte (OLG) development. For example, fibroblast growth factor-2 (FGF-2) and platelet-derived growth factor (PDGF) impact proliferation of oligodendrocyte lineage cells (OLCs) and inhibit their differentiation (Calver et al. 1998; McKinnon et al. 1991; Raff et al. 1988). Other mitogens for OLCs include insulin growth factor-I (IGF-I) and interleukin-2 (IL-2), which also promote OLG maturation (Benveniste and Merrill 1986; McMorris et al. 1993). Of particular interest to us, members of the neurotrophin family of growth factors, nerve growth factor (NGF), neurotrophin-3 (NT-3), and neurotrophin-4/5 (NT-4/5), have also been implicated in OLG development. NGF promotes OLG survival (Cohen et al. 1996), while NT-4/5 promotes OLG proliferation (Scarisbrick et al. 2000) and NT-3 promotes survival, proliferation, and differentiation of OLGs (Barres et al. 1994; Heinrich et al. 1999).
In this study we investigate the role of brain derived neurotrophic factor (BDNF) on OLGs in the basal forebrain (BF). The BF region is sensitive to BDNF, NGF and NT-3 (Friedman et al. 1993). NGF promotes the differentiation and NT-3 enhances the survival of BF cholinergic neurons in culture, while BDNF promotes both their survival and differentiation in culture (Alderson et al. 1990), as well as in vivo (Grosse et al. 2005; Ward and Hagg 2000). Our analysis focuses on the effects of BDNF on BF OLGs and considers the possibility that the same growth factor may impact both neurons and their associated myelinating cells. Previous work analyzed BDNF’s actions on BF OLCs in vitro. It was shown that BDNF increases DNA synthesis in OLG progenitors and increases their proliferation in the presence of FGF-2 (Van’t Veer et al. 2009). In addition, BDNF treatment of post-mitotic OLGs increases the expression of several myelin proteins including myelin basic protein (MBP), myelin-associated glycoprotein (MAG), and proteolipid protein (PLP) without affecting cell number, indicating that BDNF may enhance the differentiation of BF OLGs in culture (Du et al. 2006a). These effects on OLGs are mediated by the trkB, tyrosine kinase receptor. The present work extends previous observations and analyzes BDNF’s effects on OLGs in vivo utilizing BDNF knockout animals.
Materials and Methods
Experimental Animals
Breeding pairs of BDNFTm1Jae mice on a 129/BalbC/C57 background or embryonic day 17 (E17) BDNF +/+ and −/− brains were generously provided by Barbara Hempstead (Weill Cornell Medical School, NY). Food and water were available ad libita. The animals were managed by the UMDNJ/Robert Wood Johnson Animal Facility, accredited by AAALAC. Animal maintenance, husbandry, transportation, and housing are in compliance with the Laboratory Animal Welfare Act (PL 89-544; PL-91-579). Moreover, animal use is in compliance with NIH guidelines (NIH Manual Chapter 4206). Breeding pairs were maintained by crossing wildtype and heterozygous animals. Mouse genotypes were determined by PCR from tail-derived DNA as described by (Ernfors et al. 1994).
Immunohistochemistry
E17 brains were fixed in 3% paraformaldehyde (PFA) in PBS (18 hours) followed by 30% sucrose/PBS. Postnatal day 1 (P1) brains were fixed in 2% PFA containing 0.1M lysine and 0.01M NaIO2 or 4% PFA (24 hours) followed by 30% sucrose/PBS. Postnatal day 14 (P14) or adult male and female mice were perfused transcardially with 2% PFA containing 0.1M lysine and 0.01M NaIO2 or 4% PFA and post-fixed (24 hours) in the same fixative followed by 30% sucrose/PBS. Brains were embedded in OCT (Tissue Tek, Sakura, CA) and frozen at −80°C for immunohistochemistry.
E17, P1, P14, and adult (6–8 months) mouse brains were sectioned coronally (14 um serial sections) using a Leica cryostat. The BF assessed includes the medial septal nucleus and surrounding tissue medial to the lateral ventricles, caudal to the optic chiasm, and rostral to the hypothalamus. For NG2 staining, sections fixed with 2% PFA containing 0.1M lysine and 0.01M NaIO2 were blocked with 30% goat serum/0.3% Triton/PBS and incubated with polyclonal anti-NG2 (1:750, 72 hours; Chemicon). Sections were incubated with biotinylated anti-rabbit antibody (1:200; Vector) and cells were visualized using the avidin-biotin complex (ABC) technique (Vectastain Elite Kit; Vector) and the 3,3′-diaminobenzidine tablet set (DAB; Sigma). For CC1 staining, sections fixed with 4% PFA were microwaved in 0.01M citrate buffer, blocked with 10% goat serum/PBS, and incubated with monoclonal anti-CC1 (1:200, 72 hours; Calbiochem). For MBP staining, sections fixed with 4% PFA were blocked with 10% goat serum/PBS, and incubated with monoclonal anti-MBP (Serotec, 72 hours; 1:50). Sections were incubated with biotinylated anti-mouse antibody (1:200; Vector) and cells were visualized using the ABC technique and DAB. Sections from BDNF +/+ and BDNF −/− or +/− mice from specific ages were stained identically, side by side.
Double immunostaining for anti-CC1/trkB was accomplished using 4% PFA fixed sections from the adult mouse. Sections were microwaved in 0.01M citrate buffer, blocked in 33% goat serum/1% bovine serum albumin (BSA)/0.1% Triton/PBS, and incubated with anti-CC1 (72 hours). Sections were re-blocked with 33% goat serum/1% BSA/0.1% Triton/PBS and incubated with polyclonal anti-trkB (1:250, 72 hours; Santa Cruz). The trkB antibody recognizes an epitope corresponding to amino acids 794 to 808 of mouse trkB and is reportedly specific for the full-length form of the receptor (Klein et al. 1989). Sections were incubated with Alexa Fluor 594 anti-mouse antibody (1:750; Molecular Probes). After washing with PBS, sections were incubated with biotinylated anti-rabbit antibody followed by Alexa Fluor 488 streptavidin (1:800; Molecular Probes).
Double immunostaining for anti-PDGFRa/trkB was accomplished using 4% PFA fixed floating sections (25 um) from P14 mouse. Sections were microwaved in 0.01M citrate buffer, blocked with 33% goat serum/1% BSA/0.1% Triton/PBS and incubated with polyclonal anti-trkB (72 hours). Sections were re-blocked with 30% rabbit serum/0.1% Triton/PBS and incubated with polyclonal anti-PDGFRa (1:100, 72 hours; R&D) and then incubated with Alexa Fluor 594 anti-rabbit antibody (1:750; Molecular Probes). After washing with PBS, sections were incubated with biotinylated anti-goat antibody followed by Alexa Fluor 488 streptavidin (1:800; Molecular Probes).
Quantification
Images of 14 um serial sections separated by 56 um of the BF of E17, P1, P14, and adult BDNF +/+ and +/− or −/− littermates were captured and analyzed at 400X using a Leica microscope. A total of 9 sections at E17, 11 sections at P1, 14 sections at P14, and 20 sections at adult were counted for each animal. All positive cells were counted and the diameters of the cell bodies were measured using an Olympus digital camera and ImagePro software. Double counting was corrected for based on cell body diameter and section thickness allowing for an estimation of cell number (Abercrombie 1946). Basal forebrain volumes of +/+ and +/− or −/− mice were quantitated. No difference was found between these groups.
Western Blot
For MBP, MAG, PLP, and neurofilament light (NF-L) Western blots, the BFs of P14 and adult BDNF +/+ and +/− littermates were dissected and frozen at −80°C. Tissue was lysed with buffer containing 50 mM Tris-Hcl, 150 mM NaCl, 10 mM EDTA, 2 mM EGTA, 1% CHAPS, 0.5% NP-40, 1% Triton, 10ug/ml leupeptin, 10 ug/ml aprotinin, 20 ug/ml soybean trypsin inhibitor, 50 mM NaF, 1 mM PMSF, 0.5 uM microcystin, and 1 mM ortho-vanadate. Protein concentration was quantified using a BCA protein assay kit (Pierce). Both BDNF +/+ and +/− samples contained 10 ug of protein plus loading buffer containing 20% glycerol, 12% 2-mercaptoethanol, 8.7% SDS, 0.1% bromophenol blue, and 0.35 M Tris-HCl. Protein was run on 12% Bis-Tris gels for MBP, PLP, and NF-L or 4–12% Tris-Glycine gels for MAG.
Protein was transferred to a PVDF membrane (Millipore). Membranes were blocked with 4% BSA/TBS-T followed by overnight incubation with primary antibody, monoclonal anti-MBP (1:200; Serotec), polyclonal anti-MAG (1:1000; Chemicon), polyclonal anti-PLP (1:500; Chemicon), or polyclonal anti-NF-L (1:1000; Chemicon). Membranes were then incubated in horseradish peroxidase-linked anti-rabbit antibody (1:3000; Amersham) for MAG and NF-L or anti-mouse antibody (1:3000) for MBP. Membranes were incubated in biotinylated anti-goat (1:500) followed by horseradish peroxidase-linked streptavidin (1:3000) for PLP. Bands were visualized with a chemiluminescence system (Amersham). Membranes were stripped and reprobed with anti-GAPDH (1:1000; Biodesign) as a loading control. Western blots were analyzed using Quantity One V 4.2.1 software (Bio-Rad).
Specificity of the trkB Antibody
To test the molecular weight of trkB recognized by the antibody, Western blot was used. Rat BF OLG cultures were scraped from 35 mm dishes and harvested in lysis buffer. BF OLG samples contained 20 ug of protein plus loading buffer. Protein was run on a 4–12% Tris-glycine gel and transferred as above. Membranes were blocked with 4% BSA/TBS-T followed by overnight incubation with anti-trkB (1:400;Santa Cruz). Membranes were then incubated in horseradish peroxidase-linked anti-rabbit antibody (1:3000) and visualized as above.
Results
Expression of trkB in BF OLG lineage cells (OLCs)
Previous studies in culture indicated that BDNF enhances DNA synthesis in BF OPCs and differentiation of post-mitotic BF OLGs. These effects are mediated through the trkB, tyrosine kinase receptor (Du et al. 2006a; Van’t Veer et al. 2009). To begin to determine whether BDNF may similarly have direct actions on BF OLCs in vivo, we assessed their expression of full-length, biologically relevant trkB. We used an antibody reported to recognize only the full-length receptor (Klein et al. 1989) and confirmed by Western blot that it did so (Figure 1A). Only the 145 kDa molecular weight form of trkB was recognized.
Figure 1.
OLCs of the BF express full-length trkB. (A) Western blot of cultured BF OLGs shows the molecular weight of the antibody at 145 kD. The antibody is specific for the full-length form of the receptor, as it does not recognize the truncated form (90 kD). (B) trkB is present on OPCs in the BF. Immunohistochemical analysis indicates that PDGFRa+ cells (green) colocalize with trkB (red) in the P14 BF. Arrows show two co-localized cells. (C) trkB is present on mature OLGs in the mouse BF. Immunohistochemistry shows that CC1+ cells (red) colocalize with trkB (green) in the adult BF of +/+ BDNF mice. The arrow shows a co-localized cell and the arrowhead indicates a cell positive for only one antigen. Scale bar represents 50um.
We used this antibody to evaluate both immature OLGs labeled with PDGF receptor alpha (PDGFRa) and mature, post-mitotic OLGs labeled with CC1. Co-labeling of OLCs with anti-trkB indicated that they express the receptor in vivo (Figure 1B and C), suggesting that BF OLCs are responsive to BDNF.
Oligodendrocyte precursor cells (OPCs) are reduced in BDNF −/− and +/− BF
In order to examine the role of BDNF on OLCs in vivo, we evaluated BDNF −/− and +/− mice. We were limited in our ability to analyze postnatal BDNF −/− mice because the BDNF mutation results in a lethal phenotype. However, a number of studies now reveal that BDNF +/− mice exhibit a 40% decrease in BDNF in the brain (Chourbaji et al. 2004), deficits in hippocampal function (Bartoletti et al. 2002), and behavioral deficits (Lyons et al. 1999), making these +/− mice a useful model with which to study effects of reduced BDNF levels. In our hands, BDNF +/− mice exhibit a decrease in BDNF in the BF of approximately 40% (Figure 2), suggesting that these mice are useful in examining BDNF effects on OLCs in this region.
Figure 2.
BDNF +/− mice exhibit reduced BDNF levels in the BF. (A) Western blot shows BDNF protein in the BF of adult BDNF +/+ and +/− mice. (B) Graph is a densitometric analysis of Western blot in (A). Anti-GAPDH was used to normalize the protein. N=5. * Significantly different from control at p < .01. Data were analyzed by Matched-pair t-test.
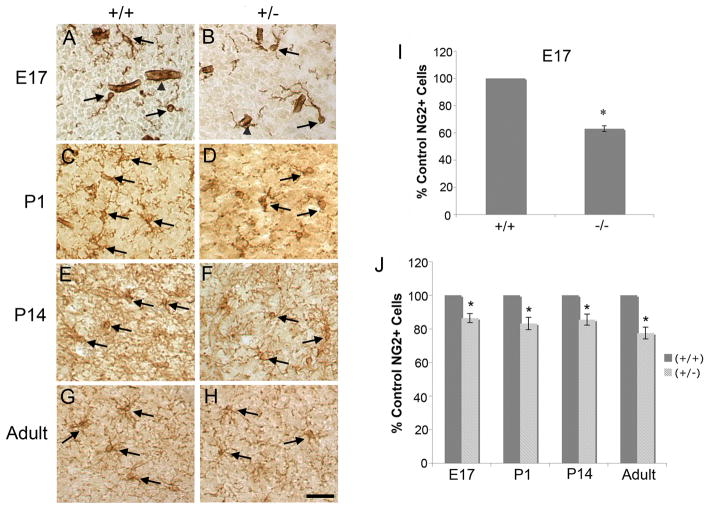
In order to analyze the proliferating population of OLGs in the BF, we used NG2 as a marker of progenitor populations in the developing and adult brain (Nishiyama et al. 1996). NG2+ cells were visualized using an anti-NG2 antibody (Figure 3A–H) and counted in the BF of BDNF +/+ and +/− or −/− littermates throughout development. Numbers of NG2+ cells in E17, P1, P14 and adult BDNF −/− or +/− mice were quantitated and compared with wildtype littermates. At E17, BDNF −/− mice exhibit a 38% reduction in NG2+ cells compared to BDNF +/+ mice (Figure 3I) suggesting that BDNF may influence OPCs early in development. Decreases in NG2+ cells were similarly observed in the BF of E17, P1, P14, and adult BDNF +/− mice (Figure 3J). These results indicate that reduced levels of BDNF may result in a significant decrease in the pool of OPCs in the BF at E17 and this reduction is maintained through development and into adulthood. BDNF, then, impacts the development of OPCs in the BF. These results are consistent with culture studies that found that BDNF enhances DNA synthesis in BF OPCs (Van’t Veer et al. 2009).
Figure 3.
NG2+ cells are reduced in BDNF −/− and +/− BF. Representative images of BDNF +/+ and +/− mice are shown for E17 (A, B), P1 (C, D), P14 (E, F), and adult (G, H) BF. Scale bar represents 50um. Arrows represent NG2+ cells. Arrowheads represent blood vessels stained in E17 samples. The NG2 antibody stained blood vessels in tissues not perfused, but these were easily distinguishable from NG2+ progenitor cells. E17 BDNF −/− mice (I), as well as E17, P1, P14, and adult BDNF +/− mice (J) exhibit a reduction in numbers of NG2+ cells in the BF. Serial coronal sections of BF were captured every 56 um and every cell was counted. E17 - N=3; P1 - N=6; P14 - N=3; adult - N=3. *Significantly different from control at p < 0.05. Data in (I) were analyzed by Matched-pair t-test and data in (J) were analyzed by ANOVA followed by Fisher’s protected least significant difference posthoc test.
The differentiation of mature OLGs in the BF is reduced in BDNF +/− mice
In culture, BDNF does not influence numbers of mature OLGs, but does increase expression of MAG, MBP and PLP (Du et al. 2006a), suggesting that it influences differentiation. To define the effects of BDNF on mature OLGs in the BF in vivo, numbers of CC1+ post-mitotic cells were counted in P1, P14, and adult mice. Numbers of CC1+ cells were similar between BDNF +/+ and +/− BFs (Figure 4).
Figure 4.
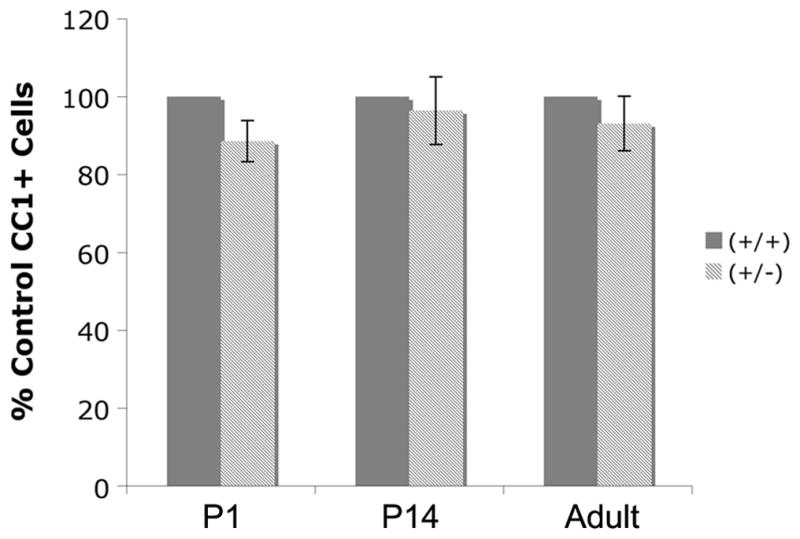
P1, P14, and adult BDNF +/+ and +/− mice exhibit similar numbers of CC1+ cells in the BF. Serial coronal sections of BF were captured every 56 um and every cell was counted. P1 - N=3; P14 - N=3; adult - N=4. Data were analyzed by ANOVA followed by Fisher’s protected least significant difference posthoc test.
To determine whether BDNF influences differentiation, we examined levels of myelin proteins by Western blot. In P14 and adult BDNF +/− BF, MBP levels were reduced by 35% and 24% respectively (Figure 5A). MAG was reduced by 46% at P14 and 19% in the adult (Figure 5B). PLP was reduced by 46% at P14 and 23% in the adult (Figure 5C). The finding that several myelin proteins are reduced without a coincident loss of OLG numbers suggests that, as was true in culture, BDNF may influence the expression of differentiated traits per cell. It is interesting to note that the effect of the BDNF +/− genotype on MAG and PLP protein was more severe at P14 than in the adult. It is possible that BDNF may have more of an impact during the initial establishment of myelin in the BF and less of an effect on the maintenance of the myelinated state. This is similar to the situation in the peripheral nervous system where neuregulin/erbB signaling is critical for the establishment of myelin but plays no role in the maintenance of already established myelinated peripheral nerves (Atanasoski et al. 2006).
Figure 5.
Myelin protein levels are reduced in BDNF +/− BF. Western blot was used to examine the expression of MBP (A), MAG (B), and PLP (C) protein levels in P14 and adult BF. Anti-GAPDH was used to normalize to total protein. The graphs represent densitometric analysis of the blots. MBP: N=5 at P14, N=6 at adult. MAG: N=4 at P14 and N=7 at adult. PLP: N=4 at P14 and N=5 at adult. *Significantly different from control +/+ at p < 0.05. **Significantly different from adult +/− at p< 0.05. Data were analyzed using ANOVA followed by Fisher’s protected least significant difference posthoc test.
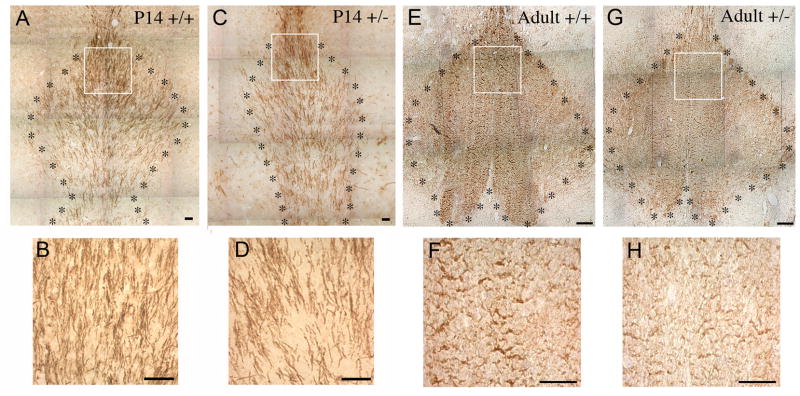
To support the Western blot data, subsequent work assessed MBP immunocytochemically. Coronal sections of P14 and adult BDNF +/+ and +/− brains were stained with anti-MBP and the myelinated tracts of the fimbria-fornix in the BF were analyzed. In both the developing P14 as well as the adult, BDNF +/− mice exhibited reduced MBP immunoreactivity (Figure 6). Thus, as in culture, BDNF appears to play a critical role in OLC differentiation.
Figure 6.
MBP immunoreactivity is reduced in BDNF +/− BF. Coronal sections of the BF of P14 BDNF +/+ (A) and +/− (C) mice and adult BDNF +/+ (E) and +/− (G) mice were stained with anti-MBP. The myelinated tracts in the BF were compared. Panels (B, D, F, H) are magnified images taken from the boxes in panels (A, C, E, G), respectively. Scale bar represents 50 um. * outlines the area of the fimbria-fornix.
BDNF has been well described for its effects on neuronal survival and differentiation (Huang and Reichardt 2001). In particular in the BF, BDNF enhances the survival and differentiation of cholinergic neurons (Alderson et al. 1990; Friedman et al. 1993). In vivo, BDNF −/− mice exhibit a deficit in the number and size of cholinergic neurons of the BF early in development (Grosse et al. 2005; Ward and Hagg 2000). However, in postnatal and adult BDNF +/− mice, numbers of cholinergic neurons are similar to wildtype littermates (Ward and Hagg 2000), suggesting that a partial loss of BDNF is not enough to affect cholinergic neurons in vivo. Nevertheless, it is possible that the deficits in the BDNF +/− mice in myelin protein expression are due to deficits in general axon density that then impact OLC differentiation. To test this possibility, we examined levels of neurofilament light (NF-L) protein in P14 and adult BF by Western blot. No differences were seen in NF-L protein levels between BDNF +/+ and +/− BF (Figure 7). This, together with the work of others, suggests that myelin protein deficits are more sensitive to losses of BDNF than are either the BF cholinergic population or axons in general. The deficits on OLCs appear to occur in a manner distinct from effects on axons and possibly are mediated by trkB on the OLCs themselves, a possibility that will be explored in greater detail in the future.
Figure 7.

NF-L protein levels are similar in BDNF +/+ and +/− BF. Western blots were used to examine the expression of NF-L protein in P14 and adult BF (A). Anti-GAPDH was used to normalize to total protein. (B) The graph represents densitometric analysis of blots. N=3 at P14 and N=3 at adult stages. Data were analyzed using ANOVA followed by Fisher’s protected least significant difference posthoc test.
Discussion
Previous studies indicate that BDNF, through trkB and the MAP kinase pathway enhances DNA synthesis and differentiation of BF OLCs in culture (Du et al. 2006a; Du et al. 2006b; Van’t Veer et al. 2009). The present work suggests that BDNF also plays a role in the development of BF OLCs in vivo. BDNF +/− mice exhibit deficits in numbers of NG2+ cells in the BF early in development and into adulthood, indicating that BDNF may impact this progenitor population. In contrast, numbers of CC1+ OLGs in the BF are similar between BDNF +/+ and +/− mice. However, BDNF +/− mice exhibit deficits in the myelin proteins, MBP, MAG, and PLP, suggesting that BDNF affects the differentiation of mature OLGs.
The role of BDNF on OLGs in vivo
Our data are supported by several studies indicating a role for BDNF in OLG development in vivo. BDNF −/− mice exhibit decreased numbers of myelinated axons in the optic nerve (Cellerino et al. 1997). In addition, PLP and MBP mRNA are decreased in the hippocampus and cortex of BDNF −/− mice at postnatal day 20 (P20) and BDNF -/− mice exhibit a decrease in MBP in the hippocampus and raphe (Djalali et al. 2005), indicating that BDNF may play a role in the development of OLGs in these brain regions as well. Moreover, the injection of BDNF into the lateral ventricles at P10 and P12 can increase PLP mRNA in the hippocampus at P14 (Cellerino et al. 1997). These studies are consistent with our contention that BDNF plays an active role in the development of OLGs in the brain. However, this study is the first to define effects of reduced BDNF on OLCs in the BF.
The reduction of NG2+ cells does not correlate with a loss of post-mitotic OLGs
In this study, BDNF +/− mice exhibit a reduction in NG2+ cells that does not correlate with a loss of mature cells in the BF. This was an unexpected finding, but may provide insights into the development of the OLG lineage. A number of possibilities may explain this result. First, it has been recently appreciated that NG2+ cells are a mixed population, some of which develop into OLGs, while others may play other roles. NG2 cells have been shown to produce trophic factors to support surrounding cells or to influence synaptic transmission (Baracskay et al. 2007; Nishiyama 2007). BDNF may impact a sub-population of OLG progenitors that do not become mature OLGs, but instead serve another function. This possibility will be explored in future studies.
Alternatively, it is established that OLGs are initially over-produced during the development of the CNS. In the optic nerve, over 50% of OLG progenitors undergo programmed cell death (Barres et al. 1992). In addition, 20% of OLG progenitors undergo cell death in the brain during early myelination (Trapp et al. 1997), suggesting that more OLGs are produced than are needed. A reduction in BDNF may reduce the initial production of OLG progenitors resulting in no subsequent effect on normal cell death that occurs with maturity.
The BDNF +/− mouse under study
This study utilized a BDNF knockout mouse to examine the effects of BDNF on OLGs in vivo. BDNF −/− mice were generated with a deletion of exon 5 of the BDNF gene, which contains the coding sequence (Ernfors et al. 1994). BDNF −/− mice have a lethal phenotype, so studies evaluating later postnatal time points focused on the BDNF +/− mice. We and others (Chourbaji et al. 2004) have found that BDNF +/− mice exhibit a loss of about 40% of endogenous BDNF. Therefore, BDNF +/− mice have become a useful model to study the effects of reduced levels of BDNF. Others have found that although these mice do not exhibit neuronal loss in the hippocampus (Ernfors et al. 1994; Jones et al. 1994), they do exhibit a deficit in choline acetyltransferase activity in the hippocampus (Chourbaji et al. 2004). In addition, BDNF +/− mice exhibit deficits in hippocampal synaptic function (Bartoletti et al. 2002; Chourbaji et al. 2004). These studies and our own indicate that although BDNF +/− mice survive as well as their wildtype littermates, they exhibit subtle deficits that may provide insight into BDNF’s role on brain development and function.
Caveats with respect to the BDNF +/− mouse model
The strengths of the BDNF +/− model must be considered together with the following caveats. First, it is not clear when BDNF signaling is critical for OLG function. Is a lack of BDNF signaling early in development responsible for effects seen in adulthood, or is constant BDNF signaling required throughout development? These questions must be addressed in the future with conditional knockout mice that delete BDNF at specific developmental time points.
It is also unclear whether BDNF acts on OLGs directly to impact their development. BDNF signals through multiple cells in the CNS, such as neurons, microglia, and endothelial cells (Elkabes et al. 1996; Kermani and Hempstead 2007; Segal and Greenberg 1996) and its actions may be mediated through these cell types to indirectly affect OLGs. Importantly, we now report that axons appear not to be affected in the BDNF +/− mice, while OLCs are. This observation complements the work of others that found these mice do not exhibit effects on numbers of cholinergic BF neurons (Ward and Hagg 2000). Together the data suggest that effects on the OLCs may be independent from neuronal signals, at least, and possibly be direct on OLCs.
Our culture work indicates that BDNF enhances the DNA synthesis of OPCs and the differentiation of post-mitotic OLGs by acting directly on the cells through the trkB receptor. The fact that OLCs in vivo express trkB suggests they are directly responsive to BDNF. Moreover, others have found that mice with a conditional deletion of trkB from the embryonic forebrain exhibit a decrease in MBP and numbers of myelinated axons in the corpus callosum and hippocampus (Medina et al. 2004), suggesting that trkB signaling may be involved in OLG development and myelination in vivo. However, because this deletion was from all cells in the forebrain and not specifically OLGs, it is still unclear whether trkB signaling in OLGs is responsible for the effects in vivo. To address this issue, future work will analyze conditional knockout mice that delete trkB signaling specifically from OLGs.
OLGs are heterogeneous
This work focused on OLGs of the BF. It is important to note that OLCs are heterogeneous and that effects of neurotrophins on OLGs may vary with the region being studied. It is established that OLGs in different brain regions and even within some brain regions exhibit differences in morphology (Bjartmar et al. 1994; Del Rio-Hortega 1928). OLGs from the white matter tracts of the optic nerve, corpus callosum, cerebellum, or spinal cord exhibit differences in somal shape and size as well as in the number of internodes and thickness of myelinated fibers (Weruaga-Prieto et al. 1996). OLGs also display biochemical differences. Concentrations of myelin proteins differ depending on the thickness of the myelin sheath. Thick myelinated fibers express higher levels of MBP, while thin myelinated fibers express higher levels of PLP (Hartman et al. 1982). In addition, it has been shown in the spinal cord that OLGs differ in their transcription factor profile (Kitada and Rowitch 2006), implying differences in their function.
Moreover, OLG populations differ in their response to growth factors (Mason and Goldman 2002; Power et al. 2002). This difference in response is true for neurotrophins as well. For example, BF OLGs express trkB and respond to BDNF in culture, while cortical OLGs do not (Du et al. 2003). In addition, optic nerve OLGs express trkC and increase proliferation in response to NT-3 in culture, while spinal cord OLGs do not (Barres et al. 1994; Robinson and Miller 1996). The heterogeneity of response to neurotrophins indicates the importance of analyzing OLGs as distinct populations within the brain.
The relevance of this work to diseases of the BF
OLGs of the BF impact normal communication between the BF and hippocampus. This tract is impacted in such degenerative diseases as Alzheimer’s disease, Down’s syndrome, and Multiple Sclerosis. Alzheimer’s disease (AD) as well as Down’s Syndrome (DS) are associated with a loss of BF cholinergic neurons (Auld et al. 2002; Casanova et al. 1985; Mann et al. 1984) as well as a dysfunction of associated OLGs and a loss of myelin (Bartzokis 2004; Soderberg et al. 1992; Vlkolinsky et al. 2001). In addition, approximately half of patients with Multiple Sclerosis (MS), a disease primarily involving OLG degeneration, are estimated to have some degree of cognitive impairment (Peyser et al. 1990). The most common cognitive deficit involves difficulties in learning and memory (Bobholz and Rao 2003; Rao et al. 1991). In one case of MS, reduced short term memory and learning impairments were correlated with plaques seen in the columns of the fornix (Fontaine et al. 1994).
Our data suggest that BDNF impacts the differentiation of OLGs, that may ameliorate demyelination associated with AD, DS and MS. Interestingly, recent clinical studies evaluating drugs that alleviate symptoms of AD or MS, such as ladostigil (Weinreb et al. 2007), donepezil (Leyhe et al. 2008), and glatiramer acetate (GA) (Azoulay et al. 2005; Tsai 2007), found that BDNF levels were increased. A randomized trial of GA for the treatment of MS found that although cognitive performance was not improved over placebo-controls after 10 years, most MS patients had stable cognitive function, some of which may be due to the therapeutic effect of GA (Schwid et al. 2007). These data suggest there may be clinical relevance to the study of BDNF’s role on OLGs of the BF.
Acknowledgments
We thank Lauren D. Lercher for excellent technical assistance.
This project is supported by NIH NS036647 and the NMSS.
References
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Alderson RF, Alterman AL, Barde YA, Lindsay RM. Brain-derived neurotrophic factor increases survival and differentiated functions of rat septal cholinergic neurons in culture. Neuron. 1990;5(3):297–306. doi: 10.1016/0896-6273(90)90166-d. [DOI] [PubMed] [Google Scholar]
- Atanasoski S, Scherer SS, Sirkowski E, Leone D, Garratt AN, Birchmeier C, Suter U. ErbB2 signaling in Schwann cells is mostly dispensable for maintenance of myelinated peripheral nerves and proliferation of adult Schwann cells after injury. J Neurosci. 2006;26(7):2124–31. doi: 10.1523/JNEUROSCI.4594-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auld DS, Kornecook TJ, Bastianetto S, Quirion R. Alzheimer’s disease and the basal forebrain cholinergic system: relations to beta-amyloid peptides, cognition, and treatment strategies. Prog Neurobiol. 2002;68(3):209–45. doi: 10.1016/s0301-0082(02)00079-5. [DOI] [PubMed] [Google Scholar]
- Azoulay D, Vachapova V, Shihman B, Miler A, Karni A. Lower brain-derived neurotrophic factor in serum of relapsing remitting MS: reversal by glatiramer acetate. J Neuroimmunol. 2005;167(1–2):215–8. doi: 10.1016/j.jneuroim.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Baracskay KL, Kidd GJ, Miller RH, Trapp BD. NG2-positive cells generate A2B5-positive oligodendrocyte precursor cells. Glia. 2007;55(10):1001–10. doi: 10.1002/glia.20519. [DOI] [PubMed] [Google Scholar]
- Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70(1):31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC, Gaese F, Bartke I, Dechant G, Barde YA. A crucial role for neurotrophin-3 in oligodendrocyte development. Nature. 1994;367:371–375. doi: 10.1038/367371a0. [DOI] [PubMed] [Google Scholar]
- Bartoletti A, Cancedda L, Reid SW, Tessarollo L, Porciatti V, Pizzorusso T, Maffei L. Heterozygous knock-out mice for brain-derived neurotrophic factor show a pathway-specific impairment of long-term potentiation but normal critical period for monocular deprivation. J Neurosci. 2002;22:10072–10077. doi: 10.1523/JNEUROSCI.22-23-10072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiol Aging. 2004;25(1):5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. author reply 49–62. [DOI] [PubMed] [Google Scholar]
- Benveniste EN, Merrill JE. Stimulation of oligodendroglial proliferation and maturation by interleukin-2. Nature. 1986;321(6070):610–3. doi: 10.1038/321610a0. [DOI] [PubMed] [Google Scholar]
- Bjartmar C, Hildebrand C, Loinder K. Morphological heterogeneity of rat oligodendrocytes: electron microscopic studies on serial sections. Glia. 1994;11(3):235–44. doi: 10.1002/glia.440110304. [DOI] [PubMed] [Google Scholar]
- Bobholz JA, Rao SM. Cognitive dysfunction in multiple sclerosis: a review of recent developments. Curr Opin Neurol. 2003;16(3):283–8. doi: 10.1097/01.wco.0000073928.19076.84. [DOI] [PubMed] [Google Scholar]
- Calver AR, Hall AC, Yu WP, Walsh FS, Heath JK, Betsholtz C, Richardson WD. Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron. 1998;20(5):869–82. doi: 10.1016/s0896-6273(00)80469-9. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Walker LC, Whitehouse PJ, Price DL. Abnormalities of the nucleus basalis in Down’s syndrome. Ann Neurol. 1985;18(3):310–3. doi: 10.1002/ana.410180306. [DOI] [PubMed] [Google Scholar]
- Cellerino A, Carroll P, Thoenen H, Barde YA. Reduced size of retinal ganglion cell axons and hypomyelination in mice lacking brain-derived neurotrophic factor. Mol Cell Neurosci. 1997;9(5–6):397–408. doi: 10.1006/mcne.1997.0641. [DOI] [PubMed] [Google Scholar]
- Chourbaji S, Hellweg R, Brandis D, Zorner B, Zacher C, Lang UE, Henn FA, Hortnagl H, Gass P. Mice with reduced brain-derived neurotrophic factor expression show decreased choline acetyltransferase activity, but regular brain monoamine levels and unaltered emotional behavior. Brain Res Mol Brain Res. 2004;121:28–36. doi: 10.1016/j.molbrainres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Cohen RI, Marmur R, Norton WT, Mehler MF, Kessler JA. Nerve growth factor and neurotrophin-3 differentially regulate the proliferation and survival of developing rat brain oligodendrocytes. J Neurosci. 1996;16(20):6433–6442. doi: 10.1523/JNEUROSCI.16-20-06433.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio-Hortega P. Tercera aportacion al conocimiento morfologico e interpretacion funcional de la oligodendroglia. Mem Real Soc Esp Hist Nat. 1928;14:40–122. [Google Scholar]
- Djalali S, Holtje M, Grosse G, Rothe T, Stroh T, Grosse J, Deng DR, Hellweg R, Grantyn R, Hortnagl H, et al. Effects of brain-derived neurotrophic factor (BDNF) on glial cells and serotonergic neurones during development. J Neurochem. 2005;92(3):616–27. doi: 10.1111/j.1471-4159.2004.02911.x. [DOI] [PubMed] [Google Scholar]
- Du Y, Fischer TZ, Clinton-Luke P, Lercher LD, Dreyfus CF. Distinct effects of p75 in mediating actions of neurotrophins on basal forebrain oligodendrocytes. Mol Cell Neurosci. 2006a;31(2):366–75. doi: 10.1016/j.mcn.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Du Y, Fischer TZ, Lee LN, Lercher LD, Dreyfus CF. Regionally specific effects of BDNF on oligodendrocytes. Dev Neurosci. 2003;25(2–4):116–26. doi: 10.1159/000072261. [DOI] [PubMed] [Google Scholar]
- Du Y, Lercher LD, Zhou R, Dreyfus CF. Mitogen-activated protein kinase pathway mediates effects of brain-derived neurotrophic factor on differentiation of basal forebrain oligodendrocytes. J Neurosci Res. 2006b;84(8):1692–702. doi: 10.1002/jnr.21080. [DOI] [PubMed] [Google Scholar]
- Elkabes S, DiCicco-Bloom EM, Black IB. Brain microglia/macrophages express neurotrophins that selectively regulate microglial proliferation and function. J Neurosci. 1996;16(8):2508–21. doi: 10.1523/JNEUROSCI.16-08-02508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368(6467):147–50. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- Fontaine B, Seilhean D, Tourbah A, Daumas-Duport C, Duyckaerts C, Benoit N, Devaux B, Hauw JJ, Rancurel G, Lyon-Caen O. Dementia in two histologically confirmed cases of multiple sclerosis: one case with isolated dementia and one case associated with psychiatric symptoms. J Neurol Neurosurg Psychiatry. 1994;57(3):353–9. doi: 10.1136/jnnp.57.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman WJ, Ibanez CF, Hallbook F, Persson H, Cain LD, Dreyfus CF, Black IB. Differential actions of neurotrophins in the locus coeruleus and basal forebrain. Exp Neurol. 1993;119(1):72–8. doi: 10.1006/exnr.1993.1007. [DOI] [PubMed] [Google Scholar]
- Grosse G, Djalali S, Deng DR, Holtje M, Hinz B, Schwartzkopff K, Cygon M, Rothe T, Stroh T, Hellweg R, et al. Area-specific effects of brain-derived neurotrophic factor (BDNF) genetic ablation on various neuronal subtypes of the mouse brain. Brain Res Dev Brain Res. 2005;156(2):111–26. doi: 10.1016/j.devbrainres.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Hartman BK, Agrawal HC, Agrawal D, Kalmbach S. Development and maturation of central nervous system myelin: comparison of immunohistochemical localization of proteolipid protein and basic protein in myelin and oligodendrocytes. Proc Natl Acad Sci U S A. 1982;79(13):4217–20. doi: 10.1073/pnas.79.13.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich M, Gorath M, Richter-Landsberg C. Neurotrophin-3 (NT-3) modulates early differentiation of oligodendrocytes in rat brain cortical cultures. Glia. 1999;28(3):244–55. [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermani P, Hempstead B. Brain-derived neurotrophic factor: a newly described mediator of angiogenesis. Trends Cardiovasc Med. 2007;17(4):140–3. doi: 10.1016/j.tcm.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada M, Rowitch DH. Transcription factor co-expression patterns indicate heterogeneity of oligodendroglial subpopulations in adult spinal cord. Glia. 2006;54(1):35–46. doi: 10.1002/glia.20354. [DOI] [PubMed] [Google Scholar]
- Klein R, Parada LF, Coulier F, Barbacid M. trkB, a novel tyrosine protein kinase receptor expressed during mouse neural development. Embo J. 1989;8(12):3701–9. doi: 10.1002/j.1460-2075.1989.tb08545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyhe T, Stransky E, Eschweiler GW, Buchkremer G, Laske C. Increase of BDNF serum concentration during donepezil treatment of patients with early Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci. 2008;258(2):124–8. doi: 10.1007/s00406-007-0764-9. [DOI] [PubMed] [Google Scholar]
- Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96(26):15239–44. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DM, Yates PO, Marcyniuk B. Alzheimer’s presenile dementia, senile dementia of Alzheimer type and Down’s syndrome in middle age form an age related continuum of pathological changes. Neuropathol Appl Neurobiol. 1984;10(3):185–207. doi: 10.1111/j.1365-2990.1984.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Mason JL, Goldman JE. A2B5+ and O4+ Cycling progenitors in the adult forebrain white matter respond differentially to PDGF-AA, FGF-2, and IGF-1. Mol Cell Neurosci. 2002;20(1):30–42. doi: 10.1006/mcne.2002.1114. [DOI] [PubMed] [Google Scholar]
- McKinnon RD, Matsui T, Aranda M, Dubois-Dalcq M. A role for fibroblast growth factor in oligodendrocyte development. Ann N Y Acad Sci. 1991;638:378–86. doi: 10.1111/j.1749-6632.1991.tb49048.x. [DOI] [PubMed] [Google Scholar]
- McMorris FA, Mozell RL, Carson MJ, Shinar Y, Meyer RD, Marchetti N. Regulation of oligodendrocyte development and central nervous system myelination by insulin-like growth factors. Ann N Y Acad Sci. 1993;692:321–34. doi: 10.1111/j.1749-6632.1993.tb26247.x. [DOI] [PubMed] [Google Scholar]
- Medina DL, Sciarretta C, Calella AM, Von Bohlen Und Halbach O, Unsicker K, Minichiello L. TrkB regulates neocortex formation through the Shc/PLCgamma-mediated control of neuronal migration. Embo J. 2004;23(19):3803–14. doi: 10.1038/sj.emboj.7600399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A. Polydendrocytes: NG2 cells with many roles in development and repair of the CNS. Neuroscientist. 2007;13(1):62–76. doi: 10.1177/1073858406295586. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996;43(3):299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Peyser JM, Rao SM, LaRocca NG, Kaplan E. Guidelines for neuropsychological research in multiple sclerosis. Arch Neurol. 1990;47(1):94–7. doi: 10.1001/archneur.1990.00530010120030. [DOI] [PubMed] [Google Scholar]
- Power J, Mayer-Proschel M, Smith J, Noble M. Oligodendrocyte precursor cells from different brain regions express divergent properties consistent with the differing time courses of myelination in these regions. Dev Biol. 2002;245(2):362–75. doi: 10.1006/dbio.2002.0610. [DOI] [PubMed] [Google Scholar]
- Raff MC, Lillien LE, Richardson WD, Burne JF, Noble MD. Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature. 1988;333(6173):562–5. doi: 10.1038/333562a0. [DOI] [PubMed] [Google Scholar]
- Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology. 1991;41(5):685–91. doi: 10.1212/wnl.41.5.685. [DOI] [PubMed] [Google Scholar]
- Robinson S, Miller R. Environmental enhancement of growth factor-mediated oligodendrocyte precursor proliferation. Molecular and Cellular Neuroscience. 1996;8:38–52. doi: 10.1006/mcne.1996.0042. [DOI] [PubMed] [Google Scholar]
- Scarisbrick IA, Asakura K, Rodriguez M. Neurotrophin-4/5 promotes proliferation of oligodendrocyte-type-2 astrocytes (O-2A) Brain Res Dev Brain Res. 2000;123(1):87–90. doi: 10.1016/s0165-3806(00)00077-8. [DOI] [PubMed] [Google Scholar]
- Schwid SR, Goodman AD, Weinstein A, McDermott MP, Johnson KP. Cognitive function in relapsing multiple sclerosis: minimal changes in a 10-year clinical trial. J Neurol Sci. 2007;255(1–2):57–63. doi: 10.1016/j.jns.2007.01.070. [DOI] [PubMed] [Google Scholar]
- Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci. 1996;19:463–89. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- Soderberg M, Edlund C, Alafuzoff I, Kristensson K, Dallner G. Lipid composition in different regions of the brain in Alzheimer’s disease/senile dementia of Alzheimer’s type. J Neurochem. 1992;59(5):1646–53. doi: 10.1111/j.1471-4159.1992.tb10994.x. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Nishiyama A, Cheng D, Macklin W. Differentiation and death of premyelinating oligodendrocytes in developing rodent brain. J Cell Biol. 1997;137(2):459–68. doi: 10.1083/jcb.137.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SJ. Glatiramer acetate could be a potential therapeutic agent for Parkinson’s disease through its neuroprotective and anti-inflammatory effects. Med Hypotheses. 2007;69(6):1219–21. doi: 10.1016/j.mehy.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Van’t Veer A, Du Y, Fischer TZ, Boetig DR, Wood MR, Dreyfus CF. Brain-derived neurotrophic factor effects on oligodendrocyte progenitors of the basal forebrain are mediated through trkB and the MAP kinase pathway. J Neurosci Res. 2009;87(1):69–78. doi: 10.1002/jnr.21841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlkolinsky R, Cairns N, Fountoulakis M, Lubec G. Decreased brain levels of 2′,3′-cyclic nucleotide-3′-phosphodiesterase in Down syndrome and Alzheimer’s disease. Neurobiol Aging. 2001;22(4):547–53. doi: 10.1016/s0197-4580(01)00218-4. [DOI] [PubMed] [Google Scholar]
- Ward NL, Hagg T. BDNF is needed for postnatal maturation of basal forebrain and neostriatum cholinergic neurons in vivo. Exp Neurol. 2000;162(2):297–310. doi: 10.1006/exnr.1999.7346. [DOI] [PubMed] [Google Scholar]
- Weinreb O, Amit T, Bar-Am O, Youdim MB. Induction of neurotrophic factors GDNF and BDNF associated with the mechanism of neurorescue action of rasagiline and ladostigil: new insights and implications for therapy. Ann N Y Acad Sci. 2007;1122:155–68. doi: 10.1196/annals.1403.011. [DOI] [PubMed] [Google Scholar]
- Weruaga-Prieto E, Eggli P, Celio MR. Topographic variations in rat brain oligodendrocyte morphology elucidated by injection of Lucifer Yellow in fixed tissue slices. J Neurocytol. 1996;25(1):19–31. doi: 10.1007/BF02284783. [DOI] [PubMed] [Google Scholar]