Abstract
People with central vision loss must use peripheral vision for visual tasks. It is well known that performance for almost all spatial tasks is worse in the normal periphery than in the normal fovea. The primary goal of my ongoing research is to understand the limiting factors and the potential for enhancing vision for people with central vision loss. Here I review previous work related to understanding the limiting factors on reading, a task that is the primary complaint of many patients with age-related macular degeneration, the leading cause of visual impairment in the elderly. I also review my work related to enhancing visual functions in the normal periphery and how it may be applied to people with central vision loss.
Keywords: low vision, central vision loss, age-related macular degeneration, reading, visual performance
Vision is an important sensory modality for human interaction with the world. To more than three million Americans who suffer from impaired vision that cannot be corrected optically,1,2 vision loss presents a major challenge for daily living. A leading cause of visual impairment in the United States is age-related macular degeneration (AMD), which often culminates in irreversible central vision loss. Recent estimates indicate that at least 1.75 million Americans have AMD, with the number projected to reach three million by the year 2020.3 To date, there is no promising cure for AMD. Recent breakthroughs in using anti vascular endothelial growth factor therapeutic agents to treat wet AMD have shown great promises in stabilizing vision in many patients, and in some cases, even improving their acuities. Yet the majority of patients with wet AMD, and many more with dry AMD, continue to suffer from impaired vision.
People with central vision loss must use peripheral vision for visual tasks. It is well known that performance for almost all spatial tasks is worse in the normal periphery than in the normal fovea. For instance, the finest details that can be resolved are approximately four times larger at 10° eccentricity than at the fovea.4 Another example is reading. Even when print size is not a limiting factor, reading speed is still a factor of six slower at 20° eccentricity than at the fovea.5
Reading is the most frequent clinical complaint as well as the primary goal for AMD patients seeking visual rehabilitation.6–9 Therefore, the understanding of why reading is slower in peripheral vision is very important for visual rehabilitation of patients with AMD. In this paper, I briefly summarize my efforts in the search of the limiting factors on reading speed, and methods to enhance reading speed in the peripheral vision. My general approach was to first test a hypothesis in the normal periphery (the “control”). This allowed me to systematically evaluate the effects of various text parameters such as print size and contrast on reading speed. From the control data, I then chose the most promising parameters to evaluate their effects on reading speed in people with central vision loss.
SENSORY LIMITATIONS ON PERIPHERAL READING SPEED
Previous research has examined and refuted various hypotheses used to explain why reading is slower in peripheral vision. In terms of motor aspects, eye-movement control has been shown to be poorer in peripheral vision.10,11 However, when reading speed was measured using the rapid serial visual presentation (RSVP) paradigm in which text is presented one word at a time for a given duration at the same location on a display,5,12–17 the improvement in reading speed over conventional page reading was smaller in peripheral than central vision,14,15 and also smaller in patients with central vision loss than those with intact central field.14 These findings are indications that oculomotor control cannot fully account for slow peripheral reading. In terms of sensory aspects, the difference in foveal and peripheral reading speeds is not a consequence of the differences in properties between cone- and rod-photoreceptors that are predominantly found in central and peripheral vision respectively.18 I briefly describe my work examining some other sensory factors that might limit peripheral reading speed.
Print Size
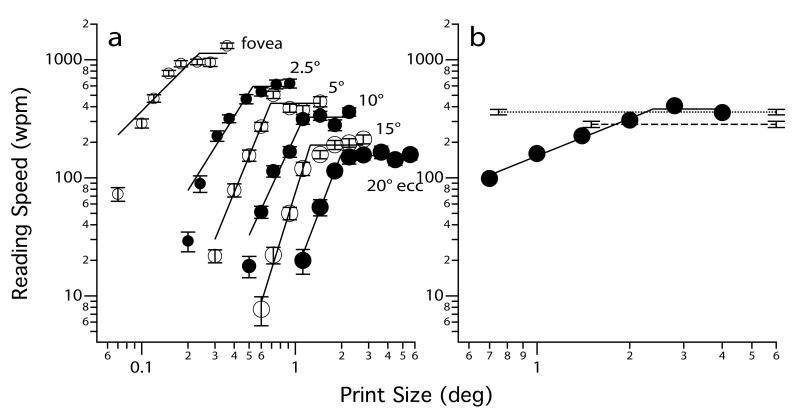
In the clinic, magnification helps low vision patients. Is print size the key limiting factor on reading speed in the normal periphery and in patients with central vision loss? In other words, can we equate peripheral reading speed with foveal reading speed if the print size is large enough? To address this question, we determined the dependence of reading speed on print size at six retinal locations from the fovea to 20° eccentricity in the inferior visual field in a group of young observers with normal vision.5 We presented text using RSVP, so that the words were more localized on the retina and were closer to the intended eccentricity. For peripheral testing, a fixation line was provided for observers. Figure 1a summarizes the main results of this study. At any given eccentricity, reading speed improves with print size up to the critical print size, beyond which reading speed remains at the maximum reading speed. Although the critical print size progressively increases with eccentricity, implying that larger print is required to attain the maximum reading speed, the maximum reading speed is always higher at the fovea and falls off with eccentricity. Averaged across the observers, the maximum reading speed drops from 807 words per minute (wpm) at the fovea to 135 wpm at 20° eccentricity.5
Figure 1.
Reading speed (wpm) is plotted as a function of print size (deg) for (a) an observer with normal vision, tested at the fovea and five other eccentricities (2.5 – 20°) in the inferior visual field; and (b) an observer with AMD whose preferred retinal locus was 7° directly below the anatomical fovea (in the visual field domain). For each set of reading speed vs. print size data, a two-line fit (on log-log axes) was used to fit the data where the intersection of the two lines represents the critical print size, the smallest print size that can be read at the maximum reading speed. In (b), we included the maximum reading speeds, averaged across six young adults with normal vision, at 5 (upper dotted line) and 10° (lower dashed line) eccentricity for comparison. The left starting point of each of these two lines represents the averaged critical print size at the respective eccentricity in the normal periphery. Error bars represent ± 1 S.E.M and are smaller than the size of the symbols when not shown. Panel (a) was modified from Chung et al.5
Figure 1b shows a similar function of RSVP reading speed vs. print size from an observer with AMD. His preferred retinal locus, the presumed retinal region adopted by the observer to function as the oculomotor reference or reference for other tasks, was 7° directly below the anatomical fovea in the visual field domain as determined using the Rodenstock scanning laser ophthalmoscope. Therefore, for comparison, the reading speed vs. print size functions obtained at 5 and 10° eccentricity in the normal periphery are included in the plot for comparison. For this AMD observer, his maximum reading speed (382.0 ± 17.4 wpm) was close to the averaged maximum reading speed obtained at 5° eccentricity (360.9 ± 19.7 wpm) and higher than that at 10° eccentricity (284.2 ± 17.6 wpm). The similar reading speeds for people with AMD and at an eccentricity-matched location in the normal periphery are found for some, but not all observers with AMD whom we had tested in our lab. For some observers with AMD, their reading speeds are lower than at eccentricity-matched locations in the normal periphery. The important point here, is that even with magnification (larger print sizes), reading speed in people with central vision loss is still lower than that at the normal fovea. This result does not imply that patients with central vision loss do not benefit from magnification. Magnification always helps patients read small print (smaller than the critical print size) better but it cannot restore the maximum reading speed to the same level as before the loss of central vision.
Crowding
It is common clinical wisdom that when patients have difficulty reading letters on a full letter chart, isolating the chart into single lines of letters often improves the measured acuity. Further improvement in acuity can be attained when letters on a line are isolated individually so that patients only see letters one at a time. The difficulty in reading letters in the presence of nearby letters is the well-known crowding effect.19–21 Previous studies showed that the magnitude and the spatial extent of crowding are greater in the periphery than at the fovea.22,23 As such, crowding has been a prime suspect for causing slow peripheral reading. In the following, I review a few studies that examined whether or not peripheral reading speed would improve if crowding between letters and words were reduced.
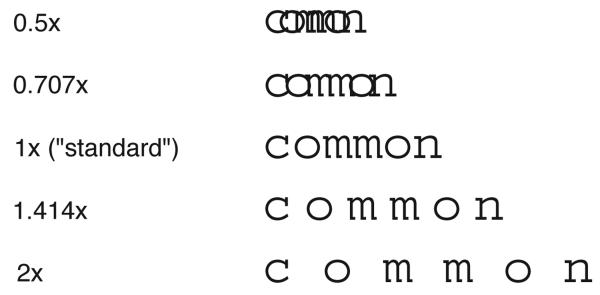
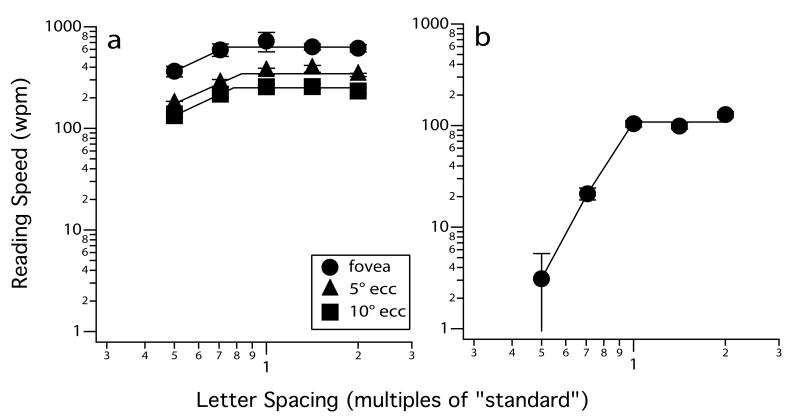
The first study evaluated whether larger letter spacings, which presumably reduce letter crowding, improve peripheral reading speed.24 Five letter spacings ranging from 0.5 to 2× the standard letter spacing were tested (Figure 2). If crowding limits peripheral reading speed, then we expect that peripheral reading speed would benefit from larger letter spacings beyond the standard. Figure 3a presents the averaged data for a group of six observers with normal vision. Reading speeds for the five letter spacings were determined using the RSVP paradigm at the fovea, 5 and 10° eccentricity in the inferior visual field. Contrary to our expectation, peripheral reading speeds, just like the foveal reading speed, are invariant with letter spacings beyond the standard. Figure 3b shows the data from one AMD observer, who showed a similar lack of benefit for reading speed with larger letter spacing (five other AMD observers showed similar results).25 This result implies that the simple trick of printing letters at larger letter spacing is not a solution to slow reading for patients with central vision loss.
Figure 2.
The word “common” rendered at the five letter spacings used in Chung.24 The illustration was modified from Chung.24
Figure 3.
Reading speed (wpm) is plotted as a function of letter spacing (see Figure 2), expressed as multiples of the standard letter spacing used for Courier font (the font used in the study). Panel (a) shows the averaged reading speed for six young adults with normal vision tested at the fovea and 5 and 10° in the inferior visual field. Panel (b) shows the reading speed for an observer with AMD. In all cases, the “critical spacing” was close to the standard letter spacing. Error bars represent ± 1 S.E.M. Panel (a) was modified from Chung.24
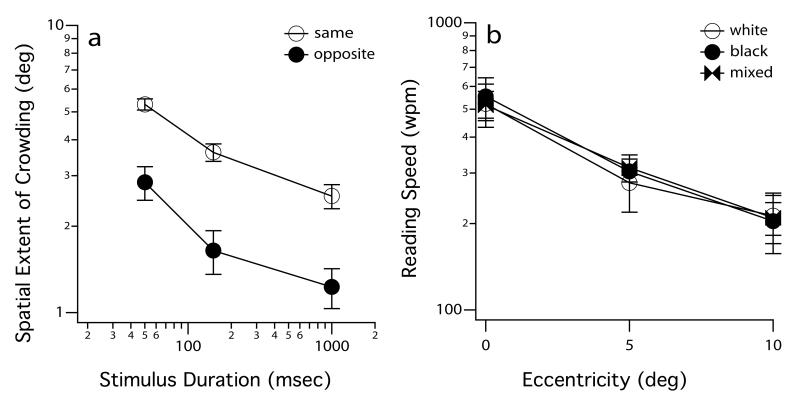
A reason for the lack of a benefit of increased letter spacing on reading speed is that the visual span, the number of characters that can be recognized during a fixation (for more information on the concept of visual span, see the next section), is reduced for text with larger-than-standard spacing.26 Therefore, we attempted a different method of reducing crowding among letters to see if reading speed could be improved while keeping the letter spacing (and thus the size of the visual span) the same. Considering that the spatial extent of crowding in peripheral vision is reduced when a target letter and its flanking letters have opposite contrast polarity,27,28 we examined whether a mixture of white and black letters against a mid-gray background (mixed contrast polarity text) could yield faster peripheral reading speed. Figure 4 summarizes the key findings from this experiment. We replicated the result that the crowding extent is reduced when the contrast polarity of the target differs from that of its flankers in peripheral vision (Figure 4a); however, mixed-polarity text does not have any advantage over same-polarity text and does not lead to improved peripheral reading speed (Figure 4b).29 Similarly, mixed-polarity text does not improve reading speed over same-polarity text in a group of observers with AMD.25
Figure 4.
Panel (a) shows how the spatial extent of crowding (deg) varies as a function of stimulus duration (msec). The spatial extent of crowding was defined as the separation between a target letter T and one of its four flanking Ts that allowed observers to identify the orientation of the target T at an accuracy of 62.5%. The contrast polarity of the target could be the same as, or opposite to that of the flankers. Panel (b) plots reading speed (wpm) as a function of eccentricity (deg), for text rendered as all white letters, all black letters, or interleaved white and black letters. Data shown in both panels represent the averaged values for the same three observers and are modified from Chung & Mansfield.29 Error bars represent ± 1 S.E.M.
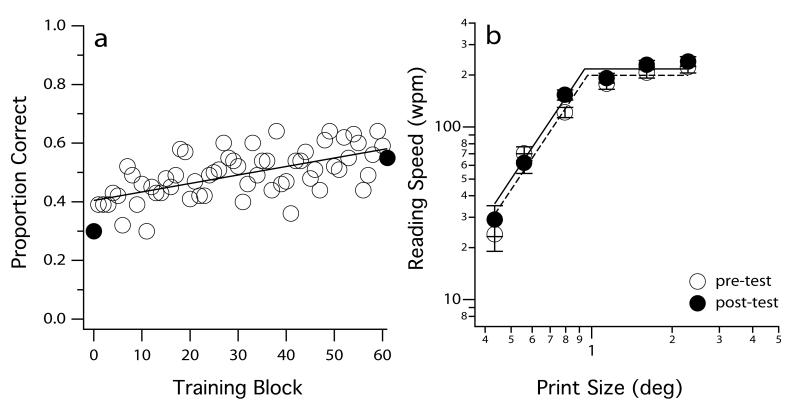
In addition to reducing letter crowding by manipulating text attributes (letter spacing, contrast polarity), we also attempted to reduce crowding in peripheral vision through perceptual learning, and to determine if this leads to improved reading speed. Perceptual learning refers to “any relatively permanent and consistent change in the perception of a stimulus array, following practice or experience with this array”.30 Practically, it refers to repeated practice on a task. Other experiments utilizing perceptual learning as a tool to improving visual function in the normal periphery will be discussed later. For the purpose of reducing crowding, we trained a group of observers with normal vision to identify crowded letters for six consecutive days (a total of 6000 trials).31 Following training, the performance accuracy for identifying crowded letters improved by 88% for the trained condition, and was associated with a 38% reduction in the spatial extent of crowding. However, reading speed (an untrained task) only improved by a mere 7.2%. In other words, even though crowding is reduced through perceptual learning, it does not benefit peripheral reading speed (Figure 5). Once again, these results are inconsistent with the common belief that crowding is a primary limiting factor on peripheral reading speed.
Figure 5.
Proportion-correct of identifying crowded letters is plotted as a function of training block for an observer in Chung31 in panel (a). Each symbol represents the averaged performance for a block of 100 trials. His reading speeds (wpm) for different print sizes (deg), determined before (pre-test) and after (post-test) training, are plotted in panel (b). The presentation of these data is modified from Chung.31
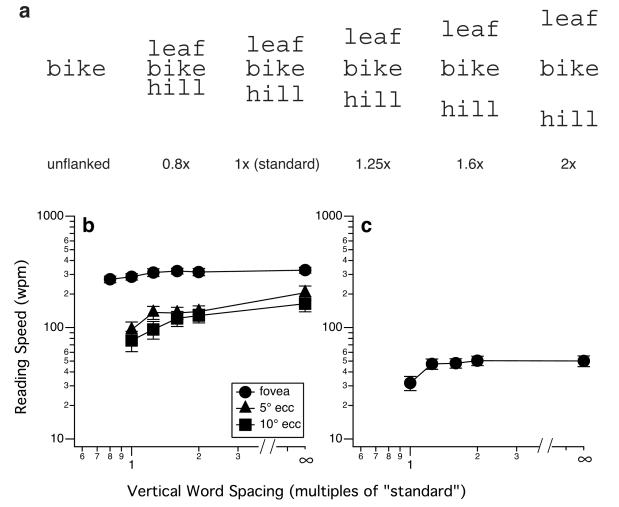
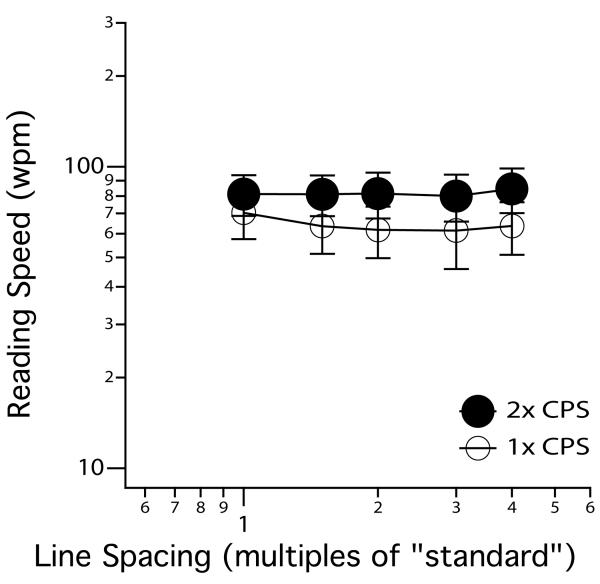
Crowding occurs at the letter level, but it also occurs at the word level. Does peripheral reading speed benefit from increased word spacing? We addressed this question by measuring RSVP reading speed for sequences of unrelated words that were flanked above and below by other words of the same word length.32 The prediction was that peripheral reading speed would benefit from larger word spacing (in this case, the vertical spacing between words), beyond the standard spacing, if crowding were the limiting factor for peripheral reading speed. Figure 6a illustrates the different vertical word spacings tested. Averaged across a group of five observers with normal vision, foveal reading speed does not benefit from increased vertical word spacing beyond the standard; while peripheral reading speed improved with larger word spacing at least up to 2× the standard vertical spacing (Figure 6b). Unfortunately, this benefit of reading speed on larger vertical word spacing is not replicated in observers with AMD. Figure 6c shows the averaged reading speed for four observers with AMD, as a function of vertical word spacing. This result clearly illustrates that for AMD observers, reading speed does not benefit from increased word spacing beyond 1.25× the standard vertical spacing, contrary to our prediction.33 To ensure that the lack of a benefit is not limited to the task of reading sequences of unrelated words, in a separate experiment we measured reading speed for passages (100 words each) printed at different line spacings. Consistent with the result for sequences of unrelated words, passage reading speed does not benefit from increased line spacing in observers with AMD (Figure 7).33
Figure 6.
The different vertical word spacings (expressed as multiples of the standard line spacing for the Courier font used) used in the studies of Chung32 and Chung et al.33 are shown in (a). Reading speeds measured for the conditions illustrated in (a) are plotted as average values for (b) a group of five young adults with normal vision tested at the fovea and 5 and 10° in the inferior visual field and (c) a group of four AMD observers. The infinity symbol on the abscissa represents the unflanked condition. Error bars represent ± 1 S.E.M. Panel (a) was modified from Chung32 and panel (b) was modified from Chung et al.33
Figure 7.
Reading speed (wpm) for 100-word passages, averaged across eight AMD observers, is plotted as a function of line spacing (multiples of “standard”), for two print sizes — 1× and 2× the critical print size (CPS). Error bars represent ± 1 S.E.M. Data were from Chung et al.33
Visual Span
When we read, even though we may have an impression of seeing the entire page of text, the number of characters that can be recognized during a fixation averages 7–11 at the fovea.17,34–38 Following O’Regan,39,40 we refer to this as the visual span — the number of characters that can be recognized at a fixation.38 In the periphery, we found that the visual span shrinks in size; for example, using comparable testing parameters, the visual span averages approximately 13 characters at the fovea and only 5 characters at 10° eccentricity.38 Using a computer simulation, we demonstrated the possible linkage of the size of the visual span with reading, implying that the shrinkage of the visual span in the periphery might be the factor accounting for slow peripheral reading.38 Additional empirical evidence supporting the link between the size of the visual span and reading speed comes from our investigations of the effect of print size, contrast and letter spacing on the size of the visual span and reading speed.26,41 In short, there exists a strong correlation between the size of the visual span and reading speed, thus supporting our proposition that visual span is a bottleneck on reading speed in peripheral vision. A recent study on the relationship between the visual span and reading speed in observers with AMD suggested that the reduced visual span, combined with the slower temporal processing of letters, is a better predictor of reading speed in people with AMD than the size of the visual span alone.42
Perceptual Learning
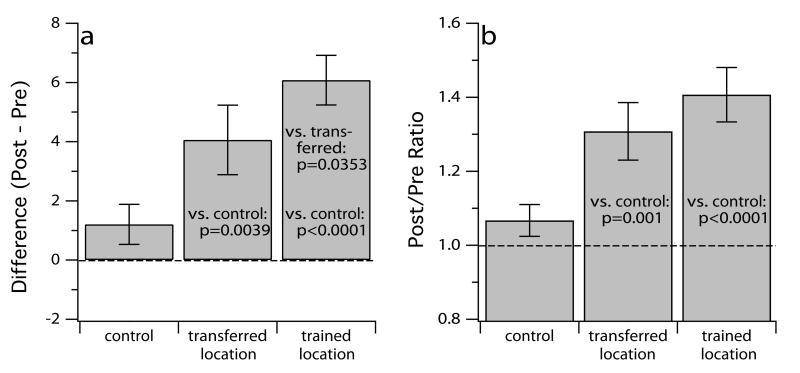
Performance for a variety of visual tasks improves with practice.43–53 In the previous section, we described findings suggesting a strong correlation between the size of the visual span and reading speed. If visual span is indeed the bottleneck on reading speed in peripheral vision, then it is important to ask whether we can enlarge the size of the visual span in peripheral vision, and if so, whether there is an associated increase in peripheral reading speed. To do so, we had observers practice a task that was designed to expand the visual span.54 Training lasted for four consecutive days (improvements occurred mostly on the first and second day of training of many perceptual tasks). To assess whether the improvements, if any, are specific to the trained retinal location, we trained some observers at 10° inferior visual field and others at 10° superior visual field. All observers were tested at both 10° inferior and superior visual fields before and after training, when we also measured reading speeds at the two locations. For comparison, we also included some observers who were only tested before and after a four-day no-training period. Figure 8 summarizes the key findings of this study. Compared with the no-training control group, both training groups showed significant improvements in the size of the visual span following training. The improvements were found not only at the trained retinal location for the respective group (averaged 6.08 bitsa), but also at the untrained retinal location (averaged 4.06 bits). In other words, observers trained at 10° inferior visual fields showed larger visual spans at 10° inferior visual field (trained location) following training, and also larger visual spans at 10° superior visual field (transferred location). More importantly, these improvements were accompanied by an improvement in reading speed, even though observers were only trained on a letter identification task but not on reading (Figure 8b). These results provide hope that we may be able to use perceptual learning as a tool to improve functional vision, including reading speed, in people with central vision loss.
Figure 8.
A comparison of the size of the visual span and maximum reading speed before and after perceptual learning of a letter-recognition task designed to expand the visual span.53 Panel (a) plots the difference in the size of the visual span, expressed as bits of information transmitted, for the no-training control group, and at the transferred (untrained) and trained retinal locations. Panel (b) plots the ratio of the maximum reading speed before and after training. Values plotted are averaged across the observers. Error bars represent ± 1 S.E.M. Pairwise comparisons among the three categories that are statistically significant are listed in the corresponding histogram. The presentation of these data is modified from Chung et al.53
However, there are at least two factors that could render perceptual learning ineffective in improving visual function of people with central vision loss. First, training-induced improvements might be smaller in older observers, compared with younger observers. This difference is usually attributed to the visual system being much less plastic (and thus less likely to show improvement) in older observers.55,56 To ensure that our training paradigm would be beneficial to people who are older, as most people with central vision loss are, we trained a group of older adults (55 – 76 years of age) using the same paradigm as described in the previous paragraph but only at 10° in the inferior visual field.57 In general, older adults also benefited from the same perceptual learning task such that the size of their visual span at 10° inferior visual field also enlarged following training, although the magnitude of the improvement is smaller. Averaged across observers, the visual span increased by 3.34 bits at the trained location (10° inferior visual field) and 3.15 bits at the untrained transferred location (10° superior visual field) for the older adults, compared with 5.86 bits (trained location) and 4.55 bits (transferred location) for younger adults who were trained at the same retinal location. The smaller amount of improvement shown by the older adults, when compared with younger adults, is not due to insufficient amount of training58 but instead, is attributed to the day-to-day lapse in the learning effect toward the end of the training period,57 which is not found in the younger adults. Also unlike for younger observers, the improvement in the size of the visual span following training was only accompanied by a faster reading speed at the trained retinal location and for the same print size used during training.
The second issue relates to the frequency of the training sessions. Most previous studies on perceptual learning had training sessions scheduled on consecutive days, or as close to consecutive days as possible, reasoning that the effectiveness of learning would fall off when the sessions are scheduled days apart. From a practical point of view, daily training sessions are challenging for people with central vision loss because most of them no longer drive and thus have to rely on alternative means for transportation in order to attend the training sessions in our lab. Is it really necessary for training sessions to be scheduled on a daily basis? How does the effectiveness of training affected by the inter-session duration? To address these questions, we trained three groups of observers with normal vision on a letter identification task. Training consisted of six sessions. The three groups differed in their training schedule — one group was trained daily, the second group was trained weekly and the third group was trained bi-weekly. In general, the amount of improvement following training was very similar for the daily and weekly groups, but was smaller for the bi-weekly group.59 These results suggest that weekly training could be as effective as daily training, making it easier for people with central vision loss to participate in perceptual learning experiments in the lab. Given this information, we have just begun a perceptual learning experiment with observers with central vision loss attending weekly training sessions.
SUMMARY AND FUTURE DIRECTIONS
The journey of understanding the limiting factors on reading in the normal periphery and in people with central vision loss has been revealing. From a clinical perspective, we know that magnification would certainly help patients read small print better, but it is never going to be able to restore the reading speed to the same level as before the loss of central vision. We also learned that simple manipulation of text typography or typesetting such as increasing the letter or line spacing is unlikely to improve reading speed in people with central vision loss. To date, the most promising way to improve reading and other types of related visual functions in peripheral vision is perceptual learning. This information is useful for low vision rehabilitation. As noted, we are pursuing the use of perceptual learning as a tool to improve the visual function of people with central vision loss.
Besides the clinical relevance, most of the findings described here, and many elsewhere, provide knowledge on how the visual system responds to central vision loss. For example, the lack of a benefit of increased vertical word spacing on reading found in observers with central vision loss,33 when compared with the normal observers,32 suggests that the properties of the visual system in the presence of central vision loss might resemble those of the normal fovea, instead of the normal periphery. We are now studying the many properties of the visual system in people with central vision loss, to determine if these properties are modified in response to central vision loss. This knowledge will help us better understand how people with central vision loss process visual information, which may in turn, help devise more effective devices or techniques for low vision rehabilitation.
ACKNOWLEDGMENTS
Ten years have lapsed since I was awarded the Irvin M. and Beatrice Borish Award, I am still humbled and honored to have received this prestigious award. Many fine colleagues contributed to the work presented here and I am grateful for their contributions. The preparation of this manuscript and most of my work described in this manuscript were supported by NIH grant R01-EY012810.
Footnotes
We typically quantify the size of a visual-span profile as bits of information transmitted through the visual span by converting percent-correct letter recognition at any given letter position to bits of mutual information transmitted. To do so, we computed the mutual information associated with confusion matrices for letter recognition.60 A plot of mutual information vs. percent-correct letter recognition was well fit by a straight line that ranged from 0 bits (for chance accuracy of 3.8% correct) to 4.7 bits (for 100% accuracy). The accuracy performance at each letter position can then be converted into bits of information and the sum of the total bits of information transmitted across all letter positions represents the size of the visual span. An increase in 4.7 bits of information transmitted through the visual span is equivalent to adding one letter slot of perfect identification (9.4 bits is equivalent to adding two letter slots of perfect identification etc).
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Congdon N, O’Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–85. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 2.Vitale S, Cotch MF, Sperduto RD. Prevalence of visual impairment in the United States. Jama. 2006;295:2158–63. doi: 10.1001/jama.295.18.2158. [DOI] [PubMed] [Google Scholar]
- 3.Friedman DS, O’Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 4.Westheimer G. The spatial sense of the eye. Proctor lecture. Invest Ophthalmol Vis Sci. 1979;18:893–912. [PubMed] [Google Scholar]
- 5.Chung ST, Mansfield JS, Legge GE. Psychophysics of reading. XVIII. The effect of print size on reading speed in normal peripheral vision. Vision Res. 1998;38:2949–62. doi: 10.1016/s0042-6989(98)00072-8. [DOI] [PubMed] [Google Scholar]
- 6.Kleen SR, Levoy RJ. Low vision care: correlation of patient age, visual goals, and aids prescribed. Am J Optom Physiol Opt. 1981;58:200–5. [PubMed] [Google Scholar]
- 7.Bullimore MA, Bailey IL. Reading and eye movements in age-related maculopathy. Optom Vis Sci. 1995;72:125–38. doi: 10.1097/00006324-199502000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Elliott DB, Trukolo-Ilic M, Strong JG, Pace R, Plotkin A, Bevers P. Demographic characteristics of the vision-disabled elderly. Invest Ophthalmol Vis Sci. 1997;38:2566–75. [PubMed] [Google Scholar]
- 9.Leat SJ, Legge GE, Bullimore MA. What is low vision? A re-evaluation of definitions. Optom Vis Sci. 1999;76:198–211. doi: 10.1097/00006324-199904000-00023. [DOI] [PubMed] [Google Scholar]
- 10.White JM, Bedell HE. The oculomotor reference in humans with bilateral macular disease. Invest Ophthalmol Vis Sci. 1990;31:1149–61. [PubMed] [Google Scholar]
- 11.Whittaker SG, Cummings RW, Swieson LR. Saccade control without a fovea. Vision Res. 1991;31:2209–18. doi: 10.1016/0042-6989(91)90173-3. [DOI] [PubMed] [Google Scholar]
- 12.Forster KI. Visual perception of rapidly presented word sequences of varying complexity. Percept Psychophys. 1970;8:215–21. [Google Scholar]
- 13.Rubin GS, Turano K. Reading without saccadic eye movements. Vision Res. 1992;32:895–902. doi: 10.1016/0042-6989(92)90032-e. [DOI] [PubMed] [Google Scholar]
- 14.Rubin GS, Turano K. Low vision reading with sequential word presentation. Vision Res. 1994;34:1723–33. doi: 10.1016/0042-6989(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 15.Fine EM, Peli E. Scrolled and rapid serial visual presentation texts are read at similar rates by the visually impaired. J Opt Soc Am (A) 1995;12:2286–92. doi: 10.1364/josaa.12.002286. [DOI] [PubMed] [Google Scholar]
- 16.Latham K, Whitaker D. A comparison of word recognition and reading performance in foveal and peripheral vision. Vision Res. 1996;36:2665–74. doi: 10.1016/0042-6989(96)00022-3. [DOI] [PubMed] [Google Scholar]
- 17.Legge GE, Ahn SJ, Klitz TS, Luebker A. Psychophysics of reading. XVI. The visual span in normal and low vision. Vision Res. 1997;37:1999–2010. doi: 10.1016/s0042-6989(97)00017-5. [DOI] [PubMed] [Google Scholar]
- 18.Chaparro A, Young RS. Reading with rods: the superiority of central vision for rapid reading. Invest Ophthalmol Vis Sci. 1993;34:2341–7. [PubMed] [Google Scholar]
- 19.Stuart JA, Burian HM. A study of separation difficulty. Its relationship to visual acuity in normal and amblyopic eyes. Am J Ophthalmol. 1962;53:471–7. [PubMed] [Google Scholar]
- 20.Flom MC, Weymouth FW, Kahneman D. Visual resolution and contour interaction. J Opt Soc Am. 1963;53:1026–32. doi: 10.1364/josa.53.001026. [DOI] [PubMed] [Google Scholar]
- 21.Bouma H. Interaction effects in parafoveal letter recognition. Nature. 1970;226:177–8. doi: 10.1038/226177a0. [DOI] [PubMed] [Google Scholar]
- 22.Loomis JM. Lateral masking in foveal and eccentric vision. Vision Res. 1978;18:335–8. doi: 10.1016/0042-6989(78)90168-2. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs RJ. Visual resolution and contour interaction in the fovea and periphery. Vision Res. 1979;19:1187–95. doi: 10.1016/0042-6989(79)90183-4. [DOI] [PubMed] [Google Scholar]
- 24.Chung ST. The effect of letter spacing on reading speed in central and peripheral vision. Invest Ophthalmol Vis Sci. 2002;43:1270–6. [PubMed] [Google Scholar]
- 25.Chung ST. Two approaches to reduce crowding do not lead to improved reading speed in patients with age-related macular degeneration. Lecture presented at VISION 2005; London, England. April 5.2005. [Google Scholar]
- 26.Yu D, Cheung SH, Legge GE, Chung ST. Effect of letter spacing on visual span and reading speed. J Vis. 2007;7(2):1–10. doi: 10.1167/7.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kooi FL, Toet A, Tripathy SP, Levi DM. The effect of similarity and duration on spatial interaction in peripheral vision. Spat Vis. 1994;8:255–79. doi: 10.1163/156856894x00350. [DOI] [PubMed] [Google Scholar]
- 28.Chakravarthi R, Cavanagh P. Temporal properties of the polarity advantage effect in crowding. J Vis. 2007;7(11):1–3. doi: 10.1167/7.2.11. [DOI] [PubMed] [Google Scholar]
- 29.Chung ST, Mansfield JS. Contrast polarity differences reduce crowding but do not benefit reading performance in peripheral vision. Vision Res. 2009;49 doi: 10.1016/j.visres.2009.08.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gibson EJ. Perceptual learning. Annu Rev Psychol. 1963;14:29–56. doi: 10.1146/annurev.ps.14.020163.000333. [DOI] [PubMed] [Google Scholar]
- 31.Chung ST. Learning to identify crowded letters: does it improve reading speed? Vision Res. 2007;47:3150–9. doi: 10.1016/j.visres.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung ST. Reading speed benefits from increased vertical word spacing in normal peripheral vision. Optom Vis Sci. 2004;81:525–35. doi: 10.1097/00006324-200407000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung ST, Jarvis SH, Woo SY, Hanson K, Jose RT. Reading speed does not benefit from increased line spacing in AMD patients. Optom Vis Sci. 2008;85:827–33. doi: 10.1097/OPX.0b013e31818527ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rayner K, Bertera JH. Reading without a fovea. Science. 1979;206:468–9. doi: 10.1126/science.504987. [DOI] [PubMed] [Google Scholar]
- 35.Fine EM, Rubin GS. Reading with central field loss: number of letters masked is more important than the size of the mask in degrees. Vision Res. 1999;39:747–56. doi: 10.1016/s0042-6989(98)00142-4. [DOI] [PubMed] [Google Scholar]
- 36.McConkie GW, Rayner K. The span of the effective stimulus during a fixation in reading. Percept Psychophys. 1975;17:578–86. [Google Scholar]
- 37.Rayner K. Eye movements in reading and information processing: 20 years of research. Psychol Bull. 1998;124:372–422. doi: 10.1037/0033-2909.124.3.372. [DOI] [PubMed] [Google Scholar]
- 38.Legge GE, Mansfield JS, Chung ST. Psychophysics of reading. XX. Linking letter recognition to reading speed in central and peripheral vision. Vision Res. 2001;41:725–43. doi: 10.1016/s0042-6989(00)00295-9. [DOI] [PubMed] [Google Scholar]
- 39.O’Regan JK. Eye movements and reading. In: Kowler E, editor. Eye Movements and Their Role in Visual and Cognitive Processes. Elsevier; New York: 1990. pp. 395–453. [Google Scholar]
- 40.O’Regan JK. Understanding visual search and reading using the concept of stimulus ‘grain’. IPO Annual Progress Report. 1991;26:96–108. [Google Scholar]
- 41.Legge GE, Cheung SH, Yu D, Chung ST, Lee HW, Owens DP. The case for the visual span as a sensory bottleneck in reading. J Vis. 2007;7(9):1–15. doi: 10.1167/7.2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheong AM, Legge GE, Lawrence MG, Cheung SH, Ruff MA. Relationship between visual span and reading performance in age-related macular degeneration. Vision Res. 2008;48:577–88. doi: 10.1016/j.visres.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKee SP, Westheimer G. Improvement in vernier acuity with practice. Percept Psychophys. 1978;24:258–62. doi: 10.3758/bf03206097. [DOI] [PubMed] [Google Scholar]
- 44.Ball K, Sekuler R. A specific and enduring improvement in visual motion discrimination. Science. 1982;218:697–8. doi: 10.1126/science.7134968. [DOI] [PubMed] [Google Scholar]
- 45.Ball K, Sekuler R. Direction-specific improvement in motion discrimination. Vision Res. 1987;27:953–65. doi: 10.1016/0042-6989(87)90011-3. [DOI] [PubMed] [Google Scholar]
- 46.Beard BL, Levi DM, Reich LN. Perceptual learning in parafoveal vision. Vision Res. 1995;35:1679–90. doi: 10.1016/0042-6989(94)00267-p. [DOI] [PubMed] [Google Scholar]
- 47.Fahle M, Edelman S. Long-term learning in vernier acuity: effects of stimulus orientation, range and of feedback. Vision Res. 1993;33:397–412. doi: 10.1016/0042-6989(93)90094-d. [DOI] [PubMed] [Google Scholar]
- 48.Fiorentini A, Berardi N. Perceptual learning specific for orientation and spatial frequency. Nature. 1980;287:43–4. doi: 10.1038/287043a0. [DOI] [PubMed] [Google Scholar]
- 49.Fiorentini A, Berardi N. Learning in grating waveform discrimination: specificity for orientation and spatial frequency. Vision Res. 1981;21:1149–58. doi: 10.1016/0042-6989(81)90017-1. [DOI] [PubMed] [Google Scholar]
- 50.Karni A, Sagi D. Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. Proc Natl Acad Sci U S A. 1991;88:4966–70. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poggio T, Fahle M, Edelman S. Fast perceptual learning in visual hyperacuity. Science. 1992;256:1018–21. doi: 10.1126/science.1589770. [DOI] [PubMed] [Google Scholar]
- 52.Saarinen J, Levi DM. Perceptual learning in vernier acuity: what is learned? Vision Res. 1995;35:519–27. doi: 10.1016/0042-6989(94)00141-8. [DOI] [PubMed] [Google Scholar]
- 53.Chung ST, Levi DM, Tjan BS. Learning letter identification in peripheral vision. Vision Res. 2005;45:1399–412. doi: 10.1016/j.visres.2004.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chung ST, Legge GE, Cheung SH. Letter-recognition and reading speed in peripheral vision benefit from perceptual learning. Vision Res. 2004;44:695–709. doi: 10.1016/j.visres.2003.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smirnakis SM, Brewer AA, Schmid MC, Tolias AS, Schuz A, Augath M, Inhoffen W, Wandell BA, Logothetis NK. Lack of long-term cortical reorganization after macaque retinal lesions. Nature. 2005;435:300–7. doi: 10.1038/nature03495. [DOI] [PubMed] [Google Scholar]
- 56.Sunness JS, Liu T, Yantis S. Retinotopic mapping of the visual cortex using functional magnetic resonance imaging in a patient with central scotomas from atrophic macular degeneration. Ophthalmology. 2004;111:1595–8. doi: 10.1016/j.ophtha.2003.12.050. [DOI] [PubMed] [Google Scholar]
- 57.Cheung SH, Yu D, Legge GE, Chung ST. Reading speed in the peripheral vision of older adults: does it benefit from perceptual learning? Vision Res. doi: 10.1016/j.visres.2010.02.006. under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Richards E, Bennett PJ, Sekuler AB. Age related differences in learning with the useful field of view. Vision Res. 2006;46:4217–31. doi: 10.1016/j.visres.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 59.Truong S, Arunkumar A, Yu D, Chung ST. A comparison of the effectiveness of perceptual learning on a daily vs. weekly basis. Optom Vis Sci. 2009;86 E-abstract 90842. [Google Scholar]
- 60.Beckmann PJ. Unpublished doctoral thesis. University of Minnesota; 1998. Preneural factors limiting letter identification in central and peripheral vision. [Google Scholar]