Abstract
Oceanic high-nitrate, low-chlorophyll environments have been highlighted for potential large-scale iron fertilizations to help mitigate global climate change. Controversy surrounds these initiatives, both in the degree of carbon removal and magnitude of ecosystem impacts. Previous open ocean enrichment experiments have shown that iron additions stimulate growth of the toxigenic diatom genus Pseudonitzschia. Most Pseudonitzschia species in coastal waters produce the neurotoxin domoic acid (DA), with their blooms causing detrimental marine ecosystem impacts, but oceanic Pseudonitzschia species are considered nontoxic. Here we demonstrate that the sparse oceanic Pseudonitzschia community at the high-nitrate, low-chlorophyll Ocean Station PAPA (50° N, 145° W) produces approximately 200 pg DA L−1 in response to iron addition, that DA alters phytoplankton community structure to benefit Pseudonitzschia, and that oceanic cell isolates are toxic. Given the negative effects of DA in coastal food webs, these findings raise serious concern over the net benefit and sustainability of large-scale iron fertilizations.
Keywords: carbon sequestering, domoic acid, ocean iron fertilization, Pseudonitzschia, toxicity
Mesoscale iron fertilization experiments have been performed in all of the major high-nitrate, low-chlorophyll (HNLC) regions (1–3) (Table 1), and the biological and chemical consequences of the resultant diatom blooms have substantially improved our understanding of marine biogeochemical cycles, including their complexities and links to climate processes. The success of these scientific efforts has led to policy proposals and emerging commercial endeavors that enhance atmospheric carbon sequestration in the deep ocean using iron-induced diatom blooms with high sinking potential. Debate on the wisdom of these ecological manipulations has focused mainly on the magnitude and quantification of stimulated carbon export (1, 4) and the resultant effects on deepwater chemistry and biological communities (5). However, there has been little discussion whether the iron-enhanced diatom community composition itself may have unintended consequences to surface-dwelling organisms.
Table 1.
Comparison of the present, deck-board Fe-enrichment experiments with previous open-ocean mesoscale Fe enrichment experiments
| HNLC Region Project | Year | Dominant phytoplankton within bloom | Initial Chl a (μg L−1) | Peak Chl a (μg L−1) | Refs. |
| Equatorial Pacific | |||||
| IronEx-I | 1933 | Synechococcus red-fluorescing picoplankton autotrophic dinoflagellates | 0.24 | >0.65 | 37, 38 |
| IronEx-II | 1995 | Pseudonitzschia spp. (Initially reported as Nitzschia) | 0.15–0.20 | <4.0 | 6, 7, 39 |
| Northwest Pacific | |||||
| SEEDS-I | 2001 | Chaetoceros debilis | 0.8–0.9 | 21.8 | 40, 41 |
| SEEDS-II | 2004 | Pseudonitzschia spp., Neodenticula semina | 0.80 | 2.48 | 42 |
| Northeast Pacific | |||||
| SERIES | 2002 | Pseudonitzschia spp., Neodenticula semina, Chaetoceros spp. | 0.27–0.43 | 6.30 | 43, 44 |
| PAPA-SEEDS | 2006 | Pseudonitzschia turgidula | 0.2–0.3 | – | Present study |
| Southern Ocean | |||||
| SOIREE (Australian polar waters) | 1999 | Fragillariopsis kerguelensis, Rhizosolenia spp., Pseudonitzschia sp. | 0.25 | 3.0 | 8, 45 |
| EisenEx (Atlantic polar waters) | 2000 | Pseudonitzschia lineola | 0.48–0.56 | 2.80 | 9, 15 |
| SOFeX – North (Pacific subpolar waters) | 2002 | Pseudonitzschia spp. | 0.26 | 2.60 | 10, 11 |
| SOFeX – South (Pacific polar waters) | 2002 | Fragillariopsis spp., Corethron spp., Chaetoceros spp., Rhizosolenia spp. | 0.20 | 4.0 | 10, 11 |
| EIFEX (Atlantic subpolar waters) | 2004 | Chaetoceros spp., Fragilariopsis kerguelensis, Pseudonitzschia spp., large single-celled diatoms | 0.54 | 2.85 | 46, 47 |
| SAGE (Pacific subpolar Si-depleted coastal waters) | 2004 | – | ~0.6–0.4 | 1.3 > 0.7 | 2, 48 |
The present, deck-board Fe-enrichment experiment was compared with previous open-ocean mesoscale Fe enrichment experiments conducted in HNLC regions of the Equatorial and North Pacific Oceans, and Southern Oceans with respect to initial and peak biomass [as chlorophyll a (Chl a)], and the dominant phytoplankton species or genera that increased in response to alleviation of in situ Fe-deficient conditions.
Mesoscale iron enrichment experiments have focused on studying the broader issue of carbon cycling, rather than assessing the potential ecological impacts of larger-scale and longer-term geoengineering-designed fertilizations. Here we consider that the large diatom blooms generated by previous iron enrichment experiments have in most cases been dominated by diatoms belonging to the genus Pseudonitzschia (Table 1), including studies conducted in the equatorial Pacific [IronEx II (6, 7)] and Southern Ocean [SOIREE (8), EisenEx (9), and SoFeX (10, 11)], and may have the capacity to create unanticipated ecological consequences. Pseudonitzschia species have the capacity to produce the potent neurotoxin domoic acid (DA), sometimes generating massive toxic harmful algal blooms (HABs) in coastal waters (12–14). To our knowledge, there have been only two attempts to measure DA production during iron fertilization experiments: Assmy et al. (15) reported that there were no measureable quantities of DA during the EisenEx fertilization, and Marchetti et al. (16) found no DA production in laboratory cultures of oceanic Pseudonitzschia isolates obtained after iron fertilization. However, the EisenEx assessment used stored, preserved phytoplankton samples, and the longer-term stability of DA in these samples is unknown. Furthermore, Pseudonitzschia spp. are notoriously variable with respect to toxin production in laboratory cultures, with supposedly “non-toxic” isolates suddenly revealing their capacity to produce DA and vice versa. Nevertheless, these negative results, combined with recognition that some coastal Pseudonitzschia produce low concentrations of DA (17), has led to a perception that oceanic Pseudonitzschia are nontoxic and that iron-generated blooms in HNLC regions will not negatively impact surface dwelling organisms of the pelagic ecosystem. This position would require reassessment if oceanic Pseudonitzschia indeed had the capacity to produce DA.
We present here results from in situ measurements and shipboard culture experiments demonstrating that oceanic Pseudonitzschia species produce DA and retain that capacity upon iron and copper amendment. The findings demonstrate that toxin production can occur with iron fertilization of HNLC waters, that the specific composition of commercial iron substrates is a critical parameter in the degree of toxin production, and that the total toxin production potentially could reach ecologically harmful levels during large-scale iron fertilization programs.
Results
Measurement of Cellular Domoic Acid in Subarctic Pacific Surface Waters.
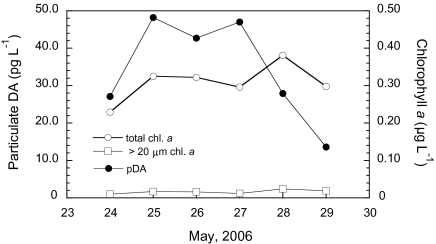
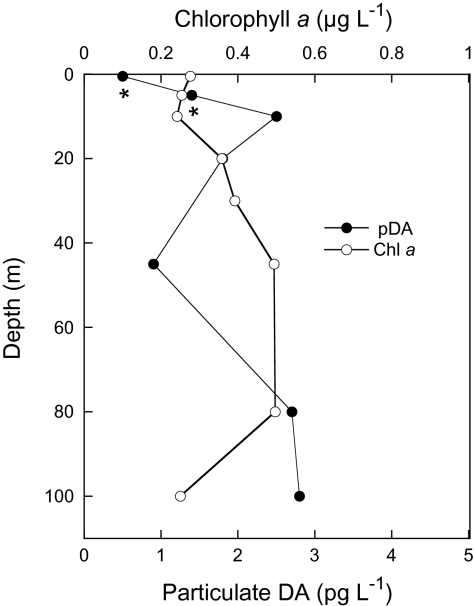
Biological and physical data were collected at Ocean Station PAPA (OSP; 50° N, 145° W) in the eastern subarctic Pacific HNLC region during May and June 2006 in a project studying the regulation of phytoplankton community structure by low-level iron amendments. Pseudonitzschia turgidula and the smaller P. cf granii were present in low concentrations (<1,000 cells L−1) at the surface (5 m) and at the chlorophyll maximum (approximately 45–60 m). No other Pseudonitzschia species were observed during this study. We collected 1-L water samples at each depth and measured particulate DA (pDA) on fresh samples on board the ship using an indirect competitive ELISA (cELISA) method (18). Particulate DA was measured at 5 m depth over 6 consecutive days (Fig. 1), often at concentrations >30 pg L−1, which is in excess of the minimum analytical method detection limit (MDL; 25.6 pg L−1 for 1-L seawater samples; 6.4 pg mL−1 detectable concentration in the extract). The vertical profile of pDA concentrations (June 5, 2006; Fig. 2) indicates that DA is found in cells throughout the photic zone, but DA concentration does not necessarily co-vary with the total phytoplankton community biomass (as measured by extracted chlorophyll a). Over the sampling period, DA concentrations periodically were substantially higher at the chlorophyll maximum (approximately 40 m): 59.0 pg L−1 on May 25, 2006, and 36.3 pg L−1 on May 27, 2006. Converting pDA concentrations to cellular DA (pDA normalized to cell abundance) by using microscopically determined total Pseudonitzschia cell abundances yields an estimated minimum cell toxin quota of approximately 15 fg DA cell−1, as only one species was observed, and assuming that all cells present were producing DA equally. These findings confirm that oceanic Pseudonitzschia species produce DA in situ and that DA accumulation by these cells is constitutive with their presence in HNLC waters.
Fig. 1.
Ambient concentrations of particulate DA and size-fractionated phytoplankton biomass as a function of time at OSP in May 2006. Discrete samples were collected using 10-L Niskin bottles; 1-L subsamples were filtered for pDA (•) and analyzed fresh at sea using a cELISA; 0.2-L and 0.4-L subsamples were filtered in parallel for phytoplankton biomass as chlorophyll a on Whatman GF/F filters (nominal pore size, 0.7 μm) for total chlorophyll a (○) and on Poretics 20 μm pore size polycarbonate filters for the >20-μm fraction (□), respectively, and analyzed fluorometrically (33). Values below the volume adjusted MDL of 25.6 pg L−1 (for 1-L samples) are reported with an asterisk.
Fig. 2.
A profile of particulate DA and phytoplankton biomass as a function of depth at OSP in June 2006. Discrete samples were collected using 10-L Niskin bottles; generally 7.3-L subsamples were filtered for pDA (•) and analyzed fresh at sea using a cELISA; 0.2-L subsamples were filtered for total phytoplankton biomass as chlorophyll a (○) on Whatman GF/F filters (nominal pore size, 0.7 μm) and analyzed fluorometrically (33). Values below the volume-adjusted MDL of 1.8 pg L−1 for all samples (7.3 L), except the 45 m depth sample (50 L) (volume-adjusted MDL, 0.5 pg L−1), are reported with an asterisk.
Effect of Iron and Copper Amendments on Toxin Production by Pseudonitzschia in Batch Cultures.
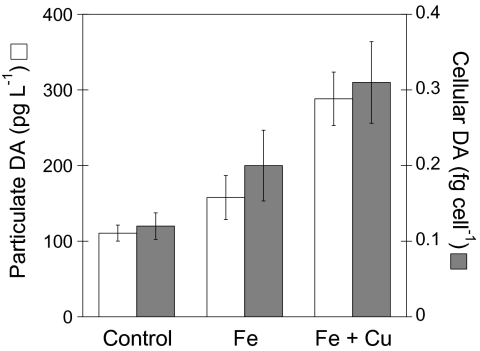
Previous studies have indicated that DA production and release into seawater by Pseudonitzschia species are linked to iron and copper nutrition of the cell (19, 20), so it is important to determine if these cells alter their toxicity or capacity to become toxic upon short-term alleviation of metal limitation. We tested this hypothesis using bottle-type growth experiments with OSP surface waters. Pseudonitzschia cell abundance and pDA concentrations increased substantially in the control bottles after 6 d (approximately 110 fg mL−1) relative to in situ levels. The pDA concentrations were further enhanced by a small (1 nM) amendment of iron (approximately 160 fg mL−1), whereas the combined addition of level Fe (1 nM) and Cu (10 nM) increased pDA by approximately 160% (approximately 290 fg mL−1) relative to that in the unamended control bottles (Fig. 3). Cellular DA concentrations followed the same trend, with the control having approximately 0.1 fg DA cell−1, which doubled to approximately 0.2 fg DA cell−1 with iron amendment and tripled to approximately 0.3 fg cell−1 with the combined iron and copper amendment (Fig. 3).Two isolates of P. turgidula were successfully returned ashore and also produced DA in laboratory cultures, although at lower concentrations (0.0052 fg DA cell−1) than those measured in the natural in situ population. These concentrations are below the analytical detection limits of the method employed by Marchetti et al. (16), who noted that DA was undetectable in their isolates.
Fig. 3.
Particulate and cellular DA concentrations in deck-board batch cultures containing P. turgidula. Basal growth media for the batch cultures was OSP seawater collected using trace metal–clean techniques. Treatments were: no metal amendments (control), amended with iron (1 nM Fe), or amended with iron and copper (1 nM Fe + 10 nM Cu). Particulate DA and Pseudonitzschia abundances were determined after 6 d of incubation when cells were in the stationary phase of growth. Open and solid bars are particulate DA (pDA) and cellular DA (pDA normalized to Pseudonitzschia cell abundance) concentrations, respectively. The volume adjusted MDL for analysis of pDA is 66 pg L−1.
Domoic Acid Production in Continuous Cultures at Ocean Station PAPA.
The chemical speciation of iron regulates its availability to phytoplankton, and organically complexed species that dominate iron speciation in the HNLC regions are poorly available to large eukaryotic phytoplankton (21). Pulsed iron input, which occurs in aerosol deposition events or rapid iron spikes to batch cultures and mesoscale iron enrichment experiments, can yield colloidal iron precipitates (2, 3) that increase iron availability to eukaryotic phytoplankton (21). In the case of mesoscale Fe enrichments, this increase is short-lived, as the bulk of these labile colloidal precipitates aggregate and sink, whereas the remaining Fe colloids dissolve and equilibrate with less available strongly complexed Fe(III) species (3). To better evaluate the effects of elevated concentrations of ambient complexed Fe(III) species, we used shipboard continuous cultures comprising duplicate controls (filtered seawater) and iron amendments. Here, iron (1 nM) was pre-equilibrated (≥24 h) with the natural suite of organic ligands in filtered seawater before being supplied to cultures at a dilution rate of 0.5 d−1 (2 d residence time). Under this growth scenario, the communities competing for the limiting nutrient are constantly replaced by being flushed from the culture vessels by new inflowing media, so that in the absence of significant grazing activity, the best-adapted (i.e., the fastest growing) species are retained in the highest numbers.
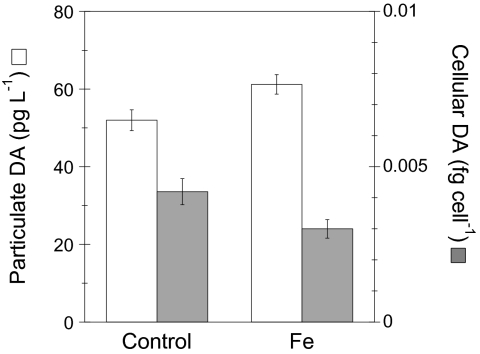
Adding iron doubled the steady-state abundance of Pseudonitzschia species after 9 d compared with controls (approximately 25,000 vs. 12,400 cells mL−1), consistent with trends in batch culture findings here and elsewhere (Table 1). In this case, the resulting Pseudonitzschia was not P. turgidula, but rather a smaller species identified by scanning electron microscopy as P. cf granii. Similar to P. turgidula, this small oceanic diatom was the only Pseudonitzschia species induced and produced DA in both control and iron-amended continuous cultures (Fig. 4). The cellular toxicity of the P. cf granii was 0.004 fg DA cell−1 in the controls and changed little in the iron treatment (0.003 fg DA cell−1; Fig. 4). Even so, previous work has shown that much of the DA produced by cells during exponential growth is released to the dissolved phase (24) (in contrast to the increasing cellular toxicity observed when cell division slows in batch cultures). Dissolved DA was not detectable in our continuous cultures as a result of the high daily medium dilution rate (0.5 d−1).
Fig. 4.
Particulate and cellular DA concentrations in deck-board continuous cultures containing P. cf granii. Basal growth media for the continuous cultures was OSP seawater collected using trace metal–clean techniques. Treatments were: not amended with metals (control) or amended with iron (1 nM Fe). Culture dilution rates were maintained at 0.5 d−1. Particulate DA and P. cf granii abundances were determined after 9 d of incubation. Open and solid bars are particulate DA (pDA) and cellular DA (pDA normalized to Pseudonitzschia cell abundance) concentrations, respectively. The volume adjusted MDL for analysis of pDA is 37 pg L−1.
Ecological Consequences of Increased Domoic Acid Production and Release by Pseudonitzschia in the HNLC Eastern Subarctic Pacific.
The metabolic function of DA in Pseudonitzschia remains to be determined, but there is evidence that it is involved in the acquisition of iron and copper. Both actively growing and senescent cells release dissolved DA (17, 19, 20), with actively growing cells releasing the majority of the DA produced (24) (i.e., intracellular DA concentrations represent a small proportion of total DA production). Field measurements of dDA have reached >100 nM in Monterey Bay, California (22), and >400 nM in Sequim Bay, Washington (23). The release of DA can increase iron uptake rates by Pseudonitzschia and increased copper availability has been shown to facilitate iron uptake in this genus (19). It is reasonable then to ask whether production and release of DA might provide oceanic Pseudonitzschia a competitive advantage over other diatoms in these iron-limited waters.
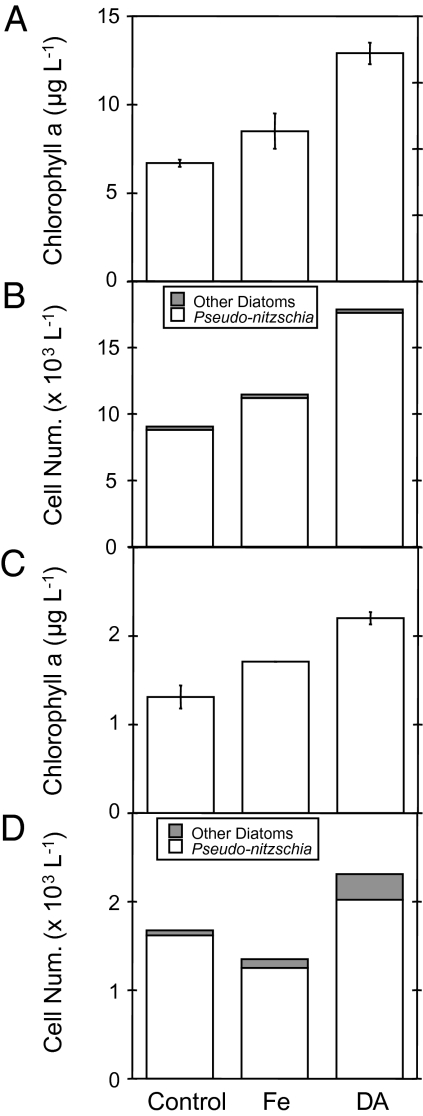
We tested the effect of dDA on diatom community response in continuous cultures at OSP in May 2007 using the same general methods described earlier. As in 2006, our experiments began before the spring bloom, and a significant increase in chlorophyll a biomass (10%) was observed in Fe-amended samples (Fig. 5A). However, addition of dissolved DA (25 nM) generated a twofold increase in total chlorophyll a biomass, apparently by increasing the relative availability of the ambient organically complexed dissolved iron. Diatoms in the control, iron, and DA treatments were dominated by Pseudonitzschia spp., with very minor contributions by Thalassiosira and Chaetoceros spp. (Fig. 5B).
Fig. 5.
Effects of Fe and dissolved DA on the relative abundance of phytoplankton and Pseudonitzschia spp. Changes in phytoplankton biomass (A) (estimated by chlorophyll a concentrations) and phytoplankton cell abundance (B) (cells mL−1) of continuous cultures grown in OSP seawater in May 2007. Changes in phytoplankton biomass (C) (estimated by chlorophyll a concentrations) and phytoplankton cell abundance (D) (cells mL−1) of continuous cultures grown in GOA mesoscale eddy (May 2007) seawater under continuous dilution rates of 0.5 d−1 for 6 d. Treatments were: not amended with metals (control), amended with iron (1 nM Fe), or amended with DA (25 nM).
We repeated this continuous culture experiment using surface waters from the central region of an anticyclonic mesoscale eddy in the Gulf of Alaska (GOA) in May 2007. These eddies advect excess nutrients and iron in their core waters from the continental margin as they propagate into the GOA and account for as much as 10% of the surface chlorophyll signal in the GOA. Adding iron to these surface waters also enhanced chlorophyll a biomass by approximately 25% over that in the control continuous cultures (Fig. 5C). Diatom biomass followed this general trend, with Pseudonitzschia again dominating numerically over the Chaetoceros and Tropodoneis spp. present in surface waters (Fig. 5D). Addition of dDA further increased chlorophyll a biomass (approximately 40%) and Pseudonitzschia abundance (approximately 20%) over that in the controls (Fig. 5 C and D), consistent with the findings at OSP.
Discussion
The issue of potential toxin production during the massive ecosystem responses to mesoscale Fe enrichments of HNLC waters has attracted little attention, primarily because the limited evidence available indicated that oceanic Pseudonitzschia species lacked the ability to produce DA (16). The findings here show unequivocally that Pseudonitzschia species in the eastern subarctic Pacific do indeed produce DA as part of their normal physiology under ambient conditions, at least during spring. Particulate DA was measured over several days in surface waters and was distributed throughout most of the photic zone, despite Pseudonitzschia comprising an exceedingly small numerical component of the phytoplankton assemblage. Moreover, growth stimulation by iron additions selected for communities enriched by two Pseudonitzschia species (P. turgidula and P. cf granii) that not only retained the capacity to produce DA but also increased their cellular DA concentrations. The experimental outcomes here are consistent with findings from coastal waters and indicate that the physiological regulation of toxin production in Pseudonitzschia is similar between oceanic and coastal species (17). The central question now is not whether oceanic Pseudonitzschia are toxigenic but whether enhanced toxicity upon iron enrichment may reach levels sufficient to cause ecosystem damage.
The cellular DA concentrations generated during our deck-board experiments lie within the range of toxic blooms in coastal waters (17), but ascertaining whether these toxin levels may generate ecosystem impacts in oceanic waters is not straightforward. Krill (24) and planktivorous fish (25) feeding on Pseudonitzschia in coastal waters accumulate DA, which affects their feeding responses (26) and increases mortality in the food web (27), but we are aware of no equivalent studies for offshore waters. Although the total toxin concentrations generated in some of these preliminary experiments (approximately 0.2 μg DA L−1) may not be enough to generate acute toxicity at higher trophic levels, it is unclear whether higher concentrations may result under conditions of large-scale and persistent iron enrichments designed to obtain carbon credits. For example, toxin concentrations increased when iron amendments included enhanced copper availability, indicating that synergistic effects with other trace metals may occur. All mesoscale enrichment experiments to date have used expensive reagent-grade (i.e., pure) iron substrates (1, 3), but costs will necessitate that industrial-grade iron substrates (that may contain Cu or other trace metals) be used for proposed larger-scale enrichment experiments, so it is conceivable that bloom toxicity might be enhanced.
Our results indicate that the manner in which iron is added to HNLC surface waters will have an effect on toxin production by Pseudonitzschia. Batch cultures, which mimic aerosol deposition and mesoscale iron dispersement strategies, yielded similar results to continuous cultures, in which added iron equilibrated with natural ligands for ≥24 h before presentation to the phytoplankton community, an equilibration time far longer than that used in voltammetric methods employed to study iron speciation in seawater. Continuous culture experiments are a better mimic for the proposed enhanced nutrient (and metal) mixing of deep waters to the surface (28). In both cases, iron additions selected for a Pseudonitzschia species over other diatoms and resulted in increased concentrations of DA.
What can the findings here tell us about the potential for acute toxicity of Pseudonitzschia blooms resulting from iron fertilization? Commercial carbon credit goals plan to use Fe enrichment of approximately 100 km2 of HNLC waters to sequester approximately 0.63 to 1.1 × 107 gC km−2 in carbon offsets (29). The cellular carbon quota (in gC/cell) of actively growing Pseudonitzschia (8) is reasonably well established at approximately 5 × 10−14 gC μm−3 cell−1. The cell volume of P. turgidula in our cultures was 400 fL cell−1 (30), corresponding to a cellular carbon quota of 2 × 10−11 gC cell−1. Assuming a mixed layer depth of 80 m (measured here) and an estimated carbon export efficiency below 500 m of 5% (deep enough to ensure multidecadal sequestration of carbon) (11), sequestration of approximately 1 × 107 gC km−2 would require blooms of approximately 6 × 107 cells L−1. These cell concentrations are comparable with large Pseudonitzschia blooms in coastal waters (12, 13) and presumably would be representative of those resulting from intensive iron fertilization. Although cellular toxin levels are highly variable, the reported cellular DA quota for P. turgidula in situ ranges between approximately 15 fg cell−1 (measured here) to approximately 30 fg cell−1 (31). Although these levels appear to be lower under rapid growth conditions, Pseudonitzschia cellular toxin loads generally increase as cell division slows in both batch and continuous cultures (32). Large-scale iron-induced blooms in these HNLC waters conceivably could yield toxin levels up to approximately 1 to 2 μg DA L−1, a level sufficient to close shellfish harvests and cause acute toxic effects on seabirds and marine mammals in near-shore waters (24–27).
Although there are clear uncertainties in this calculation, it is worth remembering that bloom toxicity in coastal waters tends to increase substantially as cells move into senescence, so actual cellular toxin quotas might be much greater than observed in these actively growing cultures. Even so, chronic sublethal exposure to DA causes substantial neurological impacts in mammals (27) and presumably other trophic levels shown to respond to DA. So if actual toxin levels generated during the blooms were closer to our lower estimated limits, persistent exposure may still impair the ecological health of the broader food web, including wild salmon and other fisheries dependent on HNLC environments.
Our findings show that oceanic Pseudonitzschia produce and accumulate DA and that they do not lose this capacity upon Fe enrichment. Although 12 major scientific field efforts have studied the role of iron in regulating ocean ecosystem and carbon cycling, they were not designed to evaluate potential negative impacts of oceanic iron fertilization as a carbon mitigation strategy. Although there remain uncertainties in extrapolating our results to large oceanic scales, the findings establish potential consequences for developing toxic phytoplankton blooms in pelagic ecosystems, which so far have not been adequately investigated. In particular, it will be important to assess the effect that these blooms may have on stressed fisheries that depend on HNLC regions. The current debate over the consequences of ocean iron fertilization as a carbon mitigation strategy must focus more closely on the impacts to organisms of the pelagic surface zone.
Methods
Water Collection.
Discrete seawater samples for in situ measures of phytoplankton biomass (as extracted chlorophyll a), phytoplankton species abundance, and DA concentrations were collected from 10-L Niskin bottles attached to an instrumented rosette. Seawater for use as the basal culture media of the deck-board, batch, and continuous cultures was collected from 5 m using a trace metal–clean, all-Teflon pumping system (19).
Chlorophyll a Measurement and Cell Enumerations.
Chlorophyll a was determined using nonacidification (33) in vitro fluorometric analyses after parallel filtration onto Whatman GF/F filters (0.7-μm nominal pore size) and Poretics 20-μm pore size polycarbonate filters. Samples were extracted at sea in 90% acetone for approximately 24 h at −20 °C, and the fluorescence subsequently measured with a 10-AU fluorometer calibrated at the beginning of the cruise with pure chlorophyll a (Turner Designs). For most analyses, fresh, nonpreserved samples were assayed for cell abundances. For the continuous culture experiments, formaldehyde-preserved samples were enumerated after the cruise. Pseudonitzschia cells were enumerated with a Palmer-Maloney counting chamber using a Zeiss Axiostar light microscope. Samples were settled for at least 24 h when required and counted at a total magnification of 200×.
Domoic Acid Measurements.
Phytoplankton cells were collected from water samples collected using Niskin bottles, and culture media by filtering onto 47-mm-diameter nitrocellulose filters (HAWP04700 MF-Membrane filters; 0.45-μm pore size; Millipore). Filters were transferred to 15-mL Falcon tubes (BD Biosciences), and 4 mL of Nanopure water was added. Samples were vortexed, bath sonicated (model 5210; Branson) for 2 h to release cellular DA, and then vortexed again to mix before syringe filtration through a 25-mm-diameter (0.45-μm pore size) mixed cellulose ester filter (Millipore) to remove particulate matter. Filtrates were analyzed for DA content via an indirect cELISA.
An eight-point standard curve was prepared with DA standard (DACs-1C; National Research Council of Canada; standard range of 0.60–50,000 pg mL−1). Standards serially diluted in Nanopure water and samples were loaded in duplicate or triplicate into wells of a 96-well plate coated with BSA with carboxyl-linked DA antigen (18). Only Nanopure water was added to wells for determination of Amax. Fifty microliters of anti-DA antibody [generated by injecting a rabbit with an imine-linked DA antigen (18)] was added to each well except blank wells, to which 50 μL of blocking buffer (3% nonfat dry milk in PBS solution) was added. The plate was sealed with plastic sealer, covered with foil, and gently mixed on an orbital shaker for 1 h at room temperature. The plate was washed four times with washing buffer (PBS-Tween), after which 100 μL of goat anti-rabbit antibody linked to horseradish peroxidase (Dako) was added to each well. After covering with plastic sealer and foil, the plates were gently rotated on an orbital mixer for 30 min at room temperature. The plate was washed four times with buffer, after which 100 μL of TMB (BioFX Laboratories) substrate was added to each well. After gentle rotation on an orbital mixer for 15 min at room temperature, 100 μL of stop solution (1N HCl) was added to each well, and after 2 min the absorbance was read at 450 nm on a Versamax plate reader (Molecular Devices). Sample concentrations were interpolated to the standard curve generated using Softmax Pro-4.8 software (Molecular Devices). The precision of reported DA concentrations, as estimated by the coefficient of variation (i.e., SD as a percentage of the mean) of duplicate analyses, averaged 33% and 12% for in situ and culture values, respectively. The DA values reported here have been corrected for the concentration factors determined for the assayed extract based on the filtered and extract sample volumes. The minimum analytical MDL of the cELISA (6.4 pg mL−1) was estimated as the product of the SD of six replicate analyses of a 5 pg mL−1 DA standard (primary standard from National Research Council of Canada), with an SD of 1.896. For a df of 5, Student t value was 3.365, the MDL is 1.896 × 3.365 = 6.4 pg mL−1, and 19.0 pg mL−1 is the limit of quantification.
Pseudonitzschia Isolation and Batch Culture Methods.
Representative phytoplankton fractions (1–2 mL) from a surface net tow (20-μm mesh size) were placed in f/10-enriched seawater medium [f/2 (Sigma) diluted 5× with sterile-filtered ambient seawater collected at 5 m] and monitored microscopically for growth. Cell isolates [P. turgidula; identified after the cruise via scanning and transmission EM (34)] from the net tow were pooled to establish a culture. This was maintained for 5 to 6 d in f/10 medium. Before enrichment experiments, f/10 culture media was replaced with ambient seawater by concentrating cells in a 20-μm mesh concentration chamber and allowing cells to grow for 3 to 4 d. Batch culture experiments were initiated in deck-board Plexiglas incubators that were maintained at ambient seawater temperature and 50% surface irradiance via constant flow with surface seawater and by covering incubators with neutral density plastic film (Cinemills). Cells were enumerated daily using Palmer-Maloney counting chambers. Cultures reached stationary growth on d 6, at which time 140 to 200 mL was filtered and extracted in 2 mL Nanopure water before particulate DA analysis.
Deck-Board Incubations and Culture Studies.
An all-Teflon sampling system was used to collect surface waters for incubation from the 50% light depth at OSP. The system comprised a plastic towfish with Kevlar-encased Teflon perfluoroalkoxyalkane (PFA) tubing, positioned 8 m outboard of the vessel using the ship's crane to avoid contamination associated with the vessel, and a double diaphragm pump that provided a continuous clean water supply to a shipboard-fabricated, positive-pressure clean room, where it was dispensed under a Class 100 HEPA bench. To reduce variance from potential small-scale surface patchiness during sample collection, a 50-L acid-cleaned polyethylene carboy was rinsed thoroughly and filled with whole water for dispersal into the batch and continuous culture vessels.
Continuous Culture Apparatus and Operation.
The Ecostat (35, 36) was comprised of fourteen 2-L custom-designed cylindrical polycarbonate culture vessels, fitted with inflow and outflow ports, supported in cradles that mechanically oscillated to keep the cultures well mixed. The bottles, cradles, and rocking assembly were contained within a transparent, enclosed Plexiglas recirculating water bath (approximately 240 L) maintained at ambient sea surface temperature. The culture media sources were filtered (<0.2 μm) ambient seawater with or without iron amendments stored in 20 L polycarbonate reservoir carboys in the dark and pumped continuously by a multichannel peristaltic pump with high-purity platinum-cured silicone pump tubing (1/16-inch outer diameter; Ismatec) to duplicate culture vessels per treatment through 15 m long 1/16” OD Teflon PFA high-purity tubing (Upchurch). Outflow from each culture vessel was carried by 1/8-inch outer diameter Teflon PFA HP tubing to a central point outside of the water bath where flow rates were measured and samples collected daily. Continuous flow to the culture vessels was set to a dilution rate of 0.5 d−1, a rate that represents approximately 50% of the ambient in situ growth rate based on previous visits to the area.
Acknowledgments
We thank N. Lundholm and R. Horner for confirmation of Pseudonitzschia species and B. T. Eberhart for assistance with the cELISA. We acknowledge the at-sea assistance of M. E. Auro, J. N. Betts, J. Herndon, and R. L. Radan (Romberg Tiburon Center for Environmental Studies, San Francisco State University), in addition to the officers and crew of the R/V Thomas G. Thompson. This research was funded through grants from the US Department of Energy–Ocean Carbon Sequestration and United States National Science Foundation–Chemical Oceanography (to W.P.C., C.G.T., and M.L.W.), an NSERC Discovery grant (C.G.T.), a grant from the West Coast Center for Oceans and Human Health as part of the National Oceanic and Atmospheric Administration Oceans and Human Health Initiative (B.D.B. and V.L.T.), and a grant from the National Marine Fisheries Service Advanced Studies program (B.D.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Buesseler KO, et al. Environment. Ocean iron fertilization—moving forward in a sea of uncertainty. Science. 2008;319:162. doi: 10.1126/science.1154305. [DOI] [PubMed] [Google Scholar]
- 2.Boyd PW, et al. Mesoscale iron enrichment experiments 1993-2005: synthesis and future directions. Science. 2007;315:612–617. doi: 10.1126/science.1131669. [DOI] [PubMed] [Google Scholar]
- 3.de Baar HJW, et al. Synthesis of iron fertilization experiments: From the iron age in the age of enlightenment. J Geophys Res. 2005;110:C09S16. [Google Scholar]
- 4.Buesseler KO, Boyd PW. Climate change. Will ocean fertilization work? Science. 2003;300:67–68. doi: 10.1126/science.1082959. [DOI] [PubMed] [Google Scholar]
- 5.Chisholm SW, Falkowski PG, Cullen JJ. Oceans. Dis-crediting ocean fertilization. Science. 2001;294:309–310. doi: 10.1126/science.1065349. [DOI] [PubMed] [Google Scholar]
- 6.Coale KH, et al. A massive phytoplankton bloom induced by an ecosystem-scale iron fertilization experiment in the equatorial Pacific Ocean. Nature. 1996;383:495–501. doi: 10.1038/383495a0. 501. [DOI] [PubMed] [Google Scholar]
- 7.Landry MR, et al. Biological response to iron fertilization in the eastern equatorial Pacific (IronEx II). I. Microplankton community abundances and biomass. Mar Ecol Prog Ser. 2000;201:27–42. [Google Scholar]
- 8.Boyd PW, et al. A mesoscale phytoplankton bloom in the polar Southern Ocean stimulated by iron fertilization. Nature. 2000;407:695–702. doi: 10.1038/35037500. [DOI] [PubMed] [Google Scholar]
- 9.Gervais F, Riebesell U, Gorbunov MY. Changes in primary productivity and chlorophyll a in response to iron fertilization in the Southern Polar Frontal Zone. Limnol Oceanogr. 2002;47:1324–1335. [Google Scholar]
- 10.Coale KH, et al. Southern Ocean iron enrichment experiment: Carbon cycling in high- and low-Si waters. Science. 2004;304:408–414. doi: 10.1126/science.1089778. [DOI] [PubMed] [Google Scholar]
- 11.Peloquin JA, Smith WO. The role of phytoplankton size on photochemical recovery during the southern ocean iron experiment. J Phycol. 2006;42:1016–1027. [Google Scholar]
- 12.Trainer VL, et al. Domoic acid production near California coastal upwelling zones, June 1998. Limnol Oceanogr. 2000;45:1818–1833. [Google Scholar]
- 13.Trainer VL, et al. An ecological study of a massive toxigenic bloom of Pseudo-nitzschia cuspidata off the Washington State coast. Limnol Oceanogr. 2009;54:1461–1474. [Google Scholar]
- 14.Scholin CA, et al. Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature. 2000;403:80–84. doi: 10.1038/47481. [DOI] [PubMed] [Google Scholar]
- 15.Assmy P, Henjes J, Klaas C, Smetacek V. Mechanisms determining species dominance in a phytoplankton bloom induced by the iron fertilization experiment EisenEx in the Southern Ocean. Deep Sea Res I. 2007;54:340–362. [Google Scholar]
- 16.Marchetti A, et al. Identification and assessment of domoic acid production in oceanic Pseudo-nitzschia (Bacillariophyceae) from iron-limited waters in the Northeast Subarctic Pacific. J Phycol. 2008;44:650–661. doi: 10.1111/j.1529-8817.2008.00526.x. [DOI] [PubMed] [Google Scholar]
- 17.Trainer VL, Hickey BM, Bates SS. Diatoms. In: Walsh PJ, et al., editors. Oceans and Human Health: Risks and Remedies from the Sea. New York: Elsevier Science Publishers; 2008. pp. 219–238. [Google Scholar]
- 18.Garthwaite I, et al. Polyclonal antibodies to domoic acid, and their use in immunoassays for domoic acid in sea water and shellfish. Nat Toxins. 1998;6:93–104. [PubMed] [Google Scholar]
- 19.Wells ML, Trick CG, Cochlan WP, Hughes M, Trainer VT. Domoic acid: The synergy of iron, copper and the toxicity of diatoms. Limnol Oceanogr. 2005;50:1908–1917. [Google Scholar]
- 20.Maldonado MT, Hughes M, Rue E, Wells ML. The effect of Fe and Cu on the growth and domoic acid production of Pseudo-nitzschia multiseries and Pseudo-nitzschia australis. Limnol Oceanogr. 2002;47:515–526. [Google Scholar]
- 21.Wells ML. Manipulating iron availability in nearshore waters. Limnol Oceanogr. 1999;44:1002–1008. [Google Scholar]
- 22.Rue E, Bruland K. Domoic acid binds iron and copper: a possible role for the toxin produced by the marine diatom Pseudo-nitzschia. Mar Chem. 2001;76:127–134. [Google Scholar]
- 23.Trainer VL, et al. Recent domoic acid closures of shellfish harvest areas in Washington State inland waterways. Harmful Algae. 2007;6:449–459. [Google Scholar]
- 24.Bargu S, et al. Krill: A potential vector for domoic acid in marine food webs. Mar Ecol Prog Ser. 2002;237:209–216. [Google Scholar]
- 25.Lefebvre KA, Silver MW, Coale SL, Tjeerdema RS. Domoic acid in planktivorous fish in relation to toxic Pseudo-nitzschia cell densities. Mar Biol. 2002;140:625–631. [Google Scholar]
- 26.Bargu S, Marinovic B, Mansergh S, Silver MW. Feeding responses of krill to the toxin-producing diatom Pseudo-nitzschia. J Exp Mar Biol Ecol. 2003;284:87–104. [Google Scholar]
- 27.Goldstein T, et al. Novel symptomatology and changing epidemiology of domoic acid toxicosis in California sea lions (Zalophus californianus): An increasing risk to marine mammal health. Proc R Soc Lond B Biol Sci. 2008;275:267–276. doi: 10.1098/rspb.2007.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lovelock JE, Rapley CG. Ocean pipes could help the Earth to cure itself. Nature. 2007;449:403–404. doi: 10.1038/449403a. [DOI] [PubMed] [Google Scholar]
- 29.Barron R. Climos seeks $10M to $12M to fertilize ocean. Greentechmedia. 2008. http://blogs.greentechmedia.com/articles/read/climos-seeks-10m-to-12m-to-fertilize-ocean-923/. Accessed July 7, 2008.
- 30.Hillebrand H, Durselen C, Kirschtel D, Pollingher U, Zohary T. Biovolume calculation for pelagic and benthic microalgae. J Phycol. 1999;35:403–424. [Google Scholar]
- 31.Rhodes L, White D, Sybre M, Atkinson M. Pseudo-nitzschia species isolated from New Zealand coastal waters: Domoic acid production in vitro and links with shellfish toxicity. In: Yasumoto T, Oshima Y, Fukuyo Y, editors. Harmful and Toxic Algal Blooms. Paris, France: UNESCO, Intergovernmental Oceanographic Commission; 1996. pp. 155–158. [Google Scholar]
- 32.Bates SS. Ecophysiology and metabolism of ASP toxin production. In: Anderson DM, Cembella AD, Hallegraeff GM, editors. Physiological Ecology of Harmful Algal Blooms. Heidelberg: Springer-Verlag; 1998. pp. 405–426. [Google Scholar]
- 33.Welschmeyer NA. Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and phaeopigments. Limnol Oceanogr. 1994;39:1985–1992. [Google Scholar]
- 34.Lundholm N, Kotaki Y, Hoef-Emden K, Scholin C, Miller P. Inter- and intraspecific variation of the Pseudo-nitzschia delicatissima complex (Bacillariophyceae) illustrated by rRNA probes, morphological data and phylogenetic analyses. J Phycol. 2006;42:464–481. [Google Scholar]
- 35.Hutchins DA, et al. A shipboard natural community continuous culture system for ecologically relevant low-level nutrient enrichment experiments. Limnol Oceanogr Methods. 2003;1:82–91. [Google Scholar]
- 36.Pickell LD, Wells ML, Trick CG, Cochlan WP. A sea-going continuous culture system for investigating phytoplankton community response to macro- and micro-nutrient (trace metal) manipulations. Limnol Oceanogr Methods. 2009;7:21–32. [Google Scholar]
- 37.Coale KH, et al. IronEx-I, an in situ iron-enrichment experiment: Experimental design, implementation and results. Deep Sea Res II. 1998;45:919–945. [Google Scholar]
- 38.Martin JH, et al. Testing the iron hypothesis in ecosystems of the equatorial pacific ocean. Nature. 1994;371:123–129. [Google Scholar]
- 39.Cavender-Bares KK, Mann EL, Chisholm SW, Ondrusek ME, Bidigare RR. Differential response of equatorial pacific phytoplankton to iron fertilization. Limnol Oceanogr. 1999;44:237–246. [Google Scholar]
- 40.Tsuda A, et al. A mesoscale iron enrichment in the western subarctic Pacific induces a large centric diatom bloom. Science. 2003;300:958–961. doi: 10.1126/science.1082000. [DOI] [PubMed] [Google Scholar]
- 41.Kudo I, et al. Primary productivity and nitrogenous nutrient assimilation dynamics during the Subarctic Pacific Iron Experiment for Ecosystem Dynamics Study. Prog Oceanogr. 2005;64:207–221. [Google Scholar]
- 42.Tsuda A, et al. Evidence for the grazing hypothesis: Grazing reduces phytoplankton responses of the HNLC ecosystem to iron enrichment in the western subarctic Pacific (SEEDS II) J Oceanogr. 2007;63:983–994. [Google Scholar]
- 43.Marchetti A, Sherry ND, Kiyosawa H, Tsuda A, Harrison PJ. Phytoplankton processes during a mesoscale iron enrichment in the NE subarctic Pacific: Part I—biomass and assemblage. Deep Sea Res II. 2006;53:2095–2113. [Google Scholar]
- 44.Boyd PW, et al. The evolution and termination of an iron-induced mesoscale bloom in the northeast subarctic Pacific. Limnol Oceanogr. 2005;50:1872–1886. [Google Scholar]
- 45.Gall MP, Boyd PW, Hall J, Safi KA, Chang H. Phytoplankton processes part 1: Community structure during the Southern Ocean Iron Release Experiment (SOIREE) Deep Sea Res II. 2001;48:2551–2570. [Google Scholar]
- 46.Hoffmann LJ, Peeken I, Lochte K. Different reactions of Southern Ocean phytoplankton size classes to iron fertilization. Limnol Oceanogr. 2006;51:1217–1239. [Google Scholar]
- 47.Jacquet SHM, Savoye N, Dehairs F, Strass VH, Cardinal D. Mesopelagic carbon remineralization during the European Iron Fertilization Experiment. Global Biogeochem Cycles. 2008;22:GB1023. [Google Scholar]
- 48.Harvey M, Hall J, Ho D. Surface ocean–lower atmosphere activities in New Zealand: The SAGE experiment. Global Change Newsletter. 2004;58:8–10. [Google Scholar]