Abstract
CD4+ helper T (Th) cells differentiate toward distinct effector cell lineages characterized by their distinct cytokine expression patterns and functions. Multiple Th cell populations secrete IL-22 that contributes to both protective and pathological inflammatory responses. Although the differentiation of IL-22-producing Th cells is controlled by the aryl hydrocarbon receptor (AhR), little is known about the regulatory mechanisms inducing physiological stimulators for AhR. Here, we show that Notch signaling enhances IL-22 production by CD4+ T cells by a mechanism involving AhR stimulation. Notch-mediated stimulation of CD4+ T cells increased the production of IL-22 even in the absence of STAT3. CD4+ T cells from RBP-J-deficient mice had little ability to produce IL-22 through T cell receptor-mediated stimulation. RBP-J-deficient mice were highly susceptible to the detrimental immunopathology associated with ConA-induced hepatitis with little IL-22 production by CD4+ T cells. Exogenous IL-22 protected RBP-J-deficient mice from ConA-induced hepatitis. Notch signaling promoted production of endogenous stimulators for AhR, which further augmented IL-22 secretion. Our studies identify a Notch–AhR axis that regulates IL-22 expression and fine-tunes immune system control of inflammatory responses.
Keywords: inflammation, cytokine
Interleukin (IL)-22 belongs to the IL-10 superfamily of cytokines and exhibits potent proinflammatory and anti-inflammatory properties (1–4). IL-22 is highly expressed in IFN-γ (Th1) and IL-17-producing CD4+ T helper cells (Th17), and recent studies have demonstrated that dendritic cells, NK cells, and lymphoid tissue-inducer cells also produce IL-22 (4). Although IL-6 and TGF-β are required for Th17 development, IL-6 induces IL-22 production, whereas TGF-β has a suppressive effect (5). Recent studies have demonstrated that ligation of the aryl hydrocarbon receptor (AhR) drives Th17 differentiation and IL-22 expression (6–8), although exogenous or endogenous ligands for AhR and inducers for endogenous ligands remain to be clarified. Furthermore, it also remains to be clarified if the same regulatory mechanism controls IL-22 expression in distinct Th subsets, including Th1 and Th17, and other cells.
Notch is an evolutionally conserved molecule and controls cell fate decision in a variety of cells (9, 10). Notch molecules are cleaved in the transmembrane region by γ-secretase after interaction with their ligands, followed by intracellular domain translocation into the nucleus (9, 10). We and other groups have demonstrated that Notch signaling controls the effector functions of both CD4+ and CD8+ T cells (11–15).
In this report we investigated the possibility that Notch controls IL-22 expression in CD4+ T cells and found that deletion of RBP-J impaired IL-22 production in CD4+ T cells. Notch signaling was able to up-regulate IL-22 even in STAT3-deficient T cells. This up-regulation of IL-22 was due to Notch-mediated production of AhR stimulators. These data indicate a regulatory mechanism of the immune system through IL-22 production by the Notch-AhR axis.
Results
Overexpression of Intracellular Domain of Notch in CD4+ T Cells Upregulates IL-22 Independent of Th17 Differentiaiton.
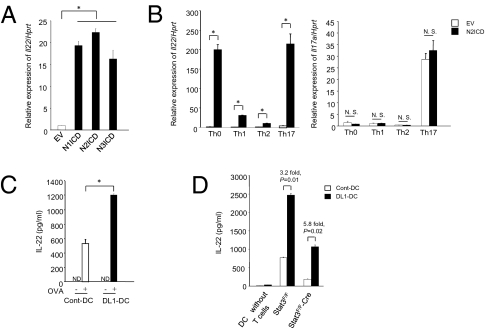
We searched by DNA microarray for genes up-regulated by the transduction of the intracellular domain of Notch2 (N2ICD), the active form of Notch2, in DO11.10 T cell hybridomas. This analysis identified a strong induction of IL-22 in N2ICD-transduced cells. To confirm IL-22 induction by Notch signaling, we introduced the intracellular domain of Notch1 (N1ICD), Notch2 (N2ICD), and Notch3 (N3ICD) into primary splenic T cells stimulated with anti-CD3 mAb and examined the expression of IL-22 in CD4+ T cells by real-time PCR after 48 h. CD4+ T cells transduced with each Notch intracellular domain had significantly increased expression of IL-22 compared with control mock-transduced cells (Fig. 1A). To confirm the contribution of CD4+ T cells in our system, we checked the purity of CD4+ T cells after MACS purification. Approximately 97% cells were positive for CD4 after MACS purification (Fig. S1A). RBP-J-deficient T cells did not show any increase in IL-22 production when transduced with N2ICD (Fig. S1B), indicating that Notch-mediated IL-22 up-regulation depends on RBP-J. We also tested the expression of other cytokines like IL-17A, IFN-γ, IL-4, TNF-α, and IL-10, along with other IL-10 family members, after transducing N2ICD into splenic T cells. Notch signaling only up-regulated the expression of IL-4 and IFN-γ (Fig. S1C), suggesting a specific effect of Notch on particular cytokines’ expression rather than a nonspecific regulatory effect of Notch on T cell effector functions. We also checked the expression of transcription factors associated with different helper T cell differentiation and found that both T-bet and Gata-3 expression was up-regulated after N2ICD transduction, whereas RORγt expression that is required for Th17 differentiation (16) was unchanged (Fig. S1D). These data indicate that Notch functions to specifically up-regulate IL-22 expression without affecting Th17 differentiation and other IL-10 family cytokines, excluding a possibility that Notch controls IL-22 expression by nonspecific regulatory effect on T cell effector functions.
Fig. 1.
Enforced expression of Notch in T cells induces IL-22. (A) Total spleen cells from C57BL/6 mice were stimulated with soluble anti-CD3 mAb (1 μg/mL) for 24 h and transduced with a retrovirus carrying N1ICD, N2ICD, N3ICD, or a control virus. Cells were further stimulated with anti-CD3 mAb (1 μg/mL) for 48 h. Then, the expression of Il22 in MACS-enriched CD4+ T cells was analyzed by real-time PCR. (B) Total spleen cells were stimulated with anti-CD3 mAb in Th0, Th1, Th2, and Th17 conditions for 24 h and then transduced with N2ICD or control virus. After 48 h of further culture under the same conditions, Il22 and Il17a expression in MACS-enriched CD4+ T cells was measured by real-time PCR. (C) ELISA for the detection of IL-22 secretion from naive CD4+ T cells (CD4+CD62L+) isolated from OT-II TCR transgenic mice stimulated with OVA peptide-pulsed Cont-DC (open) or DL1-DC (filled) for 3 days. (D) ELISA for the detection of IL-22 secretion from naive CD4+ T cells isolated from STAT3F/F or STAT3F/F-Cre transgenic mice stimulated with allogenic Cont-DC (open) or DL1-DC (filled) prepared from BALB/c mice. *, P < 0.05, indicates a statistically significant difference. Data are representative of at least four independent experiments. N.D., not detected; N.S., not significant.
Stimulation of CD4+ T Cells by Notch Ligand Upregulates IL-22 in CD4+ T Cells.
To clarify the direct contribution of Notch signaling in CD4+ T cells in terms of IL-22 secretion, we purified CD4+ T cells 48 h after transduction of total spleen cells or purified CD4+ T cells with N2ICD or EV and measured the expression of IL-22 by real-time PCR. Similar to experiments with total splenocytes, we found that N2ICD transduction caused increased expression of IL-22 in purified naive CD4+ T cells, although IL-22 expression by CD4+ T cells was higher in the presence of APC compared with purified naive CD4+ T cells (Fig. S2A). To identify the effector T cell type responding to Notch, we introduced N2ICD into primary spleen cells under Th0, Th1, Th2, and Th17-promoting conditions. We found that expression of IL-22 in CD4+ T cells was increased by Notch signaling in all culture conditions (Fig. 1B Left) but not IL-17A (Fig. 1B Right). In particular, the effect of Notch signaling was great under Th0 or Th17 condition. The expression of IL-17 was comparable among Th0, Th1, and Th2 conditions. Those data again suggest that Notch-mediated IL-22 production is not necessarily dependant on Th17 differentiation. We also checked the expression of IL-4 and IFN-γ in all culture conditions and found induced expressions of both cytokines after N2ICD transduction (Fig. S2B). The expression of Notch1 and Notch2 was comparable for each Th condition (Fig. S2C).
We further examined the effect of Notch signaling on IL-22 expression by directly stimulating T cells with Notch ligand. To induce Notch signaling in CD4+ T cells, we stimulated naïve OT-II TCR transgenic T cells with bone marrow-derived, OVA peptide-pulsed dendritic cells (BMDCs) transduced with Delta-like 1 (DL1-DC) for 3 days. DL1-DC-stimulated OT-II T cells secreted more IL-22 in the culture supernatant than when mock-transduced DCs (Cont-DC) were used (Fig. 1C). The secretion of IFN-γ was also induced with DL1-DC-stimulated OT-II T cells (Fig. S3A). We also stimulated naive OT-II CD4+ T cells with Cont-DC or DL1-DC under neutral, Th1, Th2, or Th17 conditions for 3 days and found increased expression of IL-22 by DL1-DC in all culture conditions (Fig. S3B). These data indicate that Notch-mediated stimulation of CD4+ T cells up-regulates IL-22 expression under any helper T cell culture condition.
Notch Signaling Controls IL-22 Expression in CD4+ T Cells in the Absence of STAT3.
Th17 is one T cell lineage that expresses IL-22, although Th1, γδ T cells, NK cells, NKT cells, and DC can also secrete IL-22 (17). IL-6 and TGF-β are essential cytokines for Th17 differentiation, and IL-23 is responsible for the survival and expansion of Th17 cells (17). Therefore, we examined whether these cytokines took part in Notch signaling-induced IL-22 production. For this purpose, we measured IL-22 secretion during an allo-response, coculturing DL1-DC or Cont-DC prepared from BALB/c mice with naive CD4+ T cells from STAT3flox/flox (STAT3F/F) or STAT3flox/flox crossed with lck-Cre transgenic (STAT3F/F-Cre) mice (18). IL-22 production was reduced in STAT3F/F-Cre CD4+ T cells when either Cont-DC or DL1-DC was used as stimulators, indicating that STAT3 is important for IL-22 production (Fig. 1D). We further found that DL1-DC could up-regulate IL-22 secretion even from STAT3F/F-Cre CD4+ T cells (Fig. 1D). This value is significantly higher than that of the Cont-DC-stimulated STAT3F/F-CD4+ T cells. Hence, these data indicate that forced Notch signaling can induce IL-22 production from CD4+ T cells with mechanisms distinct from STAT3 signaling pathway.
RBP-J Deficiency in CD4+ T Cells Impairs IL-22 Production.
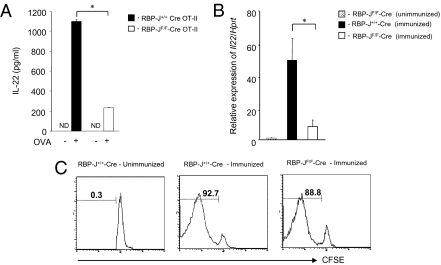
RBP-J is a transcription factor essential for Notch signaling (9). Next, we investigated IL-22 production in CD4+ T cells in the absence of RBP-J. When CD4+ T cells from RBP-Jflox/flox mice crossed with both CD4-Cre and OT-II TCR transgenic (RBP-JF/F-Cre OT-II) mice were stimulated with OVA peptide presented by BMDCs, their IL-22 secretion was impaired in contrast to RBP-J+/+ mice crossed with CD4-Cre and OT-II TCR transgenic (RBP-J+/+-Cre OT-II) mice (Fig. 2A). RBP-J deficiency also caused decreased secretion of IFN-γ in this system (Fig. S3C). We did not see any difference between T cells from RBP-J+/+-Cre OT-II and RBP-JF/F-Cre OT-II mice in their proliferative responses to peptide-pulsed DCs (Fig. S3D).
Fig. 2.
Deficiency of Notch signaling in CD4+ T cell impairs IL-22 expression. (A) Naive CD4+ T cells isolated from RBP-J+/+-Cre OT-II (filled) or RBP-JF/F-Cre OT-II (open) mice were stimulated with OVA peptide-pulsed BMDCs for 3 days. IL-22 concentrations in the supernatants were evaluated by ELISA. (B) CD4+ T cells were isolated from RBP-J+/+-Cre (filled) and RBP-JF/F-Cre (open) mice 7 days after immunization with OVA emulsified in CFA. As a control, CD4+ T cells from unimmunized RBP-JF/F-Cre mice (dot) were isolated. Il22 expression in CD4+ T cells was evaluated by real-time PCR. (C) CFSE-labeled splenic T cells from RBP-J+/+-Cre OT-II or RBP-JF/F-Cre OT-II mice were transferred into C57BL/6 Thy1.1 mice that were immunized with OVA protein emulsified in CFA soon after T cell transfer. CFSE dilution was evaluated after gating on CD4+ Thy1.2+ cells 7 days after OVA immunization. *, P < 0.05, indicates a statistically significant difference. Data in Fig. 2 are representative of at least four independent experiments.
To further confirm the contribution of Notch signaling in BMDC coculture system, we stimulated naive CD4+ T cells isolated from OT-II TCR transgenic mice with OVA-pulsed BMDCs in the presence of γ-secretase inhibitor. The γ-secretase inhibitor decreased IL-22 production from CD4+ T cells (Fig. S3E). These data indicate that Notch signaling controls IL-22 secretion from CD4+ T cells through γ-secretase-mediated cleavage of Notch and RBP-J.
To further examine the involvement of RBP-J in IL-22 production in vivo, we immunized RBP-Jflox/flox mice crossed with CD4-Cre transgenic (RBP-JF/F-Cre) mice with OVA emulsified in complete Freund’s adjuvant (CFA) and examined IL-22 expression in CD4+ T cells 7 days after immunization. IL-22 expression was highly impaired in RBP-JF/F-Cre mice compared with control RBP-J+/+-Cre mice (Fig. 2B), whereas IL-17A expression was intact (Fig. S4A). These data also reveal that Notch signaling controls IL-22 expression independent of Th17 differentiation. We also investigated the proliferation of CD4+ T cells in OVA-immunized mice, and there was no difference between RBP-JF/F-Cre and RBP-J+/+-Cre mice (Fig. 2C). To test which Notch receptor contributes to IL-22 production in vivo, we immunized Notch1F/F-Cre (Notch1flox/flox mice crossed with CD4-Cre transgenic mice), Notch2F/F-Cre (Notch2flox/flox mice crossed with CD4-Cre transgenic mice), and RBP-JF/F-Cre mice with OVA emulsified in CFA and examined IL-22 expression in CD4+ T cells 7 days after immunization. Although IL-22 expression was highly impaired in RBP-JF/F-Cre mice compared with control RBP-J+/+-Cre mice, IL-22 expression was not impaired under each Notch receptor deficiency (Fig. S4B). Taken together, including both in vitro and in vivo data, RBP-J is important for IL-22 production but not for IL-17A, indicating that Notch signaling controls IL-22 expression, although it remains unclear which Notch receptor is involved. These data also prove that the expression of IL-17 and IL-22 are differentially regulated.
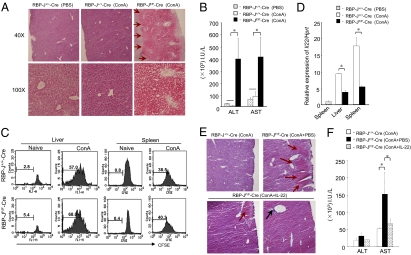
RBP-J-Deficient Mice Have High Susceptibility to ConA-Induced Hepatitis.
IL-22 is a pivotal cytokine in several inflammatory diseases, although its role remains controversial (3). That is, it is proinflammatory in psoriasis (5) and anti-inflammatory in irritable bowel disease (19) and Con A (ConA)-induced hepatic injury (20). We employed the ConA-hepatitis model in RBP-JF/F-Cre mice to further define the contribution of Notch in IL-22 secretion in the context of autoimmunity. We injected several doses of ConA into mice and quantified the severity of hepatitis 48 h after injection. Both RBP-J+/+-Cre and RBP-JF/F-Cre mice had similar hepatic damage in response to high-dose (30 μg/g) injection of ConA. However, only RBP-JF/F-Cre mice exhibited hepatic injury, including severe inflammation and bleeding, after a relatively low-dose (10 μg/g) ConA injection that did not induce hepatitis in control RBP-J+/+-Cre mice (Fig. 3A). This increased susceptibility to low-dose ConA-induced hepatitis in RBP-JF/F-Cre mice was also confirmed by the elevated liver enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (Fig. 3B). We examined CD4+ T cell proliferation in vivo, and there was no difference in ConA-induced T cell proliferation between the two groups (Fig. 3C). The IL-22 transcription was highly impaired in CD4+ T cells from RBP-JF/F-Cre mice in both liver and spleen (Fig. 3D). However, we found that the expression of other inflammatory cytokines in liver and splenic CD4+ T cells of ConA-injected mice, such as IFN-γ, IL-4, TNF-α, IL-17A, and the anti-inflammatory cytokine IL-10, was comparable between the two groups (Fig. S5A). Furthermore, expression of FasL was also comparable between two groups (Fig. S5B). To know if non-CD4+ T cells also express IL-22, we checked the expression of IL-22 in NK, NKT, or CD8+ T cells. We injected ConA (10 μg/g) to wild-type C57BL/6 mice and after 48 h of injection, we sorted CD4, CD8, NKT (NK1.1 and CD3 double positive), and NK (CD3 negative and NK1.1 positive) cells from liver and checked IL-22 expression by real-time PCR. As expected, IL-22 expression was only up-regulated in CD4+ T cells (Fig. S5C), suggesting that CD4+ T cells provide protection in ConA-induced acute hepatitis by secreting IL-22. These results suggest that the increased susceptibility to ConA-induced hepatic injury in RBP-JF/F-Cre mice might be due to decreased production of protective IL-22 by CD4+ T cells.
Fig. 3.
Notch deficiency in T cells results in susceptibility to ConA-induced hepatitis. The RBP-J+/+-Cre or RBP-JF/F-Cre mice received ConA (10 μg/g) and/or IL-22 (2 μg per mouse). As a control, RBP-JF/F-Cre mice received PBS. (A) Liver sections 48 h after ConA injection were stained with hematoxylin and eosin. Red arrows indicate bleeding. (B) The ALT and AST levels in the serum of each mouse were measured. (C) CFSE-labeled total spleen cells from RBP-J+/+-Cre or RBP-JF/F-Cre mice were transferred into C57BL/6 Thy 1.1 mice, and transferred mice received ConA. Lymphocytes were purified from liver and spleen by Lympholyte M 48 h after ConA injection, and CFSE dilution was plotted after gating on CD4+Thy1.2+ cells. (D) RBP-J+/+-Cre or RBP-JF/F-Cre mice received ConA (10 μg/g). CD4+ T cells were purified from livers and spleens 48 h after ConA injection by Lympholyte M and MACS beads and Il22 mRNA was measured by real-time PCR. (E) RBP-J+/+-Cre and RBP-JF/F-Cre mice received ConA (10 μg/g) with IL-22 (2 μg) or PBS. Liver sections 48 h after ConA and IL-22 injection were stained with hematoxylin and eosin. Red arrows indicate bleeding, and black arrow indicates cell infiltration. (F) The ALT and AST levels in the serum of each mouse were measured. *, P < 0.05, indicates a statistically significant difference. Data in Fig. 3 are representative of at least four independent experiments.
We next tested whether exogenous IL-22 administration is able to cure ConA-hepatitis in RBP-JF/F-Cre mice by compensating for low or absent endogenous IL-22. The low-dose ConA injection did not induce any pathological change in RBP-J+/+-Cre mice, whereas the injection of ConA induced severe bleeding in the liver in RBP-JF/F-Cre mice (Fig. 3E Upper). When we administered recombinant IL-22 along with ConA in RBP-JF/F-Cre mice, 25% of the mice showed mild bleeding (Fig. 3E Lower Left) and 75% had no bleeding at all in the liver (Fig. 3E Lower Right). Furthermore, serum AST levels were also significantly decreased by IL-22 injection in RBP-JF/F-Cre mice (Fig. 3F). Hence, the ConA-induced IL-22 production depends on Notch signaling, as evidenced by the ability of IL-22 to protect RBP-J-deficient mice from liver injury.
Notch Controls IL-22 by Affecting a Signaling Pathway Through AhR.
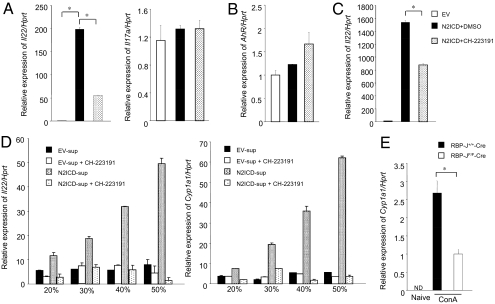
There is no consensus RBP-J binding element in the 800 bp proximal promoter of IL-22 conserved between human and mouse, suggesting that Notch indirectly regulates the induction of IL-22. Recent reports describe the essential role of the AhR in IL-22 production (6–8). Therefore, we checked whether IL-22 production due to Notch signaling depended on AhR signaling. Blocking AhR signaling by the AhR antagonist CH-223191 (2-Methyl-2H-pyrazole-3-carboxilic acid-(2-methyl-4-o-tolyl-azophenyl)-amide) (20 μM) in N2ICD-transduced T cells preferentially decreased IL-22 expression (Fig. 4A Left) and was concentration dependent (Fig. S6A), whereas IL-17A expression was unaffected (Fig. 4A Right). Next, we tested whether the expression of AhR was influenced by N2ICD transduction and found that AhR expression was comparable with mock transduction (Fig. 4B). We also examined AhR expression under different effector T cell culture conditions and found that although AhR expression was highest under Th17 conditions, N2ICD did not further up-regulate AhR expression under any of the culture conditions (Fig. S6B). These data do not suggest that Notch-mediated IL-22 production depends on AhR expression itself. Similarly, AhR antagonism significantly decreased the expression of IL-22 in N2ICD-transduced DO11.10 T cell hybridoma cells without T cell receptor-mediated signaling (Fig. 4C).
Fig. 4.
Notch signaling induces AhR stimulators. Total spleen cells were stimulated with anti-CD3 mAb for 24 h and transduced with a retrovirus carrying N2ICD or a control vector. After infection, cells were restimulated with anti-CD3 mAb for 48 h in the presence (filled) or absence (open) of an AhR antagoinst. Then Il22 and Il17a (A) or AhR expression in CD4+ T cells (B) was measured by real-time PCR. (C) The expression of Il22 after retroviral transduction of N2ICD (filled or dot) or EV (open) in DO11.10 T cells hybridoma in the presence (filled) or absence (dot) of the AhR antagonist was examined by real-time PCR. (D) The supernatant from total spleen cells transduced with N2ICD (dot or shaded) or EV (open or filled) was collected 72 h after initial stimulation. CD4+ T cells from C57BL/6 mice were stimulated with anti-CD3 mAb in the presence of different concentrations of supernatant and in the presence (open, shaded) or absence (filled, dot) of an AhR antagonist. The expression of Il22 (Left) and Cyp1a1 (Right) in mature CD4+ T cells was tested 48 h after initial stimulation. (E) The RBP-J+/+-Cre or RBP-JF/F-Cre mice received ConA (10 μg/g) and the expression of Cyp1a1 in liver CD4+ T cells purified by MACS beads 48 h after ConA injection was measured by real-time PCR. *, P < 0.05, indicates a statistically significant difference. Data in Fig. 4 are representative of at least three independent experiments.
Notch Signaling Allows CD4+ T Cells To Produce AhR Stimulators.
According to the results that IL-22 induction by Notch signaling was inhibited by AhR antagonism, and that Notch signaling does not up-regulate AhR, we hypothesized that Notch signaling might increase an endogenous AhR agonist. To test this hypothesis, we harvested the culture supernatant from total T cells transduced with N2ICD (N2ICD-sup) and added dilutions of this supernatant during the stimulation of CD4+ T cells. The CD4+ T cells with N2ICD-sup significantly increased IL-22 expression compared with that of control supernatant (EV-sup), and in a dose-dependent manner (Fig. 4D Left). The up-regulation of IL-22 by adding N2ICD-sup was suppressed by an AhR antagonist. Indeed, N2ICD-sup also increased expression of Cyp1a1, the typical downstream target gene of AhR signaling (Fig. 4D Right), indicating that N2ICD-sup contained a factor(s) competent to induce AhR signaling. Because overexpression of N2ICD in CD4+ T cells induced both IL-4 and IFN-γ expression (Figs. S1C and 2B), we checked whether N2ICD-sup affects IL-4 and IFN-γ expression in CD4+ T cells. We found that N2ICD-sup did not affect the expression of both cytokines (Fig. S6C), suggesting that Notch-AhR axis is not involved in Th1 or Th2 differentiation and specifically contributes to IL-22 secretion.
To identify the effector T cell culture conditions that best promote the production of AhR stimulators, we used N2ICD-transduced total splenocytes supernatants from different effector T cell culture conditions to stimulate spleen cells. We found that using the supernatants collected from N2ICD-transduced Th0 and Th17 conditions resulted in the highest IL-22 expression (Fig. S6D). We also considered the issue of whether DC present in the culture could induce AhR stimulators. We introduced N2ICD in total splenocytes and purified CD4+ T cells and cultured for 48 h. The collected supernatants were used to stimulate purified CD4+ T cells in the presence of anti-CD3 mAb. We observed increased expressions of both IL-22 and Cyp1a1 when N2ICD supernatant was used (Fig. S6E). This effect was stronger in the presence of APC than in the absence of APC (Fig. S6E), probably because of efficient T cell stimulation in the presence of APC.
To identify AhR stimulator production in vivo, we purified CD4+ T cells from livers 48 h after ConA injection of RBP-J+/+-Cre and RBP-JF/F-Cre mice and restimulated such cells with anti-CD3 mAb or left unstimulated for 24 h. Finally, we used such supernatant to restimulate total splenic CD4+ T cells for 48 h in the presence of anti-CD3 mAb (Fig. S6F). The supernatant of purified CD4+ T cells of RBP-J+/+-Cre mice had induced Cyp1a1 expression and IL-22 (Fig. S6F). The supernatant collected from restimulated cells of RBP-JF/F-Cre mice had no effect on either Cyp1a1 or IL-22 (Fig. S6F). To confirm this finding in vivo, we measured Cyp1a1 expression in CD4+ T cells from livers 48 h after ConA injection in RBP-J+/+-Cre and RBP-JF/F-Cre mice. The expression of Cyp1a1 was lower in T cells from RBP-JF/F-Cre mice than in those from RBP-J+/+-Cre mice (Fig. 4E). These results indicate that Notch signaling up-regulated IL-22 expression by inducing a natural ligand that resulted in AhR signaling.
We tested up-regulated genes in T cell hybridoma transduced with N2ICD by DNA microarray and evaluated genes involved in cell development, signal transduction, and metabolism (Table S1). However, we could not find any molecules related to tryptophan metabolism. Therefore, we evaluated whether the 6-formylindolo [3,2-b] carbazole (FICZ), a tryptophan photoproduct and an only known endogenous ligand for AhR signaling, is involved in Notch-mediated IL-22 production (21). Hence, we used Sep-Pak Plus C18 cartridges to track FICZ activity in our system because this cartridge can trap FICZ very efficiently (21). We stimulated total spleen cells with N2ICD supernatant, flow-through, and eluate of a Sep-Pak Plus C18 cartridge in conjunction with anti-CD3 mAb. The expression of both IL-22 and Cyp1a1 was increased by N2ICD-transduced supernatant and flow-through compared with that of EV (Fig. S7A). In contrast, we could not detect any such activity in any elute fraction, suggesting that FICZ is not involved in our system. To examine whether the AhR stimulator is a heat labile protein, we heated the flow-through to 95 °C for 10 min and used this flow-through to restimulate total spleen cells. We found that heating the flow-through collected from N2ICD-transduced supernatant abrogated the up-regulation of IL-22 and Cyp1a1 (Fig. S7B), suggesting that Notch signaling induces the production of heat labile molecules as AhR stimulators.
Discussion
Several studies have highlighted the importance of IL-22 in inflammatory responses (20, 22, 23). Likewise, the role of AhR in Th17 cells producing IL-22 has recently received much attention (6, 7), although the environmental and endogenous agents that control AhR-mediated IL-22 expression remain unclear. The present data demonstrate that Notch signaling is crucial for IL-22 production from CD4+ T cells, which depends on the production of endogenous stimulators of AhR signaling, but not STAT3. Blockade of Notch signaling in T cells increased susceptibility for ConA-induced inflammatory hepatic injury due to dramatically reduced IL-22 production, stressing the crucial role of Notch signaling in IL-22 production. Because there are many endogenous molecules able to activate AhR (24), the physiological ligands for AhR in terms of Notch-mediated IL-22 production will be an interesting issue to be addressed in the context of regulating inflammatory responses.
Mounting evidence supports that IL-22 production depends on IL-23 or IL-6 (17). The present study revealed that Notch signaling is able to up-regulate IL-22 production from CD4+ T cells even in the absence of STAT3 or up-regulation of RORγt, which are crucial for IL-6 signaling or Th17 differentiation, respectively (16). These data indicate that Notch-mediated IL-22 production is independent of Th17 differentiation, which also excludes the possibility that Notch-mediated IL-22 production results from nonspecific regulatory roles of Notch on T cell effector functions. As for the relationship between Notch and STAT3 signaling, our data suggest that IL-22 production is controlled by several signaling pathways, although each pathway might converge. It would be interesting to test whether STAT3 or Notch-AhR axis-mediated IL-22 production has distinct roles in immune responses, or whether distinct cell types use a particular signaling pathway to induce IL-22. In addition, we found in this study that overexpression among active Notch1, Notch2, or Notch3 in CD4+ T cells is able to up-regulate IL-22, indicating that any Notch signaling is able to induce IL-22. We did not observe decreased IL-22 production in Notch1- or Notch2-deficient mice, although it is possible that Notch1 and Notch2 compensate each other.
Previous studies have demonstrated that Notch signaling controls many aspects of effector function of not only CD4+ but also CD8+ T cells (10–15). We and other groups have demonstrated that Notch signaling controls cytolytic effector functions in CD8+ T cells (13, 25), and recent studies have revealed that IL-22 is also produced by CD8+ T cells (17). Because the studies described in this report used CD4-Cre transgenic mice to delete the RBP-J gene, both CD4+ and CD8+ T cells lack the RBP-J gene in our system because of thymic expression of Cre in CD4+CD8+ T cells. Therefore, we cannot completely deny the possibility that Notch also controls IL-22 production from CD8+ T cells, which may contribute to the susceptibility of ConA-induced hepatitis. However, we think such a possibility is unlikely because we showed that CD8+ T cells did not up-regulate IL-22 after ConA injection. In addition, previous papers reported the contribution of NKT cells in the pathogenesis of ConA-induced hepatitis (26, 27). Although those studies revealed that the possible contribution of NKT cells is more likely inflammatory rather than anti-inflammatory, it would be important to determine whether Notch controls IL-22 production by NKT cells.
Present studies revealed that overstimulation of Notch in CD4+ T cells is able to up-regulate IL-22 under neutral, Th1, Th2, and Th17 promoting culture conditions. Those data suggest at least three possibilities. The first possibility is that Notch signaling is able to help express IL-22 even in fixed helper T cells. The second one is that Notch signaling up-regulates IL-22 in a small fraction of naïve CD4+ T cells that did not receive enough cytokine signaling to skew toward each helper cell lineage. The third one is that Notch signaling helps differentiation of new type of helper cells such as IL-22 and IFN-γ or IL-22 and IL-4 double producer. To analyze these possibilities in the future, it is necessary to examine cytokines and transcription factors in a single cell, by establishing specific antibodies useful for flow cytometoric analysis.
Our present studies have demonstrated that Notch-mediated stimulation of CD4+ T cells helps produce AhR stimulators. The AhR stimulators interact with CD4+ T cells, which up-regulates IL-22 in CD4+ T cells as we showed in ConA-induced hepatitis model, although AhR stimulators might also interact with other cells. We found that a known endogenous AhR ligand, FICZ, is not involved in Notch-induced AhR stimulation in our model system, although heat labile proteins do appear to be candidates for AhR stimulators. The identification of such endogenous AhR stimulators would contribute not only to our understanding of the mechanism how Notch controls IL-22 production in T cells, but also to our understanding of the other physiological roles of AhR in cellular responses.
Notch is crucial for a variety of behaviors in cells, including cell proliferation, tumorigenesis, cell fate decisions, and embryogenesis. It is thought that AhR-mediated environmental signals affect tumorigenesis and cell differentiation (28). For instance, overstimulation of AhR by environmental signals might enhance physiological Notch-mediated AhR stimulation, which would contribute to Notch over-activating phenotypes such as tumor cells or aberrant cell fate decisions. Therefore, the newly identified Notch-AhR axis would suggest not only a unique regulatory mechanism for inflammatory responses, but also a close link between the broad regulation of Notch-mediated cell differentiation and environmental signals. Our findings may also advocate for the manipulation of AhR pathway components as a means to modulate cell activation or as a therapy for Notch-mediated tumorigenesis.
Methods
Mice.
Female C57BL/6 mice (6–8 weeks old) and Thy1.1 C57BL/6 mice were obtained from Japan SLC or The Jackson Laboratory, respectively). RBP-Jflox/flox mice crossed with CD4-Cre mice (15, 29) were further crossed with OT-II TCR transgenic (Taconic) mice. STAT3flox/flox mice and STAT3flox/flox crossed with lck-Cre transgenic mice were reported (18). Mice were housed in the Animal Research Center of the University of Tokushima under specific pathogen-free conditions, and all animal work was approved by the Animal Research Committee of the University of Tokushima.
Cell Culture.
Naive CD4+ T cells (CD4+CD62L+) were isolated from spleens by paramagnetic bead enrichment according to the manufacturer's protocol (Miltenyi Biotech). For purification of total CD4+ cells, lymph node cells were incubated with anti-B220, anti-CD32/16, anti-CD11b, and anti-CD8 mAbs followed by incubation with anti-rat IgG-coated Dynabeads (Dynal). CD4+ T cells were further purified by magnetic separation using biotin-conjugated anti-CD4 mAb and streptavidin microbeads (Miltenyi Biotech). DCs were generated from mouse bone marrow cells with GM-CSF (R&D Systems). Three days after the final retroviral infection, DCs were stimulated with LPS (1 μg/mL; Sigma). After overnight stimulation with LPS, CD11c+ cells were isolated by magnetic separation with CD11c microbeads (Miltenyi Biotech). In some experiments, total spleen cells were labeled by 5-(and 6-) carboxyfluorescein diacetate succinamidyl ester (CFSE; Invitrogen) as described (13). For T cell stimulation, purified naive CD4+ OT-II TCR transgenic T cells were stimulated with BMDCs pulsed with OVA323–339 peptide (Abgent). In some BMDC and CD4+ T cell coculture experiments, the 20 μM concentration of γ-secretase inhibitor (Calbiochem) was added. Retrovirus carrying N2ICD was used once to infect CD4+ T cells 1 day after stimulation with anti-CD3 mAb. As for the different helper T cell differentiation conditions, we stimulated total spleen cells with anti-CD3 mAb with several combinations of cytokines and antibodies; Th1 [IL-12 (10 ng/mL) plus anti IL-4 mAb (10 μg/mL)], Th2 [IL-4 (30 ng/mL) plus anti-IL-12 mAb (10 μg/mL)] and Th17 [IL-6 (10 ng/mL) and TGF-β (2 ng/mL) plus anti IL-4 mAb (10 μg/mL) and anti IL-12 mAb (10 μg/mL)] throughout the culture.
Retroviral Infection.
Total spleen cells were stimulated with anti-CD3 mAb (1 μg/mL) for 24 h and then retrovirus carrying N1ICD, N2ICD, or N3ICD were used once for infection. Finally, cytokine expression was analyzed 48 h after further stimulation with anti-CD3 mAb (1 μg/mL). The retroviral infection protocol under Th1, Th2, and Th17 culture conditions is the same as in neutral conditions. For naive CD4+ T cells, infection procedures were the same as that of total splenocytes except that the cells were stimulated with plate-bound anti-CD3 mAb (1 μg/mL) instead of soluble anti-CD3 mAb. The DL1 gene was transduced into DCs via retroviral gene delivery repeated a total of three times (days 0, 1, and 2) as described (13).
ELISA.
IL-22 in culture supernatants was measured by using an ELISA kit (R&D Systems). In the experiment shown in Fig. 1D, a FlowCytomix kit (Bender Medsystems) was used.
Supplementary Material
Acknowledgments
We thank Dr. Takeda (Osaka University) for providing mice, Dr. Kubo (Tokyo Science University) for critically reading our manuscript, and Ms. Yamakawa and Ms. Kinouchi for editorial and technical assistance. This work was supported by a Grant-in-Aid for Young Scientists (S) from the Japan Society for the Promotion of Science from The Ministry of Education, Culture, Sports, Science and Technology, Takeda Science Foundation, the Uehara Memorial Foundation, and the Mochida Memorial Foundation.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911755107/DCSupplemental.
References
- 1.Pestka S, et al. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 2.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zenewicz LA, Flavell RA. IL-22 and inflammation: Leukin’ through a glass onion. Eur J Immunol. 2008;38:3265–3268. doi: 10.1002/eji.200838655. [DOI] [PubMed] [Google Scholar]
- 4.Vivier E, Spits H, Cupedo T. Interleukin-22-producing innate immune cells: New players in mucosal immunity and tissue repair? Nat Rev Immunol. 2009;9:229–234. doi: 10.1038/nri2522. [DOI] [PubMed] [Google Scholar]
- 5.Zheng Y, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 6.Veldhoen M, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 7.Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 8.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci USA. 2008;105:9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radtke F, Raj K. The role of Notch in tumorigenesis: Oncogene or tumour suppressor? Nat Rev Cancer. 2003;3:756–767. doi: 10.1038/nrc1186. [DOI] [PubMed] [Google Scholar]
- 10.Osborne BA, Minter LM. Notch signalling during peripheral T-cell activation and differentiation. Nat Rev Immunol. 2007;7:64–75. doi: 10.1038/nri1998. [DOI] [PubMed] [Google Scholar]
- 11.Maekawa Y, et al. Delta1-Notch3 interactions bias the functional differentiation of activated CD4+ T cells. Immunity. 2003;19:549–559. doi: 10.1016/s1074-7613(03)00270-x. [DOI] [PubMed] [Google Scholar]
- 12.Amsen D, et al. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 13.Maekawa Y, et al. Notch2 integrates signaling by the transcription factors RBP-J and CREB1 to promote T cell cytotoxicity. Nat Immunol. 2008;9:1140–1147. doi: 10.1038/ni.1649. [DOI] [PubMed] [Google Scholar]
- 14.Tsukumo S, Yasutomo K. Notch governing mature T cell differentiation. J Immunol. 2004;173:7109–7113. doi: 10.4049/jimmunol.173.12.7109. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka S, et al. The interleukin-4 enhancer CNS-2 is regulated by Notch signals and controls initial expression in NKT cells and memory-type CD4 T cells. Immunity. 2006;24:689–701. doi: 10.1016/j.immuni.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 17.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 18.Akaishi H, et al. Defective IL-2-mediated IL-2 receptor alpha chain expression in Stat3-deficient T lymphocytes. Int Immunol. 1998;10:1747–1751. doi: 10.1093/intimm/10.11.1747. [DOI] [PubMed] [Google Scholar]
- 19.Sugimoto K, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zenewicz LA, et al. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–659. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oberg M, Bergander L, Håkansson H, Rannug U, Rannug A. Identification of the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole, in cell culture medium, as a factor that controls the background aryl hydrocarbon receptor activity. Toxicol Sci. 2005;85:935–943. doi: 10.1093/toxsci/kfi154. [DOI] [PubMed] [Google Scholar]
- 22.Zheng Y, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 23.Aujla SJ, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21:102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho OH, et al. Notch regulates cytolytic effector function in CD8+ T cells. J Immunol. 2009;182:3380–3389. doi: 10.4049/jimmunol.0802598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toyabe S, et al. Requirement of IL-4 and liver NK1+ T cells for concanavalin A-induced hepatic injury in mice. J Immunol. 1997;159:1537–1542. [PubMed] [Google Scholar]
- 27.Kaneko Y, et al. Augmentation of Valpha14 NKT cell-mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A-induced hepatitis. J Exp Med. 2000;191:105–114. doi: 10.1084/jem.191.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem. 2004;279:23847–23850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- 29.Tanigaki K, et al. Regulation of alphabeta/gammadelta T cell lineage commitment and peripheral T cell responses by Notch/RBP-J signaling. Immunity. 2004;20:611–622. doi: 10.1016/s1074-7613(04)00109-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.