Abstract
The P23H mutation within the rhodopsin gene (RHO) causes rhodopsin misfolding, endoplasmic reticulum (ER) stress, and activates the unfolded protein response (UPR), leading to rod photoreceptor degeneration and autosomal dominant retinitis pigmentosa (ADRP). Grp78/BiP is an ER-localized chaperone that is induced by UPR signaling in response to ER stress. We have previously demonstrated that BiP mRNA levels are selectively reduced in animal models of ADRP arising from P23H rhodopsin expression at ages that precede photoreceptor degeneration. We have now overexpressed BiP to test the hypothesis that this chaperone promotes the trafficking of P23H rhodopsin to the cell membrane, reprograms the UPR favoring the survival of photoreceptors, blocks apoptosis, and, ultimately, preserves vision in ADRP rats. In cell culture, increasing levels of BiP had no impact on the localization of P23H rhodopsin. However, BiP overexpression alleviated ER stress by reducing levels of cleaved pATF6 protein, phosphorylated eIF2α and the proapoptotic protein CHOP. In P23H rats, photoreceptor levels of cleaved ATF6, pEIF2α, CHOP, and caspase-7 were much higher than those of wild-type rats. Subretinal delivery of AAV5 expressing BiP to transgenic rats led to reduction in CHOP and photoreceptor apoptosis and to a sustained increase in electroretinogram amplitudes. We detected complexes between BiP, caspase-12, and the BH3-only protein BiK that may contribute to the antiapoptotic activity of BiP. Thus, the preservation of photoreceptor function resulting from elevated levels of BiP is due to suppression of apoptosis rather than to a promotion of rhodopsin folding.
Keywords: autosomal dominant retinitis pigmentosa, endoplasmic reticulum stress, adeno-associated virus, unfolded protein response
Mutations within the RHO gene, which encodes rod cell opsin, lead to retinitis pigmentosa, an inherited form of retinal degeneration. Most RHO mutations are inherited in a dominant fashion, and the condition is described as autosomal dominant retinitis pigmentosa (ADRP). More than 80 RHO mutations have been identified that account for 30% of ADRP in humans (1). Substitution of proline to histidine at position 23 (R23H RHO) was the first ADRP mutation to be identified (2). Due to problems with protein folding, P23H rhodopsin only partially reconstitutes with 11-cis retinal in vitro (3). Mutated rhodopsin also causes retinal degeneration when expressed in transgenic animals. This fact makes these animal models extremely helpful for studying the physiological and biochemical impact of defective rhodopsin and also for testing approaches to treat ADRP (4, 5).
The P23H RHO mutation exhibits characteristics of both types of dominant mutation: toxic gain-of-function and dominant negative (6). In cultured cells, P23H opsin is retained in the endoplasmic reticulum (ER) unlike wild-type rhodopsin, which is glycosylated and transported to the plasma membrane (7). The ER-retention of P23H opsin can induce the unfolded protein response (UPR) and later apoptosis (3, 8–10). However, P23H opsin is subject to degradation by the ubiquitin-proteosome system (UPS). Ubiquitinated aggregates disrupt the processing of normal rhodopsin synthesized in the same cell (11, 12). Prevention of trafficking of the wild-type protein would imply a classic dominant negative effect of P23H mutated rhodopsin.
Results concerning the pathogenic mechanism of ADRP obtained in animal models have led to conflicting interpretations. Overexpression of P23H opsin in transgenic mice led to accumulation of mutant protein at photoreceptor nerve terminals at the outer plexiform layer (13) but another transgenic mouse model showed P23H opsin trafficking to the rod outer segment (OS), as well as to the nerve terminals (4). P23H opsin that reaches the outer segments of photoreceptor cells can combine with the 11-cis retinal chromophore (14). Tam and Mortiz (15) showed that the mechanism of P23H RHO toxicity in frog photoreceptors is associated with retention of misfolded P23H rhodopsin in the ER and activation of the UPR.
The UPR is a signal transduction cascade that detects and alleviates protein-folding stress in the ER caused by physiological demands or environmental variation (16). There are three transmembrane proteins that sense prolonged protein folding stress in the ER: IRE1α, ATF6α, and PERK (16). These proteins initially produce cytoprotective stimuli resulting in reduced translation, enhanced ER protein folding capacity, and clearance of misfolded ER proteins. Cytoprotective outputs coexist along with proapoptotic signaling, outweighing it in initial stages of ER stress. The acute UPR permits cells to readjust protein synthesis and chaperone levels to cope with stress. If these steps fail to re-establish homeostasis, IRE1α signaling and then ATF6α signaling are attenuated, creating an imbalance in which proapoptotic output guides the cell toward apoptosis (10).
A resident member of the Hsp70 family of chaperones, BiP/Grp78 plays a key role in ER stress signaling. BiP is an abundant protein that binds to each of the transducers of ER stress (IRE1α, ATF6α, and PERK) and serves as a sensor of alterations of ER homeostasis. UPR activation results in the transcriptional induction of the genes encoding the ER-localized stress response proteins such as GRP94, ERp72, GRP170, calreticulin, as well as BiP itself. Although strong evidence supports the cytoprotective role of BiP (17–20), little is known about the effect of direct BiP delivery. In cells stably expressing the Swedish APP (β-amyloid precursor) mutant, transient BiP overexpression leads to a decrease in the level of Aβ4 and Aβ42 amyloids (21), indicating the role of BiP as a chaperone protein. The overexpression of BiP results in reduced apoptosis in Chinese hamster ovary cells (22) and cardiomyocytes (23). This effect is associated with formation of a complex of BiP and caspases-7 and -12 through an ATP-binding domain within BiP. The role of BiP during stress is not limited to the quality control of the misfolded proteins, but also includes an increase of the Ca2+ buffering within the ER lumen. For example, increased levels of BiP in HeLa cells lead to appreciable increases of the ER Ca2+ storage capacity (24).
These cell culture studies suggest that increasing levels of BiP may be beneficial in preventing ADRP associated with rhodopsin misfolding. However, the overexpression of BiP has not been studied yet in animal models of ADRP. We previously demonstrated that BiP plays an important role in the mechanism of degeneration in P23H RHO transgenic photoreceptors (10). Specifically, photoreceptor cells expressing P23H rhodopsin showed a selective decrease in BiP mRNA compared to wild-type photoreceptors at the same ages. This correlation suggested that insufficient BiP levels contribute to photoreceptor cell death arising from P23H rhodopsin expression.
In the current study, we tested the hypothesis that increasing the levels of BiP will protect photoreceptors from the ER stress imposed by P23H rhodopsin. We found that increased BiP expression mod-ulated the UPR to favor survival pathways in photoreceptors and suppressed apoptosis in P23H photoreceptors, reducing the rate of retinal degeneration.
Results
Overexpression of BiP Protein Does Not Promote the Trafficking of P23H RHO to the Cell Membrane.
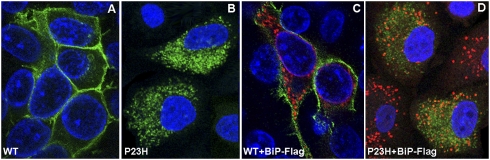
P23H opsin expressed in mammalian cells forms cytoplasmic aggregates that resemble aggresomes (12). To determine if increasing levels of BiP would promote opsin folding and thus limit cytoplasmic aggregation, we performed cotransfection of HeLa cells with plasmids expressing P23H opsin and a plasmid expressing BiP protein to observe the localization of misfolded opsin. Wild-type rhodopsin served as a control, demonstrating plasma membrane localization. The cDNA encoding the BiP gene contained the FLAG sequence that was placed in a front of a KDEL fragment as used by Murray et al. (25). Immunoblot analysis showed that the increase in levels of BiP protein was greater than 61 ± 11% (P = 0.0009) compared to mock-transfected cells (Fig. S1). By immunostaining analysis we demonstrated that the coexpression of P23H RHO and BiP proteins in cells had no effect on the distribution of misfolded rhodopsin at 48 h posttransfection (Fig.1). This experiment indicated that extra BiP protein in the ER did not permit exit of P23H rhodopsin from the ER and delivery to the cell membrane.
Fig. 1.
Immunostaining of HeLa cells cotransfected with wild-type RHO, P23H RHO, and BiP-Flag. (A) Immunostaining analysis demonstrated the localization of wild-type RHO to the cell membrane (green). (B) In contrast, P23H RHO was not able to traffic to the cell membrane and was localized in the cytoplasm (green). (C) Overexpression of BiP did not affect the trafficking of wild-type RHO, which was still associated with the plasma membrane in the presence of excess BiP–Flag (red). (D) The distribution of the P23H RHO was not affected by extra BiP (red). P23H RHO was still localized in the cytoplasm and did not traffic to the cell membrane. The average transfection efficiency was 75%.
Expression of P23H Rhodopsin Activates the UPR and BiP Elevation Moderates the UPR.
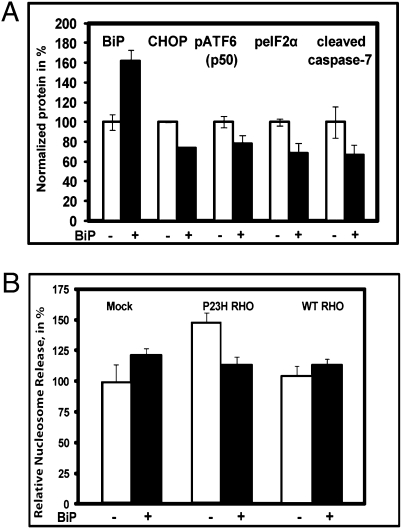
We performed cotransfection of HeLa cells with plasmids expressing P23H rhodopsin, or both P23H rhodop-sin and BiP proteins, to study effects on ER stress and UPR signaling. We previously reported that transient transfection of P23H RHO activates the UPR as demonstrated by induction of BiP transcription (10). Here, we identified additional markers of UPR activation including cleavage of ATF6 (p50) and phosphorylation of eIF2α protein in P23H RHO-transfected cells. Interestingly, the protein encoded by spliced XBP1 mRNA was not detected by immunoblotting under these conditions. When we coexpressed P23H RHO and BiP proteins, production of the cleaved pATF6 50 kDa protein and peIF2α were reduced (Fig. 2A). In BiP treated cells, we also found that the level of proapoptotic CHOP protein and cleaved caspase-7 were diminished. Thus, we confirmed that P23H rhodopsin induces the UPR and demonstrated that artificial enhancement of BiP levels reduced ER stress and prevented activation of ATF and PERK signaling.
Fig. 2.
Increased level of BiP in HeLa cells expressing mutant RHO leads to modulation of the ER stress pathways and the apoptotic signal. (A) HeLa cells were cotransfected with plasmids expressing P23H RHO or P23H RHO and BiP proteins. The level of total BiP was increased by 62% (P < 0.01) leading to decrease in CHOP/GADD153 protein by 27% (P < 0.0002), phosphorylated ATF6 protein p50 by 22% (P < 0.04), pEIF2α by 30% (P < 0.006), and cleaved caspase-7 by 33% (P < 0.04). (B) HeLa cells were transfected with either wild-type RHO, P23H RHO, both wild-type RHO, or P23H RHO plus BiP. At 48 h cell lysates were obtained to perform a nucleosome release assay. Increased expression of P23H rhodopsin led to elevation of the apoptotic signal by 48% (P < 0.007) although wild-type RHO did not induce apoptosis. The overexpression of BiP led to down-regulation of the apoptotic signal by over 30% (P < 0.003) Transfection of cells with BiP alone elevated the apoptotic signal only slightly (P = 0.47). Data were normalized to the “Mock, BiP” mean. Experiments were performed in quadruplicate. The bars show the average ± SEM.
Expression of P23H RHO Induces Apoptosis in Cell Culture.
Because increased expression of BiP down-regulated the proapoptotic protein CHOP/GADD153, we examined the ability of BiP protein to reduce apoptosis in cells expressing misfolded rhodopsin. To quantify the protective effect of BiP protein on apoptosis in HeLa cells transfected with P23H RHO, we used a nucleosome release assay that measures mono- and oligonucleosomes in the cytoplasmic fraction of cell lysates, a hallmark of cell death via apoptosis. Comparison of the apoptotic signal was performed by evaluation of ELISA signals obtained from mock transfected cells and cells expressing P23H RHO or wild-type RHO alone or in combination with BiP protein. In P23H rhodopsin expressing cells, there was an elevation in nucleosome release (Fig. 2B). However, cotransfection of cells with both P23H RHO and BiP protein expressing plasmids eliminated this increase and reduced the signal down to that observed in mock transfected cells or in cells transfected with wild-type RHO. We conclude that expression of P23H rhodopsin promotes apoptosis and that an elevated level of BiP suppresses apoptosis resulting from misfolded rhodopsin.
P23H Rhodopsin Stimulates the UPR in the Retina.
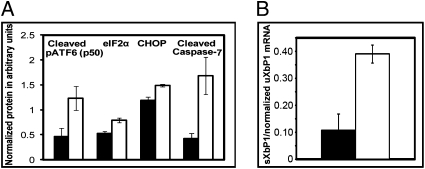
Our cell culture findings suggested that UPR signaling played an important role in retinal degeneration induced by P23H rhodopsin. To determine the role of UPR signaling in retinal degeneration in vivo, we used P23H RHO line 3 transgenic rats (P23H-3 RHO). This choice was based on the moderate rate of photoreceptor degeneration in P23H-3, which provides a relatively wide window for therapeutic intervention (26). P23H-3 RHO rats display an age-related decline of BiP mRNA and an increase of CHOP mRNA compared to wild-type rats, providing evidence that UPR signaling was elevated in vivo (10). Here, we analyzed UPR signaling status in more depth in these animals. We compared the protein extract obtained from retinas of P23H-3 RHO and wild-type Sprague-Dawley rats at postnatal day 30 (P30). P30 was selected because retinal degeneration has begun, but over 60% of photoreceptor nuclei are still intact. The result of this analysis is summarized in Figs. 3 A and B. We detected signals corresponding to a persistent ER stress response in the photoreceptors of P23H-3 RHO rats at P30 (Fig. 3A). The levels of cleaved pATF6 p50, peIF2α protein, proapoptotic CHOP protein, and cleaved capspase-7 were increased in transgenic retinas compared to wild-type. Using RT-PCR and semiquantitative analysis, we also found that the spliced Xbp1 mRNA levels were almost fourfold higher in transgenic rats compared to wild-type rats (Fig. 3B). Images of blots treated with antibodies against the ER stress markers and the levels of the XbP1 RT-PCR products from the retina RNA extract are shown in Fig. S1 A and B. Thus, all three pathways of the UPR are elevated in the retinas of P23H-3 RHO rats at P30.
Fig. 3.
Study of the UPR in P23H-3 RHO transgenic (white) and wild-type (black) rats at P30. (A) At P30 in P23H-3 RHO rats, we observed up to 2.7-fold increase in the cleaved pATF6 50kD protein (P<0.03), a 51% increase in peIF2α (P<0.01), a 26% increase in the- level of CHOP protein (P<0.003), and an almost fourfold increase in activated caspase-7 (P < 0.016) (n = 5). (B) At P30 we also observed persistence IRE1 pathway in transgenic retinas. The level of spliced Xbp1 mRNA was fourfold higher in P23H-3 RHO rats compared to wild-type rats, P < 0.012 (n = 6). The bars show the average ± SEM.
AAV BiP Delivery to Photoreceptors of P23H-3 RHO Rats.
Our cell culture studies suggested that overexpression of BiP alleviated the deleterious effect of P23H rhodopsin. To test if BiP prevents photoreceptor cell death in P23H RHO animals, we used an Adeno-associated virus (AAV) 2/5 to overexpress the BiP protein in P23H-3 RHO photoreceptors. The map of the AAV vector expressing GRP78 cDNA is shown in Fig. S2. The construct contained the full length of human GRP78 cDNA (NM_005347) inserted in AAV plasmids with serotype 2 terminal repeats. The BiP expression was controlled by the chicken β-actin promoter and CMV immediate early enhancer (the CBA promoter). Virus was packaged in serotype 5 capsids for gene delivery to photoreceptors by subretinal injection pups at P15. In addition, we also performed injections of viruses expressing BiP-Flag in P23H-3 RHO pups at P15 to study the distribution of BiP in degenerating photoreceptors. Indirect immunofluorescence analysis of representative sections showed the distribution of BiP-Flag throughout the retina (Fig. S3).
AAV Delivery of BiP Leads to Increased BiP mRNA and Protein Production in the Retina.
We quantified the level of BiP expression in treated retina by RT-PCR analysis and digestion of the PCR product with endonuclease NCO1, which has a unique site in the human cDNA. RT-PCR analysis of total retinal RNA demonstrated that the human BiP mRNA was expressed at 67 ± 2.6% (P < 0.03) of the level of rat mRNA (Fig. S4). The comparison of the normalized BiP protein by immunoblot showed that, in the injected right eyes, the total BiP level was 26 ± 5.3% higher compared to untreated left eyes (P < 0.02). This is a modest increase in BiP, but this was measured in the entire retina, not just in AAV-transduced photoreceptors.
Overexpression of BiP in P23H-3 RHO Photoreceptors Leads to the Rescue of the Scotopic Electroretinogram (ERG).
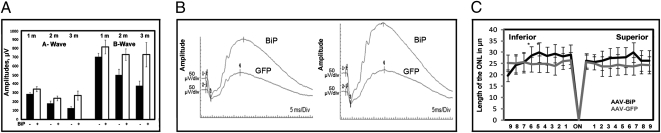
Following injection we analyzed animals over three months by ERG and then euthanized them for histological and immunochemical analysis. The results of such an experiment registered at 2.68 cd-s/m2 intensity with 60 s flash intervals are shown in Fig. 4A. It should be noted that full-field ERGs measure the response from the entire retina, and the average extent of retinal infection was 60% (Fig. S3). The ERG analysis showed that AAV5 BiP treatment led to the rescue of retinal activity in P23H RHO rats. In BiP-treated eyes, a- and b-wave response amplitudes were higher than in control (GFP-injected) eyes, and this protection was consistent over 3 months.
Fig. 4.
Subretinal delivery of AAV-BiP preserves function of P23H-3 RHO photoreceptors. (A) Expression of BiP in the right retinas of transgenic rats (n = 10) carrying P23H RHO delays the decline of ERG a- and b-wave amplitudes compared with the left eyes expressing AAV5 GFP. The a-wave amplitudes of the scotopic ERG in treated eye at 2.68 cd-s/m2 intensity increased by 43% (P < 0.048) at 1 month, 44% (P < 0.16) at 2 months, and 115% (P < 0.014) at 3 months, relative to control eyes. The b-wave amplitudes were also increased by 47% (P < 0.015), 51% (P < 0.011), and 97% (P < 0.028), at 1, 2, and 3 months, respectively. (B) Two examples of wave forms of scotopic ERGs obtained by analyzing the BiP-treated rats at 3 months. (C) Increased BiP expression in P23H-3 RHO eyes (n = 8) preserved retinal integrity of photoreceptors in inferior and superior hemispheres. Modest increase (18%; P = 0.0043) in the thickness of the ONL was observed in the central inferior hemisphere. The bars show the average ± SEM.
To determine if this increase in retinal function corresponded to an increase in rhodopsin levels, we extracted total rhodopsin from the AAV-BiP treated of eyes dark adapted P23H-3 rats and compared these levels to control injected eyes from the same animals. Based on the absorbance at 500 nm, we observed no difference between BiP treated and control eyes at 3 months following injection (Fig. S5), indicating that the ERG difference did not reflect an increase in rhodopsin holoenzyme.
Preservation of Retinal Structure in P23H-3 RHO Eyes by BiP Over-expression.
After 3 months of monitoring rats by ERG, we euthanized rats and enucleated and processed the retina for histological analysis by measuring the thickness of the outer nuclear layer (ONL). Morphometric analysis of individual retinas showed a trend toward increasing of the thickness of the ONL of BiP treated eyes in the inferior hemisphere, but the difference between treated and untreated eyes was statistically significant only in individual sectors 5 and 6 of the central inferior retina. These differences were 16% and 20% (P = 0.025 and P = 0.012, respectively), as shown in Fig. 4C. By averaging results of measurements in the central inferior and in the central superior retinas from eight animals we obtained significant difference between treated and untreated eyes. In the central inferior retinas it was 18% (P = 0.0043), and in the central superior retinas it was 13% (P = 0.005) (Fig. 4C).
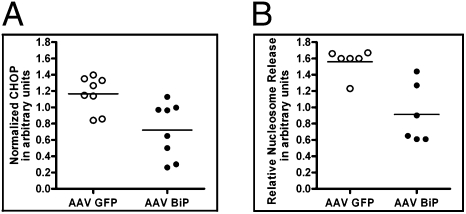
Gene Delivery of BiP Leads to Reduction of Apoptosis in P23H-3 RHO Retinas.
To study the cellular pathways involved in the therapeutic action of BiP protein, we measured the level of apoptosis at 1 month after injection. Despite the fact that the P23H-3 transgenic rats show a moderate rate of degeneration under the lighting conditions employed (<100 lx; 12-h light/dark cycle), analyzing them at later times postinjection might prove too late in the course of retinal degeneration to detect significant impact of BiP in surviving cells. Therefore at P45, a sufficient fraction of photoreceptors survived to measure postinjection modification of the retina. By analogy with cell culture experiments, we performed nucleosome release assays in P23H-3 RHO retinal lysates and found that AAV-delivery of BiP led to reduction in apoptosis (Fig. 5A). We also analyzed P23H-3 RHO protein extracts isolated at 1 month after injection and observed a reduction in production of proapoptotic CHOP protein in BiP treated retinas (Fig. 5B).
Fig. 5.
Increased expression of BiP protein in P23H-3 RHO retinas reduced apoptosis and diminished expression of CHOP 1 month after AAV-BiP delivery. (A) AAV-BiP reduced nucleosome release by 42% (n = 6), P = 0.006 compared to control (AAV-GFP) treated retina. (B) Quantification of CHOP protein. Immunoblot analysis revealed that AAV-BiP reduced the levels of proapoptotic CHOP protein by 30% (P = 0.0052) (n = 8).
To study the mechanism by which BiP inhibits apoptosis, we precipitated the protein extracts isolated from BiP-Flag-expressing retinas individually with primary antibodies against procaspase-12 and BiK (NBK) proteins. Immunoprecipitated proteins were separated by SDS polyacrylamide gel electrophoresis and transferred to PVDF membranes for reaction with anti-Flag antibodies. BiK is a proapoptotic BH3-only member of the BCL-2 family that is integrated into the ER by a single transmembrane domain. We detected bands corresponding to BiP-Flag proteins in injected retinas (Fig. S6), suggesting that AAV-delivered BiP binds to both procaspase-12 and BiK and blocks apoptosis.
Discussion
BiP/Grp78 is a key chaperone induced by UPR signaling, and we had previously demonstrated that BiP levels were reduced in vivo in animals undergoing retinal degeneration arising from P23H rhodopsin expression. Here, we sought to determine whether elevating levels of BiP in photoreceptors could be therapeutic for ADRP arising from the P23H mutation in rhodopsin, a mutation known to cause misfolding of opsin and decreased association of 11-cis retinal. We hypothesized that increasing the levels of this chaperone would promote the correct folding of opsin protein, would alleviate ER stress signaling, and, finally, would prevent apoptosis of photoreceptors in a rat model of ADRP. Here, we found that the BiP overexpression alleviated ER stress and reduced signaling from ATF6 and PERK branches of the UPR. We also determined that BiP elevation prevents apoptosis associated with ER stress in photoreceptors. Lastly, we demonstrated that moderate elevation in levels of BiP preserved the function of photoreceptors in P23H rats and trended toward preservation of photoreceptors at 3 months.
In earlier work, we demonstrated an elevation in CHOP in P23H rats and suggested that this increase could be caused by persistent stimulation of the UPR, particularly the PERK pathway (10). Indeed, we found that this hypothesis was correct: P23H-3 RHO photoreceptors are under impact of the misfolded rhodopsin, and in these animals we found an activation of ATF6, IRE, and PERK signaling pathways. The level of the 50 kDa cleavage product of pATF6 was almost 2.7-fold higher in transgenic animals compared to wild-type rats, indicating active ER stress signaling. Another ER stress marker, peIF2α protein, was also elevated by 50% in transgenic retinas, suggesting that PERK signaling persists at P30. The activation of the PERK pathway might be responsible for the elevated level of the proapoptotic CHOP protein (up 26%) in P23H-3 RHO. Yang et al. (27) demonstrated that retinal degeneration in the rd1 mouse is accompanied by an increase in both CHOP and peIF2α, reflecting ER-associated apoptosis. CHOP protein may promote apoptosis by facilitating the resumption of protein synthesis in the stressed ER and attendant production of reactive oxygen species (16). The pEIF2α/PERK pathway promotes apoptosis through activation of proapoptotic transcription factor AP-1 (27). Thus, the observation of an increased level of cleaved caspase-7 (up to fourfold) in P23H-3 RHO retina is not surprising. Photoreceptors are known to die by apoptosis in this model (28). Our studies have therefore demonstrated the importance of the UPR in clinically relevant model of a human disease.
However, AAV delivery of BiP cDNA resulted in only 26% increase in BiP protein levels in treated retinas. Part of the reason for this moderate elevation was incomplete transduction of the retina (60%) by AAV-BiP. It is also possible that expression of BiP from the AAV-delivered gene down-regulates the synthesis of endogenous BiP mRNA (29). The level of endogenous BiP protein was not elevated more than 20–40% when it was induced by BiP inducer X (BIX) (20). A discrepancy between overexpressed BiP mRNA and BiP protein levels was also reported in Saccaharomyces cerevisiae (30). In this respect, BiP behaves very differently than other ER proteins, such as calreticulin, which can be overexpressed 50- to 100-fold without a divergence between protein and mRNA levels.
Activators of BiP, such as the BIX protein and 2-deoxy-d-glucose, promote the survival of neuronal cells in vitro and in vivo, indicating that BiP can serve as neuronal survival factor. Therefore, we wanted to examine if increased levels of BiP could protect photoreceptors from degeneration caused by P23H rhodopsin. We demonstrated that the overexpression of BiP protein causes a significant physiological impact on the function in the P23H-3 RHO retina. A nearly normal electroretinogram response was maintained up to 3 months and a- and b-wave amplitudes were elevated up to twofold compared to control eyes.
Morphometric measurements of the ONL demonstrated a trend toward increased preservation of the ONL, with modest significant differences in the central inferior retina. The most parsimonious interpretation is that increased expression of BiP does not prevent loss of photoreceptor cells in the long term (3 months). Based on the electrophysiological improvement (Fig. 4A) and the 42% reduction in apoptosis in retinas treated with AAV-BiP (Fig. 5A), however, one would expect a more dramatic improvement of the retinal structure. We conclude that AAV delivery of BiP has led to an improvement in functionality of photoreceptors that exceeds its impact on cell survival, at least at 3 months. Although we detected no increase in properly reconstituted rhodopsin in BiP-treated retinas relative to control treated retinas (Fig. S5), the relief of ER stress that we observed (Fig. 5A) could affect the folding and displacement of other membrane proteins and therefore impact the visual cycle.
In cultured cells we found that the 62% increase in BiP protein led to down-regulation of the ATF6 and PERK pathways. The level of cleaved pATF6 p50 was reduced by 22% in cells transfected with both P23H RHO and BiP plasmids. We did not detect modification in the IRE pathway in vitro. Protein analysis of the PERK pathway demonstrated over 50% difference in peIF2α protein in P23H RHO and P23H RHO plus BiP transfected cells, suggesting, first, that the PERK pathway is active and, second, that BiP overexpression dampens the PERK signaling stimulated by P23H rhodopsin. In extracts of cells transfected with P23H RHO, we did not detect protein produced by the Xbp1 spliced mRNA. Failure to detect Xbp1 protein might indicate that IRE signaling is shut down in HeLa cells expressing P23H RHO cDNA by 48 h posttransfection.
Our in vitro studies indicate that the ATF6 pathway is a major ER stress signaling pathway affected by elevation of BiP in cells expressing misfolded P23H rhodopsin. Reduced level of cleaved ATF6 protein is likely to contribute to reduction of CHOP pro-tein in transfected cells. Shen et al. (31) have found that overexpression of BiP attenuates ATF6 activation by affecting its cleavage upon the ER stress. In particular, they concluded that overexpressed BiP delays ATF6 translocation to the Golgi by inhibiting Golgi localization signals found within the ATF6 sequence (31). Zeng et al. (32) showed that overexpressed BiP attenuates the ER stress that is caused by an inhibitory effect of ATF6 on translation of SREBP2-targeted genes. In the presence of elevated BiP, the inhibitory effect of ATF6 is reversed by almost fourfold by reducing ATF6 cleavage.
The PERK pathway is the second signaling pathway that is modified by BiP in P23H RHO cells that have undergone ER stress. We observed suppression of peIF2α protein by over 50% at 48 h after treatment. These results are supported by those of Lai et al. (33), who found reduced PERK signaling following transfection of cells with a BiP-expressing plasmid. The down-regulation of CHOP protein by 27% in cells expressing P23H rhodopsin and overexpressing BiP may be the result of down-regulation of ATF6 or PERK pathways, or both (34).
Several recent reports indicate that the overexpression of BiP protein reduces apoptosis in cultured cells (35, 18). Knowing this and knowing that apoptosis is a final common pathway in many cases of retinal degeneration (36, 37), we tested cells transfected with P23H RHO for the activation of apoptosis. We found that P23H rhodopsin activates the cleavage of caspase-7 by 61% and elevates nucleosome release by 48%. We also found that overexpression of BiP protein reduced the activation of caspase-7 by 33%. Apoptosis, based on nucleosome release, was diminished by 30% by elevation of BiP. The overexpression of wild-type rhodopsin did not lead to apoptosis in HeLa cells.
Treatment of photoreceptors with AAV-BiP led to down-regulation of CHOP protein by 30%. Due to the small amount of injected tissue, we were not able to establish which ER stress pathway was affected by elevated levels of BiP in photoreceptors in vivo. However, data on cultured cells expressing P23H RHO and BiP proteins suggest that both the ATF6 and PERK pathways could be down-regulated (32). Because persistent PERK signaling might be responsible for degeneration of P23H-3 RHO photoreceptors, we proposed that modest elevation of BiP down-regulates the PERK pathway, which leads to 30% reduction of the CHOP protein. By using a nucleosome release assay, we found that the AAV-BiP treatment reduces the apoptotic signal by 42% at 1 month postinjection in ADRP photoreceptors. We know of no previous reports of the antiapoptotic effects of overexpressing BiP in vivo.
Overexpression of BiP is more critically needed in cells undergoing physiological or pathological stress. In certain types of cancer, the overexpression of BiP leads to drug resistance, recurrence, and poor patient survival. The mechanism by which BiP overexpression can contribute to cancer proliferation is linked to its ability to inactivate proapoptotic pathways and activate prosurvival pathways for cancer cells (38). Therefore, inhibition of BiP activity or down-regulating of BiP expression might be useful as anticancer therapies. In the case of ADRP progression in humans, however, chronically overexpressed BiP would occur in photoreceptor cells that are preferentially targeted by AAV serotype 5. This overexpression would not provide systemic elevation of BiP protein in humans and, therefore, could be considered safe.
Because apoptosis is a final step involved in photoreceptor degeneration, we investigated the mechanism of BiP in preventing photoreceptor apoptosis. BiP binds to and inhibits ER-associated promoters of apoptosis. Rao et al. (39) have demonstrated that BiP forms a complex with caspase-7 and caspase-12 and prevents release of caspase-12 from the ER. Association of BiP with BiK protein has been reported, as well (40). We found that the AAV-delivered BiP binds to procaspase-12 and BiK proteins and, apparently, limits apoptosis in P23H-3 RHO photoreceptors.
The proposed mechanism by which increasing expression of BiP blocks photoreceptor apoptosis is presented in Fig. S7. We propose three possible pathways by which elevation of BiP may block apoptosis. Pathway 1 deals with the down-regulation of ER stress signaling pathways, such as the PERK and the ATF6 pathways, and consequent reduction in the production of CHOP. In P23H-3 RHO rats, both ATF6 and peIF2α are up-regulated at P30 and could be a potential target for the excess BiP in photoreceptors. In Pathway 2, BiP simply prevents release of caspase-12 from the ER, thus, reducing the ER-associated apoptotic cascade. Less procaspase-12 will be available for cleavage by calpain and caspase-7 in the cytosol of P23H-3 RHO photoreceptors. For therapeutic intervention it is important to understand how the caspase-7/caspase-12 pathway differs from the calpain/caspase-12 pathway, as well as the relevance of each of these pathways in the ER stress-induced cell death. Studies employing calpain and site-specific antibodies to caspase-12 might prove useful in elucidating these specific pathways. The knowledge of procaspase-12 activators in P23H RHO photoreceptors would give an additional window for therapeutic intervention.
The third pathway is linked to an ability of BiP protein to counteract cell death mediated by proapoptotic BCL-2 family proteins. We have demonstrated that BiP binds to BiK in photoreceptors and may inhibit it by forming a complex with this client protein. As a general feature of molecular chaperones, this interaction may alter BiK’s conformation or interfere with its hetero-dimerization with other interactive partners like BAX and BAK that are essential for its proapoptotic activity.
We have verified the importance of the UPR in an animal model of ADRP that faithfully recapitulates the progressive degeneration of the retina in this blinding disease. Our study also highlights pathways that could be targets for gene therapy or pharmacologic therapy for human ADRP. Reduction of CHOP, caspase-7, caspase-12 mRNA, as well as a balancing in proapoptotic BCL2 family, BIK, should be considered as possible treatments.
Materials and Methods
Transient Transfection of HeLa Cells and Immunoblot Analysis of Cell Protein Extracts.
Transfection was performed using Lipofectamine 2000 according to the manufacturer's protocol. For in vitro experiments we used pcDNA3.1 plasmids to express mouse P23H RHO, wild-type RHO, and human BiP/Grp78 cDNAs that were under control of the CMV promoter. An empty pcDNA 3.1 vector was used to maintain a constant amount of total DNA in individual transfections. We routinely obtained 75% transfection efficiency. At 48 h after transfection, cells were harvested and protein extracts were prepared.
rAAV Vectors, Rat Model, Subretinal Injection of AAV Vectors, Immunostaining Analysis.
An rAAV vector with serotype 2 terminal repeats was used to express the human BiP/GRP78 and human BiP-Flag. Viruses were packaged (pseudotyped) in serotype 5 capsids. The final titers were 4 × 1012 vector genome (vg) per ml. The study was conducted in accordance with the Association for Research in Vision and Ophthalmology Statement on the Use of Animals in Ophthalmic and Vision Research. Details of injection and immunostaining analysis are given in SI Text.
ELISA Quantification of Apoptosis.
The nucleosome release assay was done by using Cell Death Detection ELISA (Roche Diagnostics). See details in SI Text.
RNA Isolation and Reverse Transcription-PCR.
Total RNA was isolated from individual retinas of P23H-3 RHO rats treated with AAV-BiP or AAV-GFP virus using TRIzol Reagent (Invitrogen). RNase-free DNase (Ambion) was used to remove contaminating DNA. Semiquantative RT-PCR analysis was performed by comparison of individual ratio of human BiP to normalized rat BiP. Rat BiP was normalized by using β-actin RT-PCR product as an internal control. Primers and reaction conditions are described in SI Text and Table S1.
Immunoprecipitation of Retinal Protein Extract.
Immunoprecipitation was done by using a Catch and Release II System (Millipore), 500 μg of protein extract, and antibodies against Flag, caspase-12, BiK, and rhodopsin 1D4. The denatured protein solutions were applied on 12% SDS polyacrylamide gel and immunoblots were prepared by standard methods.
Retinal Tissue Preparation and Histological Quantification of Retinal Outer Nuclear Layer.
The tissue analysis was conducted by methods described in SI Text.
Statistical Analysis.
Results obtained from immunoblots, semiquantitive RT-PCR, ERG amplitudes, and histological measurements of the ONL thickness were analyzed using ANOVA or Student's t test for paired samples.
Supplementary Material
Acknowledgments
The authors thank James Thomas, Jr.; Haidong Yang; Douglas Yasumura; and Michael T. Matthes for technical assistance. We acknowledge grants from the Foundation Fighting Blindness TA-GT-4090-0479-UFL, TA-GT-0507-0384; National Institutes of Health Grants EY13729, EY11123, EY08571, EY01919, EY06842, EY02162, EY018313, and EY013280; That Man May See, Inc. (MML); and Research to Prevent Blindness (MML); Macular Vision Research Foundation; Juvenile Diabetes Research Foundation; and Hope for Vision for partial support of this work.
Footnotes
Conflict of interest statement: W.W.H. and the University of Florida have a financial interest in the use of AAV therapies and own equity in a company (AGTC Inc.) that might, in the future, commercialize some aspects of this work. All other authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911991107/DCSupplemental.
References
- 1.Dryja TP, McEvoy JA, McGee TL, Berson EL. Novel rhodopsin mutations Gly114Val and Gln184Pro in dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2000;41:3124–3127. [PubMed] [Google Scholar]
- 2.Dryja TP, Hahn LB, Cowley GS, McGee TL, Berson EL. Mutation spectrum of the rhodopsin gene among patients with autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci USA. 1991;88:9370–9374. doi: 10.1073/pnas.88.20.9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu X, Garriga P, Khorana HG. Structure and function in rhodopsin: Correct folding and misfolding in two point mutants in the intradiscal domain of rhodopsin identified in retinitis pigmentosa. Proc Natl Acad Sci USA. 1996;93:4554–4559. doi: 10.1073/pnas.93.10.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goto Y, Peachey NS, Ripps H, Naash MI. Functional abnormalities in transgenic mice expressing a mutant rhodopsin gene. Invest Ophthalmol Vis Sci. 1995;36:62–71. [PubMed] [Google Scholar]
- 5.LaVail MM, et al. Ribozyme rescue of photoreceptor cells in P23H transgenic rats: Long-term survival and late-stage therapy. Proc Natl Acad Sci USA. 2000;97:11488–11493. doi: 10.1073/pnas.210319397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendes HF, van der Spuy J, Chapple JP, Cheetham ME. Mechanisms of cell death in rhodopsin retinitis pigmentosa: Implications for therapy. Trends Mol Med. 2005;11:177–185. doi: 10.1016/j.molmed.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Chapple JP, et al. Unfolding retinal dystrophies: A role for molecular chaperones? Trends Mol Med. 2001;7:414–421. doi: 10.1016/s1471-4914(01)02103-7. [DOI] [PubMed] [Google Scholar]
- 8.Frederick JM, et al. Mutant rhodopsin transgene expression on a null background. Invest Ophthalmol Vis Sci. 2001;42:826–833. [PubMed] [Google Scholar]
- 9.Leonard KC, et al. XIAP protection of photoreceptors in animal models of retinitis pigmentosa. PLoS One. 2007;2:e314. doi: 10.1371/journal.pone.0000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin JH, et al. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Illing ME, Rajan RS, Bence NF, Kopito RR. A rhodopsin mutant linked to autosomal dominant retinitis pigmentosa is prone to aggregate and interacts with the ubiquitin proteasome system. J Biol Chem. 2002;277:34150–34160. doi: 10.1074/jbc.M204955200. [DOI] [PubMed] [Google Scholar]
- 12.Saliba RS, Munro PM, Luthert PJ, Cheetham ME. The cellular fate of mutant rhodopsin: Quality control, degradation and aggresome formation. J Cell Sci. 2002;115:2907–2918. doi: 10.1242/jcs.115.14.2907. [DOI] [PubMed] [Google Scholar]
- 13.Olsson JE, et al. Transgenic mice with a rhodopsin mutation (Pro23His): A mouse model of autosomal dominant retinitis pigmentosa. Neuron. 1992;9:815–830. doi: 10.1016/0896-6273(92)90236-7. [DOI] [PubMed] [Google Scholar]
- 14.Wu TH, et al. Opsin localization and rhodopsin photochemistry in a transgenic mouse model of retinitis pigmentosa. Neuroscience. 1998;87:709–717. doi: 10.1016/s0306-4522(98)00173-0. [DOI] [PubMed] [Google Scholar]
- 15.Tam BM, Moritz OL. Characterization of rhodopsin P23H-induced retinal degeneration in a Xenopus laevis model of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2006;47:3234–3241. doi: 10.1167/iovs.06-0213. [DOI] [PubMed] [Google Scholar]
- 16.Rutkowski DT, Kaufman RJ. That which does not kill me makes me stronger: Adapting to chronic ER stress. Trends Biochem Sci. 2007;32:469–476. doi: 10.1016/j.tibs.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Kudo T, et al. A molecular chaperone inducer protects neurons from ER stress. Cell Death Differ. 2008;15:364–375. doi: 10.1038/sj.cdd.4402276. [DOI] [PubMed] [Google Scholar]
- 18.Reddy RK, et al. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: Role of ATP binding site in suppression of caspase-7 activation. J Biol Chem. 2003;278:20915–20924. doi: 10.1074/jbc.M212328200. [DOI] [PubMed] [Google Scholar]
- 19.Inokuchi Y, et al. Effect of an inducer of BiP, a molecular chaperone, on endoplasmic reticulum (ER) stress-induced retinal cell death. Invest Ophthalmol Vis Sci. 2009;50:334–344. doi: 10.1167/iovs.08-2123. [DOI] [PubMed] [Google Scholar]
- 20.Oida Y, et al. Induction of BiP, an ER-resident protein, prevents the neuronal death induced by transient forebrain ischemia in gerbil. Brain Res. 2008;1208:217–224. doi: 10.1016/j.brainres.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 21.Hoshino T, et al. Endoplasmic reticulum chaperones inhibit the production of amyloid-beta peptides. Biochem J. 2007;402:581–589. doi: 10.1042/BJ20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris JA, Dorner AJ, Edwards CA, Hendershot LM, Kaufman RJ. Immunoglobulin binding protein (BiP) function is required to protect cells from endoplasmic reticulum stress but is not required for the secretion of selective proteins. J Biol Chem. 1997;272:4327–4334. doi: 10.1074/jbc.272.7.4327. [DOI] [PubMed] [Google Scholar]
- 23.Fu HY, et al. Overexpression of endoplasmic reticulum-resident chaperone attenu-ates cardiomyocyte death induced by proteasome inhibition. Cardiovasc Res. 2008;79:600–610. doi: 10.1093/cvr/cvn128. [DOI] [PubMed] [Google Scholar]
- 24.Lièvremont JP, Rizzuto R, Hendershot L, Meldolesi J. BiP, a major chaperone protein of the endoplasmic reticulum lumen, plays a direct and important role in the storage of the rapidly exchanging pool of Ca2+ J Biol Chem. 1997;272:30873–30879. doi: 10.1074/jbc.272.49.30873. [DOI] [PubMed] [Google Scholar]
- 25.Murray PJ, Watowich SS, Lodish HF, Young RA, Hilton DJ. Epitope tagging of the human endoplasmic reticulum HSP70 protein, BiP, to facilitate analysis of BiP—substrate interactions. Anal Biochem. 1995;229:170–179. doi: 10.1006/abio.1995.1399. [DOI] [PubMed] [Google Scholar]
- 26.Walsh N, van Driel D, Lee D, Stone J. Multiple vulnerability of photoreceptors to mesopic ambient light in the P23H transgenic rat. Brain Res. 2004;1013:194–203. doi: 10.1016/j.brainres.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 27.Yang LP, Wu LM, Guo XJ, Tso MO. Activation of endoplasmic reticulum stress in degenerating photoreceptors of the rd1 mouse. Invest Ophthalmol Vis Sci. 2007;48:5191–5198. doi: 10.1167/iovs.07-0512. [DOI] [PubMed] [Google Scholar]
- 28.White DA, Fritz JJ, Hauswirth WW, Kaushal S, Lewin AS. Increased sensitivity to light-induced damage in a mouse model of autosomal dominant retinal disease. Invest Ophthalmol Vis Sci. 2007;48:1942–1951. doi: 10.1167/iovs.06-1131. [DOI] [PubMed] [Google Scholar]
- 29.Leborgne-Castel N, Jelitto-Van Dooren EP, Crofts AJ, Denecke J. Overexpression of BiP in tobacco alleviates endoplasmic reticulum stress. Plant Cell. 1999;11:459–470. doi: 10.1105/tpc.11.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denecke J, Goldman MH, Demolder J, Seurinck J, Botterman J. The tobacco luminal binding protein is encoded by a multigene family. Plant Cell. 1991;3:1025–1035. doi: 10.1105/tpc.3.9.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell. 2002;3:99–111. doi: 10.1016/s1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- 32.Zeng L, et al. ATF6 modulates SREBP2-mediated lipogenesis. EMBO J. 2004;23:950–958. doi: 10.1038/sj.emboj.7600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai E, Bikopoulos G, Wheeler MB, Rozakis-Adcock M, Volchuk A. Differential activation of ER stress and apoptosis in response to chronically elevated free fatty acids in pancreatic beta-cells. Am J Physiol Endocrinol Metab. 2008;294:E540–E550. doi: 10.1152/ajpendo.00478.2007. [DOI] [PubMed] [Google Scholar]
- 34.Averous J, et al. Induction of CHOP expression by amino acid limitation requires both ATF4 expression and ATF2 phosphorylation. J Biol Chem. 2004;279:5288–5297. doi: 10.1074/jbc.M311862200. [DOI] [PubMed] [Google Scholar]
- 35.Miyake H, Hara I, Arakawa S, Kamidono S. Stress protein GRP78 prevents apoptosis induced by calcium ionophore, ionomycin, but not by glycosylation inhibitor, tunicamycin, in human prostate cancer cells. J Cell Biochem. 2000;77:396–408. doi: 10.1002/(sici)1097-4644(20000601)77:3<396::aid-jcb5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 36.Donovan M, Cotter TG. Caspase-independent photoreceptor apoptosis in vivo and differential expression of apoptotic protease activating factor-1 and caspase-3 during retinal development. Cell Death Differ. 2002;9:1220–1231. doi: 10.1038/sj.cdd.4401105. [DOI] [PubMed] [Google Scholar]
- 37.Sanges D, Comitato A, Tammaro R, Marigo V. Apoptosis in retinal degeneration involves cross-talk between apoptosis-inducing factor (AIF) and caspase-12 and is blocked by calpain inhibitors. Proc Natl Acad Sci USA. 2006;103:17366–17371. doi: 10.1073/pnas.0606276103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang M, Wey S, Zhang Y, Ye R, Lee AS. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal. 2009;11:2307–2316. doi: 10.1089/ars.2009.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao RV, et al. Coupling endoplasmic reticulum stress to the cell death program: Role of the ER chaperone GRP78. FEBS Lett. 2002;514:122–128. doi: 10.1016/s0014-5793(02)02289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu Y, Li J, Lee AS. GRP78/BiP inhibits endoplasmic reticulum BIK and protects human breast cancer cells against estrogen starvation-induced apoptosis. Cancer Res. 2007;67:3734–3740. doi: 10.1158/0008-5472.CAN-06-4594. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.