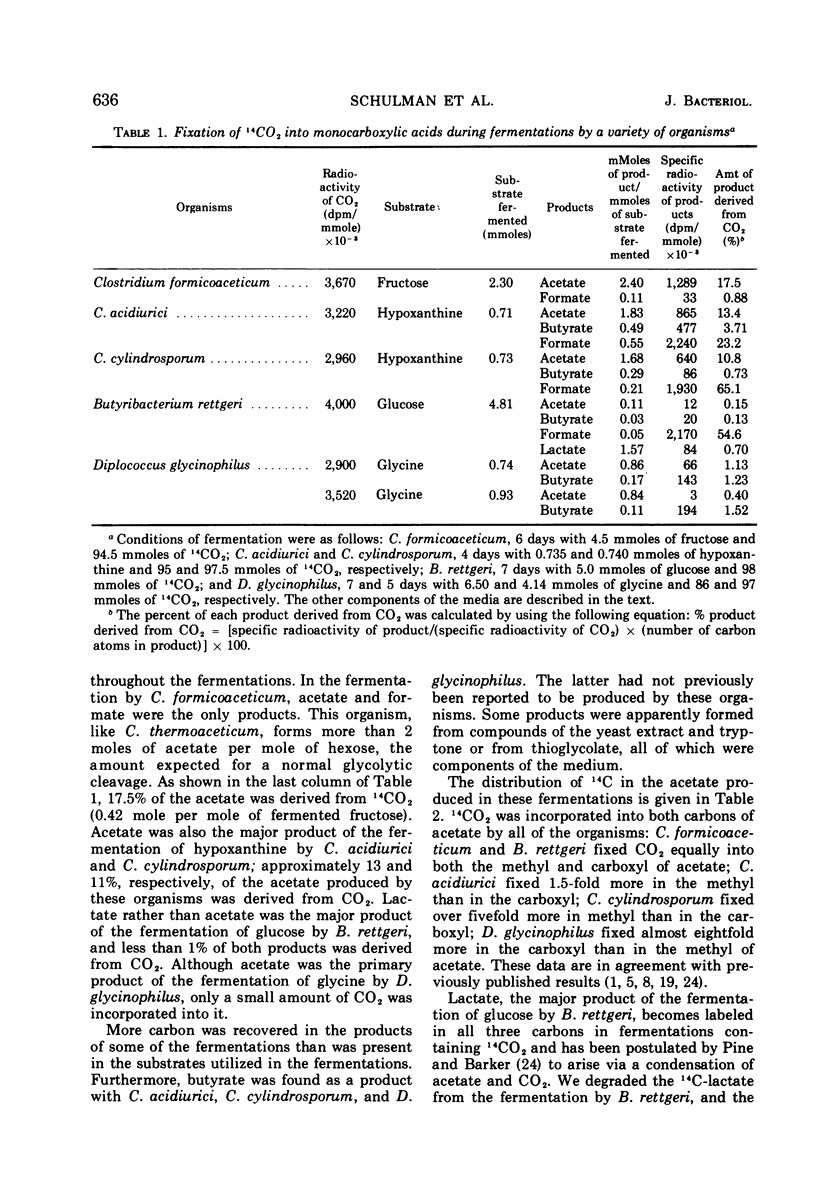
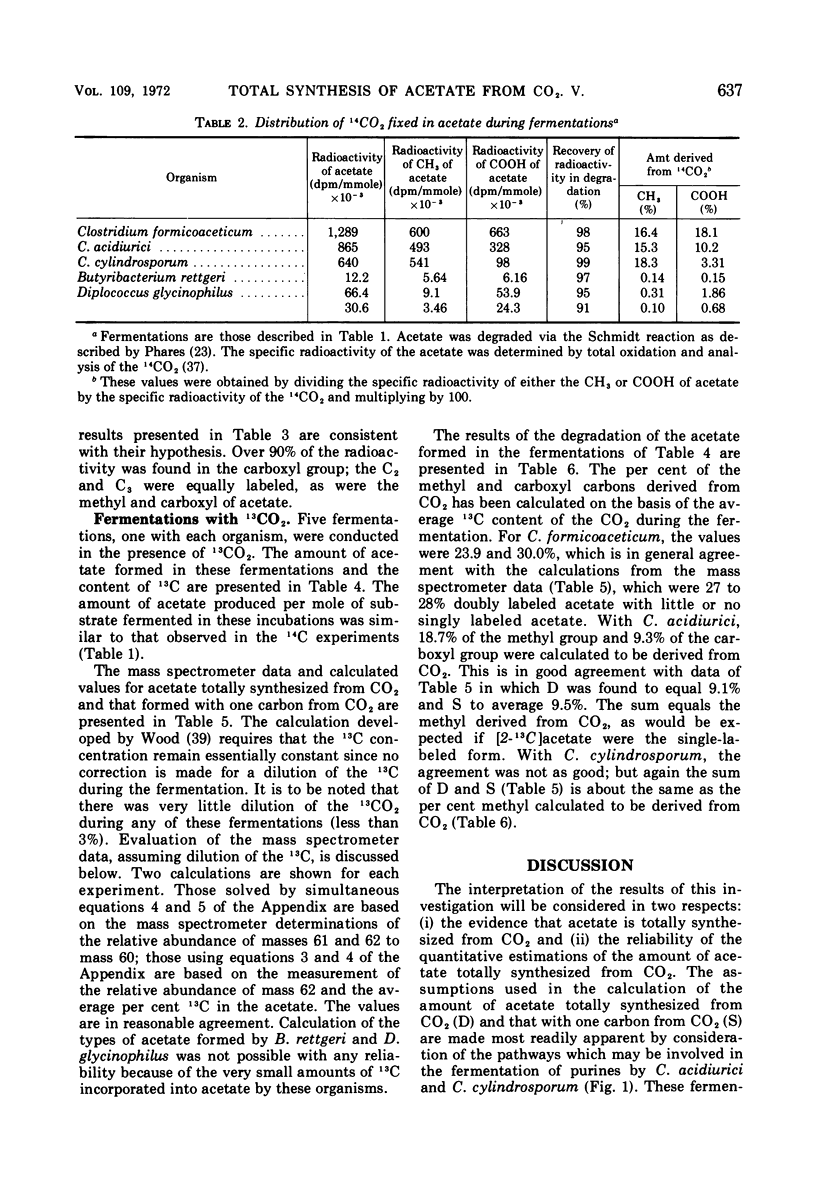
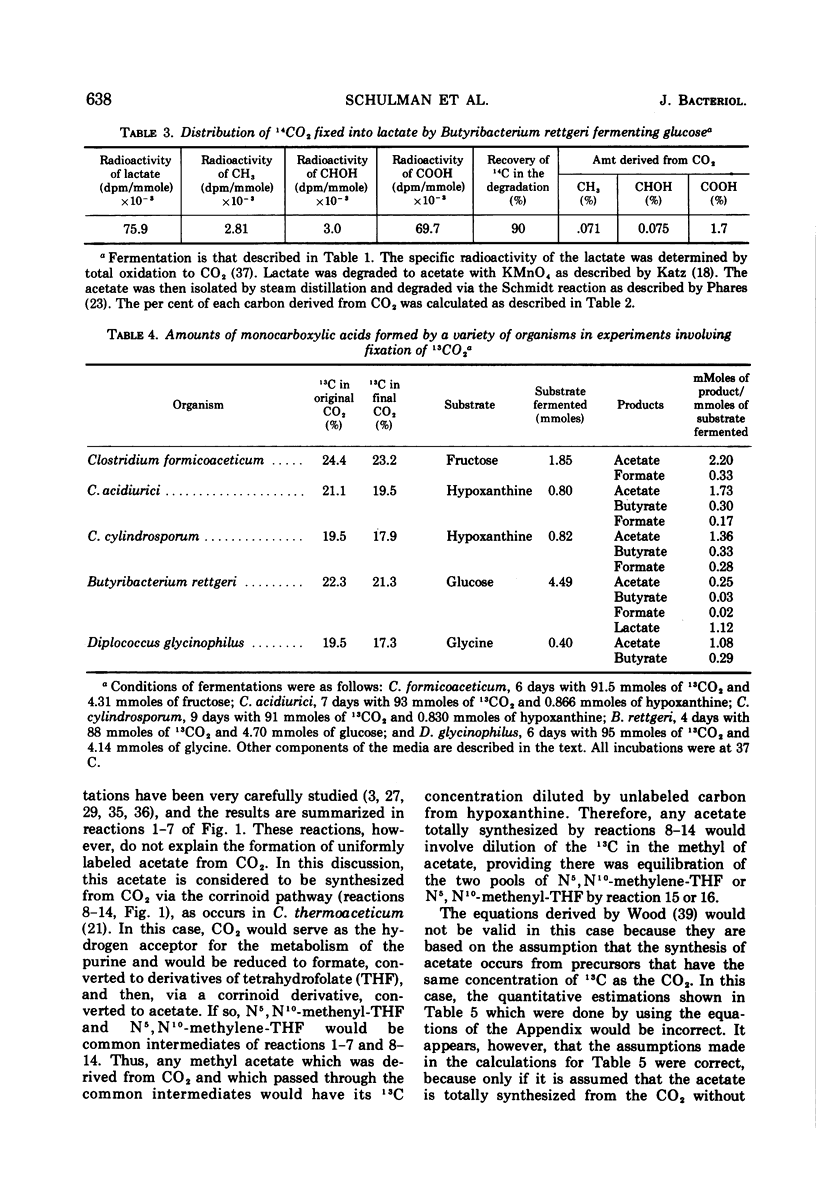
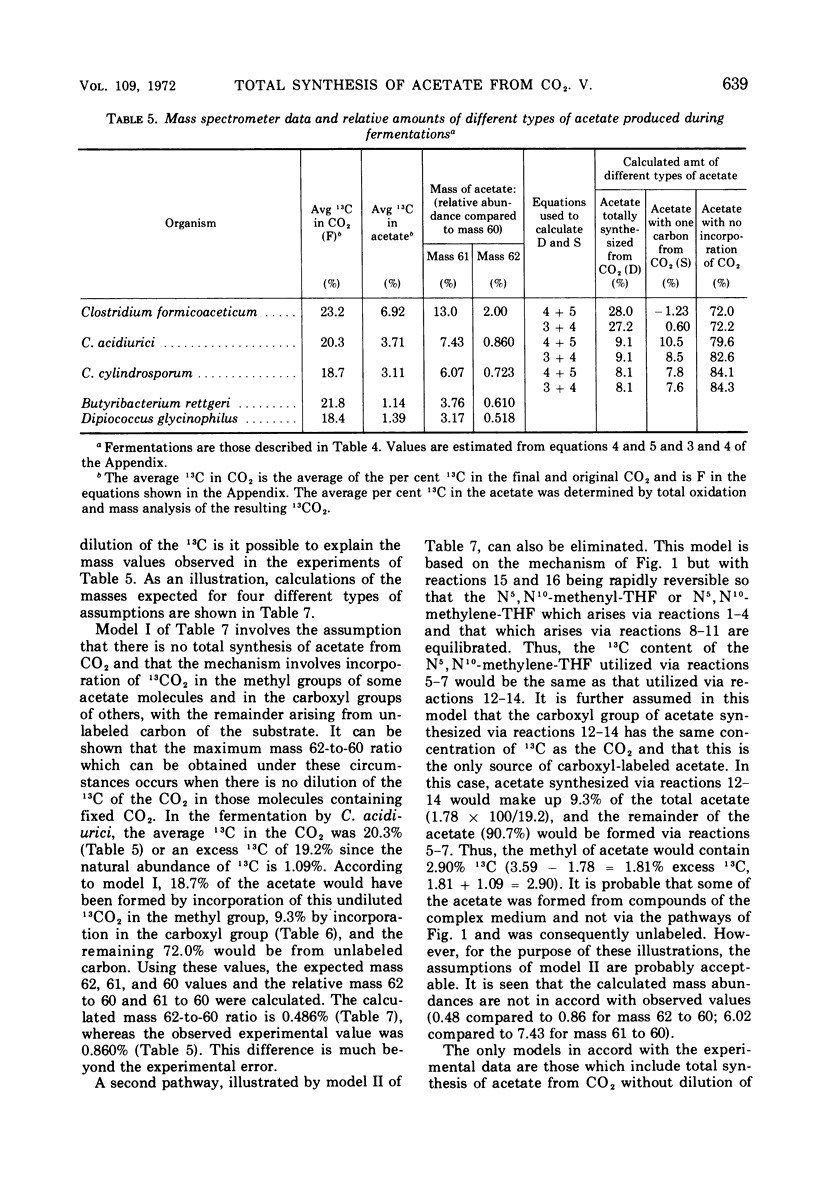
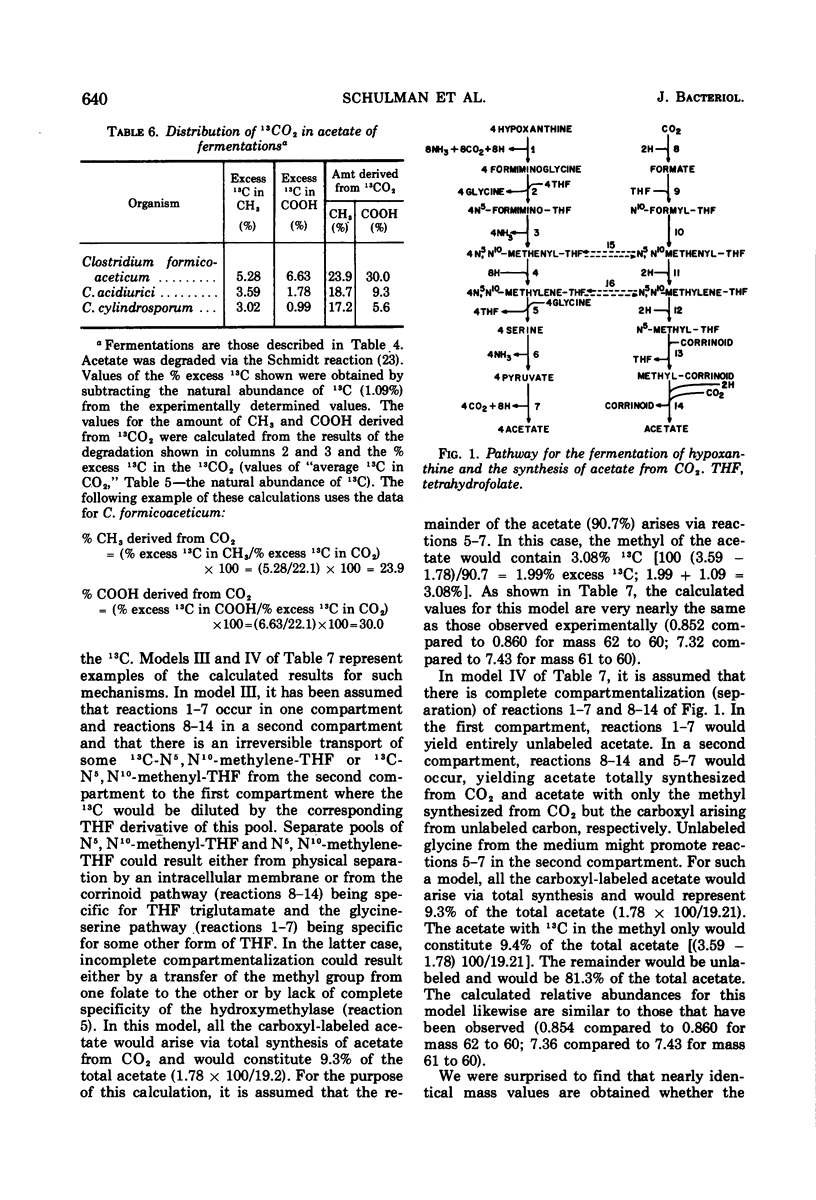
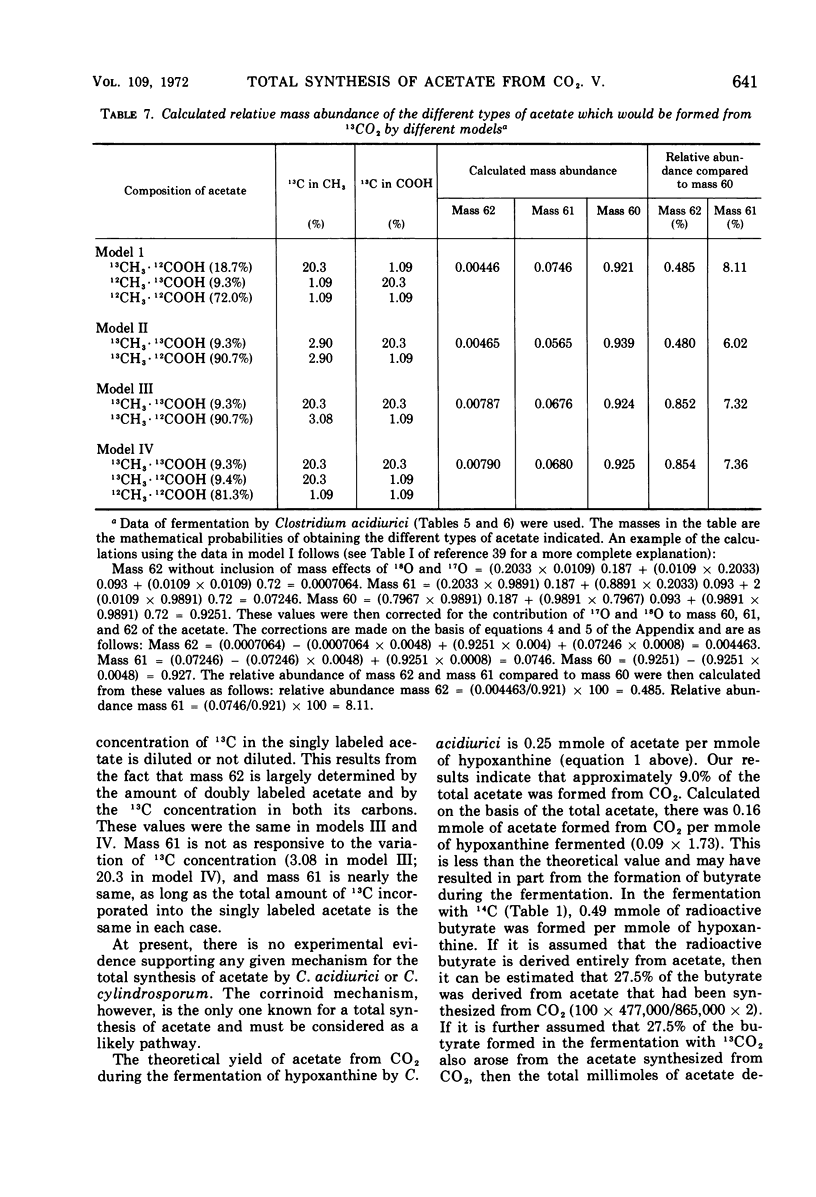
Abstract
Mass analysis was used to determine the amount of acetate which is totally synthesized from 13CO2 during fermentations by Clostridium formicoaceticum, C. acidiurici, C. cylindrosporum, Butyribacterium rettgeri, and Diplococcus glycinophilus. In the fermentation of fructose by C. formicoaceticum, 27% of the acetate was found to be totally synthesized from CO2, and the remaining acetate was unlabeled, having been formed from fructose. Evidence is presented that the purine-fermenting organisms, C. acidiurici and C. cylindrosporum, totally synthesized about 9% of the acetate from CO2, and that the methyl group of an additional 9% was formed from CO2. The remaining acetate was formed from the carbons of the purine and not via CO2. It has been postulated that the fermentation of the purines and synthesis of acetate from CO2 both occur via derivatives of tetrahydrofolate. Evidence is presented that a compartmentalization of these folate intermediates is required if both the purine degradation and the CO2 utilization involve identical intermediates. Neither B. rettgeri nor D. glycinophilus incorporated sufficient 13CO2 into acetate to allow determination of the types of acetate by mass analysis, although they did incorporate labeled 14CO2 in both positions of acetate.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreesen J. R., Gottschalk G., Schlegel H. G. Clostridium formicoaceticum nov. spec. isolation, description and distinction from C. aceticum and C. thermoaceticum. Arch Mikrobiol. 1970;72(2):154–174. doi: 10.1007/BF00409521. [DOI] [PubMed] [Google Scholar]
- BUCHANAN B. B., BACHOFEN R., ARNON D. I. ROLE OF FERREDOXIN IN THE REDUCTIVE ASSIMILATION OF CO2 AND ACETATE BY EXTRACTS OF THE PHOTOSYNTHETIC BACTERIUM, CHROMATIUM. Proc Natl Acad Sci U S A. 1964 Sep;52:839–847. doi: 10.1073/pnas.52.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker H. A., Beck J. V. Clostridium acidi-uridi and Clostridium cylindrosporum, Organisms Fermenting Uric Acid and Some Other Purines. J Bacteriol. 1942 Mar;43(3):291–304. doi: 10.1128/jb.43.3.291-304.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker H. A., Haas V. Butyribacterium, a New Genus of Gram-positive, Non-sporulating Anaerobic Bacteria of Intestinal Origin. J Bacteriol. 1944 Mar;47(3):301–305. doi: 10.1128/jb.47.3.301-305.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker H. A., Kamen M. D., Haas V. Carbon Dioxide Utilization in the Synthesis of Acetic and Butyric Acids by Butyribacterium Rettgeri. Proc Natl Acad Sci U S A. 1945 Nov;31(11):355–360. doi: 10.1073/pnas.31.11.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker H. A., Ruben S., Beck J. V. Radioactive Carbon as an Indicator of Carbon Dioxide Reduction: IV. The Synthesis of Acetic Acid from Carbon Dioxide by Clostridium Acidi-Urici. Proc Natl Acad Sci U S A. 1940 Aug 15;26(8):477–482. doi: 10.1073/pnas.26.8.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ghazzawi E. Neuisolierung von Clostridium aceticum Wieringa und stoffwechselphysiologische Untersuchungen. Arch Mikrobiol. 1967 May 17;57(1):1–19. [PubMed] [Google Scholar]
- Fontaine F. E., Peterson W. H., McCoy E., Johnson M. J., Ritter G. J. A New Type of Glucose Fermentation by Clostridium thermoaceticum. J Bacteriol. 1942 Jun;43(6):701–715. doi: 10.1128/jb.43.6.701-715.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson J. L., Volcani B. E., Barker H. A. The Nutritional Requirements of Clostridium aceticum. J Bacteriol. 1948 Dec;56(6):781–782. [PMC free article] [PubMed] [Google Scholar]
- Linke H. A. Der Fructose-Stoffwechsel von Clostridium aceticum. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. 1969;123(4):369–379. [PubMed] [Google Scholar]
- Ljungdahl L. G. Total synthesis of acetate from CO2 by heterotrophic bacteria. Annu Rev Microbiol. 1969;23:515–538. doi: 10.1146/annurev.mi.23.100169.002503. [DOI] [PubMed] [Google Scholar]
- Ljungdahl L., Irion E., Wood H. G. Total synthesis of acetate from CO2. I. Co-methylcobyric acid and CO-(methyl)-5-methoxybenzimidazolylcobamide as intermediates with Clostridium thermoaceticum. Biochemistry. 1965 Dec;4(12):2771–2780. doi: 10.1021/bi00888a030. [DOI] [PubMed] [Google Scholar]
- PHARES E. F. Degradation of labeled propionic and acetic acids. Arch Biochem Biophys. 1951 Sep;33(2):173–178. doi: 10.1016/0003-9861(51)90094-x. [DOI] [PubMed] [Google Scholar]
- PINE L., BARKER H. A. Tracer experiments on the mechanism of acetate formation from carbon dioxide by Butyribacterium rettgeri. J Bacteriol. 1954 Aug;68(2):216–226. doi: 10.1128/jb.68.2.216-226.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTON J. M., KURATOMI K., STADTMAN E. R. METHYL-VITAMIN B12 AS A SOURCE OF METHYL GROUPS FOR THE SYNTHESIS OF ACETATE BY CELL-FREE EXTRACTS OF CLOSTRIDIUM THERMOACETICUM. Ann N Y Acad Sci. 1964 Apr 24;112:804–806. doi: 10.1111/j.1749-6632.1964.tb45057.x. [DOI] [PubMed] [Google Scholar]
- RABINOWITZ J. C., BARKER H. A. Purine fermentation by Clostridium cylindrosporum. I. Tracer experiments on the fermentation of guanine. J Biol Chem. 1956 Jan;218(1):147–160. [PubMed] [Google Scholar]
- RABINOWITZ J. C., PRICER W. E., Jr An enzymatic method for the determination of formic acid. J Biol Chem. 1957 Nov;229(1):321–328. [PubMed] [Google Scholar]
- RABINOWITZ J. C., PRICER W. E., Jr Purine fermentation by Clostridium cylindrosporum. IV. 4-Ureido-5-imidazolecarboxylic acid. J Biol Chem. 1956 Jan;218(1):189–199. [PubMed] [Google Scholar]
- SAGERS R. D., BENZIMAN M., GUNSALUS I. C. Acetate formation in Clostridium acidi-urici: acetokinase. J Bacteriol. 1961 Aug;82:233–238. doi: 10.1002/path.1700820136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGERS R. D., GUNSALUS I. C. Intermediatry metabolism of Diplococcus glycinophilus. I. Glycine cleavage and one-carbon interconversions. J Bacteriol. 1961 Apr;81:541–549. doi: 10.1128/jb.81.4.541-549.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWIM H. E., KRAMPITZ L. O. Acetic acid oxidation by Escherichia coli; evidence for the occurrence of a tricarboxylic acid cycle. J Bacteriol. 1954 Apr;67(4):419–425. doi: 10.1128/jb.67.4.419-425.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardesai V. M., Provido H. S. The determination of glycine in biological fluids. Clin Chim Acta. 1970 Jul;29(1):67–71. doi: 10.1016/0009-8981(70)90222-6. [DOI] [PubMed] [Google Scholar]
- Schulman M., Wood H. G. Determination and degradation of microquantities of acetate. Anal Biochem. 1971 Feb;39(2):505–520. doi: 10.1016/0003-2697(71)90441-6. [DOI] [PubMed] [Google Scholar]
- UYEDA K., RABINOWITZ J. C. METABOLISM OF FORMIMINOGLYCINE. GLYCINE FORMIMINOTRANSFERASE. J Biol Chem. 1965 Apr;240:1701–1710. [PubMed] [Google Scholar]
- Uyeda K., Rabinowitz J. C. Metabolism of formiminoglycine. Formiminotetrahydrofolate cyclodeaminase. J Biol Chem. 1967 Jan 10;242(1):24–31. [PubMed] [Google Scholar]
- WOOD H. G. A study of carbon dioxide fixation by mass determination of the types of C13-acetate. J Biol Chem. 1952 Feb;194(2):905–931. [PubMed] [Google Scholar]



