Abstract
Recurrent group A Streptococcus (GAS) tonsillitis and associated autoimmune diseases indicate that the immune response to this organism can be ineffective and pathological. TGF-β1 is recognized as an essential signal for generation of regulatory T cells (Tregs) and T helper (Th) 17 cells. Here, the impact of TGF-β1 induction on the T-cell response in mouse nasal-associated lymphoid tissue (NALT) following intranasal (i.n.) infections is investigated. ELISA and TGF-β1-luciferase reporter assays indicated that persistent infection of mouse NALT with GAS sets the stage for TGF-β1 and IL-6 production, signals required for promotion of a Th17 immune response. As predicted, IL-17, the Th17 signature cytokine, was induced in a TGF-β1 signaling-dependent manner in single-cell suspensions of both human tonsils and NALT. Intracellular cytokine staining and flow cytometry demonstrated that CD4+ IL-17+ T cells are the dominant T cells induced in NALT by i.n. infections. Moreover, naive mice acquired the potential to clear GAS by adoptive transfer of CD4+ T cells from immunized IL-17A+/+ mice but not cells from IL-17A−/− mice. These experiments link specific induction of TGF-β1 by a bacterial infection to an in vivo Th17 immune response and show that this cellular response is sufficient for protection against GAS. The association of a Th17 response with GAS infection reveals a potential mechanism for destructive autoimmune responses in humans.
Keywords: IL-17, nasal-associated lymphoid tissue, Streptococcus pyogenes
Group A Streptococcus (GAS) causes a wide spectrum of diseases in humans, commonly pharyngeal mucosa and skin infections, such as tonsillitis (strep throat) and impetigo. Additionally, this bacterium is associated with relatively rare but severe life-threatening invasive infections, such as necrotizing fasciitis, toxic shock, and sepsis. Tonsillitis is the most frequent GAS infection in children (1). Although antibiotic treatment efficiently relieves symptoms, patients often have recurrent tonsil infections, which ultimately cause hypertrophy of tonsils and account for the fact that tonsillectomy is still the most common childhood surgical procedure in the United States (2). Moreover, recurrent tonsillitis may increase the risk for GAS-associated autoimmune diseases, such as rheumatic fever and rheumatic heart disease (3). Epidemiological and clinical data indicate that the immune response to GAS infection can be protective, can be insufficient for eliminating bacteria from tonsils and preventing subsequent infections, or can attack the host itself. Although virulence mechanisms of GAS have been intensively studied, the molecular and cellular basis for adaptive immunity to GAS is still obscure. This lack of knowledge has frustrated understanding of the basis of infection sequelae and impeded development of an efficacious and safe GAS vaccine.
Cytokines are critical for differentiation of activated antigen-specific T cells into the appropriate effector T-cell lineage. We previously demonstrated that GAS can induce TGF-β1 by cultured human tonsil fibroblasts and that exogenous TGF-β1 up-regulates expression of α5β1 integrins and fibronectin, which are required for internalization of GAS by host cells (4). Among the diverse functions of TGF-β1, this cytokine plays a pivotal role in generation of regulatory T cells (Tregs) and T helper (Th) 17 cells (5–7). Tregs prevent immune responses to self-antigens and also dampen the cellular response to foreign antigens, whereas IL-17-producing Th17 cells significantly enhance infiltration of neutrophils and macrophages into infected tissue, leading to efficient eradication of invading pathogens (8). Thus, TGF-β1-mediated differentiation of T cells dramatically influences immunity to infections.
Previous studies showed that GAS has a tropism for mouse nasal-associated lymphoid tissue (NALT) and suggested that intranasal (i.n.) infection in the mouse is a reasonable model of human tonsillitis (9). Our experiments used this model to test the hypothesis that induction of TGF-β1 by GAS infection plays an important role in T-cell immune responses. In vivo kinetics of TGF-β1 and IL-6 induction by GAS and the impact of that response on development of effector T-cell subtypes were investigated. Experiments showed that streptococci preferentially skewed CD4 T-cell development toward the Th17 lineage in NALT, and protective immunity induced by i.n. infection was demonstrated to be dependent on this lineage.
Results
Induction of TGF-β1 in Human Tonsils and Mice by GAS.
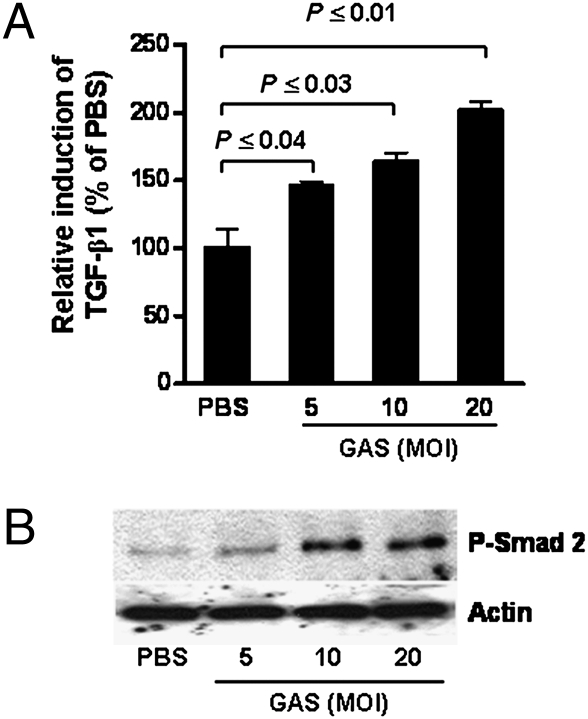
We previously demonstrated that TGF-β1 is induced in primary human tonsil fibroblasts on prolonged exposure to GAS (7–14 days) (4). TGF-β1 can be produced by many cell types and is regulated by autocrine and paracrine mechanisms (8); therefore, we postulated that induction of TGF-β1 would be greater and more rapid in mixed tonsil cell suspensions, which contain a variety of cell types with TGF-β1-producing potential. Single-cell suspensions were prepared from tonsils (from patients with recurrent tonsillitis) (10) and treated with heat-killed GAS strain 90-226 (HK-90-226). Significantly increased TGF-β1 secretion was detected by 48 hr following treatment (Fig. 1A). In addition, activation of TGF-β1 signaling (phosphorylation of Smad2) was detected in cell extracts as early as 24 hr following treatment (Fig. 1B), even though secreted TGF-β1 was not detected by this time. Both TGF-β1 secretion and TGF-β1 signaling were induced in a bacterial dose-dependent manner (Fig.1 A and B).
Fig. 1.
Induction of TGF-β1 and TGF-β1 signaling in human tonsil cells by GAS. Single-cell suspensions from a fresh human tonsil specimen were seeded into tissue culture plates. (A) Culture supernatants were collected 48 hr after treatment with HK-90-226 at the indicated multiplicity of infection (MOI) and assayed for active TGF-β1 by a TGF-β1-luciferase reporter. Data are mean ± SEM of three independent determinations. Representative results from one of three independent experiments with different tonsils are shown. (B) Western blots of extracts from tonsil cells treated with HK-90-226 at the indicated MOI for 24 hr. Phosphorylated Smad2 (P-Smad 2) was detected on Western blots using anti-P-Smad2. Antiactin (control) was used to assess total protein loaded.
Because mouse NALT is a primary streptococcal target following i.n. infection (9) and is functionally similar to human tonsils, we questioned whether NALT could serve as a model to study induction of TGF-β1 by Streptococcus further. However, unlike human tonsils, no increase in TGF-β1 secretion or phosphorylated Smad2 was detected in naive NALT cell cultures following ex vivo exposure to HK-90-226.
To determine whether the strong TGF-β1 response observed in tonsil cell cultures could be induced in the NALT in vivo model, mice were inoculated i.n. with sublethal doses of live 90-226 streptococci (Materials and Methods). Increased TGF-β1 expression was detected in extracts of NALT cells (SI Text and Fig. S1A) by 48 hr using ELISA; however, significant TGF-β1 secretion was not observed in the supernatants from NALT cells that were further cultured. A time course showed a 2-fold change in blood levels of TGF-β1 between 6 and 48 hr after infection (Fig. S1B), but concentrations were not statistically different from those of the mice before infection (0 hr) because of greater variability among the latter. The inability of NALT cells to secrete measurable TGF-β1 ex vivo, in contrast to human tonsil specimens, suggests that the latter contain larger populations of cells primed to secrete TGF-β1 on reexposure to streptococci.
Persistent GAS Infections Amplify TGF-β1 Expression in Mouse Secondary Lymphoid Tissues.
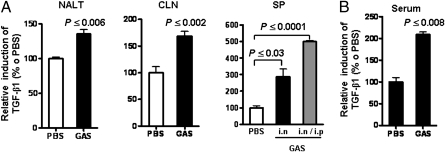
Persistent streptococcal colonization following treatment and recovery from tonsillitis is common (11, 12). The observation that human tonsil cell suspensions produce robust increases in TGF-β1 relative to that of NALT following a single stimulation suggests that persistent infection, as in recurrent tonsillitis, would amplify the potential of TGF-β1 expression. Therefore, to mimic persistent infection of tonsils, mice were infected i.n. four times with strain 90-226 at 1-week intervals. At 48 hr after the last infection, cells from lymphoid tissues were cultured for 48 hr. Compared with noninfected mice (PBS), significantly higher levels of TGF-β1 were detected in the culture supernatants of cervical lymphoid nodes (CLN), spleen, and NALT from infected mice (Fig. 2A). Even greater increases in TGF-β1 were measured in splenocytes from multiple i.n. infected mice, which had also received an i.p. inoculation along with the last i.n. inoculation (Fig. 2A, SP). Furthermore, serum TGF-β1 levels were significantly elevated following multiple infections (Fig. 2B). These results indicate that multiple streptococcal i.n. infections increase the potential for TGF-β1 expression by lymphoid tissues.
Fig. 2.
Multiple GAS infections amplify TGF-β1 expression in mouse secondary lymphoid tissues. Mice were infected i.n. four times with viable 90-226 (A) At 48 hr after the last infection, single-cell suspensions of NALT (0.5 × 106 cells per well), CLN (2.5 × 106 cells per well), and spleen (SP; 2.5 × 106 cells per well) were prepared separately from each mouse (n = 5) and cultured for 48 hr without ex vivo exposure to GAS. Culture supernatants were assayed with the TGF-β1 reporter. The gray column in SP shows data from mice that received one i.p. infection alone with the last i.n. infection. (B) Blood specimens were taken 48 hr after the last of four infections. Serum samples (n = 5) were assayed for TGF-β1 as in A. Data are mean ± SEM of five mice per group. Representative data from one of three independent experiments are shown.
GAS Induces Cytokines, Which Are Known to Promote Th17 Differentiation.
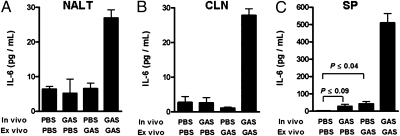
TGF-β1, in combination with other cytokines, is known to direct CD4 T-cell differentiation toward either a Th17 or Treg phenotype. In mice, both TGF-β1 and IL-6 are essential for de novo Th17 differentiation (6, 13, 14). Hence, it was of interest to evaluate whether i.n. infection induces IL-6 expression in NALT and other lymphoid tissue. Mice were inoculated once i.n and also i.p at the same time with viable 90-226 GAS to prime splenocytes better. At 48 hr after inoculation, NALT, CLN, and spleen were taken and single-cell suspensions were restimulated with HK-90-226. At 72 hr after ex vivo streptococcal restimulation, IL-6 was dramatically increased in lymphoid tissues from GAS-infected mice (P ≤ 0.001; Fig. 3, last bar in each panel), whereas no or low levels of increases in IL-6 were observed in response to a single in vivo (Fig. 3, second bar in each panel) or single ex vivo (Fig. 3, third bar in each panel) treatment with streptococci. These results indicate that the previous infection primes these tissues to secrete IL-6 on reexposure to bacteria. Higher concentrations of IL-6 in supernatants of splenocytes likely reflect the larger number of total cells relative to NALT and CLN in cultures (Fig. 3C).
Fig. 3.
GAS infection induces IL-6. Mice were inoculated i.n. (1.5 × 108 CFUs) and i.p. (1.5 × 107 CFUs) once with viable 90-226 or PBS. At 48 hr after infection, single-cell suspensions of NALT and CLN cells pooled from three mice and splenocytes from individual mice were separately incubated with HK-90-226. (A–C) Cell culture supernatants (n = 3) were collected after 72 hr and assayed for IL-6 by ELISA. Data are mean ± SEM of three independent treatments of pooled cells.
In addition to TGF-β1 and IL-6, IL-1β and TNF-α contribute to Th17 cellular expansion in mice (14) and in humans (15). Transcription of both IL-1β and TNF-α genes was significantly increased in NALT tissue 24 hr after i.n. infection (SI Text and Fig. S2).
GAS-Induced IL-17 Expression Is Dependent on TGF-β1 Signaling.
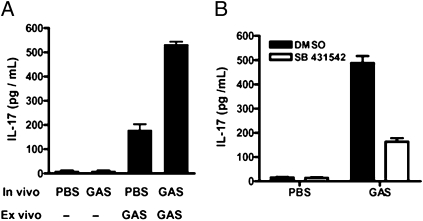
IL-17 is preferentially produced by Th17 cells in the presence of TGF-β1, IL-1β, IL-6, and IL-23 (15). The previous experiments suggested that IL-17 would be secreted by lymphoid tissue from GAS-infected mice. NALT is small in size, and the T-cell population in this tissue rapidly undergoes apoptosis when cultivated ex vivo (16); therefore, splenocytes were employed in experiments intended to measure T-cell function. Moreover, mice were infected i.p. to ensure exposure of splenocytes to streptococci. Like TGF-β1 and IL-6, IL-17 was secreted in greater amounts in splenocytes from infected mice on ex vivo restimulation with HK-90-226 (Fig. 4A). Exposure of naive splenocytes to HK-90-226 induced IL-17, but levels were significantly less than those produced by cells from previously infected mice.
Fig. 4.
IL-17 induction by GAS is dependent on TGF-β1 signaling. Mice were inoculated i.p. with viable 90-226 or PBS. At 48 hr after infection, splenocyte suspensions were prepared and incubated with HK-90-226 [multiplicity of infection (MOI) = 10] or buffer for 72 hr (A); splenocytes were first treated with TGF-β1 type I receptor inhibitor (SB 431542) or inhibitor solvent (DMSO) for 1 h before incubation with HK-90-226 at an MOI of 10 for 72 hr (B). Supernatants of splenocytes from mice (n = 3) were collected and assayed for IL-17 by ELISA. Data are mean ± SEM of three independent treatments from one of three experiments.
To confirm that IL-17 production requires TGF-β1 signaling, cultures of splenocytes from mice infected i.p. were first treated with an inhibitor (SB 431542) of TGF-β1 signaling by inhibiting TGF-β1 type I receptor phosphorylation (4) before exposure to HK-90-226. Blockage of TGF-β1 signaling significantly reduced IL-17 production by up to 66.6% (Fig. 4B), suggesting that TGF-β1 is a critical signal for the IL-17 response to GAS. Although the target of SB 431542 is well defined and Smad2 is a primary component of TGF-β1 signaling, it is possible that other minor functions of the receptor could be inhibited and unexpectedly restrict the IL-17 response.
GAS Infection Induces Th17 Differentiation in Mouse NALT.
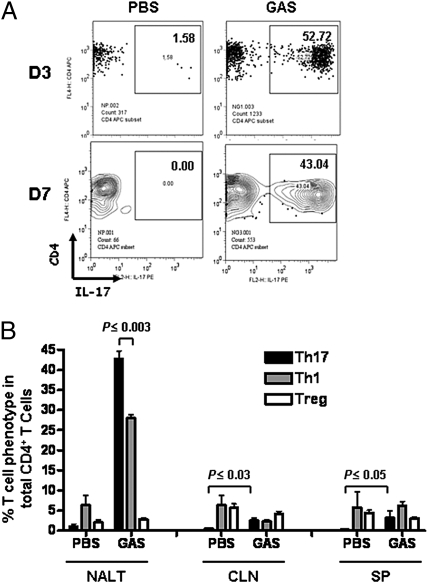
The cytokine profile induced by GAS infection suggested that the cellular immune response to GAS is directed toward a Th17 phenotype. This was confirmed by quantitation of lineage-specific CD4+ effector T cells. Mice were infected i.n. four times with viable 90-226 as described. Single-cell preparations of NALT, CLN, and spleen were stained for CD4, CD25, and FoxP3 as well as for intracellular cytokines IFN-γ and IL-17 and were then analyzed by flow cytometry. Remarkable increases in CD4+IL-17+ T cells in NALT were observed by 3 and 7 days after the last infection (Fig. 5A). CD4+IFN-γ+ cells also increased but were significantly fewer in number relative to CD4+IL-17+ cells (P ≤ 0.003) (Fig. 5B, NALT), indicating that Th17 cells are the major effector T-cell type induced by GAS infection. CD4+IL-17+ T cells also increased in CLN (P ≤ 0.03) and in spleen (P ≤ 0.05) but represented a much smaller fraction of the total CD4+ population than observed in NALT (Fig. 5B, black bar). CD4+IFN-γ+ cells did not increase in CLN or spleen following GAS infections (Fig. 5B, gray bar). Relative to PBS controls, Tregs or CD4+CD25+Foxp3+ cells underwent little change in these tissues following infection. In preliminary experiments, CD4+ cells were also stained for intracellular IL-4 to assess the Th2 response. No increase in Th2 cells (<2.0%) was observed after i.n. infections in any of these lymphoid tissues. Infected tonsils and NALT are both immune inductive and effector sites; therefore, repeated bacterial loading may result in accumulation of larger numbers of Th17 cells.
Fig. 5.
GAS induces Th17 cell differentiation in mouse NALT in vivo. NALT, CLN, and spleen were taken 3 and 7 days following four i.n. infections. Cell suspensions were stained with allophycocyanin (APC)-CD4, followed by intracellular staining with phycoerythrin (PE)-IL-17, and were analyzed by FACS (Materials and Methods). (A) Analysis of stained NALT cells gated on CD4 T cells on day 3 and day 7. The percentages of CD4+IL-17+ cells were expressed as a fraction of total CD4+ cells (Upper Right). (B) Percentages of CD4+IL-17+, CD4+IFN-γ+, and CD4+CD25+FoxP3+ cells among total CD4+ cells are plotted (7 days following infection). Data are mean ± SEM of three mice per group.
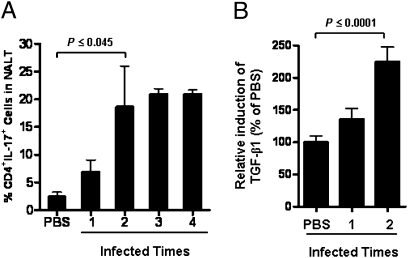
The dependence of IL-17 expression on TGF-β1 signaling and amplification of TGF-β1 expression by multiple i.n. infections suggested that persistent infection would increase the size of the CD4+IL-17+ population in NALT. This possibility was tested by comparing the T-cell populations in NALT from mice infected i.n. from one to four times with 1-week intervals. Little change in the frequency of CD4+IL-17+ cells was observed following a single infection; however, their numbers increased significantly following the second infection and stayed high after further infections (Fig. 6A). As before, repeated i.n. infections also further increased levels of TGF-β1 in serum samples (Fig. 6B) in parallel with increased numbers of CD4+IL-17+ cells in NALT. The increased CD4+IL-17+ cell number is consistent with the discovery of large numbers of Th17 cells in surgically removed human tonsils (17), which, in most cases, were from individuals who had persistent and recurrent GAS tonsillitis.
Fig. 6.
Th17 cell differentiation in mouse NALT paralleled TGF-β1 induction. Mice were infected i.n. one to four times. (A) Three days after the last infection, NALT cells from each group were stained and analyzed by FACS as described in Fig. 5. (B) Blood samples were collected, and serum TGF-β1 was measured by a TGF-β1-luciferase reporter. Data are mean ± SEM of three to four mice per group.
GAS and Listeria monocytogenes Skew the T-Cell Responses Toward Th17 and Th1 Phenotypes, Respectively.
The previous experiments suggested that the T-cell response to GAS infection would be distinct from that of Listeria monocytogenes (Lm), a Gram-positive pathogen known to induce strong Th1 responses in mice (18). This was confirmed in a series of experiments, which are more completely described in SI Text. As predicted, i.n. infection with GAS induced a Th17 cellular response, whereas infection with Lm via the same route predominantly induced expansion of CD4+IFN-γ+ cells (Th1) (SI Text and Fig. S3). Ex vivo restimulation of splenocytes from mice infected i.p. with streptococci produced TGF-β1 and IL-17. As anticipated, infection with Lm had little or no impact on TGF-β1 or IL-17 levels (SI Text and Fig. S4). These results suggest that TGF-β1 is the critical signal that leads to distinct T-cell phenotypes induced by these different pathogens and are in agreement with findings that IL-17-deficient mice are not more susceptible to Lm infection (20).
Multiple i.n. Infections Induce Th17-Dependent Protection Against Subsequent i.n. Challenge with GAS.
The previous experiments raised the important question of whether the Th17 cellular response could protect against i.n. infection; therefore, clearance of bacteria from NALT after immunizing mice by repeated i.n. inoculations was investigated (SI Text and Fig. S5). At 4 hr after i.n. challenge, NALT from immunized and control mice contained approximately equal numbers of bacteria (Fig. S5A); however, by 24 hr, when TGF-β1 levels were significantly elevated (Fig. S5B), immunized animals had efficiently cleared streptococci from NALT (P = 0.007). In contrast, streptococci had increased by more than 10-fold in NALT from PBS controls by 24 hr.
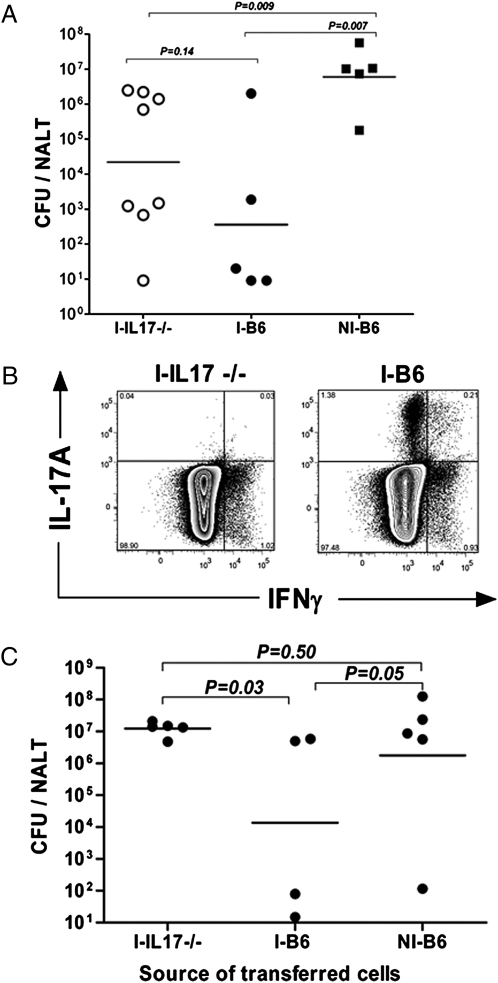
The correlation between TGF-β1 and efficient clearance of streptococci suggests that Th17 cells play a critical role in protection against infection. To test this possibility, WT B6 and IL-17A−/− mice (21) were compared for their capacity to mount protective T-cell responses. At 24 hr after the fourth immunization, NALT samples were assayed for colony-forming units (CFUs) and cells were obtained from lymph nodes for transfer experiments. Immunized WT mice (I-B6) cleared streptococci more rapidly from NALT than immunized IL-17A−/− (I-IL-17−/−) mice; however, mean differences in colonized bacteria in NALT were not significant (P = 0.14). Both I-B6 and I-IL-17−/− mice retained significantly fewer streptococci than PBS control mice (P = 0.007 and P = 0.009, respectively) (Fig. 7A), an indication of protection.
Fig. 7.
CD4+IL-17A+ T cells promote clearance of GAS from NALT. Immunized mice were euthanized, and single-cell suspensions of lymph nodes were pooled separately from each group. (A) Immunized IL-17A−/− (I-IL17A−/−) and WT B6 (I-B6) mice, and nonimmunized (NI-B6) mice (PBS sham controls) are shown. (B and C) CD4+ T cells were separately pooled from each group of immunized donor mice and then transferred to three groups of naive WT B6 mice (S7). At 24 hr after transfer, mice were challenged i.n., and they were euthanized 24 hr later. (B) Representative FACS data showing intracellular cytokine staining for IL-17A and IFNγ on enriched cells. (C) Plots shown were gated on CD4+ cells. CFU equals the number of viable bacteria per NALT. In A and C, the horizontal bars are geometrical means.
CD4+ T cells were purified by negative selection (SI Text) and characterized by intracellular staining of IL-17A and IFNγ (Fig. 7B). I-B6 preparations contained expanded nearly equal numbers of IL-17A+ and IFNγ+ T cells, whereas I-IL-17−/− preparations contained only CD4+IFNγ+ cells. At 24 hr after transfer of cells to naive WT mice (B6), recipients were challenged i.n. Mice that received cells from immunized WT mice cleared streptococci from NALT more completely (P = 0.03) by 24 hr after inoculation than those that received CD4+ T cells from I-IL-17A−/− mice (Fig. 7C). When data from this experiment and a second repeat experiment were combined, analyses indicated that a larger number of mice (4 of 9) that received IL-17A+ cells cleared streptococci from NALT (fewer than 100 CFUs per NALT) than those that received cells from IL-17−/− knockout mice (0 of 10) (Fisher's exact test, P = 0.03).
Recipients that received cells from mice inoculated with PBS did not efficiently clear streptococci. It is notable that recipients of IFNγ+IL-17A−/− cells were not protected even though the number of transferred CD4+IFNγ+ cells equaled the number of those transferred from WT immunized mice (Fig. 7B), suggesting that protection is primarily dependent on IL-17A+ T cells.
Discussion
Children commonly have multiple episodes of GAS pharyngitis and tonsillitis, and many carry streptococci in their tonsils (11, 12). After they reach the age of 12 years, most have fewer infections and have developed protective immunity. Although streptococcal-specific antibodies develop following infections, titers do not correlate with resistance to infections, questioning the role of antibodies in eliminating GAS from tonsil tissue. However, virtually nothing is known about the cellular immunity. Our study indicates that this pathogen induces unique cytokine signals that direct T cells toward the Th17 phenotype and that these cells play a critical role in protective immunity. Moreover, our experiments link the Th17 immune response to specific induction of TGF-β1 by a bacterial pathogen.
Experiments suggested that bacterial priming by a single infection changes the cellularity of lymphoid tissues, which sets the stage for enhanced TGF-β1 expression. Many types of cells can produce TGF-β1, including monocytes and dendritic cells. We observed early influxes of neutrophils and macrophages into NALT following i.n. infection of naive mice (19). Thus, amplification of TGF-β1 expression by persistent or multiple infections could be dependent on the influx of polymorphonuclear (PMN) cells and macrophages or on expansion of T cells, which can be a significant source of TGF-β1 (22).
Human peripheral T cells were shown to undergo polyclonal expansion and to produce IL-17 on exposure to superantigens from GAS and Staphylococcus aureus (23). This raised the possibility that IL-17 secretion during infection is not antigen specific but, instead, induced by superantigens. This is unlikely because SpeA, a superantigen commonly produced by serotype M1 strains, is not expressed by the 90-226 strain used in these experiments. Moreover, only antigen-specific T-cell expansion was observed in mice after i.n. infection with Ova-tagged 90-226 (24).
TGF-β1 promotes T-cell differentiation to the Th17 phenotype when accompanied by IL-6 (25, 26). Although Th17 responses to other bacteria and fungi were reported (27), a link to induction of specific cytokines by these microbes has not been established. IL-6 is an inflammatory cytokine commonly induced by a variety of pathogens, including Lm (28, 29). Experiments here show that GAS elicits TGF-β1-dependent secretion of IL-17 and a dominant Th17 response, whereas Lm fails to induce TGF-β1 and only induces a weak Th17 response, suggesting that TGF-β1 is the decisive signal for Th17 differentiation. Moreover, preliminary experiments indicate that i.n. infection of IL-6−/− knockout mice does not result in expansion of streptococcal-specific CD4+IL-17+ T cells, suggesting a requirement for this cytokine.
The role of antibody in protective immunity against mucosal bacterial pathogens is undergoing significant revision. Indeed, Th17 cells are known to be important for immune protection from several mucosal pathogens (27, 30, 31). For example, in contrast to vaccine-induced immunity, protection activated by infection against Staphylococcus pneumoniae is independent of B cells and antibody (31). Although numerous studies have shown that antibody-mediated protection against GAS infection can be induced by vaccination with recombinant proteins (32), this study shows that i.n. infection induces T-cell-mediated protection, specifically Th17 cells. Immunity was passively transferred to naive mice by CD4+ T cells only when those cells contained IL-17+ T cells.
Independence of IFNγ+ is consistent with the restrictive effect of IFNγ on Th17 cell differentiation and our observation that IFNγ−/− knockout mice recruit more PMN cells and macrophages into NALT and more effectively clear streptococci from this tissue than WT mice (19). We suggest that IL-17 promotes recruitment of neutrophils and macrophages, which are able to eradicate streptococci from NALT. Interestingly, I-IL-17A−/− mice were partially protected (Fig. 7A). This suggests that other components of adaptive immunity, such as IL-17F and IL-22, also produced by T cells and known to influence infection by other mucosal pathogens may contribute to protection (33, 34). In addition, antibody directed against streptococci could be a factor. Another unexpected result is that protective immunity did not prevent invasion of NALT during the first 4 hr after inoculation (Fig. S5). This suggests that immune protection is not critical on the nasal epithelium but primarily functions to clear streptococci from NALT.
Identification of the distinct Th17 lineage of memory and effector T cells has opened unique avenues for understanding microbial infections and associated autoimmune disease. The previously discussed findings suggest that vaccines or adjuvants that promote a Th17 response may ultimately be more effective. On the other hand, uncontrolled Th17 responses are implicated in various human autoimmune diseases, such as multiple sclerosis, rheumatoid arthritis, and psoriasis (7, 30, 35). Coincidently, high levels of TGF-β1 were found in tissues and sera from patients with GAS-associated autoimmune diseases, such as rheumatic heart disease, acute poststreptococcal glomerulonephritis, and psoriasis (36–38). The link between GAS, TGF-β1, and Th17 cellular responses could tip the balance toward autoimmunity. Although molecular mimicry between streptococci and host antigens has been proposed and reported to be one mechanism responsible for rheumatic heart disease (39), a prolonged imbalance between Th17 and Treg responses could break immune tolerance and predispose the host to poststreptococcal diseases.
Materials and Methods
Bacterial Strains and Culture Conditions.
Serotype M1 strain 90-226 of GAS (40) was maintained on sheep blood agar and grown in THB-Neo (Todd-Hewitt broth supplemented with 2% Neopeptone). WT Lm strain 1043S was grown and maintained in brain–heart infusion. Both bacteria were used at log phase (90-226, OD560 = 0.4; Lm 1043S, OD550 = 0.5). For heat-killed bacteria, log phase cultures were washed with PBS, resuspended in PBS, and heated at 60 °C for 1 hr. Viable bacteria were used for all in vivo infections, and heat-killed bacteria were used for ex vivo activation of T cells.
Mice.
Female C57BL/6 (B6) and BALB/c mice aged 5 to 6 weeks were purchased from Taconic, Inc. IL-17A knockout mice were obtained from Dr. Yoichiro Iwakura (Institute of Medical Science, University of Tokyo, Japan) (21). Mice infected with GAS were housed in biosafety level 2 facilities under pathogen-free conditions at the animal facility of the University of Minnesota, according to National Institutes of Health guidelines.
Animal Infections.
Mice were anesthetized with an isoflurane/oxygen mixture for 1 min and inoculated i.n. with a sublethal (2 × 108 CFUs per mouse) dose of live 90-226 in 10 μL PBS (5 μL per nostril) (9). In experiments that compared GAS and Lm, 2 × 107 CFUs per mouse were used for i.n. and i.p. inoculations of either bacterium. Antigen-specific T-cell responses to GAS were shown to be qualitatively very similar in NALT and spleen (24); therefore, spleen or CLN was used as the source of T cells when questions were primarily immunological in nature and the route of infection was irrelevant. In those experiments, mice were infected i.p. to maximize priming in the spleen. Blood was taken by heart puncture or by cheek bleed from the submandibular vein.
Harvesting NALT.
NALT tissues were collected as previously described (9) and then stored in RPMI containing 10% FCS (vol/vol) until further processing.
Human Tonsil Tissues.
Palatine tonsil tissue specimens were obtained and manipulated as described by Kumar et al. (10). The reader is also referred to SI Text.
Single-Cell Suspension and ex Vivo Treatment with Bacteria for Cytokine Analyses.
The reader is referred to SI Text.
Western Blot Analysis of Smad2 Phosphorylation.
Western blot analysis was performed as described in ref. 4.
Measurement of Secretion of TGF-β1, IL-6, and IL-17A by TGF-β1 Reporter and ELISAs.
The reader is referred to SI Text.
Measurement of IL-1β and TNF-α m-RNAs.
Microarrays were used to measure cytokine RNAs as previously described (19). The reader is also referred to SI Text.
Intracellular Staining and Flow Cytometry.
Single-cell suspensions of lymphoid tissue were stained as described by Pape et al. (41). The reader is also referred to SI Text.
Enrichment and Adoptive Transfer of CD4 T Cells.
Single-cell suspensions of all lymph nodes were prepared from immunized mice, and CD4− cells were removed by negative selection and transferred to naive B6 mice (42). The reader is also referred to SI Text.
Statistics.
Statistical analyses were performed with a one-tailed unpaired Mann–Whitney U nonparametric t test or with Fisher exact tests using GraphPad Prism (Version 4.03 for Windows; GraphPad Software). The data were considered significantly different at P ≤ 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Peter Southern for providing human tonsil tissues, the Flow Cytometry Core Facility of the University of Minnesota Cancer Center for assistance, and Dr. Marc Jenkins for helpful discussions of data. This work was supported by Public Health Service Grant AI059533.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904831107/DCSupplemental.
References
- 1.Brook I. Penicillin failure and copathogenicity in streptococcal pharyngotonsillitis. J Fam Pract. 1994;38:175–179. [PubMed] [Google Scholar]
- 2.Paradise JL, et al. Tonsillectomy and adenotonsillectomy for recurrent throat infection in moderately affected children. Pediatrics. 2002;110:7–15. doi: 10.1542/peds.110.1.7. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham MW. Pathogenesis of group A streptococcal infections and their sequelae. Adv Exp Med Biol. 2008;609:29–42. doi: 10.1007/978-0-387-73960-1_3. [DOI] [PubMed] [Google Scholar]
- 4.Wang B, Li S, Southern PJ, Cleary PP. Streptococcal modulation of cellular invasion via TGF-beta1 signaling. Proc Natl Acad Sci USA. 2006;103:2380–2385. doi: 10.1073/pnas.0506668103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 6.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 7.Stockinger B, Veldhoen M, Martin B. Th17 T cells: Linking innate and adaptive immunity. Semin Immunol. 2007;19:353–361. doi: 10.1016/j.smim.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Li MO, Flavell RA. TGF-beta: A master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park HS, Francis KP, Yu J, Cleary PP. Membranous cells in nasal-associated lymphoid tissue: a portal of entry for the respiratory mucosal pathogen group A streptococcus. J Immunol. 2003;171:2532–2537. doi: 10.4049/jimmunol.171.5.2532. [DOI] [PubMed] [Google Scholar]
- 10.Kumar RB, Maher DM, Herzberg MC, Southern PJ. Expression of HIV receptors, alternate receptors and co-receptors on tonsillar epithelium: Implications for HIV binding and primary oral infection. Virol J. 2006;3:25–37. doi: 10.1186/1743-422X-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osterlund A, Popa R, Nikkilä T, Scheynius A, Engstrand L. Intracellular reservoir of Streptococcus pyogenes in vivo: A possible explanation for recurrent pharyngotonsillitis. Laryngoscope. 1997;107:640–647. doi: 10.1097/00005537-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Gerber MA, et al. Potential mechanisms for failure to eradicate group A streptococci from the pharynx. Pediatrics. 1999;104:911–917. doi: 10.1542/peds.104.4.911. [DOI] [PubMed] [Google Scholar]
- 13.Yang L, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Kattah MG, Wong MT, Yocum MD, Utz PJ. Cytokines secreted in response to Toll-like receptor ligand stimulation modulate differentiation of human Th17 cells. Arthritis Rheum. 2008;58:1619–1629. doi: 10.1002/art.23497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rharbaoui F, et al. Characterization of a B220+ lymphoid cell subpopulation with immune modulatory functions in nasal-associated lymphoid tissues. J Immunol. 2005;174:1317–1324. doi: 10.4049/jimmunol.174.3.1317. [DOI] [PubMed] [Google Scholar]
- 17.Lim HW, Lee J, Hillsamer P, Kim CH. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3+ regulatory T cells. J Immunol. 2008;180:122–129. doi: 10.4049/jimmunol.180.1.122. [DOI] [PubMed] [Google Scholar]
- 18.Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 19.Hyland KA, et al. The early interferon response of nasal-associated lymphoid tissue to Streptococcus pyogenes infection. FEMS Immunol Med Microbiol. 2009;55:422–431. doi: 10.1111/j.1574-695X.2009.00540.x. [DOI] [PubMed] [Google Scholar]
- 20.Aujla SJ, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakae S, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 22.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Nooh MM, Kotb M, Re F. Commercial peptidoglycan preparations are contaminated with superantigen-like activity that stimulates IL-17 production. J Leukocyte Biol. 2008;83:409–418. doi: 10.1189/jlb.0807588. [DOI] [PubMed] [Google Scholar]
- 24.Park HS, et al. Primary induction of CD4 T cell responses in nasal associated lymphoid tissue during group A streptococcal infection. Eur J Immunol. 2004;34:2843–2853. doi: 10.1002/eji.200425242. [DOI] [PubMed] [Google Scholar]
- 25.Gutcher I, Becher B. APC-derived cytokines and T cell polarization in autoimmune inflammation. J Clin Invest. 2007;117:1119–1127. doi: 10.1172/JCI31720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korn T, et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci USA. 2008;105:18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curtis MM, Way SS. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology. 2009;126:177–185. doi: 10.1111/j.1365-2567.2008.03017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolb-Mäurer A, Kurzai O, Goebel W, Frosch M. The role of human dendritic cells in meningococcal and listerial meningitis. Int J Med Microbiol. 2003;293:241–249. doi: 10.1078/1438-4221-00266. [DOI] [PubMed] [Google Scholar]
- 29.Kwinn LA, Nizet V. How group A Streptococcus circumvents host phagocyte defenses. Future Microbiol. 2007;2:75–84. doi: 10.2217/17460913.2.1.75. [DOI] [PubMed] [Google Scholar]
- 30.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu YJ, et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 2008;4:e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bisno AL, Rubin FA, Cleary PP, Dale JB National Institute of Allergy and Infectious Diseases. Prospects for a group A streptococcal vaccine: Rationale, feasibility, and obstacles—Report of a National Institute of Allergy and Infectious Diseases workshop. Clin Infect Dis. 2005;41:1150–1156. doi: 10.1086/444505. [DOI] [PubMed] [Google Scholar]
- 33.Ishigame H, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Aujla SJ, Kolls JK. IL-22: A critical mediator in mucosal host defense. J Mol Med. 2009;87:451–454. doi: 10.1007/s00109-009-0448-1. [DOI] [PubMed] [Google Scholar]
- 35.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 36.Mezzano S, Burgos ME, Olavarría F, Caorsi I. Immunohistochemical localization of IL-8 and TGF-beta in streptococcal glomerulonephritis. J Am Soc Nephrol. 1997;8:234–241. doi: 10.1681/ASN.V82234. [DOI] [PubMed] [Google Scholar]
- 37.Nockowski P, Szepietowski JC, Ziarkiewicz M, Baran E. Serum concentrations of transforming growth factor beta 1 in patients with psoriasis vulgaris. Acta Dermatovenerol Croat. 2004;12:2–6. [PubMed] [Google Scholar]
- 38.Kim L, et al. Overexpression of transforming growth factor-beta 1 in the valvular fibrosis of chronic rheumatic heart disease. J Korean Med Sci. 2008;23:41–48. doi: 10.3346/jkms.2008.23.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cunningham MW. T cell mimicry in inflammatory heart disease. Mol Immunol. 2004;40:1121–1127. doi: 10.1016/j.molimm.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 40.Wang B, Yurecko RS, Dedhar S, Cleary PP. Integrin-linked kinase is an essential link between integrins and uptake of bacterial pathogens by epithelial cells. Cell Microbiol. 2006;8:257–266. doi: 10.1111/j.1462-5822.2005.00618.x. [DOI] [PubMed] [Google Scholar]
- 41.Pape KA, Khoruts A, Mondino A, Jenkins MK. Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J Immunol. 1997;159:591–598. [PubMed] [Google Scholar]
- 42.Winstead CJ, Fraser JM, Khoruts A. Regulatory CD4+CD25+Foxp3+ T cells selectively inhibit the spontaneous form of lymphopenia-induced proliferation of naive T cells. J Immunol. 2008;180:7305–7317. doi: 10.4049/jimmunol.180.11.7305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.