Abstract
Darkness serves as a stimulus for vertebrate photoreceptors; they are actively depolarized in the dark and hyperpolarize in the light. Here, we show that larval zebrafish essentially turn off their visual system at night when they are not active. Electroretinograms recorded from larval zebrafish show large differences between day and night; the responses are normal in amplitude throughout the day but are almost absent after several hours of darkness at night. Behavioral testing also shows that larval zebrafish become unresponsive to visual stimuli at night. This phenomenon is largely circadian driven as fish show similar dramatic changes in visual responsiveness when maintained in continuous darkness, although light exposure at night partially restores the responses. Visual responsiveness is decreased at night by at least two mechanisms: photoreceptor outer segment activity decreases and synaptic ribbons in cone pedicles disassemble.
Keywords: photoreceptors, circadian rhythm, synaptic plasticity
Tomita (1) first showed that vertebrate photoreceptors hyperpolarize in response to light. In the dark, the outer segment membranes of both rod and cone photoreceptors are permeable to Na+ and Ca2+, causing the membrane potential to depolarize (2). In light, the channels close, the conductance of the outer segment membrane decreases, and the cells hyperpolarize (3). In the dark, Na+ and Ca2+ must be continuously pumped out of the cell to maintain electrochemical gradients. This requires a significant amount of ATP and constitutes a substantial energy drain for the cell (4, 5).
Because photoreceptors are depolarized in the dark, they also release transmitter continuously at night, and this too requires energy. Photoreceptors have an unusual type of synapse in their terminals, the ribbon synapse, which is structurally and functionally specialized for a large and tonic release of neurotransmitter (6–10). The synaptic ribbon is a plate-like structure that extends from the site of transmitter release into the synaptic terminal cytoplasm and has hundreds of synaptic vesicles tethered to it (11). Ultrastructural analyses of cone pedicles in several fish, including larval zebrafish, have shown that synaptic ribbons undergo diurnal alterations in structure, with ribbons present during the day but almost completely absent at night (12–14). The disassembly and reassembly of synaptic ribbons was suggested to be circadian driven because it occurs when fish are kept in continuous darkness (14).
Studies in several vertebrate species have shown that visual sensitivity and other retinal phenomena are regulated by a circadian mech-anism (15). In adult zebrafish, rod sensitivity measured behaviorally varied by about 2.2 log units and cone sensitivity changed by 1.4 log units over the course of 24 h (16). However, visual responses of larval zebrafish are mainly cone driven until approximately 15 days of age. Here we show that zebrafish larvae become virtually blind at night and that this phenomenon is largely circadian driven. We propose that zebrafish larvae shut down their visual system at night, perhaps to conserve energy.
Results
Larval Zebrafish Exhibit a Day/Night Cycle of Retinal Responsiveness.
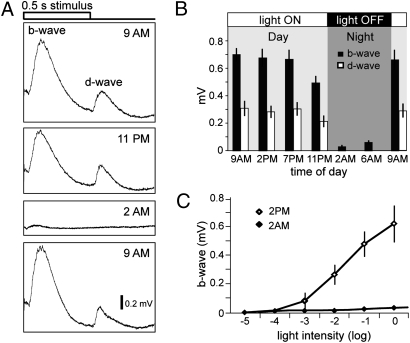
To determine whether visual responsiveness is altered during a 24-h period, we first recorded electroretinograms (ERGs) of 5-day-old zebrafish larval eyes at different times of the day and night using a light flash about 3 log units above ERG threshold [log of light intensity (I) = −1]. Because zebrafish are maintained on a 14-h light cycle in our facility, we recorded ERGs from wild-type zebrafish larvae at 9:00 AM, when the room light is first turned on in the morning, at 11 PM, just before the lights are turned off for the night, and at 2:00 AM, 3 h after lights off (Fig. 1A). At 9:00 AM, ERG amplitude in response to a 500-ms full-field light stimulus were large, indicating that ON-bipolar cells, which generate the b-wave at the onset of the light stimulus, and OFF-bipolar cells, which generate the d-wave at cessation of the light stimulus, were functioning robustly. By 11:00 PM, fish were still responsive to the light stimulus, but both b- and d-waves showed a decrease in amplitude. Three hours into the night (2:00 AM), the light-evoked ERG was profoundly reduced. By the next morning (9:00 AM), fish displayed robust ERGs, indicating that they were again fully responsive to the light stimulus (Fig. 1A).
Fig. 1.
Larval zebrafish exhibit a day/night cycle of visual responsiveness. (A) Representative ERG recordings to a 0.5 second white light stimulus are shown at four different time points: at 9:00 AM, when the ambient lights are turned ON; 11:00 PM, shortly before the lights are turned OFF; 2:00 AM, after the lights have been OFF for 3 h; and at 9:00 AM the next morning, after the lights are turned ON again. Note the large reduction of ERG amplitudes at 2:00 AM. (B) Average ERG b- (black bars) and d-wave (white bars) amplitudes taken at different time points over a 24-h period (n = 15 fish for each time point; bars indicate SD). (C) V-log I curves showing that increasing the light stimulus intensity at 2:00 AM did not increase the ERG amplitudes significantly. Average b-wave amplitudes in response to 5 log units of white light stimulus intensities at 2:00 PM and 2:00 AM (n = 5 fish for each time point).
To quantify the changes in retinal responsiveness, b- and d-wave peak amplitudes were recorded at different times during the day and at night as shown in Fig. 1B. Between 9:00 AM and 7:00 PM, the b- and d-wave average amplitudes did not change significantly (approximately 700 and 300 μV, respectively). However, by 11:00 PM at night, the b- and d-wave average amplitudes had decreased significantly (to approximately 500 and 200 μV, respectively; Fig. 1B). This downward trend in ERG amplitudes continued after the lights were turned off, and the ERG was almost completely absent by 2:00 AM (b-wave of approximately 30 μV). The d-wave amplitudes were not measurable at 2:00 AM because dark adaptation either during the day or at night abolishes the response (17). By 9:00 AM the next morning, the b- and d-wave average amplitudes had returned to normal daytime levels (approximately 670 and 300 μV, respectively).
To measure visual sensitivity, ERGs were recorded to full field light stimulation over 5 log units of I at two times, once during the day then at night. At 2:00 PM, the voltage-intensity (V-log I) function was a classic “S” shaped curve, rising from a b-wave amplitude of approximately 10 μV at log I = −4, to approximately 650 μV at log I = 0 (Fig. 1C). In contrast, at 2:00 AM the V-log I curve was essentially flat, increasing from a threshold of approximately 10 μV at log I = −2 to only about approximately 30 μV at log I = 0 (Fig. 1C). Thus, at 2:00 AM not only was ERG sensitivity decreased, but ERG responsiveness was severely depressed. That is, at 2:00 PM, a light flash of 2 log units above threshold generated a response of approximately 250 μV, whereas a light flash 2 log units above threshold at 2:00 AM generated a response of only approximately 30 μV.
Responses to Altered Lighting Conditions During Day and Night.
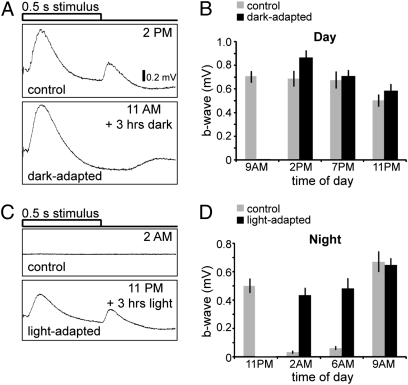
To determine whether retinal responsiveness is altered by different lighting conditions, ERGs were recorded from 5-day-old fish that were either dark-adapted during the day or light-adapted at night. When zebrafish larvae were exposed to 3 h of darkness during the day (noon plus 3 h of dark), b-wave amplitudes were similar to control animals that were left in the light (Fig. 2A). When animals were dark-adapted starting at 9:00 AM, and ERGs recorded at different times of the day, we observed a small increase in b-wave amplitude at 2:00 and 7:00 PM as compared to the control responses (Fig. 2B). At 11:00 PM, the average b-wave amplitudes of dark-adapted animals were also slightly higher than control animals but were significantly lower than the dark-adapted b-wave amplitudes measured at 2:00 PM earlier in the day (P = 0.0196).
Fig. 2.
Exposure to light inhibits the loss of visual responsiveness at night. (A) Dark-adaptation during the day causes a slight increase in b-wave amplitude. Representative ERG recordings are shown for animals exposed to normal light conditions (control) and for animals exposed to 3 h of darkness during the day (dark-adapted). (B) Comparison of average b-wave amplitudes for control fish (gray bars) and dark-adapted fish (black bars) taken at different time points throughout the day (n = 15 fish for each time point; averaged b-wave amplitudes are compared to their respective control; bars indicate SD). (C) Light-adaptation at night partially overrides the loss of visual responsiveness and prevents the severe decrease of b- and d-wave amplitudes. Representative ERGs for control animals (Upper) and for animals exposed to 3 h of light (Lower) recorded at 2:00 AM. (D) Light-adapted b-wave amplitudes during the night increased as dawn approaches and are similar to control b-wave amplitudes by 9:00 AM when the lights are turned ON. Comparison of averaged b-wave amplitudes for control fish (gray bars) and fish exposed to light (black bars) throughout the night starting at 11:00 PM taken at different time points (n = 15 fish for each time point; averaged b-wave amplitudes are compared with their respective control; bars indicate SD).
Fish were next exposed to light throughout the night and b-wave amplitudes were measured at 2:00, 6:00, and 9:00 AM (Fig. 2 C and D). Unlike control animals, which were virtually unresponsive to light stimuli during the night (2:00 and 6:00 AM), light-adapted animals had much larger b-wave amplitudes, although smaller than daytime b-wave amplitudes (compare with Fig. 1B). However, by the next morning (9:00 AM), b-wave amplitudes of the light-adapted animals had increased significantly and were similar to the control group (approximately 650 μV) (Fig. 2C).
Zebrafish Larvae Lose Visual Behavioral Responses at Night.
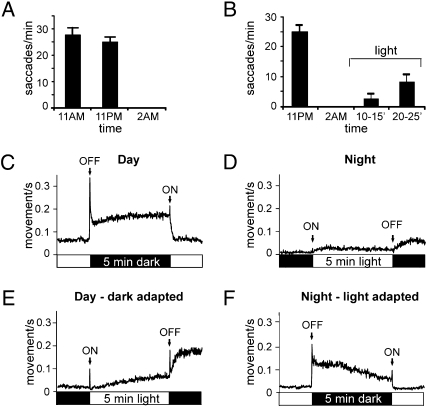
To determine whether visual behaviors of zebrafish larvae are similarly altered during night, the optokinetic reflex (OKR) was measured at 11:00 AM, 11:00 PM, and 2:00 AM. At 11:00 AM, fish robustly moved their eyes in response to a black and white moving grating. By 11:00 PM, the fish showed a slight reduction in their ability to respond in the OKR test, but by 2:00 AM (3 h after darkness onset) the response was completely abolished (Fig. 3A). To determine whether visual responsiveness can be restored at night, fish were exposed to continuous light starting at 2:00 AM (3 h after darkness). After 10 min, fish exhibited an increase in the OKR response and by 20 min, the response had increased further as shown in Fig. 3B.
Fig. 3.
Zebrafish behavioral responses to light are abolished at night but can be partially restored by continuous exposure to light. (A) Larval zebrafish exhibit a robust OKR at 11:00 AM and 11:00 PM (shortly before the lights are turned OFF at night) but not at 2 AM (3 h after the lights were turned OFF, n = 20 for each time point). (B) Light-adaptation during the night reverses the loss of OKR responses. OKR responses were taken at 11 PM, 2 AM, and at two time points after exposure to light starting at 2 AM: 10–15 and 20–25 min. (C) Larval zebrafish displayed ON and OFF visual-motor responses VMR during the day but fail to exhibit transient startle responses at night (each trace represents an average of 240 responses from 80 individual fish). During the daytime, motor output increased in response to a 5-min light off stimulus with prominent OFF and ON responses (first stimulus was introduced at 2:00 PM). (D) At night, the VMR responses to a 5-min light stimulus were abolished (first stimulus was introduced at 2:00 AM). (E) Dark-adaptation during the day does not abolish VMR responses. Fish, dark-adapted for 3 h, were then exposed to a 5-min light stimulus (first light stimulus was introduced at 2:00 PM). (F) Light-adaptation at night prevents the loss of visual responsiveness and restores VMR responses. Fish were first light-adapted for 3 h starting at 11:00 PM and then exposed to 5-min of darkness (first lights off stimulus was introduced at 2:00 AM).
During the day, zebrafish larvae also dramatically increase their motor activity in response to both light increments and decrements (18, 19). To determine whether these visual-motor responses (VMRs) are abolished at night, 5-day-old zebrafish larvae were tested with this assay at 11:00 and 2:00 AM. During the day (11:00 AM), fish significantly increased their motor activity in response to a 5-min dark stimulus and also displayed prominent OFF and ON responses at the cessation and onset of the light, respectively (Fig. 3C). However, at night, when 5-min periods of light were presented after the lights had been turned OFF for 3 h (2:00 AM), zebrafish larvae failed to display these transient ON and OFF responses (Fig. 3D). The fish did increase their motor activity gradually in response to both the onset and offset of the light stimulus, but the overall level of activity at night was much lower than the activity observed during the day.
We also determined whether dark-adaptation during the day affects the VMR responses. When 5 min of light was presented after 3 h of dark-adaptation during the day (11:00 AM to 2:00 PM), the fish exhibited prominent ON and OFF responses (Fig. 3E). Similarly, fish exhibited transient OFF and ON responses when 5 min of darkness was presented at night after the animals had been light-adapted for 3 h (starting at 11:00 PM) (Fig. 3F). Thus, exposure to light inhibits the loss of visual responsiveness at night, although the increase in activity to light decrements at night after light-adaptation was less than that observed during the day (compare Fig. 3 F and C).
Loss and Recovery of ERG Responsiveness Occurs Within Tens of Minutes.
The results from our initial experiments, described in Fig. 1, suggest that after 3 h of darkness, outer retinal function is essentially lost as indicated by a flat ERG trace. How short a period of dark exposure is necessary to initiate the changes in visual responsiveness at night? To address this question, ERGs were recorded at 11:00 PM, shortly before the lights were turned off, and at several time points after the lights were turned OFF (Fig. 4A). b-wave amplitudes gradually decreased over 1 h of darkness and were virtually abolished after 90 min of darkness (Fig. 4B). To determine how rapidly the recovery of visual sensitivity occurs, the lights were turned on at 2:00 AM, and ERGs were recorded at several time points (Fig. 4C). b-wave amplitudes increased steadily for 35 min, at which point increases in amplitudes became more gradual (Fig. 4D). Thus, both the loss of visual responsiveness in darkness and its recovery following the onset of light at night is relatively rapid; significant changes were observed within 10–20 min.
Fig. 4.
Loss and recovery of visual responsiveness occurs within tens of minutes. (A) Visual responsiveness gradually decreases over 1 h of darkness and is abolished after 90 min of darkness at night. Representative ERG traces are shown for 11:00 PM, recorded right before the ambient light is turned OFF at night, and at different time points after the lights are turned OFF. (B) Averaged b-wave amplitudes at different time points (n = 5 per time point) after the lights are turned OFF at night. (C) Visual responsiveness is partially restored within 35 min after the lights are turned ON during the night. Representative ERGs are shown for 2:00 AM at night, and at different time points after the lights are turned ON. (D) Averaged b-wave amplitudes at different time points (n = 5 per time points) after the lights are turned ON at 2:00 AM.
Visual Responsiveness Follows a Circadian Rhythm.
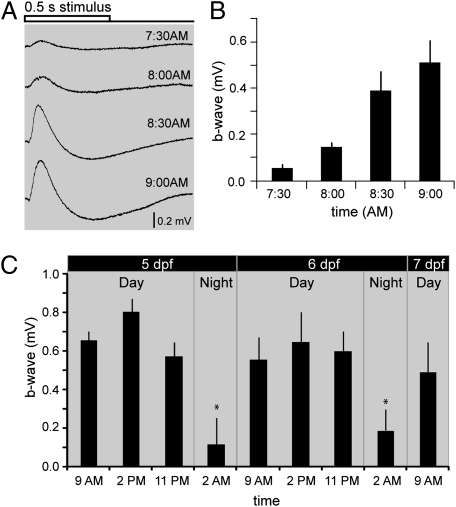
Because the loss of ERG amplitude at 11:00 PM (Figs. 1 and 2) suggested that fish anticipated the offset of light, we asked if fish might also anticipate the onset of light. To address this question, ERGs were recorded every 30 min starting at 7:30 AM until 9:00 AM at log I = −1. Fig. 5A shows representative ERG traces recorded over this period. Average b-wave amplitudes increased steadily in the dark before the lights were turned ON in the morning (Fig.5B). Taken together, these results indicate that zebrafish larvae anticipate day and night, suggesting that visual responsiveness in larval zebrafish is regulated, at least partially, by a circadian mechanism.
Fig. 5.
A circadian clock regulates visual responsiveness in larval zebrafish. (A) Zebrafish larvae anticipate sunrise and exhibit an increase in b-wave amplitudes before the lights are turned ON (at 9:00 AM). Representative ERG recordings taken at different time points before the lights are turned ON in the morning. (B) Averaged b-wave amplitudes at different time points (n = 5 per time point) before the lights are turned ON in the morning. c) Visual responsiveness is regulated by a circadian cycle. Averaged b-wave amplitudes at different time points (n ≥ 10) over 48 h of darkness starting at day 5 before the lights were turned on. A consistent cycle of visual responsiveness is observed: b-wave amplitudes are high during the day (9:00 AM, 2:00 PM, 11:00 PM), become significantly smaller at night (2:00 AM), but are increased by the next morning (9:00 AM) for several days.
To determine whether visual responsiveness follows a circadian rhythm, animals were raised in normal light/dark cycles for 4 days and then kept in darkness for 48 h. Average b-wave amplitudes (at log I = −1) were measured at different times during the subjective days and nights (Fig. 5C). During subjective day 5, b-wave amplitudes were significantly higher at 9:00 AM and at 2:00 PM than at 2:00 AM during the subjective night. Similar results were obtained for subjective day 6, where b-wave amplitudes were high during the subjective day, but were decreased significantly by 2:00 AM during the subjective night. On subjective day 7, b-wave amplitudes were once again significantly increased by 9:00 AM. These results clearly demonstrate that visual responsiveness follows a circadian cycle in the absence of any light stimulation. However, some “run down” of b-wave amplitude was observed over the 48 h of dark-adaptation, such that b-wave amplitudes became gradually smaller during the subjective day and larger during the subjective night. Such a run down in continuous darkness (or light) is seen commonly in circadian rhythm phenomena (16, 20).
Photoreceptor Sensitivity Is Reduced at Night.
What possible mech-anisms could account for the decrease in b-wave amplitudes at night? The ERG a-wave, which reflects hyperpolarization of the outer segments in response to light, is largely masked by the depolarizing b-wave in larval zebrafish recordings and, thus, difficult to evaluate (Fig. 1). To isolate the a-wave, 5-day-old zebrafish larvae were incubated in fish water containing 0.4 mM L(+)-2amino-4-phosphonobutyric acid (L-AP4) and 0.2 mM DL-threo-β-benzyloxyaspartate (TBOA) for 2 h. TBOA (an excitatory amino acid transporter blocker) and L-AP4 (a metabotropic glu-tamate receptor agonist) have been shown to completely elim-inate the b-wave in zebrafish (19, 21). Although control animals displayed normal b-waves, animals treated with the drug mixture did not show any b-wave response, thus revealing the a-wave (Fig. 6A Left). The drug mixture, on the other hand, did not appreciably affect the d-wave in control fish, as has been shown previously (21). To determine whether outer segment sensitivity is altered at night, ERGs were recorded at 2:00 AM with and without the drug mixture at log I = −1(Fig. 6A Right). The ERG was essentially flat at 2:00 AM in control animals, but in animals treated with the drug mixture, an a-wave was recorded that was significantly reduced at night as compared to the daytime a-wave (approximately 130 μV at 2:00 PM and approximately 47 μV at 2:00 AM; Fig. 6B). As an additional control, fish were exposed to 3 h of light at night starting at 11 PM, and a-wave amplitudes were measured. The a-wave persisted in these fish and was similar to the daytime a-wave amplitudes (Fig. 6B).
Fig. 6.
Photoreceptor function is altered at night. (A) ERG recordings from eyes of fish incubated in fish water (control) and in fish water containing a drug mixture to block the b-wave. The a-wave, reflecting photoreceptor outer segment hyperpolarization and revealed after drug treatment, was significantly smaller at night (2 AM) than during the day (2:00 PM). (B) Averaged a-wave amplitudes (n = 15) taken from drug-treated fish at a 12-h interval (2:00 PM and 2:00 AM); and from fish that were exposed to light for 3 h at night. A-wave amplitudes at 2 AM were reduced by about 65% from those measured at 2:00 PM. Three hours of light adaptation at night increased a-wave amplitudes to control level (*, P < 0.01 and bars indicate SD). (C) EM sections of cone pedicles at different times of the day and under different exposures to light. Most cone pedicles exhibit prominent synaptic ribbons during the day (2:00 PM), but are essentially devoid of synaptic ribbons at night (2:00 AM). When fish are exposed to darkness for 3 h during the day (dark-adapted 2:00 PM) synaptic ribbons are still observed. Exposure to 3-h day light conditions at night (light-adapted 2:00 AM) prevents the degradation of synaptic ribbons that usually occurs at night and synaptic ribbons are observed. Arrows denote synaptic ribbons.
Photoreceptors Synaptic Ribbons Disassemble at Night.
Although the a-wave is clearly reduced in amplitude at night, it was still present, and thus, other factors that might alter photoreceptor function were examined. Synaptic ribbons in fish disassemble at night and reform in the morning (12, 14, 22). Indeed, Allwardt et al. (14) examined 6-day-old zebrafish and showed that the plasticity of synaptic ribbons in cone pedicles is a circadian phenomenon. To determine whether such structural changes in the photoreceptor terminals might relate to the loss of visual responsiveness in our animals, retinas were studied by electron microscopy at appropriate times of the day and night. As expected, cone pedicles exhibited prominent synaptic ribbons (arrows) during the day (2:00 PM) but were essentially devoid of synaptic ribbons at night (2:00 AM; Fig. 6C). When fish were examined after exposure to 3 h of darkness during the day (11 AM to 2 PM) prominent synaptic ribbons were observed, showing that dark-adaptation during the day does not initiate the disassembly of synaptic ribbons (Fig. 6C). However, exposure to 3 h of continuous light conditions at night (11:00 PM to 2:00 AM) prevented the disassembly of synaptic ribbons (Fig. 6C), indicating, in accord with our electrophysiological and behavioral measurements (Figs. 2D and 3F), that prolonged light exposure at night prevents the loss of visual responsiveness.
Discussion
In this study we recorded ERGs from 5- and 6-day-old zebrafish larval eyes at various times of the day and night and observed striking differences in outer retinal function. ERG responses in larval zebrafish were normal in terms of waveform and amplitude throughout the day, but the ERG was almost completely absent after 90 min of darkness at night. Two behavioral tests, the OKR and VMR also indicated that larval zebrafish become virtually unresponsive to light stimuli at night. When we maintained larval fish in constant darkness for several days, we observed similar changes in visual sensitivity suggesting that this fluctuation of visual responsiveness is largely circadian driven, although light exposure of larvae at night restores visual responsiveness. We determined that visual responsiveness is decreased at night by at least two mechanisms: photoreceptor outer segment function is decreased at night as measured by a-wave amplitudes and synaptic ribbons in the cone pedicles disassemble.
What might underlie the almost complete disappearance of the ERG at night and loss of visual function in larval fish? We suggest that at least two mechanisms can contribute to the loss of visual responsiveness at night. First, a-wave amplitude, reflecting outer segment activity, is reduced at night. Whereas b-wave amplitude is reduced by nearly 20-fold at night, a-wave amplitude is reduced by about 3-fold. Thus, the reduction of a-wave amplitude can account for part of the reduction in b-wave amplitude but probably not all. A second phenomenon that could contribute to the shut-down of retinal function at night is the disassembly of the synaptic ribbons in the photoreceptor terminals. Although vertebrate photoreceptors make two types of synapses (ribbon synapses and basal synapses that drive the ON and OFF bipolar cells, respectively), it is generally thought that transmitter is released only at the ribbon synapse (23).
Why do zebrafish larvae shut down their visual system at night? One possibility is that it is metabolically too costly to keep it operating fully at night. At 5 days, zebrafish larvae have essentially depleted their yolk sac, are just beginning to eat, and may need to conserve energy. For example, motor activity of zebrafish larvae also varies significantly between day and night. Larvae are active during the day but substantially decrease their motor activity at night, spending much of their time on the bottom (18). This was evident in our VMR studies performed during the night. The animals showed much less maintained activity in both light and dark, as well as giving virtually no ON and OFF light responses (Fig. 3D). Thus, zebrafish larvae generally reduce their activity at night.
A recent study showed that mouse rods consume 108 ATP molecules/sec−1 in the dark, and most of this energy consumption is required to extrude Na+ and Ca2+ from the cells. In the light, when the cyclic nucleotide gated channels are closed, energy consumption of the rods is reduced by 75% (5). Ca2+enters both the outer segments as well as the synaptic terminals (in response to depolarization) and ATP is required for the synthesis of the photoreceptor transmitter (glutamate) as well as for the recycling of synaptic vesicles and other functions of the cell including the synthesis of cyclic GMP, the second messenger of the visual transduction process. Furthermore, horizontal and many bipolar cells are maintained in a highly depolarized state in the dark, and they too are likely to be consuming more energy than if they were at resting membrane voltage. Thus, decreasing photoreceptor activity in the dark is likely to decrease energy consumption significantly in larval zebrafish.
What mechanisms might underlie the decrease in photoreceptor function at night in zebrafish larvae? It has long been known that photoreceptors contain cAMP and that this second messenger molecule is a component of multiple signaling pathways that show circadian rhythmicity. These include melatonin secretion (24), retinomotor movements (25, 26), and regulation of cGMP-gated cationic channels (27). cAMP, of course, affects many signaling cascades through PKA/Ras, Rap/Raf/Erk, or PI3K (27–31). It is conceivable that various photoreceptor activities from outer segment function to synaptic ribbon maintenance are regulated by circadian mechanisms in this way.
Methods
Zebrafish Maintenance.
Zebrafish were maintained on a 14-h light (1.2 × 102 μW/cm2 at 500 nm) and 10-h dark cycle (32). All experiments were performed on AB wild-type strain larvae at 5 dpf.
Electroretinography.
All ERG recordings were performed using the isolated eye preparation as previously described (21, 33). Briefly, one eye was removed using a fine tungsten wire loop, placed on 2% agarose, and superfused with Ringer’s solution. The Ringer’s solution was maintained at pH 7.8 by continuously gassing it with approximately 97% O2 and approximately 3% CO2. For ERG measurements during the night and for dark-adapted animals, the eye surgery was performed under dim far red (670 nm) light. Responses were recorded as previously described (33). Briefly, the isolated eye was placed with the cornea facing up at the center of the stimulus light (diameter 5 mm) from a halogen light source. Most recordings were performed using a flash intensity of 5.3 × 103 μW/cm2 at 500 nm (log I = −1), unless otherwise noted. Responses were recorded by placing an electrode with a tip diameter of 2–6 μm under the lens on the surface of the retina by using an anterior transscleral approach. The reference electrode was placed within the agarose in the recording chamber. Using custom-written software in IGOR Pro (Wave Matrics) three to six consecutively elicited ERGs were typically averaged in response to 500-ms flashes of light presented at 10-s intervals. ERGs were amplified at 1,000 total gain and low-pass filtered at 300 Hz.
Behavioral Assays.
The OKR test was performed as previously described (34). Briefly, fish were immobilized in 3% methylcellulose and placed within a rotating drum with black and white gratings. A camera was placed directly above the drum. OKR tests at night were performed at the same light intensities as during the day. The VMR test was also performed as previously described (19, 33). Briefly, individual fish were transferred into 1 of 80 wells of a 96-well plate and placed in a recording box, in which the camera, infrared light, and white light were housed inside. The box was constantly illuminated with infrared light a-wavelength that the animals do not see. For all experiments, fish were placed inside the box for at least 3 h in the dark or light before the experiment to allow the animals to settle and reach a baseline activity level. After the initial light or darkness exposure, motor output in response to four consecutive periods of 5-min light stimulation followed by 1-h darkness or 5-min darkness followed by 1-h light stimulation was measured.
Drug Treatment.
L-AP4 and TBOA were purchased from Sigma and Tocris, respectively. Stock solutions of 100 mM were prepared by dissolving L-AP4 in 0.1 M NaOH and TBOA in DMSO and were diluted in the fish water to achieve final concentrations. Zebrafish larvae were incubated in fish water containing 0.2 mM TBOA and 0.4 mM L-AP4 for 2 h in either room lighting condition (1.81 × 102 μW/cm2 at 500 nm) or darkness before recording ERG responses.
Electron Microscopy Sections.
Tissues were processed as previously described (14, 35). Briefly, zebrafish larvae were collected at various time points of the day and night and anesthetized. Specimens were fixed with 1% paraformaldehyde, 1.6% glutaraldehyde prepared in 0.06 M phosphate buffer with 3% sucrose, pH 7.4, for 1 h (4 °C). Samples were then rinsed and postfixed in 1% osmium tetroxide in phosphate buffer for 2 h at 4 °C. After rinsing, samples were dehydrated in a graded series of ethanol-water mixture and infiltrated with epon/araldite resin overnight (20 °C). The next day, specimens were oriented and embedded for sectioning. Ultrathin (50 ηm) transverse sections of the retina through the optic nerve from three fish for each time point were stained with uranyl acetate and lead citrate. Sections were viewed and photographed with a FEI Tecnai G2 Spirit transmission electron microscope.
Acknowledgments
We thank Dr. Edward Soucy for his generous technical and intellectual help. We also thank Stephan Neuhauss and David R. Copenhagen for reviewing the manuscript and providing a number of helpful comments. This work was supported by National Institute of Health Grants EY000811 (J.E.D.) and F32 EY018044-01A2 (to F.E.). J.R. is a Bristol-Myers Squibb Fellow of the Life Sciences Research Foundation.
Footnotes
The authors declare no conflict of interest.
References
- 1.Tomita T. Electrophysiological study of the mechanisms subserving color coding in the fish retina. Cold Spring Harb Symp Quant Biol. 1965;30:559–566. doi: 10.1101/sqb.1965.030.01.054. [DOI] [PubMed] [Google Scholar]
- 2.Hagins WA, Penn RD, Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970;10:380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomita T. Electrical activity of vertebrate photoreceptors. Q Rev Biophys. 1970;3:179–222. doi: 10.1017/s0033583500004571. [DOI] [PubMed] [Google Scholar]
- 4.Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: Fundamental and clinical aspects. Arch Ophthalmol. 2003;121:547–557. doi: 10.1001/archopht.121.4.547. [DOI] [PubMed] [Google Scholar]
- 5.Okawa H, Sampath AP, Laughlin SB, Fain GL. ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr Biol. 2008;18:1917–1921. doi: 10.1016/j.cub.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heidelberger R, Heinemann C, Neher E, Matthews G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature. 1994;371:513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- 7.Heidelberger R, Thoreson WB, Witkovsky P. Synaptic transmission at retinal ribbon synapses. Prog Retin Eye Res. 2005;24:682–720. doi: 10.1016/j.preteyeres.2005.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens CF, Tsujimoto T. Estimates for the pool size of releasable quanta at a single central synapse and for the time required to refill the pool. Proc Natl Acad Sci USA. 1995;92:846–849. doi: 10.1073/pnas.92.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagnado L, Gomis A, Job C. Continuous vesicle cycling in the synaptic terminal of retinal bipolar cells. Neuron. 1996;17:957–967. doi: 10.1016/s0896-6273(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 10.von Gersdorff H, Vardi E, Matthews G, Sterling P. Evidence that vesicles on the synaptic ribbon of retinal bipolar neurons can be rapidly released. Neuron. 1996;16:1221–1227. doi: 10.1016/s0896-6273(00)80148-8. [DOI] [PubMed] [Google Scholar]
- 11.Rao-Mirotznik R, Harkins AB, Buchsbaum G, Sterling P. Mammalian rod terminal: Architecture of a binary synapse. Neuron. 1995;14:561–569. doi: 10.1016/0896-6273(95)90312-7. [DOI] [PubMed] [Google Scholar]
- 12.Wagner HJ. Darkness-induced reduction of the number of synaptic ribbons in fish retina. Nat New Biol. 1973;246:53–55. doi: 10.1038/newbio246053a0. [DOI] [PubMed] [Google Scholar]
- 13.Wagner HJ, Ali MA. Cone synaptic ribbons and retinomotor changes in the brook trout, Salvelinus fontinalis (Salmonidae, Teleostei), under various experimental conditions. Can J Zool. 1977;55:1684–1691. doi: 10.1139/z77-217. [DOI] [PubMed] [Google Scholar]
- 14.Allwardt BA, Lall AB, Brockerhoff SE, Dowling JE. Synapse formation is arrested in retinal photoreceptors of the zebrafish nrc mutant. J Neurosci. 2001;21:2330–2342. doi: 10.1523/JNEUROSCI.21-07-02330.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cahill GM, Besharse JC. Circadian rhythmicity in vertebrate retinas: Regulation by a photoreceptor oscillator. Prog Retin Eye Res. 1995;14:267–291. [Google Scholar]
- 16.Li L, Dowling JE. Zebrafish visual sensitivity is regulated by a circadian clock. Vis Neurosci. 1998;15:851–857. doi: 10.1017/s0952523898155050. [DOI] [PubMed] [Google Scholar]
- 17.Ren JQ, Li L. A circadian clock regulates the process of ERG b- and d-wave dominance transition in dark-adapted zebrafish. Vision Res. 2004;44:2147–2152. doi: 10.1016/j.visres.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Prober DA, Rihel J, Onah AA, Sung RJ, Schier AF. Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J Neurosci. 2006;26:13400–13410. doi: 10.1523/JNEUROSCI.4332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emran F, et al. OFF ganglion cells cannot drive the optokinetic reflex in zebrafish. Proc Natl Acad Sci USA. 2007;104:19126–19131. doi: 10.1073/pnas.0709337104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurd MW, Cahill GM. Entraining signals initiate behavioral circadian rhythmicity in larval zebrafish. J Biol Rhythms. 2002;17:307–314. doi: 10.1177/074873002129002618. [DOI] [PubMed] [Google Scholar]
- 21.Wong KY, Gray J, Hayward CJC, Adolph AR, Dowling JE. Glutamatergic mechanisms in the outer retina of larval zebrafish: Analysis of electroretinogram b- and d-waves using a novel preparation. Zebrafish. 2004;1:121–131. doi: 10.1089/zeb.2004.1.121. [DOI] [PubMed] [Google Scholar]
- 22.Abe H, Yamamoto TY. Diurnal changes in synaptic ribbons of rod cells of the turtle. J Ultrastruct Res. 1984;86:246–251. doi: 10.1016/s0022-5320(84)90104-7. [DOI] [PubMed] [Google Scholar]
- 23.Sterling P, Demb JB. Retina. The Synaptic Organization of the Brain. In: Shepherd GM, editor. 5th Ed. New York: Oxford Univ Press; 2004. pp. 217–269. [Google Scholar]
- 24.Ivanova TN, Iuvone PM. Circadian rhythm and photic control of cAMP level in chick retinal cell cultures: A mechanism for coupling the circadian oscillator to the melatonin-synthesizing enzyme, arylalkylamine N-acetyltransferase, in photoreceptor cells. Brain Res. 2003;991:96–103. doi: 10.1016/j.brainres.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Besharse JC, Dunis DA, Burnside B. Effects of cyclic adenosine 3′,5′-monophosphate on photoreceptor disc shedding and retinomotor movement. Inhibition of rod shedding and stimulation of cone elongation. J Gen Physiol. 1982;79:775–790. doi: 10.1085/jgp.79.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burnside B. Light and circadian regulation of retinomotor movement. Prog Brain Res. 2001;131:477–485. doi: 10.1016/s0079-6123(01)31038-5. [DOI] [PubMed] [Google Scholar]
- 27.Ko GY, Ko ML, Dryer SE. Circadian regulation of cGMP-gated channels of vertebrate cone photoreceptors: Role of cAMP and Ras. J Neurosci. 2004;24:1296–1304. doi: 10.1523/JNEUROSCI.3560-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vossler MR, et al. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- 29.Dugan LL, et al. Differential effects of cAMP in neurons and astrocytes. Role of B-raf. J Biol Chem. 1999;274:25842–25848. doi: 10.1074/jbc.274.36.25842. [DOI] [PubMed] [Google Scholar]
- 30.Ambrosini A, et al. cAMP cascade leads to Ras activation in cortical neurons. Brain Res Mol Brain Res. 2000;75:54–60. doi: 10.1016/s0169-328x(99)00294-6. [DOI] [PubMed] [Google Scholar]
- 31.Iida N, et al. Requirement of Ras for the activation of mitogen-activated protein kinase by calcium influx, cAMP, and neurotrophin in hippocampal neurons. J Neurosci. 2001;21:6459–6466. doi: 10.1523/JNEUROSCI.21-17-06459.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish. Eugene, OR: Univ of Oregon Press; 2000. [Google Scholar]
- 33.Emran F, Rihel J, Dowling JE. A behavioral assay to measure responsiveness of zebrafish to changes in light intensities. J Vis Exp. 2008 doi: 10.3791/923. 10.3791/923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brockerhoff SE, et al. A behavioral screen for isolating zebrafish mutants with visual system defects. Proc Natl Acad Sci USA. 1995;92:10545–10549. doi: 10.1073/pnas.92.23.10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmitt EA, Dowling JE. Early retinal development in the zebrafish, Danio rerio: Light and electron microscopic analyses. J Comp Neurol. 1999;404:515–536. [PubMed] [Google Scholar]