Abstract
Fractionation of carbon isotopes by plants during CO2 uptake and fixation (Δleaf) varies with environmental conditions, but quantitative patterns of Δleaf across environmental gradients at the global scale are lacking. This impedes interpretation of variability in ancient terrestrial organic matter, which encodes climatic and ecological signals. To address this problem, we converted 3,310 published leaf δ13C values into mean Δleaf values for 334 woody plant species at 105 locations (yielding 570 species-site combinations) representing a wide range of environmental conditions. Our analyses reveal a strong positive correlation between Δleaf and mean annual precipitation (MAP; R2 = 0.55), mirroring global trends in gross primary production and indicating stomatal constraints on leaf gas-exchange, mediated by water supply, are the dominant control of Δleaf at large spatial scales. Independent of MAP, we show a lesser, negative effect of altitude on Δleaf and minor effects of temperature and latitude. After accounting for these factors, mean Δleaf of evergreen gymnosperms is lower (by 1–2.7‰) than for other woody plant functional types (PFT), likely due to greater leaf-level water-use efficiency. Together, environmental and PFT effects contribute to differences in mean Δleaf of up to 6‰ between biomes. Coupling geologic indicators of ancient precipitation and PFT (or biome) with modern Δleaf patterns has potential to yield more robust reconstructions of atmospheric δ13C values, leading to better constraints on past greenhouse-gas perturbations. Accordingly, we estimate a 4.6‰ decline in the δ13C of atmospheric CO2 at the onset of the Paleocene-Eocene Thermal Maximum, an abrupt global warming event ∼55.8 Ma.
Keywords: biogeochemistry, ecophysiology, fractionation, PETM
Human perturbation of the global carbon (C) cycle is potentially far greater in rate and magnitude than variations in the recent past, pushing predictions of future climate beyond the calibration range of models based on modern and near-modern observations. Robust predictions of future impacts of rising CO2 require not only extrapolation of ecological patterns along modern environmental gradients but also insights gained from changing ecological patterns at times of high CO2 and hot climate in the geologic past (1 and 2). Global patterns of variation in leaf carbon isotope (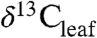 ) values potentially record climate-driven changes in modern plant physiology and biogeochemistry. An understanding of factors controlling plant fractionation (Δleaf) at the global scale will improve interpretations of past changes in climate and ecology recorded in ancient terrestrial sedimentary organic carbon (2). Patterns in
) values potentially record climate-driven changes in modern plant physiology and biogeochemistry. An understanding of factors controlling plant fractionation (Δleaf) at the global scale will improve interpretations of past changes in climate and ecology recorded in ancient terrestrial sedimentary organic carbon (2). Patterns in 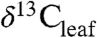 of living plants at the global scale, however, are unresolved in spite of abundant published data at smaller spatial scales.
of living plants at the global scale, however, are unresolved in spite of abundant published data at smaller spatial scales.
In living plants, 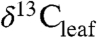 values reflect the balance of photosynthesis and stomatal conductance and their coupled response to the environment (3). Edaphic factors (e.g., water availability, altitude, temperature) and plant attributes (e.g. phylogeny and leaf traits) can influence
values reflect the balance of photosynthesis and stomatal conductance and their coupled response to the environment (3). Edaphic factors (e.g., water availability, altitude, temperature) and plant attributes (e.g. phylogeny and leaf traits) can influence 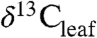 values (4–6). The relative importance of these factors at the global scale is not known, nor is it clear how they might drive the variations in ancient
values (4–6). The relative importance of these factors at the global scale is not known, nor is it clear how they might drive the variations in ancient 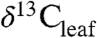 values recorded in either terrestrial organic carbon (
values recorded in either terrestrial organic carbon (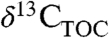 ) or δ13C values of plant biomarkers.
) or δ13C values of plant biomarkers.
During extreme climate events in the past, changes in the isotope ratio of atmospheric CO2 (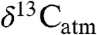 ) reflect perturbations in atmospheric C fluxes, therefore accurate estimates of
) reflect perturbations in atmospheric C fluxes, therefore accurate estimates of 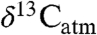 are central to estimating past climate sensitivity to changes in pCO2 (7). Values of
are central to estimating past climate sensitivity to changes in pCO2 (7). Values of 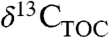 from sedimentary rocks are widely used to estimate
from sedimentary rocks are widely used to estimate 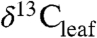 of ancient plants and infer changes in
of ancient plants and infer changes in 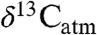 (8). However, the offset between
(8). However, the offset between 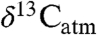 and
and 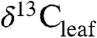 (i.e., Δleaf) varies with climate and plant characteristics and such variations must be accounted for when estimating atmospheric C isotope excursions (CIE) from ancient plant-derived C. For example, during the Paleocene-Eocene Thermal Maximum (PETM), a period of rapid global warming 55.8 million years ago (9), the CIEs in terrestrial organic carbon (TOC) and atmospheric CO2 likely differ because of changes in Δleaf that accompanied plant community shifts, warming (∼5 °C), decreasing precipitation and increases in atmospheric CO2 (2).
(i.e., Δleaf) varies with climate and plant characteristics and such variations must be accounted for when estimating atmospheric C isotope excursions (CIE) from ancient plant-derived C. For example, during the Paleocene-Eocene Thermal Maximum (PETM), a period of rapid global warming 55.8 million years ago (9), the CIEs in terrestrial organic carbon (TOC) and atmospheric CO2 likely differ because of changes in Δleaf that accompanied plant community shifts, warming (∼5 °C), decreasing precipitation and increases in atmospheric CO2 (2).
In this study, we provide predictive relationships for Δleaf variability of woody C3 plants at the global scale from analysis of published 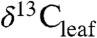 values, plant functional types, biome, climate, and geography. We use these relationships to show how environmental and ecologic change during the PETM influenced plant fractionation and therefore the CIE recorded by fossil leaf waxes.
values, plant functional types, biome, climate, and geography. We use these relationships to show how environmental and ecologic change during the PETM influenced plant fractionation and therefore the CIE recorded by fossil leaf waxes.
Results and Discussion
Leaf Carbon Isotope Fractionation.
We used Δleaf in our analyses as it controls for variation in 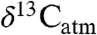 (10). Δleaf in C3 plants is a function of the fractionation associated with CO2 diffusion (4.4%) and photosynthetic fractionation by Rubisco (27%). For C3 plants, the concentration of CO2 in the substomatal cavities of leaves (ci) is directly proportional to Δleaf when the atmospheric CO2 concentration (ca) is held constant (10) (but see ref. 11). In turn, ci depends on the flux of CO2 into the leaf, which is largely regulated by stomatal conductance (gs) and the flux of CO2 removed from the leaf for C fixation by assimilation (A) (10). Values of Δleaf generally decrease with reductions in water availability, reflecting a down-regulation of gs and increased water-use efficiency (WUE) (10, 12). Thus, Δleaf is a time-integrated measure of WUE, although leaf temperature (T), mesophyll conductance and differences in ca are potential confounding factors (11 and 13). Confounding effects are reduced, however, because mesophyll conductance is correlated with A (and with gs) (11 and 14) and trees appear to maintain leaf T within a narrow range (21.4 ± 2.2 °C) across a wide range of ambient T (15). A and gs also vary with plant phylogeny, growth form and leaf traits, thus Δleaf and its response to the environment may differ for plant functional types (PFTs) defined by these attributes (4).
(10). Δleaf in C3 plants is a function of the fractionation associated with CO2 diffusion (4.4%) and photosynthetic fractionation by Rubisco (27%). For C3 plants, the concentration of CO2 in the substomatal cavities of leaves (ci) is directly proportional to Δleaf when the atmospheric CO2 concentration (ca) is held constant (10) (but see ref. 11). In turn, ci depends on the flux of CO2 into the leaf, which is largely regulated by stomatal conductance (gs) and the flux of CO2 removed from the leaf for C fixation by assimilation (A) (10). Values of Δleaf generally decrease with reductions in water availability, reflecting a down-regulation of gs and increased water-use efficiency (WUE) (10, 12). Thus, Δleaf is a time-integrated measure of WUE, although leaf temperature (T), mesophyll conductance and differences in ca are potential confounding factors (11 and 13). Confounding effects are reduced, however, because mesophyll conductance is correlated with A (and with gs) (11 and 14) and trees appear to maintain leaf T within a narrow range (21.4 ± 2.2 °C) across a wide range of ambient T (15). A and gs also vary with plant phylogeny, growth form and leaf traits, thus Δleaf and its response to the environment may differ for plant functional types (PFTs) defined by these attributes (4).
Data.
Our dataset, provided in Dataset S1, includes 334 species from 75 woody plant families (including trees and shrubs) present at 105 geographic sites distributed across 5 continents (representing 3,310 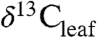 measurements of individual plants). The result is 570 unique species-site combinations represented by a mean Δleaf value calculated from multiple individuals of each species at a site. These sites represent eight biomes with mean annual temperature ranging from -10° to 28 °C and mean annual precipitation (MAP) from 147 to 3,700 mm per year, capturing the broad range in which higher plants occur (SI Appendix).
measurements of individual plants). The result is 570 unique species-site combinations represented by a mean Δleaf value calculated from multiple individuals of each species at a site. These sites represent eight biomes with mean annual temperature ranging from -10° to 28 °C and mean annual precipitation (MAP) from 147 to 3,700 mm per year, capturing the broad range in which higher plants occur (SI Appendix).
Precipitation.
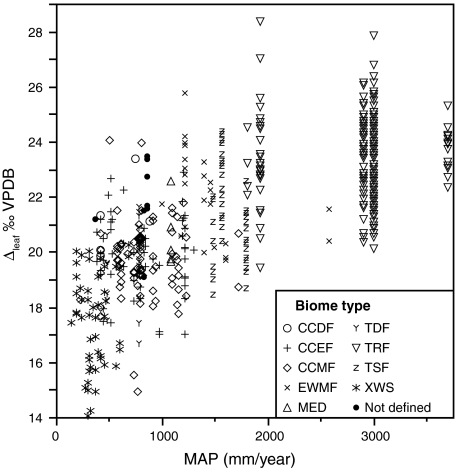
MAP is positively correlated to global Δleaf values (p < 0.0001, R2 = 0.55; Fig. 1) and is the strongest predictor of Δleaf among the environmental parameters in our dataset. To our knowledge this study represents the largest spatial scale at which this correlation has been documented, though it has been seen in some, but not all, regional studies and with considerably lower explanatory power (16–20). The strong correlation of MAP with Δleaf suggests that as water availability increases, stomatal limitations on ci and A relax and WUE declines (i.e., gs increases relative to A), leading to greater absolute C fixation (3 and 10). These apparent stomatal limitations on leaf-level A likely scale up to the ecosystem level and help explain global variation in gross and net primary production, which also varies with MAP (21).
Fig. 1.
Effect of mean annual precipitation (MAP) on Δleaf (n = 506). MAP accounts for 55% (p < 0.0001) of the variability in Δleaf in a linear regression model. Points are coded by biome: tropical rain forest (TRF), evergreen warm mixed forest (EWMF), tropical seasonal forest (TSF), cool-cold deciduous forest (CCDF), cool-cold evergreen forest (CCEF), cool-cold mixed forest (CCMF), tropical deciduous forest (TDF), xeric woodland scrubland (XWS). In a regression model using site-means (Δleaf averaged by geographic location), MAP accounts for 52% (p < 0.0001) of the variability in Δleaf (SI Appendix).
The relationship between MAP and Δleaf is consistently strong within the continents of North America (NA) and Asia (SI Appendix), but not Europe (EU; R2 = 0.025, p = 0.125). Extensive analysis of European data does not reveal any single environmental variable (or simple combination of variables) that accounts for more than 20% of the variation in Δleaf across all plant types. The lack of strong MAP effects on Δleaf in EU (see also ref. 16) and elsewhere (17–19) may arise from heterogeneity in growing season water availability that is not captured by MAP, due to differences in the seasonality of rainfall across sites (i.e. EU includes areas with wet winters in the south and wet summers in the north) or the presence of sites where soil water does not come from local precipitation. Better measures of water availability during growth may yield different results. For example, in our NA dataset, summer precipitation (June to August) is a better estimate of water availability than MAP for deciduous angiosperms and is more strongly correlated with Δleaf (R2 = 0.83 and 0.57 respectively, p < 0.0001, n = 36 species-site combinations, excluding tropical sites and one California site with a wet winter).
Altitude and Other Factors.
We assessed the influence of other factors on Δleaf using bivariate partial regression models that account for the covariance of MAP with other predictors (SI Appendix) and its correlation with Δleaf. This approach is justified by our observation that MAP emerges as the strongest single predictor of Δleaf and because the influence of MAP on Δleaf is supported by theory and other observations. The partial regression model with altitude as a secondary predictor is the only model with notable explanatory power (R2 = 0.13, p < 0.0001) indicating that altitude has a weak, negative influence on Δleaf that is statistically independent of its covariance with MAP. The combination of MAP and altitude explains 61% of the global variability in Δleaf in a multiple regression model (p < 0.0001), and regression models for data-rich continents show similar results (SI Appendix). Elevation effects on Δleaf have been observed previously (3 and 20), although altitude is rarely evaluated independently of MAP. The responsible mechanisms remain uncertain (3) and could be related to T, vapor pressure, oxygen partial pressure, irradiance, or other factors.
MAP and altitude effects on Δleaf are evident when regressions are performed on data grouped by PFT (SI Appendix), indicating that their influence is independent of community changes along elevation or precipitation gradients. The effects of MAP and altitude are still apparent when regressions are performed separately for well represented genera in our database (SI Appendix). Phylogenetic variation is reduced within genera, making it unlikely that the global correlations of Δleaf with MAP (Fig. 1) and altitude are driven exclusively by differences in the taxonomic composition of floras in different regions. Rather, we suggest Δleaf is evolutionarily and physiologically plastic in response to changes in water availability.
Unexpectedly, other factors (e.g. T, latitude, evapotranspiration) were not significantly related to Δleaf after controlling for MAP and altitude in regression models of the entire dataset. Negligible effects of these factors could indicate that A and gs covary more tightly with T and other factors than with changes in MAP and altitude. Covariance of T with MAP, altitude, or PFT distribution may also mask its influence (SI Appendix, see below).
Plant Functional Types.
Despite the considerable variance in Δleaf explained by MAP and altitude, much remains unexplained, as evident by the ∼8% range in Δleaf at any given MAP value (Fig 1; Dataset S1). Microclimate, ecosystem structure, and differences in plant traits among the species and PFTs must explain some of this variability. Several studies have reported localized differences in Δleaf among woody PFTs and similar results have been observed in metaanalyses of wood δ13C (6). Yet previous metaanalyses of Δleaf of C3 plants have not found evidence of a PFT effect (22), with the exception that grasses differ from woody plants (4). This likely reflects inadequate control for environmental influences on Δleaf that are independent of PFT. On the other hand, accounting for the uneven distribution of PFTs along environmental gradients may reveal additional environmental controls on Δleaf. When regression models are analyzed separately for each PFT, small latitude and T effects on Δleaf are evident for deciduous angiosperms (DA) and evergreen gymnosperms (EG), the PFTs that span the greatest range in latitude and T (SI Appendix). For DAs and EGs, increases in latitude or decreases in T result in slightly higher Δleaf than predictions based on MAP and altitude alone, indicating limitations on carbon assimilation due to low T or irradiance.
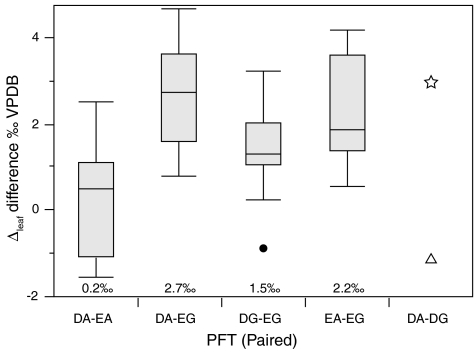
We used a multiple regression model with MAP and altitude as predictors to capture the dominant environmental influences on Δleaf, and explored whether PFTs explain any residual variation in Δleaf. This approach reveals significant differences in Δleaf among PFTs (SI Appendix), with EGs between 1.0% and 1.5% lower than the others. More strict control of confounding environmental influences on Δleaf is achieved by limiting PFT comparisons to plants at the same geographic site (Fig. 2; SI Appendix). With this constraint, DAs and evergreen angiosperms (EA) have higher Δleaf than EGs, by 2.7% and 2.2% respectively. PFT effects on Δleaf are not solely related to phylogeny or leaf habit. These differences may be related to genetic or phenotypic differences in A and gs mediated by leaf morphology, hydraulic architecture, rooting depth, leaf T, and/or mesophyll conductance (3, 10, 11, 13). These traits also vary among species within the same PFT; future studies should examine this variation.
Fig. 2.
Differences in Δleaf between cooccurring plant functional types (PFTs). For each geographic site, mean Δleaf values of each species were averaged to produce a mean Δleaf for each PFT. Box and whisker plots show the median, upper and lower quartiles, and maximum and minimum values, with outlier values shown as black dots. Number of sites per PFT comparison are as follows (deciduous angiosperm, DA; deciduous gymnosperm, DG; evergreen angiosperm, EA; evergreen gymnosperm, DG): DA - EA = 16, DA - EG = 17, DG - EG = 21, EA - EG = 12. DA-DG comparison is shown to highlight differences between Larix (triangle) and Taxodium (star).
Deciduous and evergreen species have contrasting nutrient use and retention strategies (23) that may underlie their dominance in different ecosystems. Our results support the notion that WUE differences are also an important component of plant strategies related to resource availability and likely help explain PFT dominance patterns. Lower Δleaf implies greater WUE of EGs relative to other woody plants, conferring a potential advantage where water is limiting and a limitation on A when water availability is high (24).
Biome Patterns.
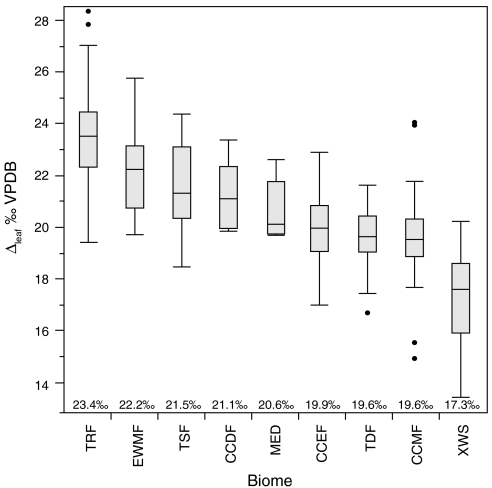
Mean Δleaf values calculated by biome type (Fig. 3; SI Appendix) differed significantly, with greatest fractionation in tropical rain forests (mean Δleaf = 23.4%) and lowest in xeric woodland/scrublands (mean Δleaf = 17.3%), which compare well with biome level Δleaf patterns predicted by Kaplan et al. (5). We observe a similar pattern when mean Δleaf for each biome is calculated separately for angiosperms and gymnosperms (SI Appendix). Biome type alone explains more Δleaf variation than MAP (R2 of 66% and 55%, respectively, SI Appendix), because it captures other factors related to Δleaf, including the spatial distribution of PFTs. PFT influences at the biome scale are readily apparent; cool-cold deciduous forests have higher Δleaf values (by 1.2%) than cool-cold evergreen forests (Fig. 3; see also Kaplan et al. (5)). The DA-EG differences in Δleaf (1.0% to 2.7%) calculated for each biome are consistent with results based on residuals of multiple regression (1.0–1.5%; SI Appendix) and the paired-site comparison (2.7%; Fig. 2).
Fig. 3.
Box and whisker plots of Δleaf values by biome. Biome accounts for 66% (p < 0.0001) of the variability in Δleaf in an ANOVA model. Number of samples per biome are as follows (see Fig. 1 caption for abbreviations): TRF = 206; EWMF = 29; TSF = 47; CCDF = 5; MED = 5; CCEF = 53; CCMF = 78; TDF = 26; XWS = 59. Statistical tests of means are shown in SI Appendix.
Implications for Models.
Kaplan et al. (5) recently extended a coupled vegetation-biogeochemical model (BIOME4) to predict Δleaf at the biome and PFT scale. Our database for C3 woody plants provides a means for full evaluation of BIOME4’s ability to reproduce global patterns in Δleaf. Since Δleaf records the time-integrated balance of A and gs, global patterns in Δleaf can be used to assess the ability of physiological models to predict the balance between leaf C and H2O fluxes. Further, incorporating Δleaf into coupled vegetation-biogeochemistry models have potential to enable predictive mapping of spatial variability in 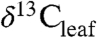 and
and 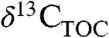 , which could improve studies of the effect of climate on modern and ancient organic matter.
, which could improve studies of the effect of climate on modern and ancient organic matter.
Geologic Implications.
The importance of water in regulating Δleaf values is often invoked in environmental and atmospheric reconstructions from 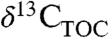 (1). More recently, the role of PFTs in controlling
(1). More recently, the role of PFTs in controlling 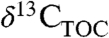 has been identified (2 and 25). Our results show that variation in PFT and MAP independently result in differences in Δleaf of up to several % (Fig. 1 and 2), and that biome may be a powerful integrator of PFT, rainfall, and other effects on Δleaf and
has been identified (2 and 25). Our results show that variation in PFT and MAP independently result in differences in Δleaf of up to several % (Fig. 1 and 2), and that biome may be a powerful integrator of PFT, rainfall, and other effects on Δleaf and 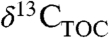 (Fig. 3). These quantitative relationships can be used to understand temporal and spatial variations in
(Fig. 3). These quantitative relationships can be used to understand temporal and spatial variations in 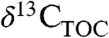 provided that MAP and PFT (or biome) can be estimated in the geologic past.
provided that MAP and PFT (or biome) can be estimated in the geologic past.
There are uniformitarian assumptions in using Δleaf patterns among extant plants to interpret the vegetation of the geological past, however two factors encourage us to proceed. First, the morphological and physiological responses of C3 plants to water stress are constrained by their fundamental anatomy and biochemistry (26), making it likely that the relationship of water availability and Δleaf has been similar in direction and magnitude over geological time. MAP and altitude effects on Δleaf are apparent within as well as among genera, implying that independent lineages respond similarly and quickly. Second, a recent study by Crisp et al. (27) shows that plant lineages rarely shift biomes during evolution, suggesting niche conservatism and supporting the careful use of biome type to constrain Δleaf values. Nonetheless, applying modern correlations of Δleaf with environment to the distant geological past may be problematic because of differences in major plant groups prior to the origin of angiosperms in the Cretaceous.
Pollen and leaf fossils document spatial and temporal changes in PFT as well as T and precipitation (9 and 28). Plant biomarkers (chemical fossils), such as n-alkanes (from leaf waxes) or terpenoids (defense compounds) are also preserved geologically (9) and have fewer diagenetic or source effects on their isotopic composition than 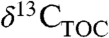 (29). Plant waxes in ancient sediments reflect productivity-weighted inputs from all PFTs contributing to that deposit. The resulting mixed molecular signal can be used to estimate a
(29). Plant waxes in ancient sediments reflect productivity-weighted inputs from all PFTs contributing to that deposit. The resulting mixed molecular signal can be used to estimate a 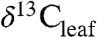 value that integrates plant biomass within the catchment. In contrast, terpenoids provide PFT specificity because tricyclic diterpenoids are unique to woody gymnosperms and pentacyclic triterpenoids are unique to woody angiosperms. Therefore, ratios of these terpenoids, after accounting for production and preservational biases, may provide estimates of PFT (30). Also, terpenoid δ13C values can be used to calculate PFT-specific Δleaf values (25).
value that integrates plant biomass within the catchment. In contrast, terpenoids provide PFT specificity because tricyclic diterpenoids are unique to woody gymnosperms and pentacyclic triterpenoids are unique to woody angiosperms. Therefore, ratios of these terpenoids, after accounting for production and preservational biases, may provide estimates of PFT (30). Also, terpenoid δ13C values can be used to calculate PFT-specific Δleaf values (25).
Heterogeneity in rainfall and PFT dominance across ancient landscapes presumably created spatial patterns in Δleaf just as they do now, even at small spatial scales. To understand differences in 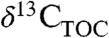 ,
, 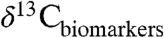 and the magnitude of CIEs recorded in different regions at the same time, and improve chemostratigraphic correlations using
and the magnitude of CIEs recorded in different regions at the same time, and improve chemostratigraphic correlations using 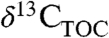 , we should account for biome (5), precipitation (9), and PFT (2 and 25) effects on Δleaf values using relationships like those reported above.
, we should account for biome (5), precipitation (9), and PFT (2 and 25) effects on Δleaf values using relationships like those reported above.
An important goal in studies that document temporal variation in 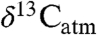 is to reconstruct the sources and fluxes of C to the ancient atmosphere (7).
is to reconstruct the sources and fluxes of C to the ancient atmosphere (7). 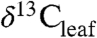 and
and 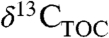 have been used to directly represent
have been used to directly represent 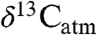 (4 and 31), but this approach is fraught with uncertainty (32). It has been suggested that using a simple numerical offset to convert
(4 and 31), but this approach is fraught with uncertainty (32). It has been suggested that using a simple numerical offset to convert 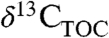 to
to 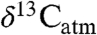 under nonlimiting water conditions is sufficient to control for environmental impacts (4). To the contrary, we argue that secular changes in PFT and climate must also result in significant—but often unaccounted—effects on Δleaf. Previous studies averaged multiple
under nonlimiting water conditions is sufficient to control for environmental impacts (4). To the contrary, we argue that secular changes in PFT and climate must also result in significant—but often unaccounted—effects on Δleaf. Previous studies averaged multiple 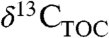 values to remove variation caused by environmental factors (4, 31, 32), ignoring insights about paleoecology and climate that might be inferred from
values to remove variation caused by environmental factors (4, 31, 32), ignoring insights about paleoecology and climate that might be inferred from 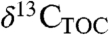 variations.
variations.
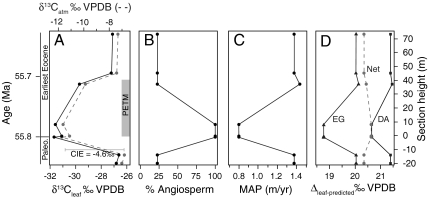
We use an example from the PETM to illustrate how our study of modern Δleaf can improve interpretation of ancient 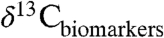 and
and 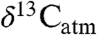 (Fig. 4; see SI Appendix). Smith et al. (2) documented a shift from a mixed angiosperm-conifer flora (∼25% angiosperm) in the late Paleocene to an angiosperm flora (∼100%) at the onset of the CIE, and inferred that the 5% decrease in
(Fig. 4; see SI Appendix). Smith et al. (2) documented a shift from a mixed angiosperm-conifer flora (∼25% angiosperm) in the late Paleocene to an angiosperm flora (∼100%) at the onset of the CIE, and inferred that the 5% decrease in 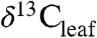 (estimated from n-alkanes) reflected both a decrease in
(estimated from n-alkanes) reflected both a decrease in 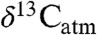 (3–4%) and an increase in Δleaf caused by a shift from conifers to angiosperms (1–2%, (2, 9)). Although the dominant late Paleocene conifers in the Bighorn Basin (Metasequoia and Glyptostrobus (9 and 33)) were deciduous, they probably had low Δleaf, as do their extant relatives (34 and 35) (Fig. 2). Yet the PETM in Wyoming is also marked by a change from wetter conditions (MAP ∼ 1,380 mm/yr) in the late Paleocene (28) to drier conditions (MAP ∼ 800 mm/yr) at the onset of the event (9), which should have decreased Δleaf. When both PFT and MAP effects on Δleaf are taken into account (Fig. 4; see SI Appendix), the decrease in Δleaf from declining MAP is countered by the increase in angiosperms, resulting in a net Δleaf increase of 0.2% and an estimated negative CIE in
(3–4%) and an increase in Δleaf caused by a shift from conifers to angiosperms (1–2%, (2, 9)). Although the dominant late Paleocene conifers in the Bighorn Basin (Metasequoia and Glyptostrobus (9 and 33)) were deciduous, they probably had low Δleaf, as do their extant relatives (34 and 35) (Fig. 2). Yet the PETM in Wyoming is also marked by a change from wetter conditions (MAP ∼ 1,380 mm/yr) in the late Paleocene (28) to drier conditions (MAP ∼ 800 mm/yr) at the onset of the event (9), which should have decreased Δleaf. When both PFT and MAP effects on Δleaf are taken into account (Fig. 4; see SI Appendix), the decrease in Δleaf from declining MAP is countered by the increase in angiosperms, resulting in a net Δleaf increase of 0.2% and an estimated negative CIE in 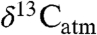 of 4.6%. This estimate of the atmospheric CIE is larger than that calculated by Smith et al. (2), who argued the CIE in
of 4.6%. This estimate of the atmospheric CIE is larger than that calculated by Smith et al. (2), who argued the CIE in 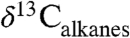 was higher than the atmospheric CIE because of the loss of conifers, and that marine sediments recorded the true CIE. Our Δleaf correction for both PFT and MAP effects on
was higher than the atmospheric CIE because of the loss of conifers, and that marine sediments recorded the true CIE. Our Δleaf correction for both PFT and MAP effects on 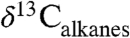 suggests the relatively large CIE recorded in the Bighorn Basin and other terrestrial sites (36) may equal the atmospheric CIE. This larger CIE falls within the range recently reported from well-preserved shallow marine carbonate records (-3.5% to -5.0%) and supports the notion that -3.5% is too small (37). A larger atmospheric CIE implies the carbon released at the onset of the PETM was more depleted or greater in mass than presently thought. Adjusting other terrestrial CIE records for biome, MAP, and PFT effects should further refine and reconcile estimates of the magnitude of the CIE.
suggests the relatively large CIE recorded in the Bighorn Basin and other terrestrial sites (36) may equal the atmospheric CIE. This larger CIE falls within the range recently reported from well-preserved shallow marine carbonate records (-3.5% to -5.0%) and supports the notion that -3.5% is too small (37). A larger atmospheric CIE implies the carbon released at the onset of the PETM was more depleted or greater in mass than presently thought. Adjusting other terrestrial CIE records for biome, MAP, and PFT effects should further refine and reconcile estimates of the magnitude of the CIE.
Fig. 4.
Conceptual diagram of 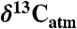 reconstruction for latest Paleocene to earliest Eocene sediments in the Bighorn Basin (WY). The figures are constructed as follows: (A)
reconstruction for latest Paleocene to earliest Eocene sediments in the Bighorn Basin (WY). The figures are constructed as follows: (A) 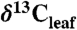 values as inferred from δ13C values of n-C31 alkanes (2), (B) percent of fossil leaves that are angiosperms, (C) mean annual precipitation (MAP) derived from fossil leaf metrics (9, 28), and (D) modeled net Δleaf from our Δleaf-MAP expressions for evergreen gymnosperm (EG; triangles) and deciduous angiosperm (DA; squares) PFTs (see SI Appendix). Each model was scaled by % angiosperm to derive a net Δleaf (dashed line).
values as inferred from δ13C values of n-C31 alkanes (2), (B) percent of fossil leaves that are angiosperms, (C) mean annual precipitation (MAP) derived from fossil leaf metrics (9, 28), and (D) modeled net Δleaf from our Δleaf-MAP expressions for evergreen gymnosperm (EG; triangles) and deciduous angiosperm (DA; squares) PFTs (see SI Appendix). Each model was scaled by % angiosperm to derive a net Δleaf (dashed line). 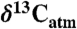 is denoted with a dashed line on A and is derived from the net Δleaf and
is denoted with a dashed line on A and is derived from the net Δleaf and 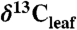 values. The negative CIE in
values. The negative CIE in 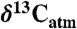 at the base of the Paleocene-Eocene Thermal Maximum (PETM) is 4.6% (difference between latest Paleocene value and average of initial PETM values). See SI Appendix for discussion of calculations and suggested use of these models.
at the base of the Paleocene-Eocene Thermal Maximum (PETM) is 4.6% (difference between latest Paleocene value and average of initial PETM values). See SI Appendix for discussion of calculations and suggested use of these models.
Relationships based on modern plants may not fully quantify the response of Δleaf to greenhouse climate conditions (high T and pCO2) such as those of the PETM (12, 13, 38). Given the multiple functions of stomata (water loss prevention, CO2 provision for A, evaporative cooling), it is unlikely that plants could simultaneously maintain homeostasis with respect to leaf T (15) and ci or Δleaf (12) during periods of rapid global change. Studies on Δleaf of extant relatives of ancient plants under high temperature and pCO2 conditions and varying degrees of water stress are needed to better interpret changes in 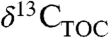 and
and 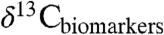 during the PETM and other events.
during the PETM and other events.
Conclusions.
We document global patterns in Δleaf among woody plants, including: (i) a strong positive correlation with MAP (R2 = 0.55), (ii) Δleaf values 1% to 2.7% lower for EGs than other woody PFTs, and (iii) differences up to 6% in Δleaf among biomes (R2 = 0.66). The relationship of Δleaf with MAP shows that the balance of leaf gas-exchange in modern plants is largely driven by water availability, consistent with the role of water in driving global trends in ecosystem-scale primary productivity. By revealing global-scale relationships of MAP, PFT, and biome with Δleaf, our results uniquely enable applications of these patterns for understanding global-scale processes, such as extrapolation of the distribution of PFTs and leaf carbon and water fluxes under future climate scenarios. In geologic studies, our models of Δleaf provide tools for interpreting spatial and temporal variation in 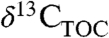 and
and 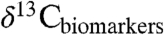 , leading to enhanced understanding of paleoecology and atmospheric CO2 during climate events that serve as analogs of the near future. Our results warn against the assumption in some geologic studies that Δleaf is invariant in space and time and emphasize that estimating
, leading to enhanced understanding of paleoecology and atmospheric CO2 during climate events that serve as analogs of the near future. Our results warn against the assumption in some geologic studies that Δleaf is invariant in space and time and emphasize that estimating 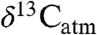 from
from 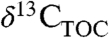 or
or 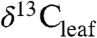 requires information about biome, PFT, and paleoclimate. We use this approach to produce a refined estimate of the atmospheric CIE (-4.6%) during the PETM and suggest that it be extended to other PETM CIE records to better estimate T sensitivity to greenhouse gases. Such work will aid our understanding of plant response to extreme climate change both in the geologic past and in the coming century.
requires information about biome, PFT, and paleoclimate. We use this approach to produce a refined estimate of the atmospheric CIE (-4.6%) during the PETM and suggest that it be extended to other PETM CIE records to better estimate T sensitivity to greenhouse gases. Such work will aid our understanding of plant response to extreme climate change both in the geologic past and in the coming century.
Methods
We extracted 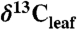 values for woody trees and shrubs from 45 publications and one new study (SI Appendix). We excluded
values for woody trees and shrubs from 45 publications and one new study (SI Appendix). We excluded 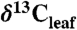 values from juvenile, fertilized, or watered plants, immature or shaded leaves, and understory shrubs. All
values from juvenile, fertilized, or watered plants, immature or shaded leaves, and understory shrubs. All 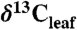 values were converted to
values were converted to 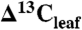 using the Farquhar et al. (10) equation (
using the Farquhar et al. (10) equation (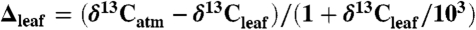 ) and
) and 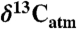 values estimated for the year of sampling (39) unless reported. Estimating
values estimated for the year of sampling (39) unless reported. Estimating 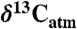 may cause bias in Δleaf due to seasonal and latitudinal gradients in
may cause bias in Δleaf due to seasonal and latitudinal gradients in 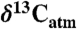 . However, this gradient is < 0.5% during the growing season (40). Species means were calculated for each geographic site to remove within-species variability. For a subset of sites where
. However, this gradient is < 0.5% during the growing season (40). Species means were calculated for each geographic site to remove within-species variability. For a subset of sites where 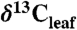 values from multiple PFTs were reported (n = 53), we calculated paired PFT differences at each site by averaging all species in each PFT and then calculating differences between PFT. For each geographic site, environmental factors were extracted from the publication or derived from global databases (SI Appendix) and biome classifications were assigned based on the BIOME4 model, descriptions in the original publication, and environmental factors. We analyzed the data using linear, least squares regression and ANOVA (SAS JMP 7.0). Δleaf values and latitude are approximately normally distributed. MAP was log 10-normally distributed and transformed accordingly. Altitude was approximately normally distributed after square root transformation. We performed pairwise comparisons of means using the Tukey-Kramer HSD. Statistics reported within the text and appendices were performed at the site-species combination level, unless otherwise noted (n = 570 or less, depending on the model). Similar results were obtained from statistical models at the site level (SI Appendix) using means for all species at a site (n = 73 or less).
values from multiple PFTs were reported (n = 53), we calculated paired PFT differences at each site by averaging all species in each PFT and then calculating differences between PFT. For each geographic site, environmental factors were extracted from the publication or derived from global databases (SI Appendix) and biome classifications were assigned based on the BIOME4 model, descriptions in the original publication, and environmental factors. We analyzed the data using linear, least squares regression and ANOVA (SAS JMP 7.0). Δleaf values and latitude are approximately normally distributed. MAP was log 10-normally distributed and transformed accordingly. Altitude was approximately normally distributed after square root transformation. We performed pairwise comparisons of means using the Tukey-Kramer HSD. Statistics reported within the text and appendices were performed at the site-species combination level, unless otherwise noted (n = 570 or less, depending on the model). Similar results were obtained from statistical models at the site level (SI Appendix) using means for all species at a site (n = 73 or less).
Supplementary Material
Acknowledgments.
We thank the editors and reviewers for helpful comments. We thank Josh Dorin, Heather Graham, and Clayton Magill for data collection assistance. We also thank David Williams for providing data. This research was supported by the National Science Foundation Grant EAR-0844212 (to K.H.F.), fellowship awards from the Penn State Biogeochemical Research Initiative for Education (BRIE) funded by National Science Foundation Integrative Graduate Education and Research Traineeship (IGERT) Grant DGE-9972759 (to A.F.D. and K.E.M.), and a Department of Energy Graduate Research Environmental Fellowship (GREF) award (to K.E.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910513107/DCSupplemental.
References
- 1.Bowen GJ, Beerling DJ, Koch PL, Zachos JC, Quattlebaum T. A humid climate state during the Palaeocene/Eocene thermal maximum. Nature. 2004;432:495–499. doi: 10.1038/nature03115. [DOI] [PubMed] [Google Scholar]
- 2.Smith FA, Wing SL, Freeman KH. Carbon and hydrogen isotope compositions of plant lipids during the PETM as evidence for the response of terrestrial ecosystems to rapid climate change. Earth Planet Sci Lett. 2007;262:50–65. [Google Scholar]
- 3.Marshall JD, Brooks JR, Lajtha K. Sources of variation in the stable isotopic composition of plants. In: Michener R, Lajtha K, editors. Stable isotopes in ecology and environmental science. 2nd Ed. USA: Wiley-Blackwell; 2007. pp. 22–60. [Google Scholar]
- 4.Arens NC, Jahren AH, Amundson R. Can C3 plants faithfully record the carbon isotopic composition of atmospheric carbon dioxide? Paleobiology. 2000;26:137–164. [Google Scholar]
- 5.Kaplan JO, Prentice C, Buchmann N. The stable carbon isotope composition of the terrestrial biosphere: Modeling at scales from the leaf to the globe. Global Biogeochem Cycles. 2002;16:1060. doi: 10.1029/2001GB001403. [Google Scholar]
- 6.Leavitt SW, Newberry T. Systematics of stable-carbon isotopic differences between gymnosperm and angiosperm trees. Plant Physiol. 1992;11:257–262. [Google Scholar]
- 7.Pagani M, Caldeira K, Archer D, Zachos JC. ATMOSPHERE: An ancient carbon mystery. Science. 2006;314:1556–1557. doi: 10.1126/science.1136110. [DOI] [PubMed] [Google Scholar]
- 8.Hayes JM, Strauss H, Kaufman AJ. The abundance of 13C in marine organic matter and isotopic fractionation in the global biogeochemical cycle of carbon during the past 800 Ma. Chem Geol. 1999;161:103–125. [Google Scholar]
- 9.Wing SL, et al. Transient floral change and rapid global warming at the Paleocene-Eocene boundary. Science. 2005;310:993–996. doi: 10.1126/science.1116913. [DOI] [PubMed] [Google Scholar]
- 10.Farquhar GD, Ehleringer JR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annu Rev Plant Phys. 1989;40:503–537. [Google Scholar]
- 11.Seibt U, Rajabi A, Griffiths H, Berry J. Carbon isotopes and water use efficiency: sense and sensitivity. Oecologia. 2008;155:441–454. doi: 10.1007/s00442-007-0932-7. [DOI] [PubMed] [Google Scholar]
- 12.Ehleringer JR, Cerling TE. Atmospheric CO2 and the ratio of intercellular to ambient CO2 concentrations in plants. Tree Physiol. 1995;15:105–111. doi: 10.1093/treephys/15.2.105. [DOI] [PubMed] [Google Scholar]
- 13.Ehleringer JR, Phillips SL, Comstock JP. Seasonal variation in the carbon isotopic composition of desert plants. Funct Ecol. 1992;6:396–404. [Google Scholar]
- 14.Warren CR, Adams MA. Internal conductance does not scale with photosynthetic capacity: Implications for carbon isotope discrimination and the economics of water and nitrogen use in photosynthesis. Plant Cell Environ. 2006;29:192–201. doi: 10.1111/j.1365-3040.2005.01412.x. [DOI] [PubMed] [Google Scholar]
- 15.Helliker BR, Richter SL. Subtropical to boreal convergence of tree-leaf temperatures. Nature. 2008;454:511–514. doi: 10.1038/nature07031. [DOI] [PubMed] [Google Scholar]
- 16.Hemming D, et al. Pan-european δ13C values of air and organic matter from forest ecosystems. Glob Change Biol. 2005;11:1065–1093. [Google Scholar]
- 17.Miller JM, Williams RJ, Farquhar GD. Carbon isotope discrimination by a sequence of Eucalyptus species along a subcontinental rainfall gradient in Australia. Funct Ecol. 2001;15:222–232. [Google Scholar]
- 18.Schulze E-D, et al. Carbon and nitrogen isotope discrimination and nitrogen nutrition of trees along a rainfall gradient in northern Australia. Australian J Plant Physiol. 1998;25:413–425. [Google Scholar]
- 19.Swap RJ, Aranibar JN, Dowty PR, Gilhooly WP, III, Macko SA. Natural abundance of 13C and 15N in C3 and C4 vegetation of southern Africa: patterns and implications. Glob Change Biol. 2004;10:350–358. [Google Scholar]
- 20.Warren CR, McGrath JF, Adams MA. Water availability and carbon isotope discrimination in conifers. Oecologia. 2001;127:476–486. doi: 10.1007/s004420000609. [DOI] [PubMed] [Google Scholar]
- 21.Chapin FS, Matson PA, Mooney HA. Principles of terrestrial ecosystem ecology. New York: Springer; 2002. p. 472. [Google Scholar]
- 22.Kelly CK, Woodward FI. Ecological correlates of carbon isotope composition of leaves: A comparative analysis testing for the effects of temperature, CO2 and O2 partial pressures and taxonomic relatedness on δ13C. J Ecol. 1995;83:509–515. [Google Scholar]
- 23.Aerts R. The advantages of being evergreen. Trends Ecol Evol. 1995;10:402–407. doi: 10.1016/s0169-5347(00)89156-9. [DOI] [PubMed] [Google Scholar]
- 24.Givnish TJ. Adaptive significance of evergreen vs. deciduous leaves: solving the triple paradox. Silva Fenn. 2002;36:703–743. [Google Scholar]
- 25.Schouten S, et al. The Paleocene-Eocene carbon isotope excursion in higher plant organic matter: Differential fractionation of angiosperms and conifers in the Arctic. Earth Planet Sci Lett. 2007;258:581–592. [Google Scholar]
- 26.Meinzer F. Functional convergence in plant responses to the environment. Oecologia. 2003;134:1–11. doi: 10.1007/s00442-002-1088-0. [DOI] [PubMed] [Google Scholar]
- 27.Crisp MD, et al. Phylogenetic biome conservatism on a global scale. Nature. 2009;458:754–756. doi: 10.1038/nature07764. [DOI] [PubMed] [Google Scholar]
- 28.Wilf P. Late Paleocene-early Eocene climate changes in southwestern Wyoming: Paleobotanical analysis. Geol Soc Am Bull. 2000;112:292–307. [Google Scholar]
- 29.Brocks JJ, Summons RE, Heinrich DH, Karl KT. Treatise on Geochemistry. Oxford: Pergamon; 2003. Sedimentary hydrocarbons, biomarkers for early life; pp. 63–115. [Google Scholar]
- 30.Bechtel A, Sachsenhofer RF, Zdravkov A, Kostova I, Gratzer R. Influence of floral assemblage, facies and diagenesis on petrography and organic geochemistry of the Eocene Bourgas coal and the Miocene Maritza-East lignite (Bulgaria) Org Geochem. 2005;36:1498–1522. [Google Scholar]
- 31.Peters-Kottig W, Strauss H, Kerp H. The land plant δ13C record and plant evolution in the Late Palaeozoic. Palaeogeogr Palaeocl. 2006;240:237–252. [Google Scholar]
- 32.Beerling DJ, Royer DL. Fossil plants as indicators of the Phanerozoic global carbon cycle. Annu Rev Earth Pl Sc. 2002;30:527–556. [Google Scholar]
- 33.Wing S, Boucher LD. Ecological aspects of the Cretaceous flowering plant radiation. Annu Rev Earth Pl Sc. 1998;26:379–421. [Google Scholar]
- 34.Jagels R, Day ME. The adaptive physiology of Metasequoia to Eocene high-latitude environments. In: Hemsley AR, Poole I, editors. The evolution of plant physiology. New York: Linnean Society of London; 2004. pp. 401–425. [Google Scholar]
- 35.McArthur JV, Moorhead KK. Characterization of riparian species and stream detritus using multiple stable isotopes. Oecologia. 1996;107:232–238. doi: 10.1007/BF00327907. [DOI] [PubMed] [Google Scholar]
- 36.Handley L, Pearson PN, McMillan IK, Pancost RD. Large terrestrial and marine carbon and hydrogen isotope excursions in a new Paleocene/Eocene boundary section from Tanzania. Earth Planet Sci Lett. 2008;275:17–25. [Google Scholar]
- 37.McCarren H, Thomas E, Hasegawa T, Röhl U, Zachos JC. Depth dependency of the Paleocene-Eocene carbon isotope excursion: Paired benthic and terrestrial biomarker records (Ocean Drilling Program Leg 208, Walvis Ridge) Geochem Geophy Geosyst 9. 2008. p. Q10008. doi: 10.1029/2008GC002116.
-
38.Jahren AH, Arens NC, Harbeson SA. Prediction of atmospheric
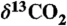 using fossil plant tissues. Rev Geophys. 2008;46 RG1002, doi: 10.1029/2006RG000219. [Google Scholar]
using fossil plant tissues. Rev Geophys. 2008;46 RG1002, doi: 10.1029/2006RG000219. [Google Scholar] - 39.McCarroll D, Loader NJ. Stable isotopes in tree rings. Quaternary Science Reviews. 2004;23:771–801. [Google Scholar]
- 40.GLOBALVIEW-CO2C13. Boulder, Colorado: NOAA ESRL; 2008. Cooperative atmospheric data integration project—δ13C of carbon dioxide. http://www.esrl.noaa.gov/gmd/ccgg/globalview/co2c13/co2c13_intro.html. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.