Tumors are characterized by excessive proliferation and, therefore, chemotherapeutics that target proteins involved in tumor cell division can be effective anticancer agents. However, as many of these proteins are involved in core mechanisms in dividing and nondividing cells, these therapeutic agents do not selectively kill tumor cells and their efficacy is limited by general toxicity. As new therapeutics that target proteins involved in these core processes are developed, the likelihood increases of identifying a protein that cancer cells depend on more than normal cells. Wood et al. report the discovery and characterization of an inhibitor of a mitotic motor protein, centromere-associated protein-E (CENP-E, kinesin-7) (1). This inhibitor for a protein whose functions are limited to cell division has the potential to lead to improved cancer chemotherapies.
Currently used antimitotic therapeutics target the cytoskeletal protein tubulin, which polymerizes to form microtubules (2). During cell division, segregation of chromosomes requires a microtubule-based bipolar spindle. Only after all chromosome pairs have been attached to the opposite ends of the bipolar spindle through microtubules, the spindle assembly checkpoint is satisfied, chromosomes are separated, and the cell cycle progresses. When normal tubulin polymerization dynamics are disrupted, proper chromosome-spindle attachments are not established, and the cell cycle is blocked by the checkpoint. Through poorly understood mechanisms, this cell-cycle arrest can lead to cell death (3). However, microtubules have essential roles in other cellular processes, such as neuronal transport. Therefore, the use of tubulin-targeting antimitotic agents is associated with side effects, including neurotoxicity.
A decade ago, a small-molecule inhibitor, monastrol, was reported for kinesin-5 (also called KSP or Eg5) (4). Kinesins are motor proteins that can use ATP hydrolysis to drive transport of cellular cargoes along microtubules. Monastrol was the first chemical inhibitor that targeted a protein, other than tubulin, needed for mitotic spindle assembly. This initial “hit” helped catalyze the development of drugs against kinesin-5, a protein that was not known to have key functions in nondividing cells such as neurons. The kinesin superfamily includes 14 different families and more than 40 individual kinesin genes in humans (5). Different mitotic kinesins have crucial roles in distinct aspects of spindle assembly and function, including microtubule organization, chromosome movement, and cytokinesis (6). Although more work is needed to determine whether kinesin-5 inhibitors will be more effective than currently used tubulin-targeting chemotherapeutics, the development of multiple kinesin-5-targeted chemicals suggests that members of the kinesin superfamily are “druggable” (7).
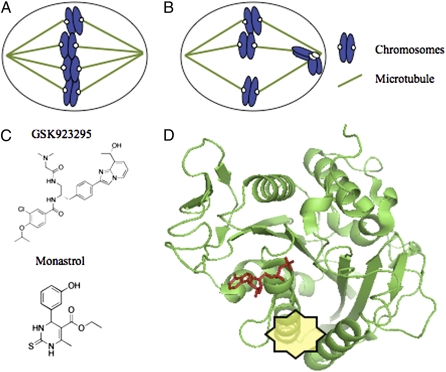
CENP-E is a kinetochore-associated kinesin with an essential role in metaphase chromosome alignment that does not function in nondividing cells (8). Depletion of CENP-E from cultured human cells is characterized by a bipolar mitotic spindle with chromosomes clustered close to either end of the bipolar spindle (i.e., the spindle pole), rather than aligning at the metaphase plate (Fig. 1 A and B) (9). It has been proposed that CENP-E contributes to a cooperative chromosome alignment mechanism in which the motor protein drives the movement of chromosomes from spindle poles to the metaphase plate alongside microtubules that are properly oriented, as these filaments link other aligned chromosomes to spindle poles (10, 11). Recently, Wood et al. conducted an in vitro high-throughput screen to identify inhibitors of CENP-E ATPase activity. Optimization of initial “hits” resulted in GSK923295, a potent inhibitor of CENP-E (1). In dividing cells treated with GSK923295, chromosomes are clustered at the spindle poles as would be expected for CENP-E inhibition. This finding goes beyond previous studies by showing that CENP-E’s motor activity is needed for chromosome alignment.
Fig. 1.
GSK923295, an inhibitor of the mitotic kinesin CENP-E. (A) Normal cell division requires alignment of chromosomes at the equator of the bipolar spindle (the metaphase plate). (B) Loss of CENP-E function results in a few chromosomes that remain stuck at spindle poles. (C) Chemical structures of GSK923295, the CENP-E inhibitor, and monastrol, the kinesin-5 inhibitor. These inhibitors bind at an allosteric site (yellow), which is ∼10 Å from the ADP-binding site (red), in the kinesin ATPase domain, as depicted on the CENP-E motor domain (PDB: 1T5C) (D).
CENP-E also has an important role in the spindle assembly checkpoint (12). Specifically, CENP-E has been hypothesized to be the receptor protein responsible for silencing the spindle assembly checkpoint after proper microtubule-kinetochore attachment. It has been reported that microtubule capture by the CENP-E motor domain is sufficient to silence activity of a kinase required for the spindle assembly checkpoint (13). Based on these data, and the fact that GSK923295 forces CENP-E to tightly bind microtubules (a “rigor” state) (1), cells treated with this inhibitor should progress through mitosis, as the checkpoint would be satisfied. Therefore, it is difficult to explain the observation that cells treated with GSK923295 are arrested in mitosis. It is possible that the mitotic arrest could be due to off-target effects of the chemical inhibitor (e.g., inhibition of proteins other than the kinesins tested). Alternatively, this inconsistency could simply be revealing our incomplete understanding of the pathways that lead to a spindle assembly checkpoint-dependent block. Another intriguing possibility is that inhibition of CENP-E by GSK923295 keeps the protein and, therefore, the chromosomes bound to microtubule tracks, effectively generating a “roadblock” for other transport processes that are needed for checkpoint silencing, such as the transport of checkpoint proteins away from kinetochores (12, 14). Additional experiments are needed to explain these findings, and it is likely that GSK923295 will be a powerful tool for dissecting these complex and dynamic cellular mechanisms.
Wood et al. report the discovery and characterization of an inhibitor of a mitotic motor protein.
To clarify the mechanism of CENP-E inhibition by GSK923295, crosslinking and mutagenesis studies were conducted. GSK923295 binding was mapped to a region in CENP-E that is ∼10 Å away from the nucleotide-binding pocket, between helices α2 and α3, adjacent to loop L5 (1). Remarkably, this site is similar to that bound by many of the known kinesin-5 inhibitors (15), suggesting that this site may be a “hotspot” targeted by kinesin inhibitors (Fig. 1 C and D). These findings also raise the intriguing possibility that native cellular ligands may exist that bind at this site and regulate kinesins. Another notable point is that this class of CENP-E inhibitors can switch between ATP-uncompetitive and ATP-competitive inhibition with chemical modifications as small as a single carbon extension (16). Determining how these changes in the mechanism of inhibition by a small organic molecule occur will likely require high-resolution structural studies. As kinesins structurally resemble GTPases, such studies may provide insight into how drugs may be designed for this important class of anticancer targets for which good chemical inhibitors have been difficult to develop.
Recent studies have shown that mice with only one functional CENP-E allele have decreased tumor incidence, suggesting inhibition of CENP-E may present a viable strategy to treat cancer (17). In fact, GSK923295 displayed dose-dependent antitumor activity in vivo against mice bearing xenografts of human tumor cell lines, including the induction of partial and complete regressions (1). However, the responses of tumor cell lines to treatment with GSK923295 are variable and there were no obvious features common to the resistant tumor lines (1). This likely reflects a gap in our understanding of the link between mitotic perturbation and cell death (3). Encouragingly, the novel CENP-E inhibitors offer an exciting tool to bridge this knowledge gap.
The mitotic spindle has proven to be an important target for cancer chemotherapy (2, 18). The new generation of drugs that target proteins whose functions are limited to cell division offers the promise of improved efficacy with reduced side effects. We await the findings from clinical studies with GSK923295.
Footnotes
The authors declare no conflict of interest.
See companion article on page 5839.
References
- 1.Wood KW, et al. Antitumor activity of an allosteric inhibitor of centromere-associated protein-E (CENP-E) Proc Natl Acad Sci USA. 2010;107:5839–5844. doi: 10.1073/pnas.0915068107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 3.Rieder CL, Maiato H. Stuck in division or passing through: What happens when cells cannot satisfy the spindle assembly checkpoint. Dev Cell. 2004;7:637–651. doi: 10.1016/j.devcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Mayer TU, et al. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science. 1999;286:971–974. doi: 10.1126/science.286.5441.971. [DOI] [PubMed] [Google Scholar]
- 5.Miki H, Okada Y, Hirokawa N. Analysis of the kinesin superfamily: Insights into structure and function. Trends Cell Biol. 2005;15:467–476. doi: 10.1016/j.tcb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Wordeman L. How kinesin motor proteins drive mitotic spindle function: Lessons from molecular assays. Semin Cell Dev Biol. 2010 doi: 10.1016/j.semcdb.2010.01.018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergnes G, Brejc K, Belmont L. Mitotic kinesins: Prospects for antimitotic drug discovery. Curr Top Med Chem. 2005;5:127–145. doi: 10.2174/1568026053507697. [DOI] [PubMed] [Google Scholar]
- 8.Wood KW, Chua P, Sutton D, Jackson JR. Centromere-associated protein E: A motor that puts the brakes on the mitotic checkpoint. Clin Cancer Res. 2008;14:7588–7592. doi: 10.1158/1078-0432.CCR-07-4443. [DOI] [PubMed] [Google Scholar]
- 9.Yao X, Abrieu A, Zheng Y, Sullivan KF, Cleveland DW. CENP-E forms a link between attachment of spindle microtubules to kinetochores and the mitotic checkpoint. Nat Cell Biol. 2000;2:484–491. doi: 10.1038/35019518. [DOI] [PubMed] [Google Scholar]
- 10.Kapoor TM, et al. Chromosomes can congress to the metaphase plate before biorientation. Science. 2006;311:388–391. doi: 10.1126/science.1122142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai S, O’Connell CB, Khodjakov A, Walczak CE. Chromosome congression in the absence of kinetochore fibres. Nat Cell Biol. 2009;11:832–838. doi: 10.1038/ncb1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 13.Mao Y, Desai A, Cleveland DW. Microtubule capture by CENP-E silences BubR1-dependent mitotic checkpoint signaling. J Cell Biol. 2005;170:873–880. doi: 10.1083/jcb.200505040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howell BJ, et al. Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J Cell Biol. 2001;155:1159–1172. doi: 10.1083/jcb.200105093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan Y, et al. Inhibition of a mitotic motor protein: Where, how, and conformational consequences. J Mol Biol. 2004;335:547–554. doi: 10.1016/j.jmb.2003.10.074. [DOI] [PubMed] [Google Scholar]
- 16.Qian X, et al. Discovery of GSK923295, the first potent and selective inhibitor of centromere-associated protein E (CENP-E) ACS Med Chem Lett. doi: 10.1021/ml900018m. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weaver BA, Silk AD, Montagna C, Verdier-Pinard P, Cleveland DW. Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer Cell. 2007;11:25–36. doi: 10.1016/j.ccr.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Jackson JR, Patrick DR, Dar MM, Huang PS. Targeted anti-mitotic therapies: Can we improve on tubulin agents? Nat Rev Cancer. 2007;7:107–117. doi: 10.1038/nrc2049. [DOI] [PubMed] [Google Scholar]