Abstract
The spectrin membrane skeleton controls the disposition of selected membrane channels, receptors, and transporters. In the brain βIII spectrin binds directly to the excitatory amino acid transporter (EAAT4), the glutamate receptor delta, and other proteins. Mutations in βIII spectrin link strongly to human spinocerebellar ataxia type 5 (SCA5), correlating with alterations in EAAT4. We have explored the mechanistic basis of this phenotype by targeted gene disruption of Spnb3. Mice lacking intact βIII spectrin develop normally. By 6 months they display a mild nonprogressive ataxia. By 1 year most Spnb3−/− animals develop a myoclonic seizure disorder with significant reductions of EAAT4, EAAT1, GluRδ, IP3R, and NCAM140. Other synaptic proteins are normal. The cerebellum displays increased dark Purkinje cells (PC), a thin molecular layer, fewer synapses, a loss of dendritic spines, and a 2-fold expansion of the PC dendrite diameter. Membrane and expanded Golgi profiles fill the PC dendrite and soma, and both regions accumulate EAAT4. Correlating with the seizure disorder are enhanced hippocampal levels of neuropeptide Y and EAAT3 and increased calpain proteolysis of αII spectrin. It appears that βIII spectrin disruption impairs synaptogenesis by disturbing the intracellular pathways selectively regulating protein trafficking to the synapse. The mislocalization of these proteins secondarily disrupts glutamate transport dynamics, leading to seizures, neuronal damage, and compensatory changes in EAAT3 and neuropeptide Y.
Keywords: cytoskeleton, membrane, spinocerebellar ataxia type 5, excitatory amino acid transporter 4, Purkinje
The mammalian nervous system expresses seven spectrin genes, two encoding α-subunits and five encoding β-subunits. All are large multifunctional molecules, and all display distinctive cellular and subcellular distributions. Their role in neuronal cells remains largely conjectural. Although generally thought to organize the membrane surface or to bestow membrane stability, their diversity implies a function beyond simple stabilization. Studies in cultured cells reveal spectrin and ankyrin as scaffolds organizing internal organelles (1–6) or receptor clusters (7–10). These proteins also facilitate protein transport in the secretory (3, 10, 11) and endocytic pathways (12–14) (reviewed in ref. 15). Consonant with these roles are observations that genetic deletion or mutation of spectrins or ankyrins may cause missorting of unique subsets of adhesion molecules, receptors, and ion channels in brain or muscle, with consequential cardiovascular, neuromuscular, and neurodegenerative disease (16–18).
In the nervous system, cortical (19) and cerebellar granular cell neurons (7) are enriched in βI spectrin, especially at the postsynaptic density (PSD) and on a subset of vesicular organelles. Spectrin βII is associated with axonal processes (20), and βIV spectrin is concentrated at the nodes of Ranvier and along the initial axon segment (21). Less is known about βIII spectrin, although studies point to an interesting pathology associated with its mutation. First identified both as an organelle-associated spectrin (22) that facilitates microtubule-mediated organelle transport (23, 24) and as a binding partner for munc13 (25), we find that it is also PSD associated in a distribution complementary to βI spectrin.
Three identified human pedigrees link mutations in βIII spectrin to cerebellar ataxia type 5 (SCA5) (26). Two relevant βIII spectrin ligands have been identified. Mutant βIII spectrin enhances the extractability of an excitatory amino acid transporter (EAAT4) and a glutamate receptor (GluRδ) (27). The mobility of EAAT4 in the plasma membrane is also reduced by the expression of wild-type (WT) but not the mutant protein, confirming yeast-two-hybrid and biochemical analyses demonstrating a direct interaction of EAAT4 with βIII spectrin.
In the present study, we use targeted deletion by exon trapping of Spnb3 to explore the mechanistic role of βIII spectrin and the pathology of its loss. Although not required for grossly normal development, we find Spnb3−/− animals prone to a mild nonprogressive ataxia and stimulus-induced seizures. Synapse morphology is altered, accompanied by an abnormal accumulation of EAAT4 within the Purkinje cell (PC) dendrite and soma. The PC apical dendrite is thickened, and the soma is filled with dilated Golgi, and other membrane compartments are expanded. The synapse-associated proteins EAAT4, EAAT1, GluRδ, IP3R, and NCAM 140 are reduced. The pathology of βIII spectrin deficiency suggests a failure to assemble transporters, receptors, and adhesion molecules at the synapse. The coappearance of dark neurons with calcium-activated proteolysis and elevations in EAAT3 and neuropeptide Y in the hippocampus indicates a disruption of glutamate neurotransmitter dynamics. We conclude that βIII spectrin deficiency is a disorder of synaptogenesis due to impaired secretory/endocytic-pathway dysfunction.
Results
Targeted Disruption of the Spnb3 Abrogates βIII Spectrin Expression.
The full-length Spnb3 gene contains 37 exons; PCR 5′-RACE analysis identified βgeo between exons 25 and 26. Biallelic insertion of βgeo in the homozygous Spnb3−/− mouse was confirmed by Southern blotting (Fig. S1). The exon-trapped gene should generate mRNA that truncates the translation product at codon 1752, yielding a protein of ≈160 kDa terminating within spectrin repeat 14. RT-PCR analysis of the Spnb3−/− mice amplified no mRNA downstream of the exon trap, but mRNA was detected upstream of exon 25 (Fig. S1). Correspondingly, a low level (<1–3%, n = 3 relative to the WT protein) of truncated βIII spectrin was detected in Spnb3−/− mice by Western blot and immunofluorescence (Fig. S1 and Fig. 1). Whereas βIII spectrin in the normal brain was concentrated in the soma, dendrites, and spines of the cerebellar Purkinje cells, the truncated protein (detected only with N-terminal antibodies) was localized in punctate accumulations in the soma, in membrane-bounded aggregates along the proximal dendrite shaft, and along the initial axon segment (Fig. S1). Conversely, C-terminal directed antibodies detected no βIII spectrin in Spnb3−/− mice, confirming the complete loss of the native protein.
Fig. 1.
βIII and βI spectrin display complimentary distributions. (A) Western blots of WT and Spnb3−/− (ko) brain lysates with antibodies to either the C- or the N-terminal regions of βIII spectrin. Full-length βIII spectrin is absent in the ko animals; a small amount of truncated βIII spectrin (Mr ≈ 160 K) is detected. Separate evaluation of the insoluble fraction from the brain lysates detected no βIII spectrin or truncation product. (B) Immunofluorescence reveals a complimentary distribution of βIII and βI spectrin in PCs. βI spectrin is largely confined to the PC soma and is abundant in granule cells (GC). βIII spectrin is concentrated in the soma and dendrites of the molecular layer (ML). A similar complementary distribution is also present in the cortex (Fig. S3).
βIII and βI Spectrin Display Complementary Distributions.
βIII spectrin is broadly expressed in the mouse cerebellum, striatum, hippocampus, and neocortical gray matter in a pattern complementary to the distribution of βI spectrin (Fig. S2 and Fig. 1). Within neurons, βIII spectrin is in the cell body, dendrites, and PSD. In the cortex, βIII-rich dendrites extend from the innermost cortical layer 6 toward the periphery, concentrating in cortical layers 5, 3, and 1 (Fig. S2). βI spectrin is enriched in layers 2 and 4 (Fig. S3). In the hippocampus, βIII spectrin is most highly expressed in CA1 and CA3 pyramidal neurons (Fig. S2). In the cerebellum, βIII spectrin is most abundant in PCs, absent from stellate and basket cells, and weakly expressed in the granular layer (Figs. S1 and S2 and Fig. 1). In the PC, βIII spectrin extends throughout the apical dendrite and spines. The distribution and abundance of other spectrins were unchanged in the Spnb3−/− mice (Fig. 1 and Fig. S4).
βIII Spectrin Loss Induces Neuronal Degeneration.
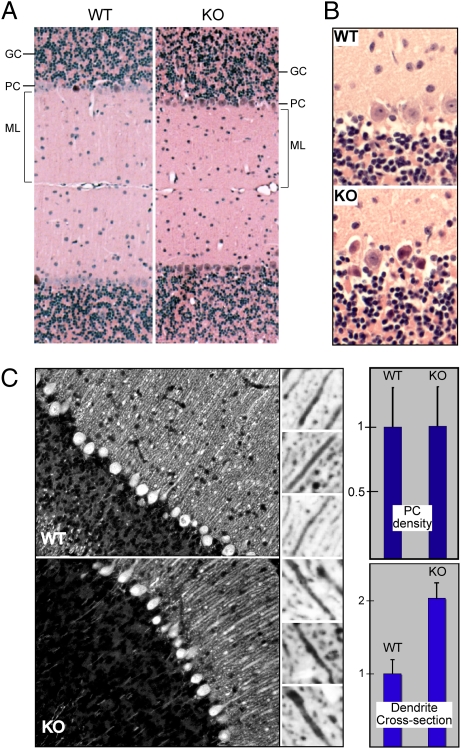
Although Spnb3−/− mice displayed grossly normal brains (Fig. S2), they harbored histological anomalies most apparent in the cerebellum. The molecular layer was 15–20% thinner, and many PCs were shrunken and dark with frequent cytoplasmic vacuoles (Fig. 2 A and B), changes characteristic of PCs (28, 29) after excitotoxic injury (30). However, PC density was unchanged relative to that in WT littermates, even in mice as old as 1.5 years [5.5 ± 0.6 vs. 5.4 ± 0.7/high-power field (±1 SD)] (Fig. 2C). Immune labeling for the endoplasmic reticulum (ER) protein calbindin-28 revealed thickened apical dendrites [2.0 ± 0.2 times normal (±1 SD)] densely packed with ER (Fig. 2C).
Fig. 2.
Purkinje cell degeneration in Spnb3−/− mice. (A) The molecular layer (ML) is ≈20% thinner in Spnb3−/− mice (KO). H&E stain is shown. (B) The loss of βIII spectrin (KO) leads to more frequent dark PCs. (C) Immunofluorescence of WT and Spnb3−/− cerebellum stained with anti-calbindin 28, an ER-resident protein. (Right) A reversed image from the molecular layer demonstrating examples of the thickened apical dendrites of the PCs. The relative abundance of PCs in cerebellum (Upper) and the relative thickness of the apical dendrites (Lower) are depicted graphically.
βIII Spectrin Loss Increases Organelle Density and Reduces PSD Density.
Ultrastructure examination revealed additional PC changes in Spnb3−/− mice. In cultured epithelial cells βIII spectrin assumes a patchy distribution on the plasma membrane and on organelles including the Golgi (22); its phosphorylation correlates with Golgi stability (5). A similar distribution was noted in the PC soma (Fig. 3A Center, arrows), where βIII spectrin concentrated in patches along the trans-Golgi network and on other organelles. The soma of Spnb3−/− PCs was also filled with swollen membrane profiles and many swollen Golgi complexes (circled) (Fig. 3A). In WT animals βIII spectrin concentrates at the PSD in PCs and on a subset of adjacent vesicular structures (Fig. 3B Center). The dendrites and postsynaptic bulbs of Spnb3−/− mice were filled with dilated ER (Fig. 3B, arrows). Four additional examples of these changes in the postsynaptic bulb are shown in Fig. 3C. The number of PSDs in the molecular layer, counted over many fields, was also reduced by 50% (P < 0.002).
Fig. 3.
Swollen Golgi and organelles in Spnb3−/− mice. (A) Normal PCs typically have multiple but indistrinct Golgi. Immuno-EM reveals a patchy concentration of βIII spectrin on the trans-Golgi network and on a subset of other vesicular and vesicular–tubular intermediates in normal cells (arrows). Spnb3−/− mice display massively dilated Golgi complexes (circled). (B) βIII spectrin is concentrated at the PSD of WT PCs (double arrowhead, Center), along with a patchy concentration on vesicular and tubular structures within the dendrite and spine. The loss of WT βIII spectrin distends the PC dendrite and spine with dilated ER or other organelles (arrows). (C) Additional examples of dilated ER within the PC spine and synaptic bulb of Spnb3−/− mice (KOs).
Disruption of βIII Spectrin Causes Defective Synaptogenesis.
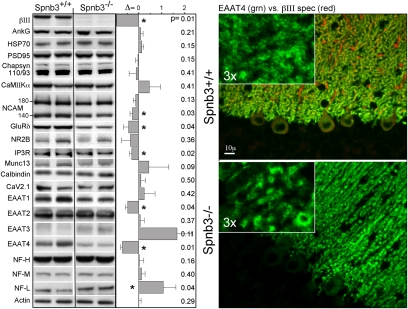
The impact of βIII spectrin depletion on 20 proteins associated with the synapse was evaluated. Six deviated significantly from controls. These proteins were EAAT4, EAAT1, NF-L, IP3R, GluRδ, and NCAM 140 (Fig. 4). All were diminished (30–70%) by βIII spectrin loss except NF-L, which more than doubled. EAAT3 also appeared to increase but this was more variable and the change did not achieve significance. Other core PSD proteins such as PSD95, CaMII kinase α, Chapsyn-110 (PSD93), and NR2B were not significantly altered. The selective loss of proteins associated with the PSD was confirmed by immunofluorescence; the results with EAAT4 are shown (Fig. 4). Both βIII spectrin and EAAT4 are normally concentrated in PC spines. In Spnb3−/− animals EAAT4 was diminished on the spines and accumulated in the distended dendrite shafts and in the cell soma.
Fig. 4.
Selective loss of synapse proteins in Spnb3−/− mice. (Left) The abundance of synapse-associated and other proteins evaluated by Western blot with antibodies to the listed proteins. The abundance (% change/100) relative to WT, normalized to actin, is depicted as Δ. Changes with P < 0.05 are marked (*). (Right) Double immunofluorescent micrograph of cerebellum: red, βIII spectrin; green, EAAT4. EAAT4 distribution is aberrant in the Spnb3−/− mice, retracting from the spines and filling the dendrite shaft.
Mice Lacking βIII Spectrin Are Ataxic and Seizure Prone.
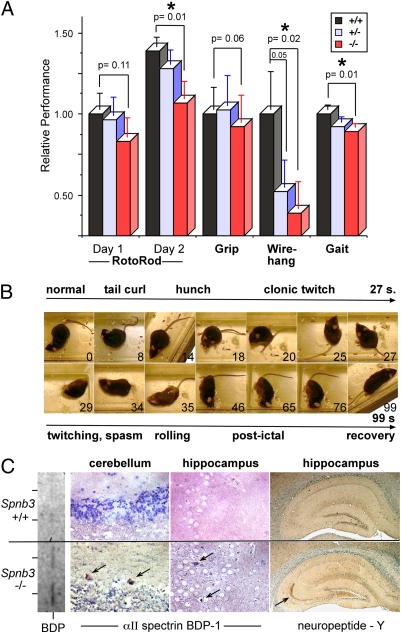
A battery of cognitive and motor tasks evaluated the Spnb3−/− animals at ages up to 2.0 years (Fig. 5). By 8 months of age, Spnb3−/− mice displayed a mild ataxia with gait disturbance and muscle weakness, but had no changes in fecundity, fur appearance, or viability. Interestingly, there was a tendency to slight motor impairment in the heterozygous animals; this reached significance (P = 0.05) in the wire-hang test, suggesting either a mild degree of haplo-insufficiency or a mild dominant-negative effect due to the residual truncated βIII spectrin in these animals. The average body weight of male Spnb3−/− mice was slightly larger (36.2 vs. 32.7 g, P = 0.04), but a similar cohort of female mice showed no difference.
Fig. 5.
Spnb3−/− mice develop ataxia and seizures. (A) Comparative performance on tasks of motor strength and coordination. The Spnb3−/− animals perform poorly in three tests. The wire-hang results of the heterozygous Spnb3+/− animals also suggest a weak dominant-negative or haplo-insufficient effect. (B) Video images of a Spnb3−/− mouse experiencing a myoclonic seizure. (C) Distribution of NPY and αII spectrin BDP-1. Both proteins, markers of seizure activity, increase in the Spnb3−/− animals. The increased BDP-1 is also apparent on the Western blot (rotated vertically, results from two WT and two Spnb3−/− animals are shown).
Spnb3−/− mice displayed no gross changes in spontaneous activity or cage behavior. However, beginning at ∼3 months and increasing in frequency as the animals aged, they experienced myoclonic seizures induced by even gentle handling or disturbance. Consecutive video frames (Fig. 5B) and Movie S1 demonstrate the seizures, characterized by tonic hindlimb extension, bilateral forelimb clonus, facial movements, chewing, head nodding, body jerks, tail curling, and running or bouncing. Seizures lasted 20–50 s, after which animals usually returned to normal activity.
The hippocampus displayed histological evidence of seizure activity, including neuropeptide Y (NPY) expression and the accumulation of αII spectrin breakdown products (BDPs) generated by calpain activation (Fig. 5C). NPY rises in the chronically epileptic human brain (31), a compensatory response reducing excitability. NPY is expressed ectopically in dentate granule cells after stimulation and in mossy fibers in hippocampus slices from kindled rats (32). Similarly, Spnb3−/− mice expressed NPY in unmyelinated axons projecting along mossy fiber pathways to the CA3 region (Fig. 5C).
A second measure of seizure activity and excitotoxicity is the accumulation of spectrin BDPs generated by the action of calpain (33). Excessive neurotransmitter activity activates calcium-dependent proteases. In the nervous system, αII spectrin is a major target of calpain; its cleavage is a sensitive measure of neuronal remodeling or neurodegeneration or neurotoxicity and is an early event in the generation of dark Purkinje cells (34). In normal littermates, no αII spectrin BDPs can be detected by either Western blot or immunohistology (Fig. 5C), even using highly sensitive antibodies to the unique epitopes generated by the cleavage process (33). Conversely, in the Spnb3−/− animals, BDPs of αII spectrin were detected by immunohistochemistry and Western blot in both PCs and hippocampus (Fig. 5C).
Discussion
The data presented reveal the native distribution of βIII spectrin within the rodent brain and establish an animal model of βIII spectrin loss that mirrors the pathology of human SCA5 as well as an unexpected seizure disorder. Given that spectrin and ankyrin contribute to intracellular protein transport and membrane biogenesis (15, 35), the pathology of βIII spectrin deficiency appears to share a similar mechanistic basis. These results extend an emerging disease paradigm in which defects in the spectrin–ankyrin skeleton present not as failures of membrane stability, but as defects in membrane protein sorting, with a resultant loss of physiological function.
The selective reduction of just a subset of synapse-associated proteins (EAAT4, EAAT1, NCAM, GluRδ, and IP3R) suggests that these proteins depend either directly or indirectly on βIII spectrin for their efficient sorting, transport, assembly, and/or retention at the PSD. Some interact directly with spectrin (EAAT4, GluRδ, NCAM) (27, 36); IP3R interacts indirectly by binding ankyrin (37). It is unknown whether EAAT1 binds spectrin directly or indirectly. Regardless, even indirect spectrin interactions can control membrane protein display, as with the loss of CD3 when CD45 is blocked from its spectrin interaction in T lymphocytes (10). Remarkably, these studies demonstrate that genetic or experimental defects in spectrin and ankyrin can produce effects on cell physiology that are comparable to knockouts of the transporters or receptors themselves.
The precise site of blockade by spectrin–ankyrin disruption may vary; in cultured cells, α-Na,K-ATPase is blocked before reaching the medial Golgi (3, 11), whereas CD45 delivery to the plasma membrane is impaired at a post-Golgi step (10). In other work, the disruption of ankyrin impairs LDL receptor dynamics along the endocytic pathway (14), and coimmunoprecipitation from brain extracts reveals that spectrin also forms a macromolecular complex with the protein machinery (clathrin, dynamin, and annexin VI) crucial to receptor-mediated endocytosis (12–14). Whereas the precise site at which EAAT4 and other synaptic proteins are blocked from assembly at the synapse in Spnb3−/− mice remains uncertain, the proliferation of Golgi and the presence of dilated ER within affected neurons suggest that the loss (or inhibition) of βIII spectrin function blocks EAAT4 transport early in the secretory pathway, probably in the intermediate compartment or Golgi. It is also interesting to speculate that the thinning of the molecular layer may be indicative of a more generalized defect in the endomembrane system.
A potentially complicating factor is the persistence of truncated βIII spectrin peptide. This peptide, albeit in low abundance, may partially function to rescue mice from a more lethal total loss of protein or conversely act as a dominant-negative inhibitor of βIII spectrin, akin to the dominant-negative effect of N-terminal peptides of βI spectrin (3, 10, 36). We favor the latter possibility, as such activity would be consistent with the phenotype of patients with SCA5 who are heterozygous for mutations in the N-terminal region of βIII spectrin (26). The phenotype observed in wire hang by the heterozygous (Spnb3+/−) mice also suggests that these animals experience either haplo-insufficiency or a mild dominant-negative effect. We cannot presently distinguish these possibilities. Nevertheless, the results reveal the pathophysiology of a disruption of βIII spectrin. The aberrant localization of the truncated protein also suggests that a strong localization signal for βIII spectrin resides in its C-terminal domain, analogous to the strong Golgi and membrane-targeting signal in the comparable region of βI spectrin (4, 36).
Finally, we consider whether the presence of truncated βIII protein or the accumulation of EAAT4 (or other PSD proteins) in the Golgi/ER might be inducing an unfolded protein response (UPR). This possibility is unlikely given (i) the paucity of truncated protein, (ii) the absence of calnexin increase by Western blot (a UPR response protein), (iii) the absence of HSP70 increase, (iv) the lack of apoptosis, (v) the invariant PC density, (vi) the lack of gliosis, as measured by GFAP staining, and (vii) normal levels of other membrane proteins.
The mild degree of ataxia is surprising, given the morphologic changes and the severity of human SCA5. Equally surprising are the myoclonic seizures. Seizure activity appears to be a consequence of deregulated glutamate neurotransmitter dynamics that follow the alterations in EAAT4 and GluRδ. The association of ataxia with seizure phenotypes has been noted. Four mouse lines with moderate to severe ataxia (ducky, lethargic, stargazer, and tottering) all exhibit 5- to 7-Hz spike wave bursts (31). EAAT4 is preferentially involved in clearing glutamate from climbing fiber synapses, preventing neurotransmitter spillage to neighboring synapses (38). Because a major determinant of extracellular glutamate levels is glutamate uptake at excitatory synaptic clefts, interest in the role of EAATs and their regulators in human epilepsy is growing (39), and changes in glutamate transporter expression characterize some animal models of epilepsy (39–41). The data presented here extend these findings and demonstrate that disruption of βIII spectrin also can cause a seizure disorder.
In future work, it will be of interest to determine the electrophysiological correlates of βIII spectrin loss, the electrophysiological basis of the seizures, and whether there are environmental factors that can precipitate more severe cerebellar degeneration and ataxia, akin to the human condition.
Materials and Methods
Generation of Exon-Trapped Spnb3 Animals.
In silico analysis identified ES cell line XK422 (Bay Genomics) that contained the gene-trap vector (βgeo) within the Spnb3 gene (Fig. S1). XK422 cells heterozygous for the insertion were microinjected into C57BL/6J blastocysts. Male chimeras were mated with C57BL/6J females to establish germ-line transmission. Subsequent matings established the trapped Spnb3 allele on a mixed 129/C57BL/6J background (as tested here). The congenic strain on B6 is currently at N10.
Genotype and Expression Analysis.
Genomic samples were digested with BsrDI and analyzed by 1% Southern blots. The 514-bp probe between exons 25 and 26 was generated by PCR: forward primer 5′-CAGAGGACAACGTCTAAGCGGTCA-3′ and reverse primer 5′-ACTGAGTCTGGACTTAAGGGTGGAAG-3′ (Fig. S1). Probes within βgeo detected the trap (forward 5′-CAAATGGCGATTACCGTTGA-3′; reverse 5′-GACAGTATCGGCCTCAGGAAGATCG-3′). WT allele detection used PCR1 (forward 5′-GACCTGCTGGAGCTGCTGG-3′; reverse 5′-CCACAGCATCAACTCTCGGAC-3′). Primers hybridizing to sequences upstream (753-bp amplimer) or downstream (255-bp amplimer) of βgeo allowed detection of truncated mRNA transcripts.
Immunostaining.
Perfusion-fixed brains were postfixed 24 h in 4% paraformaldehyde, paraffin embedded, and immunostained (42). Antibodies used were goat anti-βIII spectrin N-term (Santa Cruz), rabbit anti-βIII spectrin C-term (Santa Cruz), rabbit anti-EAAT4 (Alpha Diagnostics), anti-calbindin (Chemicon), anti-neuropeptide Y (Peninsula Laboratories), and anti-αII BDP1 (33).
Protein Expression Levels.
Homogenized brains [Dounce, eight strokes, 5 mL 20 mM Hepes (pH 7.4), 120 mM NaCl, 25 mM KCl, 2 mM EDTA, 1 mM EGTA, 1% TX-100, 1:200 Protease Arrest; Calbiochem] were sedimented at 21,000 × g, for 10 min at 4 °C. Supernatant and pellets were analyzed by SDS/PAGE and Western blots quantified using ImageJ (National Institutes of Health). Antibodies were as before (22, 33, 43), with the addition of anti-IP3R (Upstate Biotech), anti-GluRδ (Chemicon), anti-NR2B (ABR), anti-CaV2.1 (Alomone Labs), anti-CaMKIIα and anti-HSP70 (StressGen), anti-EAAC1/EAAT3 (gift from J. Rothstein, Johns Hopkins University), anti-EAAT1 and EAAT2 (Novacastra), anti-Munc13 (BD Transduction), and anti-NCAM (Developmental Studies Hybridoma Bank).
Electron Microscopy.
Sections were postfixed 12 h at 4 °C in 1% glutaraldehyde, 2% formaldehyde, 0.1 M Na cacodylate, pH 7.4; permeated 2 h in 1% OsO4, 0.1 M S-collidine, pH 7.4; dehydrated in ethanol; and embedded in Epox-812. Sections (80 nm) were stained with 2% uranyl acetate and lead citrate.
Behavioral Analysis.
At least six males and six females of wild-type and knockout animals were evaluated for gait, wire hang, grip, and rotarod. Results were analyzed for significance by ANOVA.
Supplementary Material
Acknowledgments
Ms. Jung Hwang is thanked for her assistance with the thin sections. This work was supported by National Institutes of Health Grants R01-HL28560 and R01-DK43812 (to J.S.M.) and R01-HL088468 (to L.L.P.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/1001522107/DCSupplemental.
References
- 1.Fath KR, Trimbur GM, Burgess DR. Molecular motors and a spectrin matrix associate with Golgi membranes in vitro. J Cell Biol. 1997;139:1169–1181. doi: 10.1083/jcb.139.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck KA, Buchanan JA, Malhotra V, Nelson WJ. Golgi spectrin: Identification of an erythroid beta-spectrin homolog associated with the Golgi complex. J Cell Biol. 1994;127:707–723. doi: 10.1083/jcb.127.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devarajan P, Stabach PR, De Matteis MA, Morrow JS. Na,K-ATPase transport from endoplasmic reticulum to Golgi requires the Golgi spectrin-ankyrin G119 skeleton in Madin Darby canine kidney cells. Proc Natl Acad Sci USA. 1997;94:10711–10716. doi: 10.1073/pnas.94.20.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Godi A, et al. ADP ribosylation factor regulates spectrin binding to the Golgi complex. Proc Natl Acad Sci USA. 1998;95:8607–8612. doi: 10.1073/pnas.95.15.8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddhanta A, Radulescu A, Stankewich MC, Morrow JS, Shields D. Fragmentation of the Golgi apparatus. A role for beta III spectrin and synthesis of phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2003;278:1957–1965. doi: 10.1074/jbc.M209137200. [DOI] [PubMed] [Google Scholar]
- 6.Mohler PJ, Yoon W, Bennett V. Ankyrin-B targets beta2-spectrin to an intracellular compartment in neonatal cardiomyocytes. J Biol Chem. 2004;279:40185–40193. doi: 10.1074/jbc.M406018200. [DOI] [PubMed] [Google Scholar]
- 7.Malchiodi-Albedi F, Ceccarini M, Winkelmann JC, Morrow JS, Petrucci TC. The 270 kDa splice variant of erythrocyte beta-spectrin (beta I sigma 2) segregates in vivo and in vitro to specific domains of cerebellar neurons. J Cell Sci. 1993;106:67–78. doi: 10.1242/jcs.106.1.67. [DOI] [PubMed] [Google Scholar]
- 8.Bloch RJ, Morrow JS. An unusual β-spectrin associated with clustered acetylcholine receptors. J Cell Biol. 1989;108:481–493. doi: 10.1083/jcb.108.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lencesova L, O'Neill A, Resneck WG, Bloch RJ, Blaustein MP. Plasma membrane-cytoskeleton-endoplasmic reticulum complexes in neurons and astrocytes. J Biol Chem. 2004;279:2885–2893. doi: 10.1074/jbc.M310365200. [DOI] [PubMed] [Google Scholar]
- 10.Pradhan D, Morrow J. The spectrin-ankyrin skeleton controls CD45 surface display and interleukin-2 production. Immunity. 2002;17:303–315. doi: 10.1016/s1074-7613(02)00396-5. [DOI] [PubMed] [Google Scholar]
- 11.Stabach PR, Devarajan P, Stankewich MC, Bannykh S, Morrow JS. Ankyrin facilitates intracellular trafficking of alpha1-Na+-K+-ATPase in polarized cells. Am J Physiol Cell Physiol. 2008;295:C1202–C1214. doi: 10.1152/ajpcell.00273.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson M, et al. Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor betalll spectrin. J Cell Biol. 2007;176:459–471. doi: 10.1083/jcb.200606077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips MD, Thomas GH. Brush border spectrin is required for early endosome recycling in Drosophila. J Cell Sci. 2006;119:1361–1370. doi: 10.1242/jcs.02839. [DOI] [PubMed] [Google Scholar]
- 14.Michaely P, Kamal A, Anderson RG, Bennett V. A requirement for ankyrin binding to clathrin during coated pit budding. J Biol Chem. 1999;274:35908–35913. doi: 10.1074/jbc.274.50.35908. [DOI] [PubMed] [Google Scholar]
- 15.De Matteis MA, Morrow JS. Spectrin tethers and mesh in the biosynthetic pathway. J Cell Sci. 2000;113:2331–2343. doi: 10.1242/jcs.113.13.2331. [DOI] [PubMed] [Google Scholar]
- 16.Mohler PJ, et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421:634–639. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 17.Lowe JS, et al. Voltage-gated Nav channel targeting in the heart requires an ankyrin-G dependent cellular pathway. J Cell Biol. 2008;180:173–186. doi: 10.1083/jcb.200710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirahata E, et al. Ankyrin-G regulates inactivation gating of the neuronal sodium channel, Nav1.6. J Neurophysiol. 2006;96:1347–1357. doi: 10.1152/jn.01264.2005. [DOI] [PubMed] [Google Scholar]
- 19.Riederer BM, Zagon IS, Goodman SR. Brain spectrin(240/235) and brain spectrin(240/235E): Two distinct spectrin subtypes with different locations within mammalian neural cells. J Cell Biol. 1986;102:2088–2097. doi: 10.1083/jcb.102.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine J, Willard M. Fodrin: Axonally transported polypeptides associated with the internal periphery of many cells. J Cell Biol. 1981;90:631–642. doi: 10.1083/jcb.90.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berghs S, et al. betaIV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. J Cell Biol. 2000;151:985–1002. doi: 10.1083/jcb.151.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stankewich MC, et al. A widely expressed betaIII spectrin associated with Golgi and cytoplasmic vesicles. Proc Natl Acad Sci USA. 1998;95:14158–14163. doi: 10.1073/pnas.95.24.14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holleran EA, et al. beta III spectrin binds to the Arp1 subunit of dynactin. J Biol Chem. 2001;276:36598–36605. doi: 10.1074/jbc.M104838200. [DOI] [PubMed] [Google Scholar]
- 24.Muresan V, et al. Dynactin-dependent, dynein-driven vesicle transport in the absence of membrane proteins: A role for spectrin and acidic phospholipids. Mol Cell. 2001;7:173–183. doi: 10.1016/s1097-2765(01)00165-4. [DOI] [PubMed] [Google Scholar]
- 25.Sakaguchi G, et al. A novel brain-specific isoform of beta spectrin: Isolation and its interaction with Munc13. Biochem Biophys Res Commun. 1998;248:846–851. doi: 10.1006/bbrc.1998.9067. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda Y, et al. Spectrin mutations cause spinocerebellar ataxia type 5. Nat Genet. 2006;38:184–190. doi: 10.1038/ng1728. [DOI] [PubMed] [Google Scholar]
- 27.Jackson M, et al. Modulation of the neuronal glutamate transporter EAAT4 by two interacting proteins. Nature. 2001;410:89–93. doi: 10.1038/35065091. [DOI] [PubMed] [Google Scholar]
- 28.Loebel F, Dresel AM. [Research on “light” and “dark” Purkinje cells. II] Biologica (Santiago) 1961;32:18–32. [PubMed] [Google Scholar]
- 29.Tewari HB, Bourne GH. Histochemical studies on the “dark” and “light” cells of the cerebellum of rat. Acta Neuropathol. 1963;3:1–15. doi: 10.1007/BF00684015. [DOI] [PubMed] [Google Scholar]
- 30.Barenberg P, Strahlendorf H, Strahlendorf J. Hypoxia induces an excitotoxic-type of dark cell degeneration in cerebellar Purkinje neurons. Neurosci Res. 2001;40:245–254. doi: 10.1016/s0168-0102(01)00234-6. [DOI] [PubMed] [Google Scholar]
- 31.Colmers WF, El Bahh B. Neuropeptide Y and epilepsy. Epilepsy Curr. 2003;3:53–58. doi: 10.1046/j.1535-7597.2003.03208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rizzi M, Monno A, Samanin R, Sperk G, Vezzani A. Electrical kindling of the hippocampus is associated with functional activation of neuropeptide Y-containing neurons. Eur J Neurosci. 1993;5:1534–1538. doi: 10.1111/j.1460-9568.1993.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 33.Glantz SB, et al. Sequential degradation of alphaII and betaII spectrin by calpain in glutamate or maitotoxin-stimulated cells. Biochemistry. 2007;46:502–513. doi: 10.1021/bi061504y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mansouri B, et al. Involvement of calpain in AMPA-induced toxicity to rat cerebellar Purkinje neurons. Eur J Pharmacol. 2007;557:106–114. doi: 10.1016/j.ejphar.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 35.Bennett V, Healy J. Organizing the fluid membrane bilayer: Diseases linked to spectrin and ankyrin. Trends Mol Med. 2008;14:28–36. doi: 10.1016/j.molmed.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Leshchyns'ka I, Sytnyk V, Morrow JS, Schachner M. Neural cell adhesion molecule (NCAM) association with PKCbeta2 via betaI spectrin is implicated in NCAM-mediated neurite outgrowth. J Cell Biol. 2003;161:625–639. doi: 10.1083/jcb.200303020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bourguignon LY, Jin H. Identification of the ankyrin-binding domain of the mouse T-lymphoma cell inositol 1,4,5-trisphosphate (IP3) receptor and its role in the regulation of IP3-mediated internal Ca2+ release. J Biol Chem. 1995;270:7257–7260. doi: 10.1074/jbc.270.13.7257. [DOI] [PubMed] [Google Scholar]
- 38.Mim C, Balani P, Rauen T, Grewer C. The glutamate transporter subtypes EAAT4 and EAATs 1-3 transport glutamate with dramatically different kinetics and voltage dependence but share a common uptake mechanism. J Gen Physiol. 2005;126:571–589. doi: 10.1085/jgp.200509365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rakhade SN, Loeb JA. Focal reduction of neuronal glutamate transporters in human neocortical epilepsy. Epilepsia. 2008;49:226–236. doi: 10.1111/j.1528-1167.2007.01310.x. [DOI] [PubMed] [Google Scholar]
- 40.Rothstein JD, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 41.Crino PB, et al. Increased expression of the neuronal glutamate transporter (EAAT3/EAAC1) in hippocampal and neocortical epilepsy. Epilepsia. 2002;43:211–218. doi: 10.1046/j.1528-1157.2002.35001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stankewich MC, Stabach PR, Morrow JS. Human Sec31B: A family of new mammalian orthologues of yeast Sec31p that associate with the COPII coat. J Cell Sci. 2006;119:958–969. doi: 10.1242/jcs.02751. [DOI] [PubMed] [Google Scholar]
- 43.Huh GY, Glantz SB, Je S, Morrow JS, Kim JH. Calpain proteolysis of alpha II-spectrin in the normal adult human brain. Neurosci Lett. 2001;316:41–44. doi: 10.1016/s0304-3940(01)02371-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.