Abstract
It has been argued that increases in predation over geological time should result in increases in defensive adaptations in prey taxa. Recent in situ and laboratory observations indicate that cidaroid sea urchins feed on live stalked crinoids, leaving distinct bite marks on their skeletal elements. Similar bite marks on fossil crinoids from Poland strongly suggest that these animals have been subject to echinoid predation since the Triassic. Following their near-demise during the end-Permian extinction, crinoids underwent a major evolutionary radiation during the Middle–Late Triassic that produced distinct morphological and behavioral novelties, particularly motile taxa that contrasted strongly with the predominantly sessile Paleozoic crinoid faunas. We suggest that the appearance and subsequent evolutionary success of motile crinoids were related to benthic predation by post-Paleozoic echinoids with their stronger and more active feeding apparatus and that, in the case of crinoids, the predation-driven Mesozoic marine revolution started earlier than in other groups, perhaps soon after the end-Permian extinction.
Keywords: macroecology, macroevolution, predation, escalation, cidaroids
Predator–prey interactions may represent a significant driving force of evolutionary change (1–4), but predation and its consequences are often difficult to assess in Recent communities and even more so in the fossil record. Data on fossil and extant crinoids, commonly known as sea lilies and feather stars (Echinodermata), indicate that they suffer from predation by fishes, and numerous evolutionary trends have been ascribed to such interactions (5–15). Among these are (i) crawling and swimming abilities in comatulids (6), (ii) choice of semicryptic habits and nocturnal–diurnal behavior among comatulids (6), (iii) increasing plate thickness and spinosity among Paleozoic crinoids (9), (iv) offshore displacement of late Mesozoic/Cenozoic stalked crinoids (11), and (v) origin of autotomy (shedding) planes in the stalk and arms (13). Some of these trends have served as examples of dramatic change in marine ecosystems, such as the Mesozoic marine revolution (MMR) (2, 16) and the middle-Paleozoic marine revolution (9).
Although predation by fish has received the most attention, cri-noids may be the prey of other organisms, most notably benthic invertebrates. Until recently, few data hinted at the importance of benthic predators to crinoids, including a swimming response in a comatulid when perturbed by the predatory sea star Pycnopodia helianthoides (17), the presence of crinoid pinnulars in the gut of the goniasterid Plinthaster dentatus (18), and a crinoid arm observed in the claw of the crab Oregonia gracilis (17). Recently, submersible studies of stalked crinoids belonging to the Isocrinidae have revealed that they are prey to cidaroids, or pencil urchins. Evidence for this interaction includes (i) in situ observations of cidaroids among large aggregations of motile isocrinid sea lilies (Neocrinus decorus and Endoxocrinus parrae), (ii) proximity of cidaroids to several upended isocrinids, (iii) a cidaroid perched over the stalk of an upended crinoid and (iv) another on top of disarticulated crinoid remains, and (v) gut contents of cidaroids consisting of as much as 70% crinoid skeletal material (19).
To further explore the interaction between extant cidaroids and crinoids, to test for evidence of the interaction in the geologic past, and to identify its evolutionary consequences, we conducted aquarium experiments, analyzed samples of Triassic fossil crinoids, and examined the evolutionary history of crinoids and echinoids.
Results
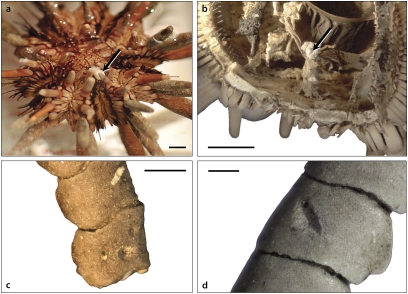
Live individuals of the commercially available cidaroid, Eucidaris sp., were placed together in aquaria with live specimens and detached arms of the shallow-water comatulid crinoid, Lamprometra palmata, as well as with isocrinid crinoid arm and stalk segments. Although cidaroids do not specialize on crinoids, every echinoid that was presented with crinoid material consumed all or part of it, and in one instance, a live L. palmata was eaten entirely (Fig. 1A and Movies S1). Ingested crinoid body parts consisting of multiple skeletal elements occasionally passed through the sea urchin gut and were excreted without disarticulating, remaining as multiple-element units bound together by soft tissue. Some crinoid elements that passed through the gut were marked by scratches and circular to oval pits with dimensions matching those of the teeth of the urchin's Aristotle's lantern chewing apparatus (Fig. 1 C and D). In one dissected cidaroid, a multiple-element segment of a crinoid's arm marked by such scratches and pits was recovered from the very distal part of the hindgut (Fig. 1B).
Fig. 1.
In-aquaria observations of the cidaroid, Eucidaris sp., consuming and processing crinoid skeletal elements. (A) Oral side of Eucidaris sp.; arrow indicates a stalk segment of the isocrinid, N. decorus, held in the Aristotle lantern. (B) A dissected section of Eucidaris sp., aboral side down; arrow indicates a multiple-element piece of a comatulid arm at the periproct, in the distal-most part of the hindgut. (C) Multiple-element comatulid arm segment that had passed through the gut of Eucidaris sp. (D) Multiple-element comatulid arm segment that had passed through the gut of Eucidaris sp. (Scale bars: 1 mm.)
The aquaria observations document that crinoids could represent sea urchin prey and demonstrate that echinoids leave characteristic scratches and pits that might be preservable in the sedimentary record. Indeed, an analysis of sediment samples collected at sites where crinoid–cidaroid interactions have been observed via submersible (19) reveal a significant proportion of crinoid elements with such pits and scratches. Furthermore, and as indicated here, an example of such markings on Jurassic crinoids has been recently described (20).
To determine whether cidaroids interacted with crinoids during the Triassic, we looked for evidence of such bite marks on more than 2,500 fragments of crinoid stalks collected from five middle Triassic localities in Poland (Fig. S1). More than 500 columnals (approximately 20%) had characteristic scratches and pits: relatively shallow, clean cuts in the stereom of the ossicle surface, 0.1 to 1.5 mm wide and as long as 2.5 mm (Fig. 2). Pits are usually oval to circular and perpendicular to the ossicle surface. These marks occur chiefly on the exposed lateral ossicle surfaces rather than on the articular faces, suggesting that they were made while skeletal elements were still articulated. This is also consistent with numerous examples of such marks on well preserved pluricolumnals—isolated stalk segments consisting of more than one skeletal ossicle. Given the rapid rates of postmortem disarticulation of crinoids in natural settings (21, 22), these data suggest that the crinoid material was fresh and most probably living when the marks were made, and we treat it as evidence of predation rather than scavenging.
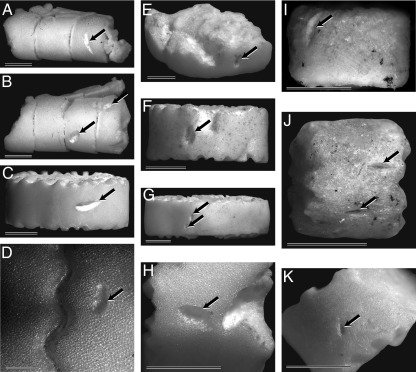
Fig. 2.
Examples of bite marks (identified by arrows) on crinoid skeletal elements. Recent examples of crinoid brachials (A and B) and columnals (C and D) extracted from the gut of extant cidaroids, Calocidaris micans and Histocidaris nuttingi (19). (E–H) Recent columnals extracted from sediment samples collected by submersible at the site where crinoids and cidaroids were observed to interact (19). (I–K) Middle Triassic dadocrinid columnals collected in the Holy Cross Mountains, Central Poland. (Scale bars: 1 mm.)
Although we cannot identify precisely which predators were responsible for all of the Triassic bite marks in our samples, many are similar to those we have retrieved from the guts and feces of extant cidaroids and from the sediment samples collected where cidaroid–crinoid interactions have been observed. As cidaroid spines and test fragments also occur with damaged crinoid elements at some of the Triassic localities, we conclude that crinoids were preyed upon by cidaroid echinoids that originated and diversified at this time (23, 24).
Discussion
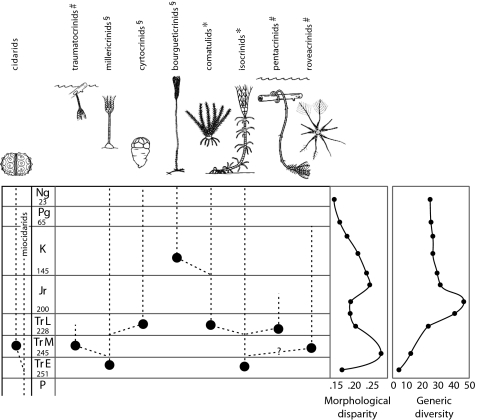
The Triassic is considered a crucial phase of post-Paleozoic echinoderm evolution. Following the end-Permian event, which led to their near extinction, both crinoids and echinoids rebounded rapidly in the Middle and Late Triassic. This radiation led to a substantial and well known ecological diversification for both groups (25–32). Among echinoids, the post-Paleozoic radiation resulted in dramatic changes in the lantern and the teeth, with the cidaroids evolving a stronger and more active jaw apparatus (27, 28). Among crinoids, in addition to a rapid expansion through morphospace (33), major morphological and behavioral innovations led to the development of both active and pas-sive motility (34, 35), a trait not found among Paleozoic crinoids and one that represents an effective escape strategy from benthic predators (Fig. 3). Taxa with a motile life habit included the Middle Triassic planktonic microcrinoids (roveacrinids) (31), pseudoplanktonic stalk-ed crinoids (traumatocrinids) (34), and benthic isocrinids we now know were capable of rapid crawling (35). The primitive free-moving crinoids (paracomatulids) and pseudoplanktonic pentacrinitids also likely originated in the early Late Triassic (37, 31). Similarly, although echinoid diversity apparently remained low through the Early Triassic, early cidaroids were widespread in carbonate ramp settings throughout low paleolatitude areas during the entire Triassic (32). We argue that the evolution of increased motility among crinoids was stimulated by their interactions with benthic predators, as suggested by our data on echinoid–crinoid interactions.
Fig. 3.
Ecological, morphological (33), and generic (19, 42) diversification history of crinoids and evolutionary history of relevant echinoids during geologic periods of the post-Paleozoic. Note that ecology and morphology peak in the Triassic (Tr E, Early Triassic; Tr M, Middle Triassic; Tr L, Late Triassic), whereas generic diversity peaks in the Jurassic. Crinoid coding: §Sessile as adults; *Capable of benthic locomotion; #Nektonic, planktonic, or pseudoplanktonic. Inferred relationships modified after published reports (27, 28, 31, 36).
The MMR represented a time of fundamental change in benthic marine communities (including trends toward diversification of infauna, increased shell strength, and environmental restriction) inferred to have been caused by intensified durophagous and grazing predation (1, 2). Although considerable debate and uncertainty surrounds its onset (38, 39), the MMR is generally thought to have begun in the Jurassic and continued to accelerate in the Cretaceous (1, 2). Our data suggest that, for crinoids at least, the predation-driven MMR started in the Triassic. We argue that the major evolutionary changes characterizing many crinoid groups in the Triassic were responses to interactions with predators such as cidaroids that have generally not been considered as major players in the MMR. Of course, even with the development of motility, crinoids did not become immune from predation later in the Mesozoic. Crinoid elements from post-Triassic localities also show signs of predation (20), some characteristic of cidaroids. Durophagous fish diversified in the later Mesozoic, accompanied by the appearance of new isocrinids with arm branching patterns well suited for reducing fish-predation damage (13, 40). Moreover, the well documented pattern of offshore displacement of stalked crinoids (11) indicates that fish might have exerted the predation pressure at this time (41). Compared with the Triassic, however, evolutionary changes in later Mesozoic crinoids were relatively minor, involving fine-tuning that predominantly led to changes in dominance (e.g., free-moving comatulids over sessile crinoids) and environmental displacement (depth restriction of stalked crinoids).
Materials and Methods
Questions pertaining to the prevalence of crinoid-echinoid interactions and the taphonomic signatures of ingested and excreted crinoid ossicles were analyzed experimentally in well established mixed-reef aquaria at Brigham Young University–Idaho. Synthetic seawater (Instant Ocean) was maintained at a relatively constant temperature of 26 °C (±0.25 °C), salinity (specific gravity, 1.025), and pH (8.2). Live comatulid crinoids (L. palmata) and cidaroid echinoids (Eucidaris sp.) were purchased from Blue Zoo Aquatics and drip-acclimated to tank conditions for 3 h. The crinoids and echinoids were initially kept in separate tanks (125 and 65 gallons, respectively). Crinoids were not initially introduced to the Eucidaris-bearing tank to give the echinoids an opportunity to establish a crinoid-free diet, which consisted mostly of red and purple coralline algae that they grazed from mature live rock.
With the purpose of observing potential echinoid–crinoid interactions, two general experiment types were performed with these specimens: (i) live crinoids were transferred to the tank containing Eucidaris, and (ii) one or two Eucidaris were transferred to a 10-gallon tank and therein confined within a plastic mesh cage that included (i) a live Lamprometra, (ii) arms and cirri naturally autotomized or mechanically severed from live Lamprometra, or (iii) frozen isocrinid (Neocrinus decorus) stalk and arm elements collected from Jamaica via submersible. Echinoids and crinoids (or crinoid parts) were transferred to a glass-bottomed 10-gallon tank, free of substrate, to (i) increase the likelihood of echinoid–crinoid encounters, (ii) facilitate photography and video capture of the interactions, and (iii) recover bitten and excreted crinoid material. Experiments were monitored first-hand and with the aid of a time-lapse video camera (using 3–7-s intervals) from approximately 3 to 14 h. Echinoids that consumed significant amounts of crinoid material were allowed to pass some of the consumed ossicles before being preserved in alcohol. This step permitted the recovery and study of crinoid ossicles as they passed through various stages of processing by the echinoids. It also permitted direct observation, via surgical dissection, of recently consumed crinoid material in the echinoids’ digestive system.
Data on fossils were obtained from field-collected samples and museum collections (Fig. S1). The collections are housed at the Faculty of Earth Sciences, University of Silesia, Sosnowiec, Poland [catalog numbers: Geological Institute of the University of Silesia (GIUS) 7–314, GIUS 7–513, and GIUS 7–43]. Bulk samples (ca. 50 kg) of poorly lithified marly limestones were washed with hot tap water and sieved using 0.315-mm-diameter mesh. The residue was then dried at 180 °C and fossils were handpicked under a binocular microscope.
Data on the evolutionary history of post-Paleozoic echinoids and crinoids (as schematized in Fig. 3) were derived from the literature (25, 31, 36, 42).
Supplementary Material
Acknowledgments
We thank B. Miljour for help with figures, and P. L. Norby, M. Martin, and D. K. Moore for assistance with the aquaria. Comments by two anonymous reviewers helped improve the manuscript . This work was funded in part by grants from the National Science Foundation (T.K.B., C.G.M.), National Geographic Society (T.K.B.), and the Foundation for Polish Science (P.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.J.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914199107/DCSupplemental.
References
- 1.Stanley SM. What has happened to the articulate brachiopods? GSA Abstracts with Programs. 1974;8:966–967. [Google Scholar]
- 2.Vermeij GJ. The Mesozoic marine revolution: Evidence from snails, predators and grazers. Paleobiology. 1977;3:245–258. [Google Scholar]
- 3.Kowalewski M, Kelley PH, editors. The Fossil Record of Predation. New Haven: Paleontological Society; 2002. [Google Scholar]
- 4.Kelley PH, Kowalewski M, Hansen TA, editors. Predator-Prey Interactions in the Fossil Record. New York: Kluwer Academic/Plenum; 2003. [Google Scholar]
- 5.Fishelson L. Ecology of northern Red Sea crinoids and their epi- and endozoic fauna. Mar Biol. 1974;26:183–192. [Google Scholar]
- 6.Meyer DL, Macurda DB., Jr Adaptive radiation of comatulid crinoids. Paleobiology. 1977;3:74–82. [Google Scholar]
- 7.Meyer DL, Ausich WI. Biotic interactions among Recent and fossil crinoids. In: Tevesz MFS, McCall PL, editors. Biotic Interactions in Recent and Fossil Benthic Communities. New York: Plenum; 1983. pp. 377–427. [Google Scholar]
- 8.Meyer DL, LaHaye CA, Holland ND, Arenson AC, Strickler JR. Time-lapse cinematography of feather stars (Echinodermata: Crinoidea) on the Great Barrier Reef, Australia: Dem-onstrations of posture changes, locomotion, spawning and possible predation by fish. Mar Biol. 1984;78:179–184. [Google Scholar]
- 9.Signor PW, III, Brett CE. The mid-Paleozoic precursor to the Mesozoic marine revolution. Paleobiology. 1984;10:229–245. [Google Scholar]
- 10.Meyer DL. Evolutionary implications of predation on Recent comatulid crinoids from the Great Barrier Reef. Paleobiology. 1985;11:154–164. [Google Scholar]
- 11.Bottjer DJ, Jablonski D. Paleoenvironmental patterns in the evolution of Post-Paleozoic benthic marine invertebrates. Palaios. 1988;3:540–560. [Google Scholar]
- 12.Meyer DL, Oji T. Eocene crinoids from Seymour Island, Antarctic Peninsula: Paleobiographic and paleoecologic implications. J Paleontol. 1993;67:250–257. [Google Scholar]
- 13.Oji T, Okamoto T. Arm autotomy and arm branching pattern as anti-predatory adaptations in stalked and stalkless crinoids. Paleobiology. 1994;20:27–39. [Google Scholar]
- 14.Nichols D. Evidence for a sacrificial response to predation in the reproductive strategy of the comatulid crinoid Antedon bifida from the English Channel. Oceanol Acta. 1996;19:237–240. [Google Scholar]
- 15.Baumiller TK, Gahn FJ. Testing predation-driven evolution using mid-Paleozoic crinoid arm regeneration. Science. 2004;305:1453–1455. doi: 10.1126/science.1101009. [DOI] [PubMed] [Google Scholar]
- 16.Walker SE, Brett CE. Post-Paleozoic patterns in marine predation: was there a Mesozoic and Cenozoic marine predatory revolution? The fossil record of predation. In: Kowalewski M, Kelley PH, editors. The Paleontological Society Papers. Vol. 8. New Haven: Paleontological Society; 2002. pp. 119–193. [Google Scholar]
- 17.Mladenov PV. Rate of arm regeneration and potential causes of arm loss in the feather star Florometra serratissima (Echinodermata: Crinoidea) Can J Zool. 1983;61:2873–2879. [Google Scholar]
- 18.Halpern JA. Miami: Univ of Miami; 1970. A monographic revision of the goniasterid sea stars of the North Atlantic. PhD thesis. [Google Scholar]
- 19.Baumiller TK, Mooi R, Messing CG. Urchins in a meadow: paleobiologial and evolutionary implications of cidaroid predation on crinoids. Paleobiology. 2008;34:22–34. [Google Scholar]
- 20.Gorzelak P, Salamon MA. Signs of benthic predation on late Jurassic stalked crinoids, preliminary data. Palaios. 2009;24:70–73. [Google Scholar]
- 21.Meyer DL, Meyer KB. Biostratinomy of Recent crinoids (Echinodermata) at Lizard Island, Great Barrier Reef, Australia. Palaios. 1986;1:294–302. [Google Scholar]
- 22.Baumiller TK, Llewellyn G, Messing CG, Ausich WI. Taphonomy of isocrinid stalks: Influence of decay and autonomy. Palaios. 1995;10:87–95. [Google Scholar]
- 23.Kier PM. Echinoids from the Triassic (St. Cassian) of Italy, their lantern supports, and a revised phylogeny of Triassic echinoids. Smithsonian Contributions to Paleobiology. 1984;56:1–41. [Google Scholar]
- 24.Smith AB, Hollingworth NTJ. Tooth structure and phylogeny of the Upper Permian echinoid Miocidaris keyserlingi. Proceedings of the Yorkshire Geological Society. 1990;48:47–60. [Google Scholar]
- 25.Kier PM. Evolutionary trends and their functional significance in the post-Paleozoic echinoids. J Paleontol. 1974;48(Suppl):1–96. [Google Scholar]
- 26.Kier PM. Triassic echinoids. Smithsonian Contributions Paleobiology. 1977;30:1–88. [Google Scholar]
- 27.Smith AB. Implications of lantern morphology for the phylogeny of post-Palaeozoic echinoids. Palaeontology. 1981;24:779–801. [Google Scholar]
- 28.Smith A. Echinoid Palaeobiology. Boston: George Allen and Unwin; 1984. p. 190. [Google Scholar]
- 29.Simms MJ. The radiation of post-Paleozoic echinoderms. In: Taylor PD, Larwood GP, editors. Major Evolutionary Radia-tions, Systematics Association Special Volume. Vol. 42. Oxford: Clarendon Press; 1990. pp. 287–304. [Google Scholar]
- 30.Simms MJ, Gale AS, Gilliland P, Rose EPF, Sevastopulo GD. Echinodermata. In: Benton MJ, editor. The Fossil Record 2. London: Chapman and Hall; 1993. pp. 491–528. [Google Scholar]
- 31.Hagdorn H. Literaturbericht Triassic crinoids. Zentralblatt für Geologie und Paläontologie. 1995;1/2:1–22. [Google Scholar]
- 32.Twitchett RJ, Oji T. Early Triassic recovery of echinoderms. C R Palevol. 2005;4:531–542. [Google Scholar]
- 33.Foote M. Morphological diversification of Paleozoic crinoids. Paleobiology. 1995;21:272–299. [Google Scholar]
- 34.Hagdorn H, Wang X, Wang C. Palaeoecology of the pseudoplanktonic Triassic crinoid Traumatocrinus from Southwest China. Palaeogeogr Palaeoclimatol Palaeoecol. 2007;247:181–196. [Google Scholar]
- 35.Baumiller TK, Messing CG. Stalked crinoid locomotion and its ecological and evolutionary implications. Palaeontol Electronica. 2007;10:1–10. [Google Scholar]
- 36.Simms MJ. Systematics, phylogeny and evolutionary history. In: Hess H, Ausich WI, Brett CE, Simms MJ, editors. Fossil Crinoids. Cambridge: Cambridge University Press; 1999. pp. 31–40. [Google Scholar]
- 37.Hagdorn H, Campbell HJ. Paracomatula triadica sp. nov.-an early comatulid crinoid from the Otapirian (Late Triassic) of New Caledonia. Alcheringa. 1993;17:1–17. [Google Scholar]
- 38.Hautmann M. Early Mesozoic evolution of alivincular bivalve ligaments and its implications for the timing of the ‘Mesozoic marine revolution’. Lethaia. 2004;37:165–172. [Google Scholar]
- 39.Vermeij GJ. Escalation and its role in Jurassic biotic history. Palaeogeogr Palaeoclimatol Palaeoecol. 2008;263:3–8. [Google Scholar]
- 40.Oji T. Early Cretaceous Isocrinus from northeast Japan. Palaeontology. 1985;28:629–642. [Google Scholar]
- 41.Jagt JWM, Salamon MA. Late Cretaceous bourgueticrinid crinoids from southern Poland: Preliminary observations. Scripta Geologica. 2007;134:61–76. [Google Scholar]
- 42.Sepkoski JJ., Jr A compendium of fossil marine animal genera. Bull Am Paleontol. 2002;363:1–560. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.