Abstract
Streptococcus mutans antigen I/II (AgI/II) is a cell surface-localized protein adhesin that interacts with salivary components within the salivary pellicle. AgI/II contributes to virulence and has been studied as an immunological and structural target, but a fundamental understanding of its underlying architecture has been lacking. Here we report a high-resolution (1.8 Å) crystal structure of the A3VP1 fragment of S. mutans AgI/II that demonstrates a unique fibrillar form (155 Å) through the interaction of two noncontiguous regions in the primary sequence. The A3 repeat of the alanine-rich domain adopts an extended α-helix that intertwines with the P1 repeat polyproline type II (PPII) helix to form a highly extended stalk-like structure heretofore unseen in prokaryotic or eukaryotic protein structures. Velocity sedimentation studies indicate that full-length AgI/II that contains three A/P repeats extends over 50 nanometers in length. Isothermal titration calorimetry revealed that the high-affinity association between the A3 and P1 helices is enthalpically driven. Two distinct binding sites on AgI/II to the host receptor salivary agglutinin (SAG) were identified by surface plasmon resonance (SPR). The current crystal structure reveals that AgI/II family proteins are extended fibrillar structures with the number of alanine- and proline-rich repeats determining their length.
Keywords: bacterial adhesion, dental caries, Streptococcus, x-ray crystallography, fibrous proteins
Streptococcus mutans is the causative agent of human dental caries (1) and its protein adhesin antigen I/II (AgI/II) is a known target of protective immunity (2). AgI/II family molecules are expressed by numerous oral streptococci (3) and homologs have also been identified in the invasive pathogens Streptococcus pyogenes and Streptococcus agalactiae (4) (Fig. S1). In addition to mediating adhesion to the tooth surface (5), AgI/II influences biofilm formation (6), promotes collagen-dependent bacterial invasion of dentin (7), and mediates adherence to human epithelial cells (8). Elimination of AgI/II results in decreased virulence (9), but despite three decades of study, a mechanistic understanding of the functional properties of the molecule has been stymied by a lack of understanding of its structure.
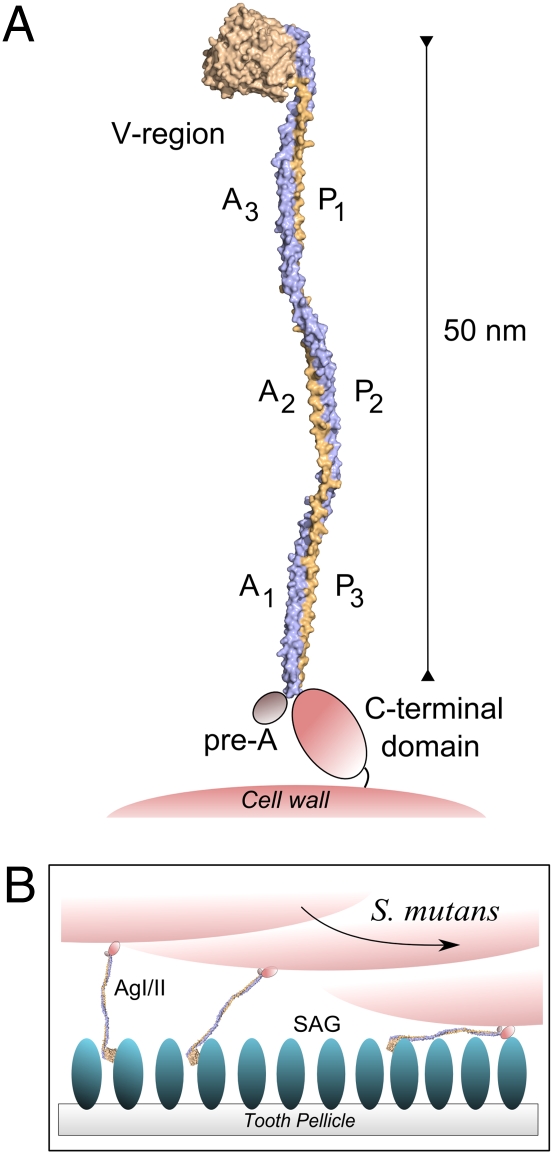
Originally identified as AgI/II (10) (also called P1, PAc, or SpaP), members of this protein family contain between 1310 and 1653 amino acids (aa) beginning with an amino-terminal signal motif that directs secretion, followed by the A, V, and P regions (Fig. 1A). The A region typically consists of 3–4 alanine-rich repeats (82 residues each) with 23–30% alanine content. The P region has 3–4 proline-rich repeats (39 residues each) with ~35% proline content. Nested between the A and P repeats is a segment commonly referred to as the V or variable region, which contains within it a stretch of ~100 amino acids where most of the sequence variation among S. mutans AgI/II molecules is clustered (11). The crystal structure of the V region adopts a globular β-stranded “super-sandwich” fold (12). Finally, the carboxy terminus contains the LPxTG sortase motif for covalent anchorage to the cell wall (13). S. mutans AgI/II possesses both low- and high-affinity binding sites for salivary agglutinin (SAG) (14), a 600-kDa oligomeric protein complex containing glycoprotein 340 (440 kDa), sIgA (25, 59, and 88 kDa), and an unknown 80-kDa polypeptide (15). Studies aimed at developing active and passive immune therapies have analyzed segments of Ag I/II that contribute to bacterial adherence and cariogenicity (2, 16). Several anti-AgI/II monoclonal antibodies (MAbs) recognize complex conformational epitopes encompassing noncontiguous sequences within the A and the P repeats (16, 17), indicating that these regions are in close proximity, but the exact nature of the intramolecular interaction required to achieve a functional adhesin was undetermined.
Fig. 1.
(A) The primary sequence layout of AgI/II is shown at the Top. The alanine-rich repeats are highlighted in shades of blue, and the proline-rich repeats are in shades of purple. Shown Below are the fragments of AgI/II used in this study, including the crystallized fragment A3VP1. Residue numbers correspond to the primary sequence of AgI/II from S. mutans strain NG8 (GenBank accession GQ456171). (B) The sequence alignments of A1, A2, and A3 repeats, where the 19 heptad motifs are highlighted in red. (C) The alignments of P1, P2, and P3 repeats, where the proline residues are shown in red.
Here we report a high-resolution (1.8 Å) crystal structure of the A3VP1 fragment of S. mutans AgI/II that demonstrates a unique fibrillar structure (155 Å) formed by the intimate association of two widely separated segments within the primary sequence. The A3 repeat of the alanine-rich domain was found to adopt a long α-helical structure that intertwines with the P1 repeat polyproline type II (PPII) helix to form a highly extended stalk. Competition ELISA experiments employing multiple adherence-inhibiting MAbs confirmed that the crystallized fragment represented a functional structure. Thermodynamic quantitation demonstrated a high-affinity interaction between the α- and PPII helices. Velocity sedimentation studies indicated that together the three A/P repeat units account for the majority of the length of AgI/II. Adherence studies identified two distinct binding sites on AgI/II for it's host receptor SAG. Finally, we propose a model for AgI/II's interaction with SAG.
Results
Crystallization and Overall Structure of A3VP1.
A3VP1 (Fig. S2) was crystallized in the P21 space group. A similar crystallization condition augmented with 50 mM fructose crystallized in the P21212 space group (Table 1). The A3VP1 structures from each space group superimpose with an average rmsd of 0.875 Å, indicating high structural similarity. A3 is an extended α-helix (110 residues) and adopts torsion angles akin to the canonical α-helix geometry, followed by the globular V region that is situated at the apex of the molecule (Fig. 2 A and B). P1 (residues 834–874) folds over to interact with A3, and conforms to the PPII helix geometry, with backbone torsion angles that are characteristic of PPII helices (Φ of −75° and the ψ of 145°) with three residues per turn (18). Viewed through an imaginary axis along this PPII helix, the α-helical segment wraps 180° around the axis to form a left-handed supercoiled structure. The resultant interaction forms an extended (~155 Å) fibrillar structure.
Table 1.
Data collection and refinement statistics
| A3VP1 | A3VP1 + fructose | |
| Data collection | ||
| Space group | P21 | P21212 |
| Cell dimensions | ||
| a, b, c (Å) | 50.03, 164.15, 67.73 | 70.73,153.06, 49.68 |
| α, β, γ (°) | 90, 91.03, 90 | 90, 90, 90 |
| Wave length (Å) | 0.97918 | 0.97918 |
| Resolution (Å) | 50–2.0 | 35–1.8 |
| Rsym* (%) | 6.2 (18.2) | 7.2 (30.5) |
| Mean I / σI | 29.3 (8.9) | 16.4 (6.3) |
| Completeness (%) | 95.9 (91.0) | 90.7 (87.7) |
| Redundancy | 7.6 (6.4) | 5.1 (4.8) |
| Solvent content (%) | 51.33 | 49.64 |
| Refinement | ||
| Rwork/Rfree (%) | 18.1/22.2 | 19.0/22.8 |
| Geometry analysis number residues (region %) | ||
| Most favored regions | 786 (91.0) | 397 (91.9) |
| Additional allowed regions | 75 (8.7) | 33 (7.6) |
| Generously allowed regions | 3 (0.3) | 2 (0.5) |
| Disallowed regions | 0 (0.0) | 0 (0.0) |
| PDB accession number | 3IPK | 3IOX |
*Rsym= ∑|Ih − <Ih>|/∑Ih, where <Ih> is the average intensity over symmetry equivalents. Highest resolution shell is shown in parentheses.
Fig. 2.
Antigen I/II layout and A3VP1 structure overview. (A) Ribbon diagram of the crystal structure of A3VP1 generated by PyMol (48). The α-helix from A3 and the PPII helix from the P1 regions interact to form the 155 Å stalk. (B) Electrostatic map of A3VP1 generated using MolMol (49). The stalk formed by the association of the A3 and P1 helices displays an extensive hydrophobic surface. (C) Superposition of the unique molecules from the P21 and P212121 crystal forms by strictly anchoring over the V region shows ~17 Å displacement at the termini of the helices.
Differences Between Crystal Forms.
When the unique molecules of the asymmetric unit from the P21 and the P21212 data sets were superimposed by strictly anchoring the residues over the V region, a latitudinal shift along the helical axis was observed (Fig. 2C). The residues of A3 (385–474) and P1 (834–874) show temperature factors increasing with distance from the V region, indicative of a global flexibility of the extended stalk. Angular deviation in the orientation of the helices of ≈7.5° between the two unique molecules of the P21 crystal begins in the vicinity of the kink at proline residue 471, resulting in a displacement of ~17.0 Å between the termini of the extended stalks. These differences in orientations of the helices resulted in alternative packing with a larger b-axis cell dimension in the P21 as compared to the P21212 crystals.
Analysis of A3–P1 Interactions.
Notable within A3 were seven heptad repeats consisting of an “AxYxAx[LV]” motif (Fig. 3A, Top), where every other residue is hydrophobic. The first, third, fifth, and seventh residues are highly conserved with the remaining positions filled with charged residues. The heptads are a defining signature of the A region with 19 present across all three repeats (Fig. 1B). The A3 heptads interact with P1 in a “knobs-into-holes” fashion (Fig. 3B) with the third tyrosine residue on each heptad facing inward into a hydrophobic pocket formed by the P1 proline side chains at “PxxP” motifs. A second hydrophobic leucine or valine residue at the seventh position also faces into this hydrophobic pocket. These interactions are reminiscent of those within the 36-amino-acid avian pancreatic polypeptide (Fig. S3), in which an 8-residue PPII helix interacts with a 17-residue α-helix (19). Similar to classical coiled-coil motifs, residues within A3 display quasiperiodicity, where hydrophobic residues are alternately three and four positions apart. Many of the A3–P1 interactions are hydrophobic as indicated from surface accessibility measurements of the A3 and P1 helices (2515.2 Å2:920.0 Å2, nonpolar:polar contact surface). Regions that intervene between the heptads are dominated by direct hydrogen bonding between asparagine residues on A3 and the main chain oxygens and nitrogens of P1 (Fig. 3C). The heptad and intervening sequences, and PxxP motifs, are highly conserved in A1A2 and P2P3 (Fig. 1 B and C), suggesting similar interactions are maintained in all repeats.
Fig. 3.
Interactions between the A3 and P1 helices. (A) The helical wheel diagram (Top) shows the interaction between the A3’s heptad motif “AxYxAx[LV]” and P1’s “PxxP” motif. The helical net of the interactions between the α- and PPII helices is also shown, where the A3-repeat heptad motifs are highlighted in blue and the P1 repeat PxxP motifs are highlighted in orange. Asparagine residues within the A3 region, intervening the heptads, that are involved in hydrogen bonding with the P1 PPII helix are highlighted in red. The conserved tyrosines and leucines (highlighted in yellow) of the heptad sequences are nestled between prolines of the P repeat. Additionally, the phenolic oxygen atom of the tyrosine residues participate in water-mediated hydrogen bonding (red-dashed lines). (B) The stereo diagram of the heptad interactions shows that tyrosine side chains nestle between the prolines in a knobs-in-holes interaction, which is highlighted by surface plots for the A and P repeats. (C) The stereo diagram of the region intervening the heptads shows a dominant direct hydrogen bonding between the asparagine side chains of A3 and the main chain oxygen and nitrogen of the PPII helix. Two prolines (Pro855 and Pro858) break this pattern and face outward.
Energetics of the A–P Interaction.
Isothermal titration calorimetry was used to measure the strength of association between the A and P repeats. The titration of VP3 by A1–3 (Fig. 4A) shows that enthalpically driven binding occurs between A1–3 and VP3 with a dissociation constant of 62.9 ± 5.1 nM at 20 °C (Table 2). The A1–3 and VP3 binding had a large enthalpy change (ΔH = −870.3 ± 5.3 kJ/mol) accompanied by a large change in entropy (TΔS = −827.5 kJ/mol), with the estimated ΔGbinding = −40.4 kJ/mol. Measurements of the VP3 and A1–3 interaction at multiple temperatures determined that the heat capacity (ΔCp) of binding as −7.95 ± 0.58 kJ K−1 mol−1.
Fig. 4.
Calorimetry, ELISA, and BIACore studies. (A) Calorimetry of the A/P interaction. (Upper) Calorimetric measurements at 20 °C, 25 injections of 10 μL A1–3 into the cell containing VP3. (Lower) The energy (kcal/mol) released during each injection. (B) Inhibition of binding of anti-AgI/II MAbs to S. mutans whole cells. Twofold serial dilutions of AgI/II polypeptides (5–0.05 μM) were incubated with each indicated MAb. Competition ELISA results are shown as the percent inhibition of binding of the indicated MAb to S. mutans whole cells in the presence of each polypeptide. Error bars indicate standard deviations. CG14 (yellow squares), A3VP1 (red diamonds), A1VP3 (blue squares), and C-terminal construct (green triangles). (C) BIAcore studies of AgI/II fragments with immobilized SAG. Full-length CG14, A3VP1, C-terminal, or V-region fragments (analytes) were flowed over a CM5 chip coated with covalently attached SAG. Analyte proteins ranged from 0.25 μM to 4 μM concentrations. Arrows indicate injection starting and stopping points. During the 4-min injection of each polypeptide, an increase in the RU is observed as AgI/II polypeptides bind to SAG. CG14 and A3VP1 showed the maximal binding responses (1 RU is equivalent to 1 pg per square millimeter of sensor surface).
Table 2.
Isothermal titration calorimetry measurements
| Temp (° C) | ΔG (kJ/mol) | Ka (M−1 × 106) | Kd (nM) | ΔH (kJ/mol) | ΔS (J K−1mol−1) | n | ΔCp (kJ K−1 mol−1) |
| 15 | −36.6 | 4.26 ± 0.48 | 234 ± 26 | −808.3 ± 10.2 | −2677.8 | 0.858 ± 0.008 | −7.95 ± 0.58 |
| 20 | −40.4 | 15.9 ± 1.3 | 62.9 ± 5.1 | −870.3 ± 5.3 | −2828.4 | 1.030 ± 0.004 | |
| 25 | −44.4 | 61 ± 4 | 16.5 ± 1.1 | −892.4 ± 2.7 | −2845.1 | 1.050 ± 0.002 | |
| 30 | −49.2 | 310 ± 60 | 3.27 ± 0.64 | −933.5 ± 5.5 | −2916.2 | 0.822 ± 0.002 |
AgI/II Is a Highly Extended Molecule.
The linear dimensions of AgI/II and its fragments in solution were estimated in velocity ultracentrifugation experiments. The sedimentation coefficients and frictional ratios of A1–3, A1VP3, A3VP1, VP3, and full-length AgI/II (CG14) exhibited high axial ratios for both prolate and oblate ellipsoids (Table 3). The ratios of the A3VP1 prolate ellipsoid axes predict an elongated shape similar to that of the A3VP1 crystal structure. Additionally, the ratio of axes of the A1VP3 prolate ellipsoid is nearly three times that of A3VP1, indicating that AgI/II forms an extended stalk of over 50 nm, consistent with the dimensions of the AgI/II “fuzzy coat” visualized on the surface of S. mutans by immunoelectron microscopy 20 years ago (20). A1–3 has elongated dimensions similar to the AP stalk, and VP3 is characterized by a shorter length. All of the polypeptides characterized are monomeric, except A1–3, which exists as a dimer.
Table 3.
Sedimentation velocity measurements
| Protein | Theoretical MW (kDa) | Fit MW (kDa) | Fit rmsd (OD) | s20 (S) | f/f0 | Stokes radius (nm) | Prolate ellipsoid a/b ratio | Prolate ellipsoid dimensions (nm × nm) |
| A3VP1 | 56.54 | 55.83 | 0.004 | 3.197 | 1.642 | 4.16 | 7.47 | 22.70 × 3.04 |
| A1VP3 | 88.25 | 92.93 | 0.005 | 2.913 | 2.548 | 7.61 | 24.56 | 59.33 × 2.42 |
| CG14 | 167.52 | 138.48 | 0.004 | 3.796 | 2.536 | 8.70 | 24.52 | 67.82 × 2.77 |
| VP3 | 56.53 | 56.61 | 0.005 | 2.840 | 1.872 | 4.76 | 12.45 | 30.65 × 2.46 |
| A1–3 | 27.63 | 55.93 | 0.007 | 2.071 | 2.545 | 6.44 | 27.33 | 51.56 × 1.89 |
Achievement of Functional Structure by A3VP1.
To confirm A3VP1 is representative of biologically active AgI/II, the ability of A3VP1 and A1VP3 to compete for binding of previously characterized anti-AgI/II MAbs against S. mutans was evaluated by ELISA (Fig. 4B). MAbs 1–6F, 4–9D, and 4–10A all react with S. mutans whole cells (21) and inhibit bacterial adherence to immobilized human SAG (22). MAbs 1–6F and 4–9D bind within the V region. The 4–10A epitope requires an interaction of A- and P-repeat-containing fragments (16, 23). MAb 3–10E also reacts with S. mutans cells, but does not inhibit bacterial adherence to SAG (21, 22). As expected, A3VP1 and A1VP3 were similar to CG14 in their ability to compete for binding of MAbs 1–6F, 4–9D, and 4–10A, whereas the C-terminal fragment did not demonstrate substantial competition. On the other hand, A3VP1 and A1VP3 did not competitively inhibit binding of MAb 3–10E, whereas the full-length CG14 molecule did. These experiments demonstrate that A3VP1 and A1VP3 reflect a functional structure in that epitopes recognized by three different anti-AgI/II MAbs known to inhibit bacterial adherence were replicated in these polypeptides to a degree comparable to that of the full-length adhesin.
To further confirm adherence properties of AgI/II fragments, binding to immobilized SAG was evaluated using surface plasmon resonance (SPR) (Fig. 4C). The binding experiments were conducted using a buffer augmented with calcium, which is necessary for AgI/II's interaction with SAG (14), while under a laminar fluid flow as found in the oral environment. CG14 and A3VP1 both interacted strongly with SAG, whereas the isolated V region demonstrated diminished binding activity. As was previously reported (24), the C-terminal fragment also bound to SAG. Modeling the association and dissociation of the AgI/II proteins with SAG using the 1:1 Langmuir binding model led to estimated KA values of 3 × 107 M−1 for A3VP1, 3 × 107 M−1 for CG14, 5 × 106 M−1 for the C-terminal construct, and 3 × 105 M−1 for the V region. The independent adherence of two nonoverlapping AgI/II fragments is consistent with previous experimental evidence indicative of multiple binding sites (14). Now viewed in the context of the A3VP1 crystal structure, two distinct regions with independent affinity for SAG appear to be widely separated by the long AgI/II stalk.
Discussion
Early work on fibrous α-keratin predicted formation of extended structures with coiled-coil α-helices, which was later witnessed in a variety of proteins (25). Similarly, a model for fibrous collagen with three left-handed polyproline type II (PPII) helices forming a right-handed supercoil was proposed (26) and then observed structurally (27). However, the formation of a unified hybrid structure composed of both α- and PPII helices was unknown. The crystal structure of A3VP1 now provides evidence for a unique interaction between α- and PPII helices that results in an elongated fibrillar form. The A–P interactions are well maintained in the molecules from two different space groups. The entire stalk flexes as a unit from the hinge-like area near proline residue 471 in the two crystal forms of A3VP1. The association of the α- and PPII helices in solution is supported by the high-affinity interaction measured between the A1–3 and VP3 fragments. A negative change in the heat capacity (ΔCP) supports the burial of hydrophobic surfaces in the association of the helices. The thermodynamic properties of the enthalpically driven association of the alanine-rich and proline-rich repeats are similar in sign and magnitude as those observed in the formation of triple-helical collagen-like peptides (28), and in the binding of SH3 to short PPII-conforming peptides (29). In the latter case, the enthalpy and entropy changes were of opposite sign from that expected of a primarily hydrophobic interaction. To explain differences between expected and measured changes of entropy and enthalpy, binding of SH3 was proposed to occur through the folding of the short PPII peptide, resulting in a large enthalpic energy release and decrease in entropy (29). It is possible that the A1–3 and VP3 interaction may follow a similar mechanism of association that is dependent on folding of the PPII helix. Differences in disordered content between VP3 and A1VP3 observed in circular dichroism (CD) studies (Table S1) support the hypothesis that the PPII helix undergoes a conformational change during its association with the A repeats. In addition, the shorter estimated length of VP3 (30 nm) compared to A1VP3 (59 nm) derived from velocity sedimentation experiments is consistent with such a change in conformation. In contrast, the CD spectrum of the unpaired A repeats (A1–3), which have nearly 100% α-helical content (Table S1), suggests that the A repeats adopt their helical fold independently and before association with the P repeats. The formation of dimers of the A1–3 fragment (Table 3) likely result in stabilization of the A repeats’ α-helix.
Although AgI/II has been implicated in S. mutans aggregation (30), a multimeric state was not prevalent in constructs with paired A/P repeats. The A3VP1, A1VP3, and CG14 fragments studied by analytical ultracentrifugation each had a single large peak with their sedimentation coefficient distributions corresponding to the mass of a monomeric molecule. However, packing of the A3VP1 fragment within the crystal (Fig. S4A) resulted in numerous hydrophobic contacts between symmetry related molecules, particularly along the A and P repeats, suggesting a possible mechanism of aggregation of AgI/II molecules. A notable symmetry interaction involved two prolines (Pro855 and Pro858, Fig. 3C) of the PPII helix that broke the dominant pattern to face outward and abut with the surface of another symmetry-related A3 (Fig. S4B). This symmetry-related interaction may mirror the reported biological interaction of the A repeats with collagen (8), where the proline and hydroxyproline residues of the classical GXY motif that face outward in collagen could potentially interact with the A repeats.
Extrapolation based upon the crystal structure of the A3 (386–474)–P1(834–847) interaction predicts that the continued association of A2 (304–385) with P2 (875–913), and subsequently A1 (222–303) with P3 (914–952), would result in a molecule that exceeds 50 nm in length. In support of this prediction, the estimated dimensions of full-length AgI/II deduced from velocity sedimentation experiments indicate that it is over 50 nm long. In addition, crystals of A1–3 (Fig. S5) diffracted poorly and displayed fiber-like diffraction patterns, but revealed the presence of one very long axis (>600 Å), agreeing well with the dimensions predicted from ultracentrifugation. It is now apparent that AgI/II is a highly extended fibrillar structure formed by a long and continuous A–P association. This architecture would position the globular V region at the tip of the stalk away from the cell surface, with the C-terminal region positioned near the cell surface and the pre-A region in close proximity (Fig. 5A). This model is consistent with the known C-terminal anchorage of LPxTG motif containing sortase substrates to the bacterial cell wall (13).
Fig. 5.
Model of the AgI/II structure and predicted binding with SAG. (A) The crystal structure of the AgI/II A3VP1 region revealed an extended stalk formed by the A3 and P1 repeats. Ultracentrifugation studies predict that the A2–P2 and A1–P3 repeats extend the stalk to a length over 50 nm. (B) Adherence studies indicated the presence of two sites, one within A3VP1 and another within the C-terminal region, which are widely separated in the AgI/II structure. The cartoon represents a possible model for AgI/II binding to SAG, where interactions occur at both the distal end through the A3VP1 region, and at a secondary adherence site mediated by the C-terminal domain.
Corresponding well with the current A3VP1 crystal structure, a single A repeat adopting an α-helix was proposed to match in length a single P repeat adopting a PPII helix (17). Alanine- and proline-rich repeats are highly conserved among AgI/II family members, although the total number of repeats varies between one and five (Fig. S1) (31). From the current structure, it is likely that all AgI/II family members have elongated structures, with the overall length of the fibrillar proteins determined by the number of A and P repeats. For example, Streptococcus intermedius Pas possesses only one A and one P repeat and would consequently be predicted to be shorter (15–17 nm).
AgI/II's relevant domains have been predicted on the basis of primary sequence and studied in isolation (14, 24, 32, 33); however, the interactions between the A and P regions as a single extended functional unit now indicate that both these segments together would share roles previously ascribed to one or the other in isolation. In addition to the anti-AgI/II MAbs described in this study, antibodies that inhibit S. mutans adherence to SAG have also been mapped to the A region (17, 34–37). Notably, two-thirds of the surface area of the A/P stalk of A3VP1 is formed by the A3 sequence. Due to the highly repetitive nature of A1–3, it is plausible that adherence-inhibiting antibodies recognize a repeated epitope and disrupt the binding via steric hindrance. SAG's gp340 also contains multiple tandem repeats of scavenger receptor cysteine-rich (SRCR) domains (38). Although a structure of tandem repeats of these SRCR domains has not been determined, gp340 may be reminiscent of fibronectin, whose repeated domains display an elongated structure (39). There would then be a potential for a longitudinal interaction of SAG with the highly extended AgI/II.
Full-length AgI/II (CG14), A3VP1, and the C-terminal fragment all demonstrated measurable adherence to immobilized SAG by surface plasmon resonance. Independent adherence to SAG by two nonoverlapping AgI/II fragments is consistent with the multiple binding sites detected by Scatchard analysis (14). In a simple model of binding based on its highly extended structure, one possibility is that high-affinity binding of AgI/II with SAG occurs via the apical fishhook-like structure observed within A3VP1, and an additional interaction occurring within the C-terminal region (Fig. 5B). The similar affinities observed for full-length AgI/II and A3VP1 suggest that AgI/II may not simultaneously interact with a single molecule of SAG at both the A3VP1 and C-terminal sites.
It appears, therefore, that Gram-positive bacteria have developed several variations of structural units that enable positioning of binding regions of adhesin proteins away from the cell surface. For example, the S. pyogenes M protein is a bacterial surface protein whose elongated shape is formed by an α-helical coiled-coil dimer structure (40). The hypervariable region at the N terminus forms a conformational binding surface that recognizes the human plasma protein C4BP (41). Similarly, the Staphylococcus aureus surface protein CNA has the N-terminal collagen binding region extended away from the cell surface by the B-repeat's inverted IgG domains (42). The current crystal structure of A3VP1 of S. mutans AgI/II displays a unique fibrillar architecture that provides insights into this widespread family of streptococcal surface proteins.
Methods
Cloning, protein expression, purification, isothermal titration calorimetry, analytical ultracentrifugation studies, CD studies, salivary agglutinin preparation, competition ELISAs, surface plasmon resonace binding studies, and solvent accessible surface area calculations are described in SI Methods.
Crystallization and Data Collection.
Crystals of A3VP1 were optimized from condition 13 of Crystal Screen II (Hampton Research) using the hanging drop vapor diffusion methodology at 22 °C. Crystals were routinely grown from a reservoir solution containing 30% (vol/vol) polyethylene-glycol monomethyl ether 2000, 200 mM of ammonium sulfate, and 50 mM of sodium cacodylate at pH 4.6. The addition of 50 mM of fructose in the reservoir solution further improved the size and appearance of the protein crystals. Crystals were flash frozen with 15% ethylene glycol and data were collected under a gaseous N2 stream maintained at 100 °K in the NE-CAT beamline at Advanced Photon Source/Argonne National Laboratory, Chicago, Illinois. Data were recorded on a Quantum CCD detector, using a wavelength (λ) of 1.0 Å, exposure time of 2.0 s, oscillation angle (ϕ) of 1.0°, and a distance (D) of 150 mm from the crystal to the detector. Diffraction data were integrated and scaled using the HKL2000 (43). A3VP1 crystals grown in the presence or absence of fructose, respectively, adopted the orthorhombic P21212 and monoclinic P21 space groups.
Structure Determination and Model Refinement.
Molecular replacement was carried out with the V region (495–828) of AgI/II (PDB 1JMM) (12) as the input model using PHASER (44). Molecular replacement yielded a strong single solution for each data set and they were further refined using CNS (45). The resulting electron density map had clear interpretable densities for the helical repeat regions, and were then built with Coot (46). Residues 495–828 of the A1VP3 structures were highly similar to the solved AgI/II V-region structure (12) (average rmsd of 0.431 Å). The final Rfactor/Rfree of the models were 18.1/22.2 (native) and 19.0/22.8 (fructose co-crystal). The quality of the final models were assessed using PROCHECK (47), where 99.5% are within the most favored and allowed regions of the Ramachandran plot with an additional 0.5% within the generously allowed region. Complete data collection and refinement statistics are shown in Table 1.
Supplementary Material
Acknowledgments
The authors thank Dr. Irina Protassevitch and Dr. Christie Brouillette for ITC, CD, and BIAcore studies, and Dr. Peter Prevelige for the analytical ultracentrifugation studies. M.L. and C.D. thank the University of Alabama at Birmingham-Comprehensive Cancer Center X-Ray core and Northeastern Collaborative Access Team facility at Advanced Photon Source/Argonne National Laboratory. This work was supported by National Institutes of Health (NIH)-National Institute of Dental and Craniofacial Research (NIDCR) grants RO1 DE017737 (to C.D.) and R01 DE013882 (to L.J.B.) and by the DART Training Grant NIDCR T-32 DE0176707 (to M.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 3IPK and 3IOX).
This article contains supporting information online at www.pnas.org/cgi/content/full/0912293107/DCSupplemental.
References
- 1.Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell MW, Childers NK, Michalek SM, Smith DJ, Taubman MA. A caries vaccine? The state of the science of immunization against dental caries. Caries Res. 2004;38:230–235. doi: 10.1159/000077759. [DOI] [PubMed] [Google Scholar]
- 3.Ma JK, Kelly CG, Munro G, Whiley RA, Lehner T. Conservation of the gene encoding streptococcal antigen I/II in oral streptococci. Infect Immun. 1991;59:2686–2694. doi: 10.1128/iai.59.8.2686-2694.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang S, Green NM, Sitkiewicz I, Lefebvre RB, Musser JM. Identification and characterization of an antigen I/II family protein produced by group A Streptococcus. Infect Immun. 2006;74:4200–4213. doi: 10.1128/IAI.00493-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenkinson HF, Demuth DR. Structure, function and immunogenicity of streptococcal antigen I/II polypeptides. Mol Microbiol. 1997;23:183–190. doi: 10.1046/j.1365-2958.1997.2021577.x. [DOI] [PubMed] [Google Scholar]
- 6.Pecharki D, Petersen FC, Assev S, Scheie AA. Involvement of antigen I/II surface proteins in Streptococcus mutans and Streptococcus intermedius biofilm formation. Oral Microbiol Immunol. 2005;20:366–371. doi: 10.1111/j.1399-302X.2005.00244.x. [DOI] [PubMed] [Google Scholar]
- 7.Love RM, McMillan MD, Park Y, Jenkinson HF. Coinvasion of dentinal tubules by Porphyromonas gingivalis and Streptococcus gordonii depends upon binding specificity of streptococcal antigen I/II adhesin. Infect Immun. 2000;68:1359–1365. doi: 10.1128/iai.68.3.1359-1365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sciotti MA, Yamodo I, Klein JP, Ogier JA. The N-terminal half part of the oral streptococcal antigen I/IIf contains two distinct binding domains. FEMS Microbiol Lett. 1997;153:439–445. doi: 10.1111/j.1574-6968.1997.tb12608.x. [DOI] [PubMed] [Google Scholar]
- 9.Crowley PJ, Brady LJ, Michalek SM, Bleiweis AS. Virulence of a spaP mutant of Streptococcus mutans in a gnotobiotic rat model. Infect Immun. 1999;67:1201–1206. doi: 10.1128/iai.67.3.1201-1206.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell MW, Bergmeier LA, Zanders ED, Lehner T. Protein antigens of Streptococcus mutans: Purification and properties of a double antigen and its protease-resistant component. Infect Immun. 1980;28:486–493. doi: 10.1128/iai.28.2.486-493.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brady LJ, et al. Restriction fragment length polymorphisms and sequence variation within the spaP gene of Streptococcus mutans serotype c isolates. Infect Immun. 1991;59:1803–1810. doi: 10.1128/iai.59.5.1803-1810.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Troffer-Charlier N, Ogier J, Moras D, Cavarelli J. Crystal structure of the V-region of Streptococcus mutans antigen I/II at 2.4 A resolution suggests a sugar preformed binding site. J Mol Biol. 2002;318:179–188. doi: 10.1016/S0022-2836(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 13.Navarre WW, Schneewind O. Proteolytic cleavage and cell wall anchoring at the LPXTG motif of surface proteins in gram-positive bacteria. Mol Microbiol. 1994;14:115–121. doi: 10.1111/j.1365-2958.1994.tb01271.x. [DOI] [PubMed] [Google Scholar]
- 14.Hajishengallis G, Koga T, Russell MW. Affinity and specificity of the interactions between Streptococcus mutans antigen I/II and salivary components. J Dent Res. 1994;73:1493–1502. doi: 10.1177/00220345940730090301. [DOI] [PubMed] [Google Scholar]
- 15.Oho T, Yu H, Yamashita Y, Koga T. Binding of salivary glycoprotein-secretory immunoglobulin A complex to the surface protein antigen of Streptococcus mutans. Infect Immun. 1998;66:115–121. doi: 10.1128/iai.66.1.115-121.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McArthur WP, et al. Characterization of epitopes recognized by anti-Streptococcus mutans P1 monoclonal antibodies. FEMS Immunol Med Microbiol. 2007;50:342–353. doi: 10.1111/j.1574-695X.2007.00260.x. [DOI] [PubMed] [Google Scholar]
- 17.van Dolleweerd CJ, Kelly CG, Chargelegue D, Ma JK. Peptide mapping of a novel discontinuous epitope of the major surface adhesin from Streptococcus mutans. J Biol Chem. 2004;279:22198–22203. doi: 10.1074/jbc.M400820200. [DOI] [PubMed] [Google Scholar]
- 18.Creamer TP, Campbell MN. Determinants of the polyproline II helix from modeling studies. Adv Protein Chem. 2002;62:263–282. doi: 10.1016/s0065-3233(02)62010-8. [DOI] [PubMed] [Google Scholar]
- 19.Blundell TL, Pitts JE, Tickle IJ, Wood SP, Wu C-W. X-ray analysis (1.4 Å resolution) of avian pancreatic polypeptide: Small globular protein hormone. Proc Natl Acad Sci USA. 1981;78:4175–4179. doi: 10.1073/pnas.78.7.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayakawa GY, et al. Isolation and characterization of monoclonal antibodies specific for antigen P1, a major surface protein of mutans streptococci. Infect Immun. 1987;55:2759–2767. doi: 10.1128/iai.55.11.2759-2767.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brady LJ, Piacentini DA, Crowley PJ, Bleiweis AS. Identification of monoclonal antibody-binding domains within antigen P1 of Streptococcus mutans and cross-reactivity with related surface antigens of oral streptococci. Infect Immun. 1991;59:4425–4435. doi: 10.1128/iai.59.12.4425-4435.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oli MW, McArthur WP, Brady LJ. A whole cell BIAcore assay to evaluate P1-mediated adherence of Streptococcus mutans to human salivary agglutinin and inhibition by specific antibodies. J Microbiol Methods. 2006;65:503–511. doi: 10.1016/j.mimet.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Crowley PJ, et al. Requirements for surface expression and function of adhesin P1 from Streptococcus mutans. Infect Immun. 2008;76:2456–2468. doi: 10.1128/IAI.01315-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly CG, et al. A synthetic peptide adhesion epitope as a novel antimicrobial agent. Nat Biotechnol. 1999;17:42–47. doi: 10.1038/5213. [DOI] [PubMed] [Google Scholar]
- 25.Parry DA, Fraser RD, Squire JM. Fifty years of coiled-coils and alpha-helical bundles: A close relationship between sequence and structure. J Struct Biol. 2008;163:258–269. doi: 10.1016/j.jsb.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Ramachandran GN, Kartha G. Structure of collagen. Nature. 1954;174:269–270. doi: 10.1038/174269c0. [DOI] [PubMed] [Google Scholar]
- 27.Brodsky B, Thiagarajan G, Madhan B, Kar K. Triple-helical peptides: An approach to collagen conformation, stability, and self-association. Biopolymers. 2008;89:345–353. doi: 10.1002/bip.20958. [DOI] [PubMed] [Google Scholar]
- 28.Venugopal MG, Ramshaw JA, Braswell E, Zhu D, Brodsky B. Electrostatic interactions in collagen-like triple-helical peptides. Biochemistry. 1994;33:7948–7956. doi: 10.1021/bi00191a023. [DOI] [PubMed] [Google Scholar]
- 29.Ferreon JC, Hilser VJ. Thermodynamics of binding to SH3 domains: The energetic impact of polyproline II (PII) helix formation. Biochemistry. 2004;43:7787–7797. doi: 10.1021/bi049752m. [DOI] [PubMed] [Google Scholar]
- 30.Lee SF, et al. Construction and characterization of isogenic mutants of Streptococcus mutans deficient in major surface protein antigen P1 (I/II) Infect Immun. 1989;57:3306–3313. doi: 10.1128/iai.57.11.3306-3313.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jakubovics NS, Stromberg N, van Dolleweerd CJ, Kelly CG, Jenkinson HF. Differential binding specificities of oral streptococcal antigen I/II family adhesins for human or bacterial ligands. Mol Microbiol. 2005;55:1591–1605. doi: 10.1111/j.1365-2958.2005.04495.x. [DOI] [PubMed] [Google Scholar]
- 32.Crowley PJ, Brady LJ, Piacentini DA, Bleiweis AS. Identification of a salivary agglutinin-binding domain within cell surface adhesin P1 of Streptococcus mutans. Infect Immun. 1993;61:1547–1552. doi: 10.1128/iai.61.4.1547-1552.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munro GH, et al. A protein fragment of streptococcal cell surface antigen I/II which prevents adhesion of Streptococcus mutans. Infect Immun. 1993;61:4590–4598. doi: 10.1128/iai.61.11.4590-4598.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brady LJ, Piacentini DA, Crowley PJ, Oyston PC, Bleiweis AS. Differentiation of salivary agglutinin-mediated adherence and aggregation of mutans streptococci by use of monoclonal antibodies against the major surface adhesin P1. Infect Immun. 1992;60:1008–1017. doi: 10.1128/iai.60.3.1008-1017.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Senpuku H, Miyauchi T, Hanada N, Nisizawa T. An antigenic peptide inducing cross-reacting antibodies inhibiting the interaction of Streptococcus mutans PAc with human salivary components. Infect Immun. 1995;63:4695–4703. doi: 10.1128/iai.63.12.4695-4703.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Senpuku H, et al. Inhibitory effects of MoAbs against a surface protein antigen in real-time adherence in vitro and recolonization in vivo of Streptococcus mutans. Scand J Immunol. 2001;54:109–116. doi: 10.1046/j.1365-3083.2001.00962.x. [DOI] [PubMed] [Google Scholar]
- 37.van Dolleweerd CJ, Chargelegue D, Ma JK. Characterization of the conforma-tional epitope of Guy's 13, a monoclonal antibody that prevents Streptococcus mutans colonization in humans. Infect Immun. 2003;71:754–765. doi: 10.1128/IAI.71.2.754-765.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holmskov U, et al. Cloning of gp-340, a putative opsonin receptor for lung surfactant protein D. Proc Natl Acad Sci USA. 1999;96:10794–10799. doi: 10.1073/pnas.96.19.10794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 40.Phillips GN, Jr, Flicker PF, Cohen C, Manjula BN, Fischetti VA. Streptococcal M protein: Alpha-helical coiled-coil structure and arrangement on the cell surface. Proc Natl Acad Sci USA. 1981;78:4689–4693. doi: 10.1073/pnas.78.8.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andre I, et al. Streptococcal M protein: Structural studies of the hypervariable region, free and bound to human C4BP. Biochemistry. 2006;45:4559–4568. doi: 10.1021/bi052455c. [DOI] [PubMed] [Google Scholar]
- 42.Deivanayagam CC, et al. Novel fold and assembly of the repetitive B region of the Staphylococcus aureus collagen-binding surface protein. Structure. 2000;8:67–78. doi: 10.1016/s0969-2126(00)00081-2. [DOI] [PubMed] [Google Scholar]
- 43.Z. Otwinowski WM. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276(A):307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 44.McCoy AJ, et al. Phaser crystallographic software. J Appl Cryst. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brunger AT. Version 1.2 of the crystallography and NMR system. Nat Protoc. 2007;2:2728–2733. doi: 10.1038/nprot.2007.406. [DOI] [PubMed] [Google Scholar]
- 46.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 12 Pt 1):2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 47.Laskowski RAMM, Moss DS, Thornton JM. PROCHECK: A program to check the stereochemical quality of protein structures. J Appl Cryst. 1993;26:283–291. [Google Scholar]
- 48.DeLano WL. The PyMOL Molecular Graphics System. Palo Alto, CA: DeLano Scientific LLC; 2009. [Google Scholar]
- 49.Koradi R, Billeter M, Wuthrich K. MOLMOL: A program for display and analysis of macromolecular structures. J Mol Graph. 1996;14(1):51–55. doi: 10.1016/0263-7855(96)00009-4. 29–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.